Investigation of Gastrointestinal Toxicities Associated with Concurrent Abdominal Radiation Therapy and the Tyrosine Kinase Inhibitor Sunitinib in a Mouse Model
Abstract
1. Introduction
2. Results
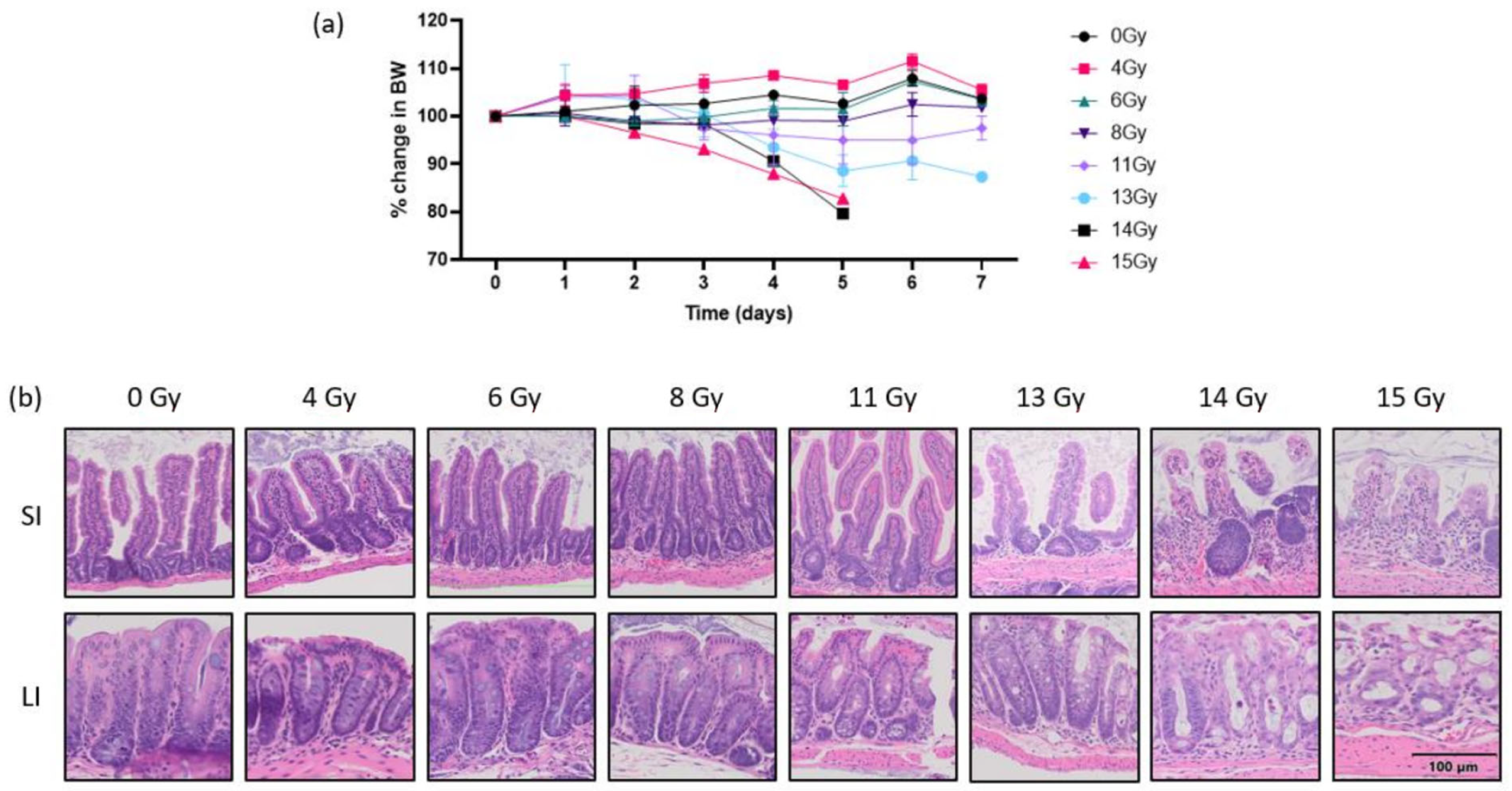
2.1. Defining Abdominal RT Dose
2.2. Radiation Therapy + Sunitinib
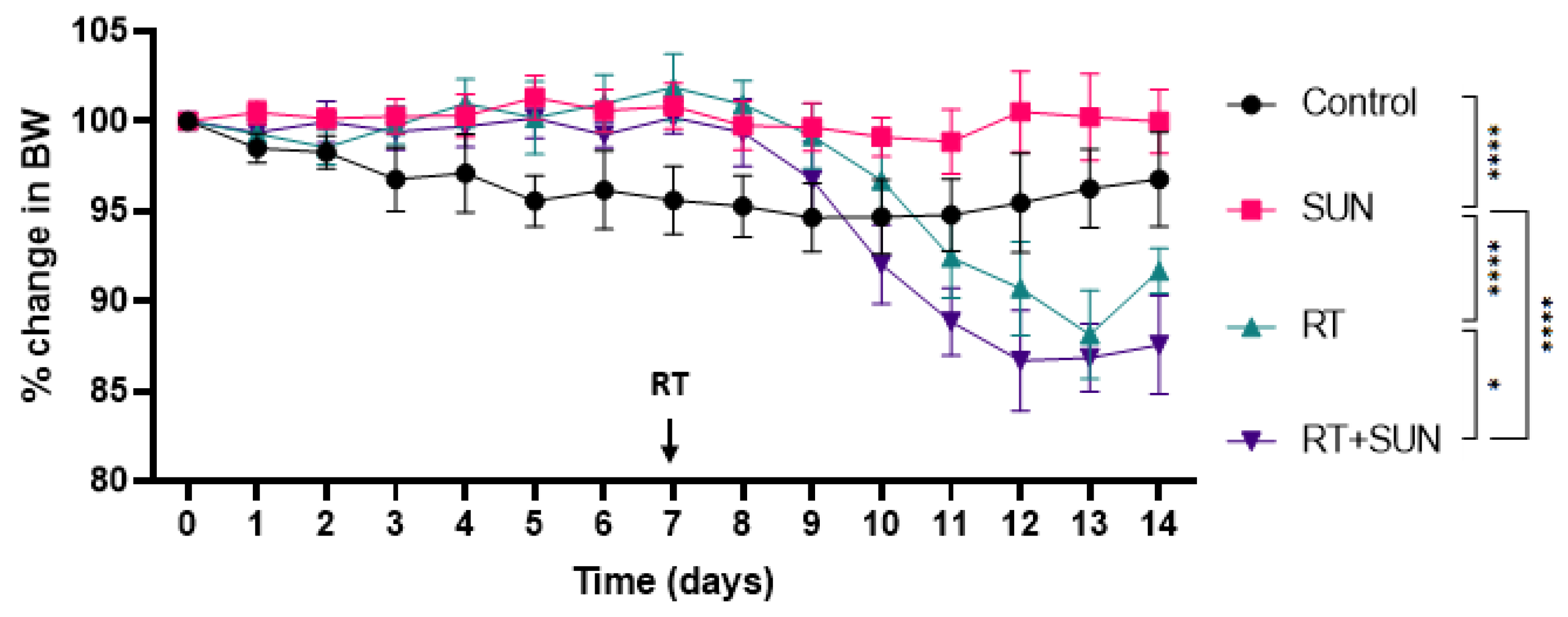
2.2.1. Weights
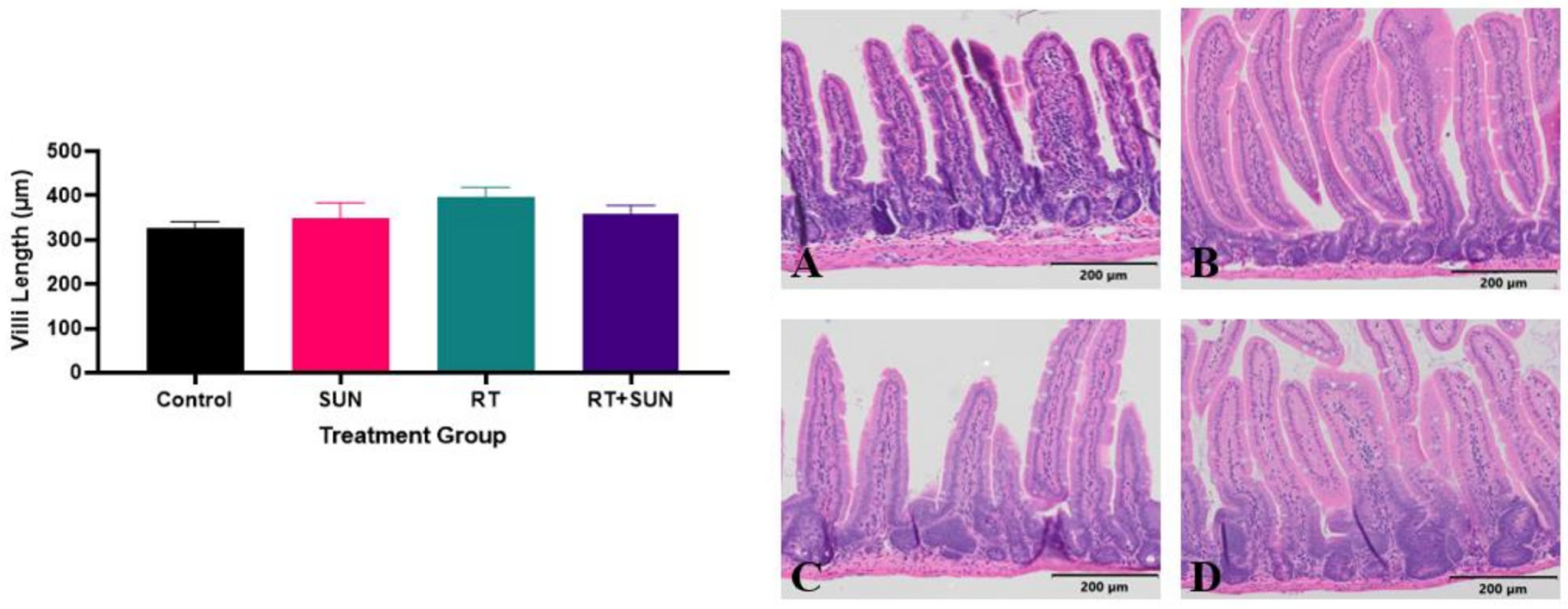
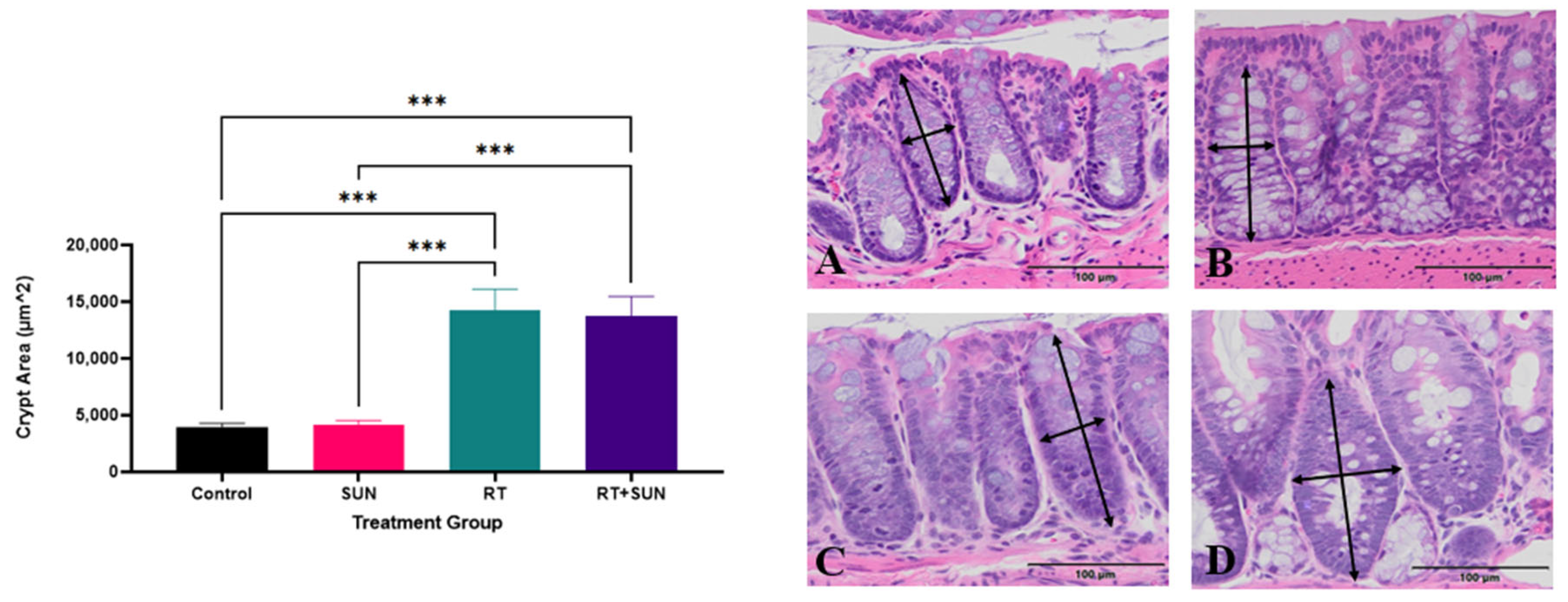
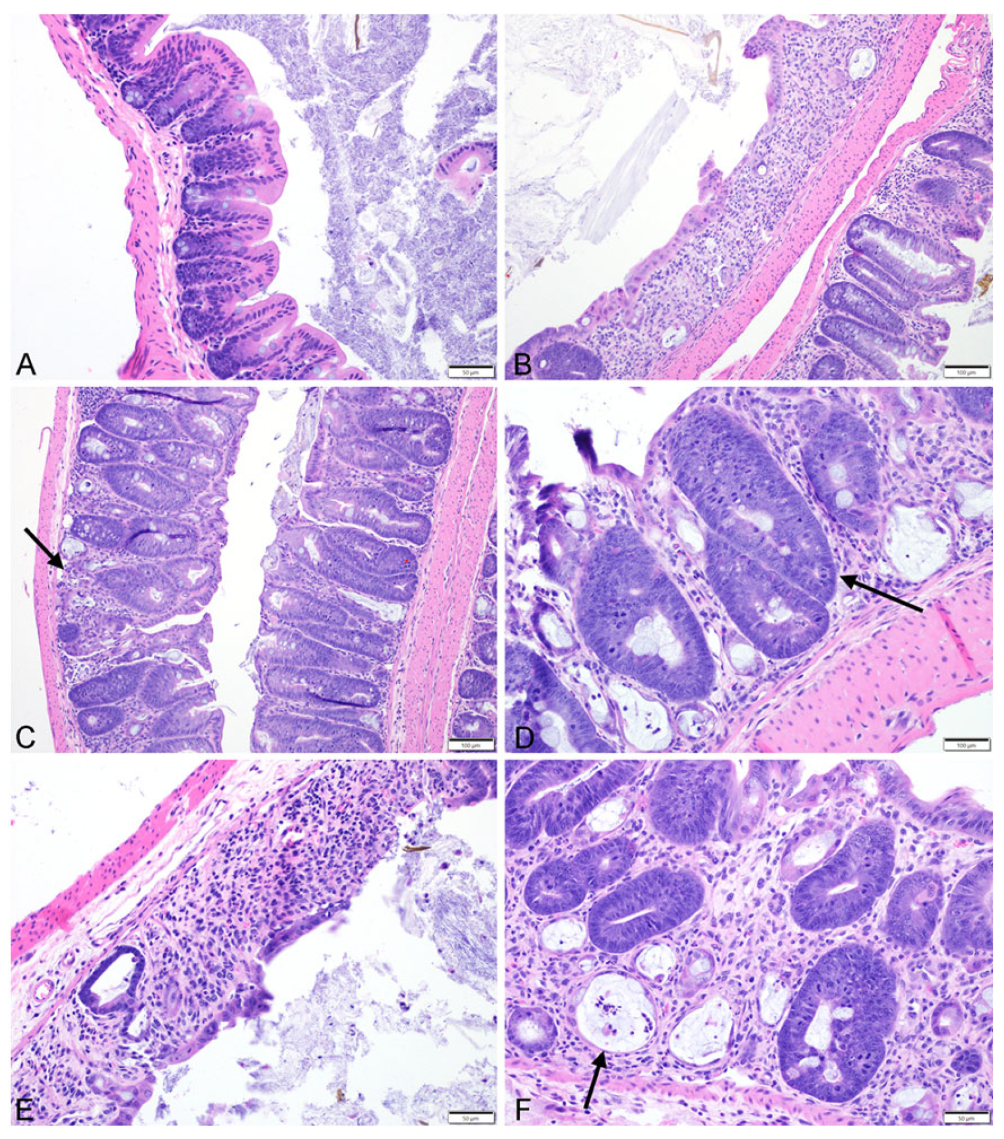
2.2.2. Quantitative Histology
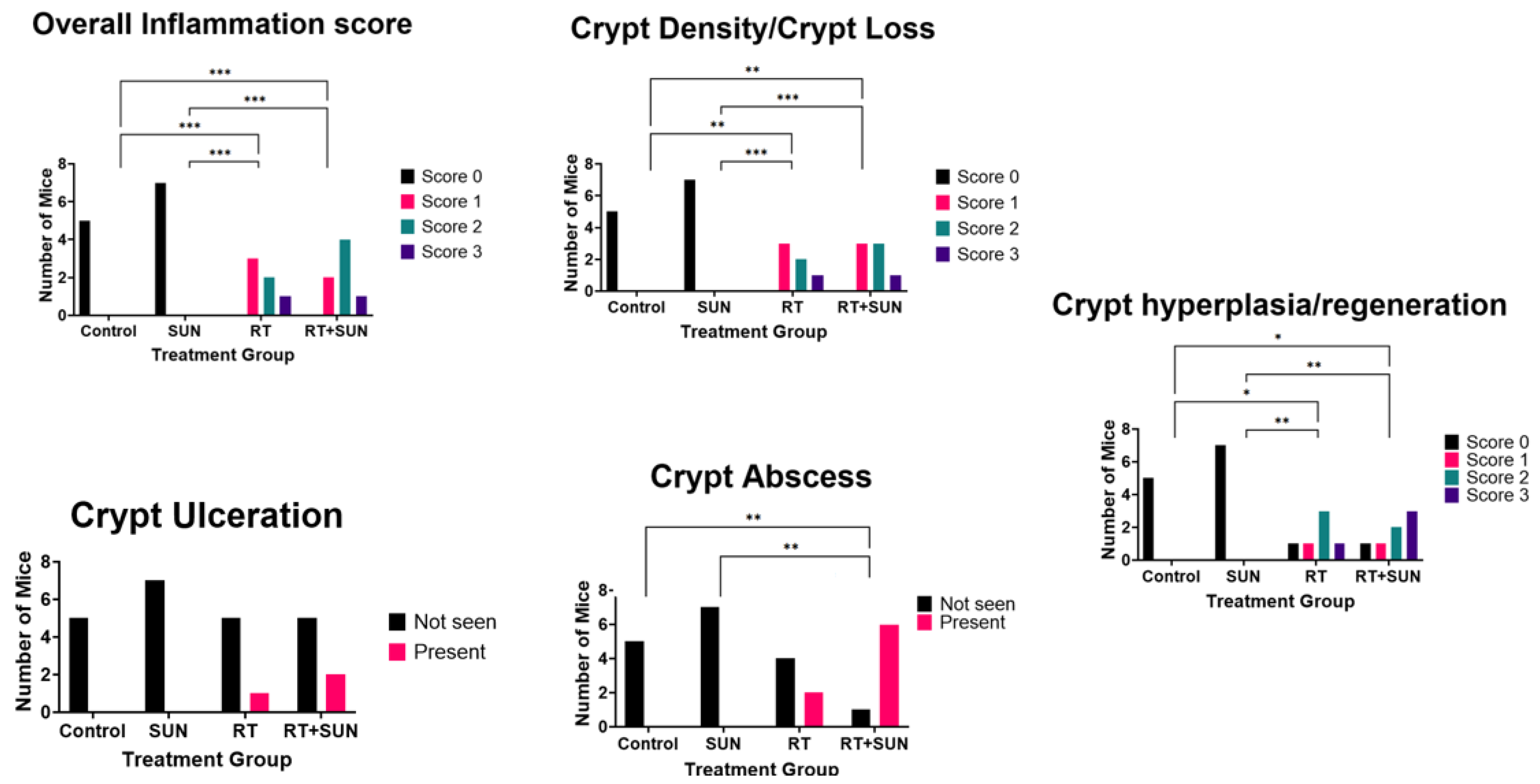
2.2.3. Semiquantitative Histopathologic Scoring of Gastrointestinal Toxicity
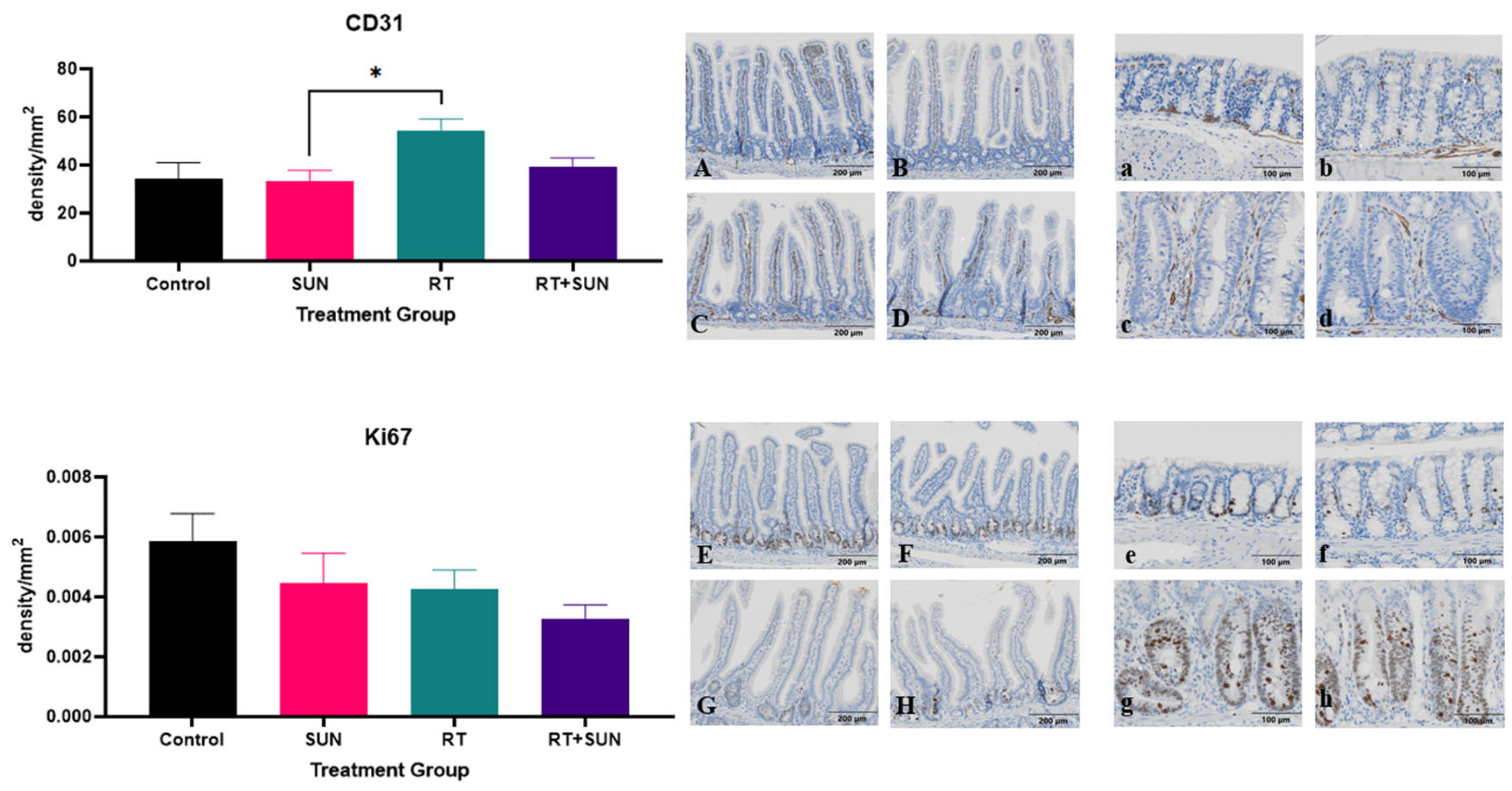
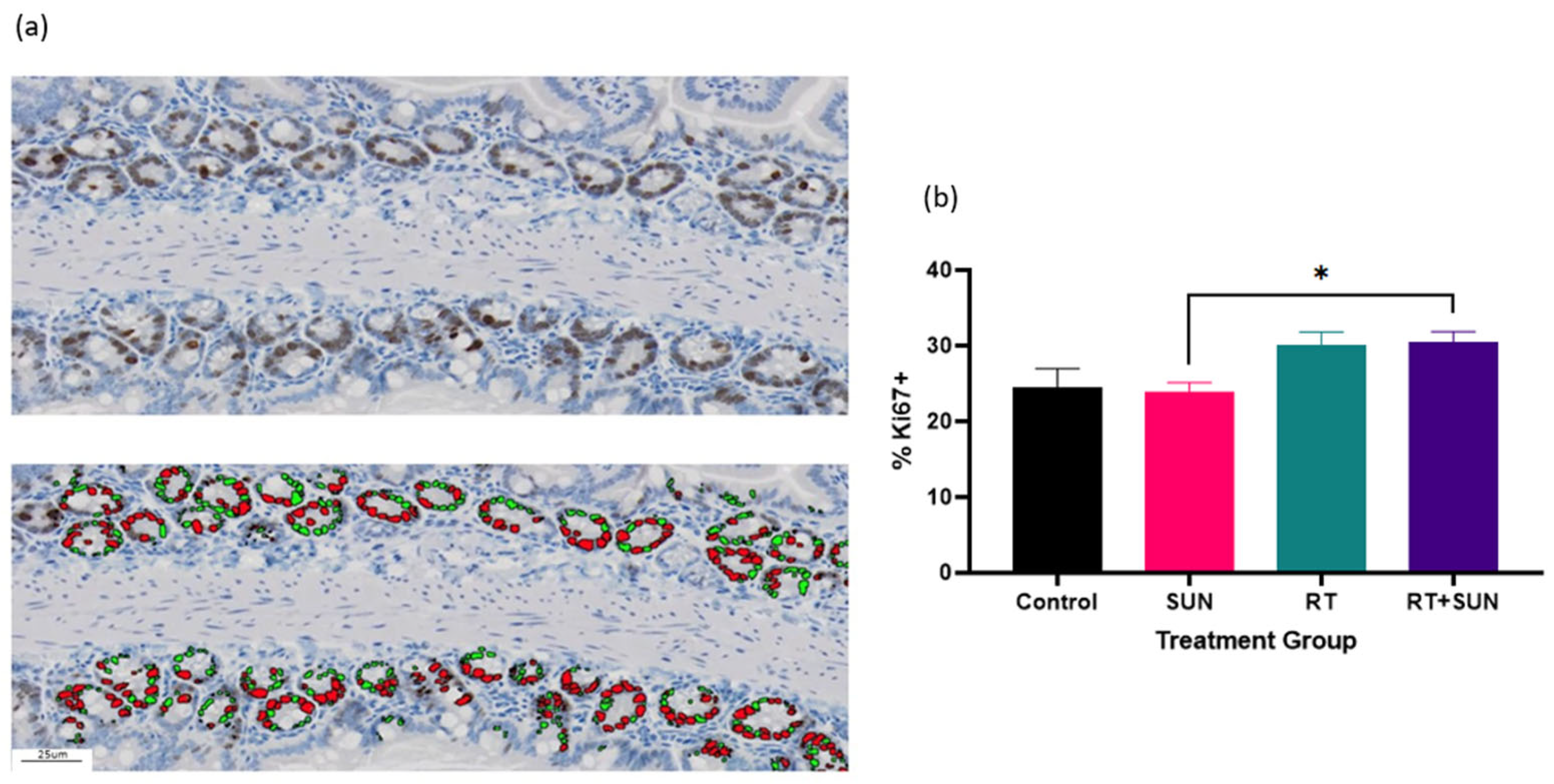
2.2.4. Immunohistochemistry
3. Discussion
4. Materials and Methods
4.1. Animal Husbandry
4.2. Sunitinib (SUN) Preparation
4.3. Irradiation
4.3.1. Abdominal Irradiation
4.3.2. Abdominal Irradiation ± Sunitinib
4.4. Experimental Endpoints
4.5. Tissue Collection
4.6. Histopathological Evaluation and Quantitative and Semiquantitative Scoring of Injury
4.7. Ki67 and CD31 Immunostaining
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kleibeuker, E.A.; ten Hooven, M.A.; Verheul, H.M.; Slotman, B.J.; Thijssen, V.L. Combining radiotherapy with sunitinib: Lessons (to be) learned. Angiogenesis 2015, 18, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Li, G.H.; Zhang, Y.P.; Tang, J.L.; Chen, Z.T.; Hu, Y.D.; Wei, H.; Li, D.Z.; Hao, P.; Wang, D.L. Effects of berberine against radiation-induced intestinal injury in mice. Int. J. Radiat. Oncol. Biol. Phys. 2010, 77, 1536–1544. [Google Scholar] [CrossRef] [PubMed]
- François, A.; Milliat, F.; Guipaud, O.; Benderitter, M. Inflammation and immunity in radiation damage to the gut mucosa. Biomed Res. Int. 2013, 2013, 123241. [Google Scholar] [CrossRef] [PubMed]
- Paris, F.; Fuks, Z.; Kang, A.; Capodieci, P.; Juan, G.; Ehleiter, D.; Haimovitz-Friedman, A.; Cordon-Cardo, C.; Kolesnick, R. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science 2001, 293, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Olcina, M.M.; Giaccia, A.J.; Olcina, M.M.; Giaccia, A.J. Reducing radiation-induced gastrointestinal toxicity—The role of the PHD/HIF axis. J. Clin. Investig. 2016, 126, 3708–3715. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T.; Elting, L.S.; Keefe, D.; Peterson, D.E.; Schubert, M.; Hauer-Jensen, M.; Bekele, B.N.; Raber-Durlacher, J.; Donnelly, J.P.; Rubenstein, E.B. Perspectives on cancer therapy-induced mucosal injury: Pathogenesis, measurement, epidemiology, and consequences for patients. Cancer 2004, 100, 1995–2025. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T. The pathobiology of mucositis. Nat. Rev. Cancer 2004, 4, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Paul, M.K.; Mukhopadhyay, A.K. Tyrosine kinase—Role and significance in cancer. Int. J. Med. Sci. 2012, 1, 101–115. [Google Scholar] [CrossRef]
- Sawyers, C.L. Rational therapeutic intervention in cancer: Kinases as drug targets. Curr. Opin. Genet. Dev. 2002, 12, 111–115. [Google Scholar] [CrossRef]
- Li, W.; Croce, K.; Steensma, D.P.; McDermott, D.F.; Ben-Yehuda, O.; Moslehi, J. Vascular and metabolic implications of novel targeted cancer therapies: Focus on kinase inhibitors. J. Am. Coll. Cardiol. 2015, 66, 1160–1178. [Google Scholar] [CrossRef]
- Farsaci, B.; Higgins, J.P.; Hodge, J.W. Consequence of dose scheduling of sunitinib on host immune response elements and vaccine combination therapy. Int. J. Cancer 2012, 130, 1948–1959. [Google Scholar] [CrossRef] [PubMed]
- Pollom, E.L.; Deng, L.; Pai, R.K.; Brown, J.M.; Giaccia, A.; Loo, B.W.; Shultz, D.B.; Le, Q.T.; Koong, A.C.; Chang, D.T. Gastrointestinal toxicities with combined antiangiogenic and stereotactic body radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Landén, N.X.; Li, D.; Ståhle, M. Transition from inflammation to proliferation: A critical step during wound healing. Cell. Mol. Life Sci. 2016, 73, 3861–3885. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-C.; Huang, C.-T.; Chang, D.-K. Anti-angiogenic therapeutic drugs for treatment of human cancer. J. Cancer Mol. 2008, 4, 37–45. [Google Scholar]
- Faivre, S.; Demetri, G.; Sargent, W.; Raymond, E. Molecular basis for sunitinib efficacy and future clinical development. Nat. Rev. Drug Discov. 2007, 6, 734–745. [Google Scholar] [CrossRef] [PubMed]
- Haznedar, J.Ö.; Patyna, S.; Bello, C.L.; Peng, G.W.; Speed, W.; Yu, X.; Zhang, Q.; Sukbuntherng, J.; Sweeny, D.J.; Antonian, L.; et al. Single- and multiple-dose disposition kinetics of sunitinib malate, a multitargeted receptor tyrosine kinase inhibitor: Comparative plasma kinetics in non-clinical species. Cancer Chemother. Pharmacol. 2009, 64, 691–706. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Guo, P.; Gallo, J.M. Impact of angiogenesis inhibition by sunitinib on tumor distribution of temozolomide. Clin. Cancer Res. 2008, 14, 1540–1549. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lu, X.; Zhou, Z.; Wang, J.; Hui, Z.; Liang, J.; Feng, Q.F.; Chen, D.; Xiao, Z.; Lv, J.; et al. The efficacy of upfront intracranial radiation with TKI compared to TKI alone in the NSCLC patients harboring EGFR mutation and brain metastases. J. Cancer 2019, 10, 1985–1990. [Google Scholar] [CrossRef]
