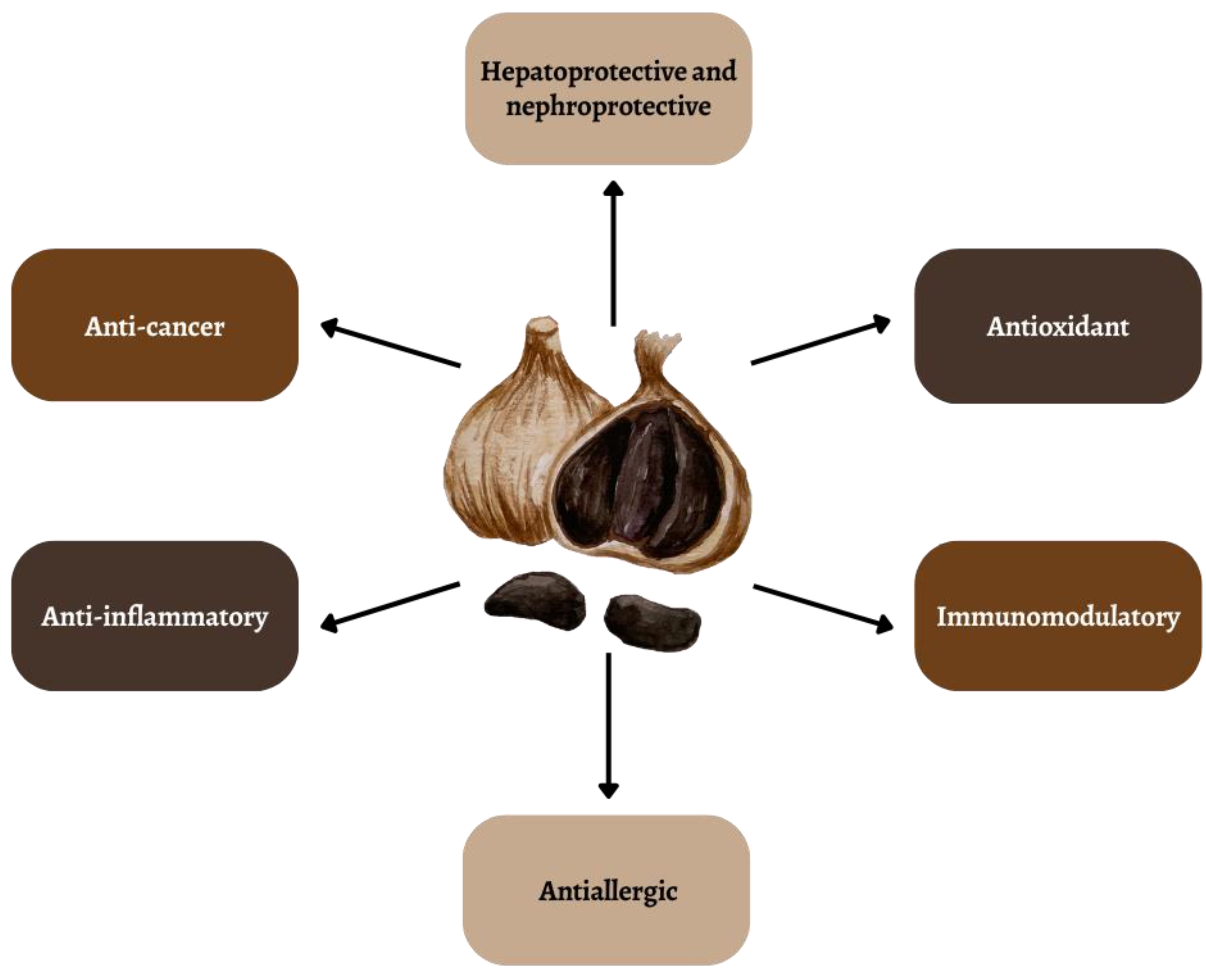
Anti-Cancer and Anti-Inflammatory Properties of Black Garlic
Abstract
1. Introduction
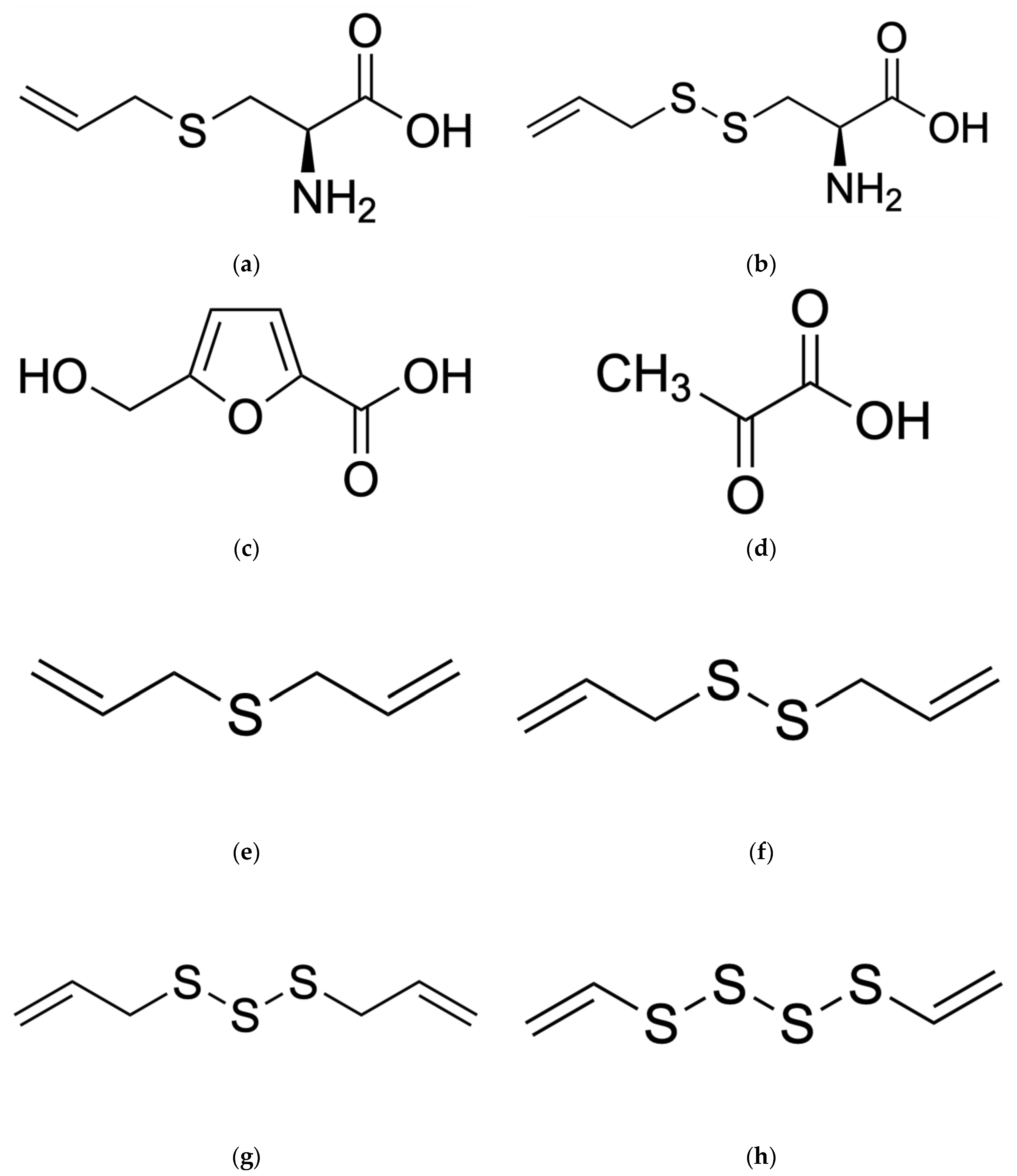
2. Active Compounds of Black Garlic
3. Antioxidative Properties of Black Garlic (BG)
4. Anti-Inflammatory Properties of Black Garlic
5. Anti-Cancer Properties of Black Garlic (BG)
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-HMF | 5-hydroxymethylfurfural |
| ABG | mature black garlic |
| ABGE | mature black garlic extract |
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt radical cation |
| Akt/mTOR | Protein kinase B/mammalian target of rapamycin |
| ALP | alkaline phosphatase |
| ALT | alanine transaminase |
| AST | alanine aminotransferase |
| BAK | Bcl-2 homologous antagonist/killer |
| BAX | bcl-2-like protein 4 |
| BCL-2 | B-cell lymphoma 2 |
| BG | black garlic |
| BIM | pro-apoptotic protein BCL2L11 |
| CEABG | chloroform extract of aged black garlic |
| COX-2 | cyclooxygenase 2 |
| DADS | diallyl disulfides |
| DAS | diallyl sulfides |
| DATS | diallyl trisulfides |
| DEN | diethylnitrosamine |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| DW | dry weight |
| FG | fresh garlic |
| FRG | fresh raw garlic |
| FM | fresh matter. |
| FW | fresh weight |
| GSTA2 | glutathione S-transferase alpha 2 |
| GAE | garlic acid equivalents |
| GSH-Px | glutathione peroxidase |
| GSH-Rd | glutathione reductase |
| HO-1 | heme oxygenase-1 |
| HUVEC | human umbilical vein endothelial cells |
| ICAM-1 | intercellular adhesion molecule |
| IL | interleukin |
| iNOS | inducible isoform nitric oxide synthases |
| JNK | c-Jun N-terminal kinases |
| LPS | lipopolysaccharides |
| MAPK | mitogen-activated protein kinases |
| MCL-1 | induced myeloid leukemia cell differentiation protein 1 |
| MDA | maldialdehyde |
| ND | not determined |
| Nrf2 | nuclear factor erythroid 2-related factor 2 |
| NF-κB | nuclear factor kappa B |
| NO | nitric oxide |
| NQO1 | qui-none-oxidoreductase-1 |
| OSC | allicin-derived organosulfur compounds |
| PGE2 | prostaglandin E2 |
| RE | rutin equivalents |
| ROS | reactive oxygen species |
| RT-PCR | reverse transcription polymerase chain reaction |
| SAC | S-Allyl-Cysteine |
| SAMC | S-allyl-Mercapto-Cysteine |
| SOD | superoxide dismutase |
| TBIL | total bilirubin |
| TEAC | Trolox Equivalent Antioxidant Capacity Assay |
| TFC | Total Flavonoid Content |
| TGF-β1 | transforming growth factor β1 |
| TNF-α | tumor necrosis factor alpha |
| TPC | Total Phenolic Content |
| VCAM-1 | vascular cell adhesion molecule-1 |
References
- Alam, K.; Hoq, O.; Uddin, S. Medicinal plant Allium sativum. A review. J. Med. Plant Stud. 2016, 4, 72–79. [Google Scholar]
- Jang, H.J.; Lee, H.J.; Yoon, D.K.; Ji, D.S.; Kim, J.H.; Lee, C.H. Antioxidant and Antimicrobial Activities of Fresh Garlic and Aged Garlic By-Products Extracted with Different Solvents. Food Sci. Biotechnol. 2018, 27, 219–225. [Google Scholar] [CrossRef]
- Ammarellou, A.; Yousefi, A.R.; Heydari, M.; Uberti, D.; Mastinu, A. Biochemical and Botanical Aspects of Allium sativum L. Sowing. BioTech 2022, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Kilic-Buyukkurt, O.; Kelebek, H.; Bordiga, M.; Keskin, M.; Selli, S. Changes in the Aroma and Key Odorants from White Garlic to Black Garlic Using Approaches of Molecular Sensory Science: A Review. Heliyone 2023, 9, e19056. [Google Scholar] [CrossRef] [PubMed]
- Saryono; Sarmoko; Nani, D.; Proverawati, A.; Taufik, A. Black Solo Garlic Protects Hepatic and Renal Cell Function in Streptozotocin-Induced Rats. Front. Nutr. 2022, 9, 962993. [Google Scholar] [CrossRef] [PubMed]