- Batchelor, T.T.; Sorensen, A.G.; di Tomaso, E.; Zhang, W.T.; Duda, D.G.G.; Cohen, K.S.; Kozak, K.R.; Cahill, D.P.; Chen, P.J.; Zhu, M.; et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell 2007, 11, 83–95. [Google Scholar] [CrossRef]
- Jain, R.K. Lessons from multidisciplinary translational trials on anti-angiogenic therapy of cancer. Nat. Rev. Cancer 2008, 8, 309–316. [Google Scholar] [CrossRef]
- Brade, A.M.; Ng, S.; Brierley, J.; Kim, J.; Dinniwell, R.; Ringash, J.; Wong, R.R.; Cho, C.; Knox, J.; Dawson, L.A. Phase 1 trial of sorafenib and stereotactic body radiation therapy for hepatocellular carcinoma. Int. J. Radiat. Oncol. Biol. Phys. 2016, 94, 580–587. [Google Scholar] [CrossRef] [PubMed]
- Peters, N.A.J.B.; Richel, D.J.; Verhoeff, J.J.C.; Stalpers, L.J.A. Bowel perforation after radiotherapy in a patient receiving sorafenib. J. Clin. Oncol. 2008, 26, 2405–2406. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Zhao, Z.; Arooj, S.; Liao, G. Impact of tyrosine kinase inhibitors (TKIs) combined with radiation therapy for the management of brain metastases from renal cell carcinoma. Front. Oncol. 2020, 10, 1246. [Google Scholar] [CrossRef] [PubMed]
- Sung, S.Y.; Lee, S.W.; Kwak, Y.K.; Kang, J.H.; Hong, S.H.; Kim, Y.S. Intracranial control and survival outcome of tyrosine kinase inhibitor (TKI) alone versus TKI plus radiotherapy for brain metastasis of epidermal growth factor receptor-mutant non-small cell lung cancer. J. Neurooncol. 2018, 139, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Sabattini, S.; Vascellari, M.; Marconato, L. The impact of toceranib, piroxicam and thalidomide with or without hypofractionated radiation therapy on clinical outcome in dogs with inflammatory mammary carcinoma. Vet. Comp. Oncol. 2018, 16, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Zheng, L.; Wang, Y.; Xu, Z.; Yang, Q.; Zhu, G.; Liao, X.; Chen, X.; Zhu, B.; Duan, Y.; Sun, J. Concurrent EGFR-TKI and thoracic radiotherapy as first-line treatment for stage IV non-small cell lung cancer harboring EGFR active mutations. Oncologist 2019, 24, 1031-e612. [Google Scholar] [CrossRef] [PubMed]
- Carlsten, K.S.; London, C.A.; Haney, S.; Burnett, R.; Avery, A.C.; Thamm, D.H. Multicenter prospective trial of hypofractionated radiation treatment, toceranib, and prednisone for measurable canine mast cell tumors. J. Vet. Intern. Med. 2012, 26, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.A.; Balagamwala, E.H.; Angelov, L.; Suh, J.H.; Rini, B.; Garcia, J.A.; Ahluwalia, M.; Chao, S.T. Spine stereotactic radiosurgery with concurrent tyrosine kinase inhibitors for metastatic renal cell carcinoma. J. Neurosurg. Spine 2016, 25, 766–774. [Google Scholar] [CrossRef]
- Bhattacharya, B.; Adhikary, S.; Basu, J.; Pal, S.; Chattopadhyay, B.; Ghosh, T. A prospective randomised controlled trial of concurrent chemoradiation versus concurrent chemoradiation along with gefitinib in locally advanced squamous cell carcinoma of head and neck. Clin. Cancer Investig. J. 2014, 3, 146. [Google Scholar] [CrossRef]
- Ehling, T.J.; Klein, M.K.; Smith, L.; Prescott, D.; Haney, S.; Looper, J.; LaDue, T.; Brawner, W.; Fidel, J.; Shiomitsu, K.; et al. A prospective, multi-centre, Veterinary Radiation Therapy Oncology Group study reveals potential efficacy of toceranib phosphate (Palladia) as a primary or adjuvant agent in the treatment of canine nasal carcinoma. Vet. Comp. Oncol. 2022, 20, 293–303. [Google Scholar] [CrossRef]
- Jia, W.; Gao, Q.; Wang, M.; Li, J.; Jing, W.; Yu, J.; Zhu, H. Overlap time is an independent risk factor of radiation pneumonitis for patients treated with simultaneous EGFR-TKI and thoracic radiotherapy. Radiat. Oncol. 2021, 16, 41. [Google Scholar] [CrossRef] [PubMed]
- Thomas, N.J.; Myall, N.J.; Sun, F.; Patil, T.; Mushtaq, R.; Yu, C.; Sinha, S.; Pollom, E.L.; Nagpal, S.; Camidge, D.R.; et al. Brain metastases in EGFR- and ALK-positive NSCLC: Outcomes of central nervous system-penetrant tyrosine kinase inhibitors alone versus in combination with radiation. J. Thorac. Oncol. 2022, 17, 116–129. [Google Scholar] [CrossRef]
- Kim, J.M.; Miller, J.A.; Kotecha, R.; Xiao, R.; Juloori, A.; Ward, M.C.; Ahluwalia, M.S.; Mohammadi, A.M.; Peereboom, D.M.; Murphy, E.S.; et al. The risk of radiation necrosis following stereotactic radiosurgery with concurrent systemic therapies. J. Neurooncol. 2017, 133, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Kinoshita, H.; Komai, Y.; Kawabata, T.; Kawa, G.; Uemura, Y.; Matsuda, T. Two cases of gastrointestinal perforation after radiotherapy in patients receiving tyrosine kinase inhibitor for advanced renal cell carcinoma. World J. Surg. Oncol. 2012, 10, 167. [Google Scholar] [CrossRef] [PubMed]