- Saikat, A.S.M.; Hossain, R.; Mina, F.B.; Das, S.; Khan, I.N.; Mubarak, M.S.; Islam, M.T. Antidiabetic Effect of Garlic. Rev. Bras. Farmacogn. 2022, 32, 1–11. [Google Scholar] [CrossRef]
- Song, X.; Xue, L.; Geng, X.; Wu, J.; Wu, T.; Zhang, M. Structural Characteristics and Immunomodulatory Effects of Melanoidins from Black Garlic. Foods 2023, 12, 2004. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Nam, S.H.; Rico, C.W.; Kang, M.Y. A Comparative Study on the Antioxidative and Anti-Allergic Activities of Fresh and Aged Black Garlic Extracts. Int. J. Food Sci. Technol. 2012, 47, 1176–1182. [Google Scholar] [CrossRef]
- Farhat, Z.; Hershberger, P.A.; Freudenheim, J.L.; Mammen, M.J.; Hageman Blair, R.; Aga, D.S.; Mu, L. Types of Garlic and Their Anticancer and Antioxidant Activity: A Review of the Epidemiologic and Experimental Evidence. Eur. J. Nutr. 2021, 60, 3585–3609. [Google Scholar] [CrossRef] [PubMed]
- Ryu, J.H.; Kang, D.; Slusarenko, A.J.; Gruhlke, M.C.H.; Mcphee, D.J. Physicochemical Properties, Biological Activity, Health Benefits, and General Limitations of Aged Black Garlic: A Review. Molecules 2017, 22, 919. [Google Scholar] [CrossRef]
- Chae, J.; Lee, E.; Oh, S.M.; Ryu, H.W.; Kim, S.; Nam, J.O. Aged Black Garlic (Allium sativum L.) and Aged Black Elephant Garlic (Allium ampeloprasum L.) Alleviate Obesity and Attenuate Obesity-Induced Muscle Atrophy in Diet-Induced Obese C57BL/6 Mice. Biomed. Pharmacother. 2023, 163, 114810. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhong, Y.; Wang, D.; Meng, F.; Li, Y.; Deng, Y.; Wang, B.; Zhong, Y.; Wang, D.; Meng, F.; et al. Formation, Evolution, and Antioxidant Activity of Melanoidins in Black Garlic under Different Formation, Evolution, and Antioxidant Activity of Melanoidins in Black Garlic under Different Storage Conditions. Foods 2023, 12, 3727. [Google Scholar] [CrossRef] [PubMed]
- Bedrníček, J.; Laknerová, I.; Lorenc, F.; Probio de Moraes, P.; Jarošová, M.; Samková, E.; Tříska, J.; Vrchotová, N.; Kadlec, J.; Smetana, P.; et al. The Use of a Thermal Process to Produce Black Garlic: Differences in the Physicochemical and Sensory Characteristics Using Seven Varieties of Fresh Garlic. Foods 2021, 10, 2703. [Google Scholar] [CrossRef] [PubMed]
- Villaño, D.; Marhuenda, J.; Arcusa, R.; Moreno-Rojas, J.M.; Cerdá, B.; Pereira-Caro, G.; Zafrilla, P. Effect of Black Garlic Consumption on Endothelial Function and Lipid Profile: A Before-and-After Study in Hypercholesterolemic and Non-Hypercholesterolemic Subjects. Nutrients 2023, 15, 3138. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Ding, Y.; Wang, D.; Deng, Y.; Zhao, Y. Effect of High Hydrostatic Pressure Conditions on the Composition, Morphology, Rheology, Thermal Behavior, Color, and Stability of Black Garlic Melanoidins. Food Chem. 2021, 337, 127790. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Li, F.; Jia, G.; Liu, R. Aged Black Garlic Extract Inhibits the Growth of Estrogen Receptor-Positive Breast Cancer Cells by Downregulating MCL-1 Expression through the ROS-JNK Pathway. PLoS ONE 2023, 18, e0286454. [Google Scholar] [CrossRef] [PubMed]
- Venir, E.; Pittia, P.; Giavon, S.; Maltini, E. Structure and Water Relations of Melanoidins Investigated by Thermal, Rheological, and Microscopic Analysis. Int. J. Food Prop. 2009, 12, 819–833. [Google Scholar] [CrossRef]
- Pakakaew, P.; Phimolsiripol, Y.; Taesuwan, S.; Kumphune, S.; Klangpetch, W.; Utama-ang, N. The Shortest Innovative Process for Enhancing the S-Allylcysteine Content and Antioxidant Activity of Black and Golden Garlic. Sci. Rep. 2022, 12, 11493. [Google Scholar] [CrossRef]
- Lawson, L.D.; Hunsaker, S.M. Allicin Bioavailability and Bioequivalence from Garlic Supplements and Garlic Foods. Nutrients 2018, 10, 812. [Google Scholar] [CrossRef]