- London, C.A.; Malpas, P.B.; Wood-Follis, S.L.; Boucher, J.F.; Rusk, A.W.; Rosenberg, M.P.; Henry, C.J.; Mitchener, K.L.; Klein, M.K.; Hintermeister, J.G.; et al. Multi-center, placebo-controlled, double-blind, randomized study of oral toceranib phosphate (SU11654), a receptor tyrosine kinase inhibitor, for the treatment of dogs with recurrent (either local or distant) mast cell tumor following surgical excision. Clin. Cancer Res. 2009, 15, 3856–3865. [Google Scholar] [CrossRef] [PubMed]
- London, C.; Mathie, T.; Stingle, N.; Clifford, C.; Haney, S.; Klein, M.K.; Beaver, L.; Vickery, K.; Vail, D.M.; Hershey, B.; et al. Preliminary evidence for biologic activity of toceranib phosphate (Palladia®) in solid tumours. Vet. Comp. Oncol. 2012, 10, 194–205. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, L.; Thamm, D.H.; Biller, B.J. Clinical and immunomodulatory effects of toceranib combined with low-dose cyclophosphamide in dogs with cancer. J. Vet. Intern. Med. 2012, 26, 355–362. [Google Scholar] [CrossRef]
- Prebble, A.R.A.R.; Weishaar, K.M.K.M.; Thamm, D.H.D.H.; Leary, D.; LaRue, S.M.S.M.; Martin, T.; Boss, M.K.M.-K. Increased incidence of gastrointestinal toxicity in canine cancer patients treated with concurrent abdominal radiation therapy and toceranib phosphate. Vet. Comp. Oncol. 2022, 20, 142–153. [Google Scholar] [CrossRef]
- Bao, P.; Kodra, A.; Tomic-Canic, M.; Golinko, M.; Ehrlich, H.P.; Brem, H. The role of vascular endothelial growth factor in wound healing. J. Surg. Res. 2009, 153, 347–358. [Google Scholar] [CrossRef]
- Waltenberger, J.; Claesson-Welsh, L.; Siegbahn, A.; Shibuya, M.; Heldin, C.H. Different signal transduction properties of KDR and Flt1, two receptors for vascular endothelial growth factor. J. Biol. Chem. 1994, 269, 26988–26995. [Google Scholar] [CrossRef]
- Baker, D.G.; Krochak, R.J. The response of the microvascular system to radiation: A review. Cancer Investig. 1989, 7, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Rodemann, H.P.; Blaese, M.A. Responses of normal cells to ionizing radiation. Semin. Radiat. Oncol. 2007, 17, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Buell, M.G.; Harding, R.K. Proinflammatory effects of local abdominal irradiation on rat gastrointestinal tract. Dig. Dis. Sci. 1989, 34, 390–399. [Google Scholar] [CrossRef] [PubMed]
- SULLIVAN, M.F. Intestinal vascular permeability changes induced by radiation or nitrogen mustard. Am. J. Physiol. 1961, 201, 951–954. [Google Scholar] [CrossRef] [PubMed]