- Ríos-Ríos, K.L.; Montilla, A.; Olano, A.; Villamiel, M. Physicochemical Changes and Sensorial Properties during Black Garlic Elaboration: A Review. Trends Food Sci. Technol. 2019, 88, 459–467. [Google Scholar] [CrossRef]
- Kimura, S.; Tung, Y.C.; Pan, M.H.; Su, N.W.; Lai, Y.J.; Cheng, K.C. Black Garlic: A Critical Review of Its Production, Bioactivity, and Application. J. Food Drug Anal. 2017, 25, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Jeong, Y.Y.; Ryu, J.H.; Shin, J.-H.; Kang, M.J.; Kang, J.R.; Han, J.; Kang, D. Comparison of Anti-Oxidant and Anti-Inflammatory Effects between Fresh and Aged Black Garlic Extracts. Molecules 2016, 21, 430. [Google Scholar] [CrossRef] [PubMed]
- Recinella, L.; Libero, M.L.; Citi, V.; Chiavaroli, A.; Martelli, A.; Foligni, R.; Mannozzi, C.; Acquaviva, A.; Di Simone, S.; Calderone, V.; et al. Anti-Inflammatory and Vasorelaxant Effects Induced by an Aqueous Aged Black Garlic Extract Supplemented with Vitamins D, C, and B12 on Cardiovascular System. Foods 2023, 12, 1558. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.E.; Wang, W. Changes in Nutritional and Bio-Functional Compounds and Antioxidant Capacity during Black Garlic Processing. J. Food Sci. Technol. 2018, 55, 479–488. [Google Scholar] [CrossRef] [PubMed]
- Botas, J.; Fernandes, Â.; Barros, L.; Alves, M.J.; Carvalho, A.M.; Ferreira, I.C.F.R. A Comparative Study of Black and White Allium sativum L.: Nutritional Composition and Bioactive Properties. Molecules 2019, 24, 2194. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.J.; Lee, S.J.; Kang, M.J.; Cho, H.S.; Sung, N.J.; Shin, J.H. Physicochemical Characteristics of Black Garlic (Allium sativum L.). J. Korean Soc. Food Sci. Nutr. 2008, 37, 465–471. [Google Scholar] [CrossRef]
- Kang, O.J. Physicochemical Characteristics of Black Garlic after Different Thermal Processing Steps. Prev. Nutr. Food Sci. 2016, 21, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, J.I.; Lu, C.; Machiya, E.; Tanahashi, M.; Hamada, K. Processed Black Garlic (Allium sativum) Extracts Enhance Anti-Tumor Potency against Mouse Tumors. Med. Aromat. J. Plant Sci. Biotechnol. 2007, 1, 271–281. [Google Scholar]
- Najman, K.; Król, K.; Sadowska, A. The Physicochemical Properties, Volatile Compounds and Taste Profile of Black Garlic (Allium sativum L.) Cloves, Paste and Powder. Appl. Sci. 2022, 12, 4215. [Google Scholar] [CrossRef]
- Najman, K.; Sadowska, A.; Hallmann, E. Evaluation of Bioactive and Physicochemical Properties of White and Black Garlic (Allium sativum L.) from Conventional and Organic Cultivation. Appl. Sci. 2021, 11, 874. [Google Scholar] [CrossRef]
- Al-Shehri, S.A. Efficacy of Black Garlic Extract on Anti-Tumor and Anti-Oxidant Activity Enhancement in Rats. Clin. Nutr. Open Sci. 2021, 36, 126–139. [Google Scholar] [CrossRef]
- Zhang, X.; Li, N.; Lu, X.; Liu, P.; Qiao, X. Effects of Temperature on the Quality of Black Garlic. J. Sci. Food Agric. 2016, 96, 2366–2372. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Lu, X.; Pei, H.; Qiao, X. Effect of Freezing Pretreatment on the Processing Time and Quality of Black Garlic. J. Food Process Eng. 2015, 38, 329–335. [Google Scholar] [CrossRef]
- Kim, J.-S.; Kang, O.-J.; Gweon, O.-C. Changes in the Content of Fat-and Water-Soluble Vitamins in Black Garlic at the Different Thermal Processing Steps. Food Sci. Biotechnol. 2013, 22, 283–287. [Google Scholar] [CrossRef]
- Shang, A.; Cao, S.Y.; Xu, X.Y.; Gan, R.Y.; Tang, G.Y.; Corke, H.; Mavumengwana, V.; Li, H. Bin Bioactive Compounds and Biological Functions of Garlic (Allium sativum L.). Foods 2019, 8, 246. [Google Scholar] [CrossRef] [PubMed]
- Stępień, A.E.; Trojniak, J.; Tabarkiewicz, J. Health-Promoting Properties: Anti-Inflammatory and Anticancer Properties of Sambucus nigra L. Flowers and Fruits. Molecules 2023, 28, 6235. [Google Scholar] [CrossRef] [PubMed]
- Javed, M.; Ahmed, W. Black Garlic: A Review of Its Biological Significance. J. Food Biochem. 2022, 46, e14394. [Google Scholar] [CrossRef] [PubMed]
- Manoonphol, K.; Suttisansanee, U.; Promkum, C.; Butryee, C. Effect of Thermal Processes on S-Allyl Cysteine Content in Black Garlic. Foods 2023, 12, 1227. [Google Scholar] [CrossRef]
- Choi, I.S.; Cha, H.S.; Lee, Y.S. Physicochemical and Antioxidant Properties of Black Garlic. Molecules 2014, 19, 16811–16823. [Google Scholar] [CrossRef]
- Nam, S.H.; Han, Y.S.; Sim, K.H.; Yang, S.O.; Kim, M.H. Changes in the Physicochemical Properties, Antioxidant Activity and Metabolite Analysis of Black Elephant Garlic (Allium ampeloprasum L.) during Aging Period. Foods 2023, 12, 43. [Google Scholar] [CrossRef]
- Chang, T.C.; Jang, H. Der Optimization of Aging Time for Improved Antioxidant Activity and Bacteriostatic Capacity of Fresh and Black Garlic. Appl. Sci. 2021, 11, 2377. [Google Scholar] [CrossRef]
- Medina, M.Á.T.; Pérez-Aparicio, J.; Moreno-Ortega, A.; Moreno-Rojas, R. Influence of Variety and Storage Time of Fresh Garlic on the Physicochemical and Antioxidant Properties of Black Garlic. Foods 2019, 8, 314. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Li, N.; Qiao, X.; Qiu, Z.; Liu, P. Composition Analysis and Antioxidant Properties of Black Garlic Extract. J. Food Drug Anal. 2017, 25, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Gavilán, J.; Mardones, C.; Oyarce, G.; Triviño, S.; Espinoza-Rubilar, N.; Ramírez-Molina, O.; Pérez, C.; Becerra, J.; Varas, P.; et al. Elephant Black Garlic’s Beneficial Properties for Hippocampal Neuronal Network, Chemical Characterization and Biological Evaluation. Foods 2023, 12, 3968. [Google Scholar] [CrossRef]
- Kim, S.; Kim, D.B.; Jin, W.; Park, J.; Yoon, W.; Lee, Y.; Kim, S.; Lee, S.; Kim, S.; Lee, O.H.; et al. Comparative Studies of Bioactive Organosulphur Compounds and Antioxidant Activities in Garlic (Allium sativum L.), Elephant Garlic (Allium ampeloprasum L.) and Onion (Allium cepa L.). Nat. Prod. Res. 2018, 32, 1193–1197. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Ross, C.F.; Powers, J.R.; Aston, D.E.; Rasco, B.A. Determination of Total Phenolic Content and Antioxidant Activity of Garlic (Allium sativum) and Elephant Garlic (Allium ampeloprasum) by Attenuated Total Reflectance-Fourier Transformed Infrared Spectroscopy. J. Agric. Food Chem. 2011, 59, 5215–5221. [Google Scholar] [CrossRef] [PubMed]
- Farhat, Z.; Scheving, T.; Aga, D.S.; Hershberger, P.A.; Freudenheim, J.L.; Blair, R.H.; Mammen, M.J.; Mu, L. Antioxidant and Antiproliferative Activities of Several Garlic Forms. Nutrients 2023, 15, 4099. [Google Scholar] [CrossRef]
- Kim, D.G.; Kang, M.J.; Hong, S.S.; Choi, Y.H.; Shin, J.H. Antiinflammatory Effects of Functionally Active Compounds Isolated from Aged Black Garlic. Phytother. Res. 2017, 31, 53–61. [Google Scholar] [CrossRef]
- Lee, Y.-M.; Gweon, O.-C.; Seo, Y.-J.; Im, J.; Kang, M.-J.; Kim, M.-J.; Kim, J.-I. Antioxidant Effect of Garlic and Aged Black Garlic in Animal Model of Type 2 Diabetes Mellitus. Nutr. Res. Pract. 2009, 3, 156–161. [Google Scholar] [CrossRef]
- Ha, A.W.; Kim, W.K. Antioxidant Mechanism of Black Garlic Extract Involving Nuclear Factor Erythroid 2-like Factor 2 Pathway. Nutr. Res. Pract. 2017, 11, 206–213. [Google Scholar] [CrossRef]