- Karamanolis, G.; Delladetsima, I.; Kouloulias, V.; Papaxoinis, K.; Panayiotides, I.; Haldeopoulos, D.; Triantafyllou, K.; Kelekis, N.; Ladas, S.D. Increased expression of VEGF and CD31 in postradiation rectal tissue: Implications for radiation proctitis. Mediat. Inflamm. 2013, 2013, 515048. [Google Scholar] [CrossRef] [PubMed]
- Schlieve, C.R.; Mojica, S.G.; Holoyda, K.A.; Hou, X.; Fowler, K.L.; Grikscheit, T.C. Vascular endothelial growth factor (VEGF) bioavailability regulates angiogenesis and intestinal stem and progenitor cell proliferation during postnatal small intestinal development. PLoS ONE 2016, 11, e0151396. [Google Scholar] [CrossRef] [PubMed]
- Brem, H.; Erlich, P.; Tsakayannis, D. Delay of wound healing by the angiogenesis inhibitor TNP-470. Surg. Forum 1997, 48, 714–716. [Google Scholar]
- Farrell, C.L.; Bready, J.V.; Rex, K.L.; Chen, J.N.; DiPalma, C.R.; Whitcomb, K.L.; Yin, S.; Hill, D.C.; Wiemann, B.; Starnes, C.O.; et al. Keratinocyte growth factor protects mice from chemotherapy and radiation-induced gastrointestinal injury and mortality. Cancer Res. 1998, 58, 933–939. [Google Scholar]
- Maj, J.G.; Paris, F.; Haimovitz-Friedman, A.; Venkatraman, E.; Kolesnick, R.; Fuks, Z. Microvascular function regulates intestinal crypt response to radiation. Cancer Res. 2003, 63, 4338–4341. [Google Scholar]
- Withers, H.R. Regeneration of intestinal mucosa after irradiation. Cancer 1971, 28, 75–81. [Google Scholar] [CrossRef]
- Chen, L.; Xu, P.; Xiao, Q.; Chen, L.; Li, S.; Jian, J.M.; Zhong, Y. Sunitinib malate inhibits intestinal tumor development in male ApcMin/+ mice by down-regulating inflammation-related factors with suppressing β-cateinin/c-Myc pathway and re-balancing Bcl-6 and Caspase-3. Int. Immunopharmacol. 2021, 90, 107128. [Google Scholar] [CrossRef]
- Patyna, S.; Arrigoni, C.; Terron, A.; Kim, T.W.; Heward, J.K.; Vonderfecht, S.L.; Denlinger, R.; Turnquist, S.E.; Evering, W. Nonclinical safety evaluation of sunitinib: A potent inhibitor of VEGF, PDGF, KIT, FLT3, and RET receptors. Toxicol. Pathol. 2008, 36, 905–916. [Google Scholar] [CrossRef]
- Northway, M.G.; Scobey, M.W.; Geisinger, K.R. Radiation proctitis in the rat: Sequential changes and effects of anti-inflammatory agents. Cancer 1988, 62, 1962–1969. [Google Scholar] [CrossRef] [PubMed]