- Kim, H.K.; Choi, Y.W.; Lee, E.N.; Park, J.K.; Kim, S.G.; Park, D.J.; Kim, B.S.; Lim, Y.T.; Yoon, S. 5-Hydroxymethylfurfural from Black Garlic Extract Prevents TNFα-Induced Monocytic Cell Adhesion to HUVECs by Suppression of Vascular Cell Adhesion Molecule-1 Expression, Reactive Oxygen Species Generation and NF-ΚB Activation. Phytother. Res. 2011, 25, 965–974. [Google Scholar] [CrossRef] [PubMed]
- Kong, F.; Lee, B.H.; Wei, K. 5-Hydroxymethylfurfural Mitigates Lipopolysaccharide-Stimulated Inflammation via Suppression of MAPK, NF-ΚB and MTOR Activation in RAW 264.7 Cells. Molecules 2019, 24, 275. [Google Scholar] [CrossRef] [PubMed]
- Basu, C.; Chatterjee, A.; Bhattacharya, S.; Dutta, N.; Sur, R. S-Allyl Cysteine Inhibits TNF-α-Induced Inflammation in HaCaT Keratinocytes by Inhibition of NF- ΚB-Dependent Gene Expression via Sustained ERK Activation. Exp. Dermatol. 2019, 28, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- You, B.R.; Yoo, J.M.; Baek, S.Y.; Kim, M.R. Anti-Inflammatory Effect of Aged Black Garlic on 12-O-Tetradecanoylphorbol-13-Acetate-Induced Dermatitis in Mice. Nutr. Res. Pract. 2019, 13, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.C.; Chen, Y.A.; Wu, J.T.; Cheng, K.C.; Lai, P.S.; Liu, K.F.; Lin, Y.K.; Huang, Y.T.; Hsieh, C.W. Extracts from Fermented Black Garlic Exhibit a Hepatoprotective Effect on Acute Hepatic Injury. Molecules 2019, 24, 1112. [Google Scholar] [CrossRef]
- Lee, T.W.; Bae, E.; Kim, J.H.; Jang, H.N.; Cho, H.S.; Chang, S.H.; Park, D.J. The Aqueous Extract of Aged Black Garlic Ameliorates Colistin-Induced Acute Kidney Injury in Rats. Ren. Fail. 2019, 41, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.N.; Choi, Y.W.; Kim, H.K.; Park, J.K.; Kim, H.J.; Kim, M.J.; Lee, H.W.; Kim, K.H.; Bae, S.S.; Kim, B.S.; et al. Chloroform Extract of Aged Black Garlic Attenuates TNF-α-Induced ROS Generation, VCAM-1 Expression, NF-ΚB Activation and Adhesiveness for Monocytes in Human Umbilical Vein Endothelial Cells. Phytother. Res. 2011, 25, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Park, S.; Chung, Y.H.; Kim, G.Y.; Choi, Y.W.; Kim, B.W.; Choi, Y.H. Induction of Apoptosis by a Hexane Extract of Aged Black Garlic in the Human Leukemic U937 Cells. Nutr. Res. Pract. 2014, 8, 132–137. [Google Scholar] [CrossRef]
- Dong, M.; Yang, G.; Liu, H.; Liu, X.; Lin, S.; Sun, D.; Wang, Y. Aged Black Garlic Extract Inhibits HT29 Colon Cancer Cell Growth via the PI3K/Akt Signaling Pathway. Biomed. Rep. 2014, 2, 250–254. [Google Scholar] [CrossRef]
- Jikihara, H.; Qi, G.; Nozoe, K.; Hirokawa, M.; Sato, H.; Sugihara, Y.; Shimamoto, F. Aged Garlic Extract Inhibits 1,2-Dimethylhydrazine-Induced Colon Tumor Development by Suppressing Cell Proliferation. Oncol. Rep. 2015, 33, 1131–1140. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, H.Y.; Zhang, Z.H.; Bian, H.L.; Lin, G. Garlic-Derived Compound S-Allylmercaptocysteine Inhibits Cell Growth and Induces Apoptosis via the JNK and P38 Pathways in Human Colorectal Carcinoma Cells. Oncol. Lett. 2014, 8, 2591–2596. [Google Scholar] [CrossRef]
- Bagul, M.; Kakumanu, S.; Wilson, T.A. Crude Garlic Extract Inhibits Cell Proliferation and Induces Cell Cycle Arrest and Apoptosis of Cancer Cells in Vitro. J. Med. Food 2015, 18, 731–737. [Google Scholar] [CrossRef]
- Medina, M.Á.T.; Merinas-Amo, T.; Fernández-Bedmar, Z.; Font, R.; Del Río-Celestino, M.; Pérez-Aparicio, J.; Moreno-Ortega, A.; Alonso-Moraga, Á.; Moreno-Rojas, R. Physicochemical Characterization and Biological Activities of Black and White Garlic: In Vivo and in Vitro Assays. Foods 2019, 8, 220. [Google Scholar] [CrossRef]
- Wang, X.; Jiao, F.; Wang, Q.W.; Wang, J.; Yang, K.; Hu, R.R.; Liu, H.C.; Wang, H.Y.; Wang, Y.S. Aged Black Garlic Extract Induces Inhibition of Gastric Cancer Cell Growth in Vitro and in Vivo. Mol. Med. Rep. 2012, 5, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Arianingrum, R.; Aznam, N.; Atun, S.; Senam, S.; Irwan, A.R.; Juhara, N.Q.; Anisa, N.F.; Devani, L.K. Antiangiogenesis Activity Of Indonesian Local Black Garlic (Allium sativum ‘Solo): Experiments And Bibliometric Analysis. Indones. J. Sci. Technol. 2023, 8, 487–498. [Google Scholar]
- Naderi, R.; Mohaddes, G.; Mohammadi, M.; Alihemmati, A.; Khamaneh, A.; Ghyasi, R.; Ghaznavi, R. The Effect of Garlic and Voluntary Exercise on Cardiac Angiogenesis in Diabetes: The Role of MiR-126 and MiR-210. Arq. Bras. Cardiol. 2019, 112, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Pan, D.; Gong, X.; Wang, X.; Li, M. Role of Active Components of Medicinal Food in the Regulation of Angiogenesis. Front. Pharmacol. 2021, 11, 594050. [Google Scholar] [CrossRef]

| Component | Fresh Garlic (FG) | Black Garlic (BG) | Change in Substance Content in Black Garlic Compared to Fresh Garlic | |
|---|---|---|---|---|
| Moisture (%) | 62.00 ± 7.00; 64.70 ± 0.50; 74.00 ± 2.00 (g/100 g FW 1) [25] | 54.00 ± 3.00 (g/100 g FW) [25] | ↓ | |
| 66.64 ± 1.31 [26] | 58.20 ± 0.39 [26] | |||
| 62.31 ± 0.57 [27] | 39.03 ± 0.01–58.48 ± 1.18 [27] | |||
| 60.30 [28] | 45.10 [28] | |||
| 62.86 ± 0.13 [29] | 43.03 ± 0.42 [29] | |||
| 59.57 ± 2.44 [13] | 38.26 ± 4.48 [13] | |||
| Dry matter (%) | 32.13 ± 0.99 [30] | 52.41 ± 0.92 [30] | ↑ | |
| 37.14 ± 0.13 [29] | 56.96 ± 0.42 [29] | |||
| pH | 6.20 ± 0.03 [30] | 3.95 ± 0.03 [30] | ↓ | |
| 6.37 ± 0.04 [29] | 3.94 ± 0.05 [29] | |||
| 6.04 ± 0.04 [13] | 4.39 ± 0.61 [13] | |||
| Nutritional characteristics | Fats | 0.47 ± 0.02; 0.67 ± 0.03; 0.74 ± 0.02 (g/100 g FW) [25] | 0.722 ± 0.001 (g/100 g FW) [25] | ↑ |
| 0.18 ± 0.01 (%) [26] | 0.58 ± 0.11 (%) [26] | |||
| 0.1 (%) [28] | 0.3 (%) [28] | |||
| Amino acids | 843.11 ± 3.75 (mg/100 g FW) [27] | 167.65 ± 1.08–372.88 ± 2.23 (mg/100 g FW) [27] | ↓ | |
| Protein | 6.50 ± 0.10; 7.80 ± 0.20; 5.20 ± 0.10 (g/100 g FW) [25] | 7.40 ± 0.10 (g/100 g FW) [25] | ↑ | |
| 0.70 ± 0.02 (%) [26] | 0.97 ± 0.07 (%) [26] | |||
| 8.40 (%) [28] | 9.10 (%) [28] | |||
| Carbohydrates | 28.00 ± 2.00; 24.20 ± 0.40; 18.00 ± 2.00 (g/100 g FW) [25] | 35.00 ± 3.00 (g/100 g FW) [25] | ↑ | |
| 28.7% [28] | 47% [28] | |||
| Ash | 2.90 ± 0.10; 2.70 ± 0.20; 1.60 ± 0.04 (g/100 g FW) [25] | 3.20 ± 0.10 (g/100 g FW) [25] | ↑ | |
| 0.92 ± 0.62 (%) [26] | 1.81 ± 0.05 (%) [26] | |||
| 73.59 ± 0.89 (mg/100 g) [27] | 75.36 ± 0.02–114.36 ± 8.65 (mg/100 g) [27] | |||
| ND [28] | 2.10 (%) [28] | |||
| Energy (kcal/100 g FW 1) | 141.00 ± 8.00; 134.00 ± 2.00; 100.00 ± 9.00 [25] | 177.00 ± 8.00 [25] | ↑ | |
| 138.00 [28] | 227.10 [28] | |||
| Free sugars | Total | 1.32 ± 0.05; 1.48 ± 0.01; 0.70 ± 0.01 (g/100 g FW) [25] | 33.6 ± 0.7 (g/100 g FW) [25] | ↑ |
| 4.47 ± 0.11 (%) [26] | 6.19 ± 0.02 (%) [26] | |||
| 292.54 ± 2.01 (mg/100 g) [27] | 754.51 ± 4.05–4726.04 ± 15.74 [27] | |||
| Xylose | 0.82 ± 0.01 (g/100 g FW) [25] | ND 2 | ↓ | |
| Fructose | 0.45 ± 0.01; 0.09 ± 0.01; 0.20 ± 0.01 (g/100 g FW) [25] | 30.4 ± 0.7 (g/100 g FW) [25] | ↑ | |
| 63.89 ± 3.42 (mg/100 g) [26] | 2043.73 ± 4.99 (mg/100 g) [26] | |||
| 7.07 ± 0.08 (mg/g) [22] | 40.02 ± 0.71 (mg/g) [22] | |||
| 31.40 ± 0.96 (mg/100 g) [27] | 486.75 ± 11.72–3383.23 ± 44.03 (mg/100 g) [27] | |||
| 9.36 ± 0.13 (g/100 g DW 3) [30] | 31.05 ± 1.34 (g/100 g DW) [30] | |||
| Glucose | 0.28 ± 0.02; 0.04 ± 0.01; 0.12 ± 0.01 (g/100 g FW) [25] | 2.14 ± 0.03 (g/100 g FW) [25] | ↑ | |
| 2.12 ± 0.05 (g/100 g DW) [26] | 4.84 ± 0.28 (g/100 g DW) [26] | |||
| Saccharose | 0.58 ± 0.01; 1.35 ± 0.01; 0.38 ± 0.01 (g/100 g FW) [25] | 0.23 ± 0.05 (g/100 g FW) [25] | ↓/↑ | |
| 76.31 ± 0.05 (mg/100 g) [26] | 119.14 ± 3.51 (mg/100 g) [26] | |||
| 0.02 ± 0.00 (g/100 g DW) [30] | ||||