- Hovdenak, N.; Fajardo, L.F.; Hauer-Jensen, M. Acute radiation proctitis: A sequential clinicopathologic study during pelvic radiotherapy. Int. J. Radiat. Oncol. Biol. Phys. 2000, 48, 1111–1117. [Google Scholar] [CrossRef] [PubMed]
- Odabasi, M.; Gokdemir, S.; Muftuoglu, T.; Aktekin, A.; Saglam, A.; Aker, F. Prophylactic and therapeutic effects of oral budesonide for acute radiation-induced enteritis and colitis in rats. Int. J. Clin. Exp. Med. 2014, 7, 940–946. [Google Scholar] [PubMed]
- Liu, C.; Amin, R.; Shatila, M.; Short, N.; Altan, M.; Shah, A.; Alhalabi, O.; Okhuysen, P.; Thomas, A.S.; Wang, Y. Clinical characteristics and outcomes of tyrosine kinase inhibitor-related lower GI adverse effects. J. Cancer Res. Clin. Oncol. 2022, 149, 3965–3976. [Google Scholar] [CrossRef] [PubMed]
- Gelfand, M.D.; Tepper, M.; Katz, L.A.; Binder, H.J.; Yesner, R.; Floch, M.H. Acute irradiation proctitisi in man: Development of eosinophilic crypt abscesses. Gastroenterology 1968, 54, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Cheng, J.; Wu, H.; Liang, W.; Cui, Y. The dynamic cellular and molecular features during the development of radiation proctitis revealed by transcriptomic profiling in mice. BMC Genom. 2022, 23, 431. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kucharavy, H.C.; Hajj, C.; Zhao, L.; Hua, G.; Glass, R.; Paty, P.B.; Fuks, Z.; Kolesnick, R.; Hubbard, K.; et al. Radiation-induced gastrointestinal (GI) syndrome as a function of age. Cell Death Discov. 2023, 9, 31. [Google Scholar] [CrossRef] [PubMed]
- Laprie, C.; Abadie, J.; Amardeilh, M.F.; Raymond, I.; Delverdier, M. Detection of the Ki-67 proliferation associated nuclear epitope in normal canine tissues using the monoclonal antibody MIB-1. Anat. Histol. Embryol. 1998, 27, 251–256. [Google Scholar] [CrossRef]
- Gerdes, J.; Lemke, H.; Baisch, H.; Wacker, H.H.; Schwab, U.; Stein, H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J. Immunol. 1984, 133, 1710–1715. [Google Scholar] [CrossRef] [PubMed]
- Potten, C.S.; Booth, C. The role of radiation-induced and spontaneous apoptosis in the homeostasis of the gastrointestinal epithelium: A brief review. Comp. Biochem. Physiol.-B Biochem. Mol. Biol. 1997, 118, 473–478. [Google Scholar] [CrossRef] [PubMed]
- Schuller, B.W.; Binns, P.J.; Riley, K.J.; Ma, L.; Hawthorne, M.F.; Coderre, J.A. Selective irradiation of the vascular endothelium has no effect on the survival of murine intestinal crypt stem cells. Proc. Natl. Acad. Sci. USA 2006, 103, 3787–3792. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, K.; Suzuki, K. Differences in radiation dose response between small and large intestinal crypts. Radiat. Res. 2016, 186, 302–314. [Google Scholar] [CrossRef]