| Total Phenolic Content (TPC) | 0.59 ± 0.08 (mg/100 g) [26] | 1.56 ± 0.14 (mg/100 g) [26] | ↑ | |
| 3.65 ± 0.17 (mg GAE 4 100 g−1) [8] | 22.17 ± 0.75 (mg GAE 100 g−1) [8] | |||
| 3.82 ± 0.37 [13] | 14.03 ± 4.44 [13] | |||
| Total Flavonoid Content (TFC) | 0.14 ± 0.01 (mg/100 g) [26] | 0.77 ± 0.03 (mg/100 g) [26] | ↑ | |
| 37.75 ± 0.54 (mg/100 g DW) [30] | 57.80 ± 0.55 (mg/100 g DW) [30] | |||
| Pyruvate (μM/g) | 486.71 ± 12.08 [22] | 2456.54 ± 23.93 [22] | ↑ | |
| 188.47 ± 3.03 [26] | 277.85 ± 2.57 [26] | |||
| S-Allyl-Cysteine (SAC) | 1.24 ± 9.22 (mg/g) [22] | 2.12 ± 10.17 (mg/g) [22] | ↑ | |
| 42.7 (μg/g) [31] | 656.5 (μg/g) [31] | |||
| 23.7 (μg/g) [28] | 194.3 (μg/g) [28] | |||
| 5-Hydroxymethylfurfural (5-HMF) | ND | 4.82 ± 0.06 (g/kg) [32] | ↑ | |
| 0.25 ± 0.04 (g/kg) FM 5 [33] | ||||
| 6–8 (g/kg) [24] | ||||
| Thiosulfan | 6.50 ± 0.29 (μM/g) [22] | 91.22 ± 0.54 (μM/g) [22] | ↑ | |
| Allicin | 3.62 ± 0.01 (mg/g) [22] | ND | ↓ | |
| Vitamins | 6632.91 ± 18.62 mg/kg [34] | 7618.24 ± 28.47–9010.44 ± 30.61 mg/kg [34] | ↑ | |
| Minerals (mg/100 g) | 567.88 ± 4.48 [26] | 969.12 ± 19.31 [26] | ↑ | |
| 1173.50 ± 2.43 [27] | 1314.68 ± 2.76–1337.71 ± 2.77 [27] | |||
| Method | Aging Conditions | Aging Period (Weeks) | Source | |||||
|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | ||||
| Temperature | Moisture | |||||||
| Total Phenolic Content (mg GAE 1/g) | 70 °C | 90% | 13.91 ± 1.62 | 25.81 ± 1.59 | 35.28 ± 0.32 | 58.33 ± 1.90 | 55.25 ± 0.70 | [39] |
| 4.62 ± 0.48 | - | 11.84 ± 0.14 | 23.43 ± 0.41 | 27.08 ± 0.14 | [40] | |||
| 70 °C | 85% | 5.85 ± 0.14 | 5.98 ± 0.16 | 7.45 ± 0.22 | 9.89 ± 0.21 | 15.48 ± 0.53 | [41] | |
| 72 ± 2 °C | ∼90% | 3.26 ± 0.29; 4.86 ± 0.24; 3.56 ± 0.2 | - | 4.42 ± 0.2; 6.75 ± 0.3; 9.97 ± 0.91 | 10 ± 0.4; 13.64 ± 0.52; 9.36 ± 0.25 | 12.63 ± 0.26; 15.79 ± 0.41; 12.65 ± 0.64 | [42] | |
| Total Flavonoid Content (mg RE 2/g) | 70 °C | 90% | 3.22 ± 0.07 | 5.38 ± 0.06 | 8.34 ± 0.61 | 15.37 ± 0.52 | 16.26 ± 1.69 | [39] |
| 0.86 ± 0.03 | - | 2.48 ± 0.05 | 7.27 ± 0.10 | 8.75 ± 0.21 | [40] | |||
| S-Allyl-Cysteine (SAC) (mg/100 g DW 3) | 60 °C | 65% | 104.34 ± 10.38 | 1772.15 ± 48.98 | 313.22 ± 63.75 | 387.42 ± 17.25 | 174.65 ± 9.65 | [18] |
| 60 °C | 80% | 1750.29 ± 49.63 | 360.00 ± 33.43 | 228.79 ± 31.94 | 200.54 ± 6.19 | |||
| 80 °C | 65% | 654.50 ± 22.95 | 104.24 ± 14.77 | 113.43 ± 2.197 | ND | |||
| 80 °C | 80% | 874.26 ± 57.27 | 123.97 ± 14.19 | 41.53 ± 4.49 | 4.34 ± 0.09 | |||
| (5-HMF) (mg/100 g DW) | 60 °C | 65% | ND 4 | ND | ND | 1.81 ± 0.05 | 2.34 ± 0.02 | [18] |
| 60 °C | 80% | 1.19 ± 0.06 | 3.20 ± 0.03 | |||||
| 80 °C | 65% | 57.44 ± 0.29 | 673.41 ± 7.62 | 832.13 ± 1.46 | 511.24 ± 0.88 | |||
| 80 °C | 80% | 50.03 ± 0.04 | 724.60 ± 0.95 | 1721.41 ± 4.17 | 400.10 ± 0.23 | |||
| DPPH test (%) | 60 °C | 65% | 146.96 ± 36.09 | 146.29 ± 25.72 | 374.55 ± 25.80 | 944.95 ± 35.92 | 1153.14 ± 44.61 | [18] |
| 60 °C | 80% | 150.11 ± 15.98 | 413.07 ± 23.97 | 953.70 ± 36.60 | 1290.14 ± 61.62 | |||
| 80 °C | 65% | 4308.06 ± 114.87 | 4814.81 ± 127.55 | 4163.51 ± 205.50 | 3275.61 ± 154.13 | |||
| 80 °C | 80% | 5390.02 ± 180.03 | 5643.58 ± 61.98 | 5194.55 ± 197.98 | 3969.23 ± 275.51 | |||
| 70 °C | 90% | 20.27 ± 0.13 | - | 50.49 ± 0.47 | 82.49 ± 0.26 | 90.98 ± 0.23 | [40] | |
| ABTS test (%) | 60 °C | 65% | 2768.94 ± 176.53 | 4681.05 ± 338.07 | 4862.37 ± 15.73 | 6564.28 ± 91.96 | 8753.91 ± 200.25 | [18] |
| 60 °C | 80% | 5836.87 ± 151.82 | 4972.23 ± 271.51 | 7350.95 ± 151.56 | 8534.50 ± 433.20 | |||
| 80 °C | 65% | 20,405.95 ± 858.88 | 22,759.16 ± 912.09 | 20,614.96 ± 598.57 | 16,611.14 ± 312.57 | |||
| 80 °C | 80% | 25,421.11 ± 262.39 | 22,112.16 ± 856.43 | 23,300.72 ± 278.24 | 20,644.76 ± 776.78 | |||
| 72 ± 2 °C | ∼90% | 23.9 ± 2.79; 62.61 ± 3.37; 32.62 ± 2.76 | - | 52 ± 2.5; 81.85 ± 3.52; 116.14 ± 4.49 | 103.11 ± 4.0; 135.74 ± 5.37; 114.31 ± 4.98 | 97.21 ± 1.6; 113.77 ± 5.49; 81.99 ± 3.32 | [42] | |
| Type of Malignancy | Cell Line | In Vitro/Animal Model | Effect | Possible Molecular Mechanism | Reference |
|---|---|---|---|---|---|
| ER+ breast cancer | MCF-7 and MDA-MB-361 | in vitro | inhibition of the proliferation, migration, invasion and metastasis, induction of apoptosis | inhibition of the expression of anti-apoptotic proteins MCL-1 and BCL-2, while stimulating the expression of pro-apoptotic proteins BIM and BAK | [16] |
| histiocytic lymphoma | U937 | in vitro | concentration- and time-dependent growth inhibition by induction of apoptosis, | upregulation of death receptor 4 and Fas legend, and an increase in the ratio of Bax/Bcl-2 protein expression | [58] |
| colon cancer | HT29 | in vitro | induction of apoptosis and cell cycle arrest | regulation of the function of the PI3K/Akt pathway through upregulating PTEN and downregulating Akt and p-Akt expression, as well as suppressing its downstream target, 70-kDa ribosomal protein S6 kinase 1, at the mRNA and protein levels | [59] |
| colorectal cancer | 1,2-dimethylhydrazine (DMH)-induced primary cancer, DLD-1 and MRC-5 cell lines | animal model (F344 rats) and in vitro study | decrease in the number of aberrant crypt foci, suppression of the proliferative activity in adenoma and adenocarcinoma lesions, but no effect on normal colon mucosa. | delayed cell cycle progression by downregulating cyclin B1 and cdk1 expression via inactivation of NF-κB in the human colorectal cancer cells but no induction of apoptosis. | [60] |
| colorectal cancer | SW620 | in vitro | reduction in the malignant cell viability in a dose- and time-dependent manner, partially through the induction of apoptosis | induction of apoptosis through JNK and p38 signaling pathways, increased tumor protein p53 (p53), and Bax activation | [61] |
| breast cancer, prostate cancer, liver cancer, and colon cancer | MCF-7 (breast) PC-3 (prostate) Hep-G2 (liver) Caco-2 (colon) | in vitro | inhibition of cell proliferation was observed for Hep-G2, MCF-7, TIB-71, and PC-3 cells, induction of apoptosis, and G1 cell cycle arrest. | activation of caspase-3 and -7 | [62] |
| acute promyelocytic leukemia | HLA-50 | in vitro | strong cytotoxic effect and induction of DNA proapoptotic internucleosomal fragmentation against | not examined | [63] |
| gastric cancer | SGC-7901 | in vitro and animal model using male white mice of the Kunming strain inoculated with murine forestomach cells | dose-dependent induction of apoptosis growth inhibition of inoculated tumors | significantly increased the activity of SOD and GSH-Px in a dose-dependent manner, increased activity of Il-2 | [64] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stępień, A.E.; Trojniak, J.; Tabarkiewicz, J. Anti-Cancer and Anti-Inflammatory Properties of Black Garlic. Int. J. Mol. Sci. 2024, 25, 1801. https://doi.org/10.3390/ijms25031801
Stępień AE, Trojniak J, Tabarkiewicz J. Anti-Cancer and Anti-Inflammatory Properties of Black Garlic. International Journal of Molecular Sciences. 2024; 25(3):1801. https://doi.org/10.3390/ijms25031801
Chicago/Turabian StyleStępień, Agnieszka Ewa, Julia Trojniak, and Jacek Tabarkiewicz. 2024. "Anti-Cancer and Anti-Inflammatory Properties of Black Garlic" International Journal of Molecular Sciences 25, no. 3: 1801. https://doi.org/10.3390/ijms25031801
APA StyleStępień, A. E., Trojniak, J., & Tabarkiewicz, J. (2024). Anti-Cancer and Anti-Inflammatory Properties of Black Garlic. International Journal of Molecular Sciences, 25(3), 1801. https://doi.org/10.3390/ijms25031801