- Kil, W.J.; Tofilon, P.J.; Camphausen, K. Post-radiation increase in VEGF enhances glioma cell motility in vitro. Radiat. Oncol. 2012, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Metcalfe, C.; Kljavin, N.M.; Ybarra, R.; De Sauvage, F.J. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell 2014, 14, 149–159. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Gallo, J.M. Differential effect of sunitinib on the distribution of temozolomide in an orthotopic glioma model. Neuro-Oncol. 2009, 11, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Koelink, P.J.; Wildenberg, M.E.; Stitt, L.W.; Feagan, B.G.; Koldijk, M.; van ’t Wout, A.B.; Atreya, R.; Vieth, M.; Brandse, J.F.; Duijst, S.; et al. Development of reliable, valid and responsive scoring systems for endoscopy and histology in animal models for inflammatory bowel disease. J. Crohn’s Colitis 2018, 12, 794–803. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prebble, A.R.; Latka, B.; Burdekin, B.; Leary, D.; Harris, M.; Regan, D.; Boss, M.-K. Investigation of Gastrointestinal Toxicities Associated with Concurrent Abdominal Radiation Therapy and the Tyrosine Kinase Inhibitor Sunitinib in a Mouse Model. Int. J. Mol. Sci. 2024, 25, 1838. https://doi.org/10.3390/ijms25031838
Prebble AR, Latka B, Burdekin B, Leary D, Harris M, Regan D, Boss M-K. Investigation of Gastrointestinal Toxicities Associated with Concurrent Abdominal Radiation Therapy and the Tyrosine Kinase Inhibitor Sunitinib in a Mouse Model. International Journal of Molecular Sciences. 2024; 25(3):1838. https://doi.org/10.3390/ijms25031838
Chicago/Turabian StylePrebble, Amber R., Bailey Latka, Braden Burdekin, Del Leary, Mac Harris, Daniel Regan, and Mary-Keara Boss. 2024. "Investigation of Gastrointestinal Toxicities Associated with Concurrent Abdominal Radiation Therapy and the Tyrosine Kinase Inhibitor Sunitinib in a Mouse Model" International Journal of Molecular Sciences 25, no. 3: 1838. https://doi.org/10.3390/ijms25031838
APA StylePrebble, A. R., Latka, B., Burdekin, B., Leary, D., Harris, M., Regan, D., & Boss, M.-K. (2024). Investigation of Gastrointestinal Toxicities Associated with Concurrent Abdominal Radiation Therapy and the Tyrosine Kinase Inhibitor Sunitinib in a Mouse Model. International Journal of Molecular Sciences, 25(3), 1838. https://doi.org/10.3390/ijms25031838





