Presenilin: A Multi-Functional Molecule in the Pathogenesis of Alzheimer’s Disease and Other Neurodegenerative Diseases
Abstract
1. Introduction
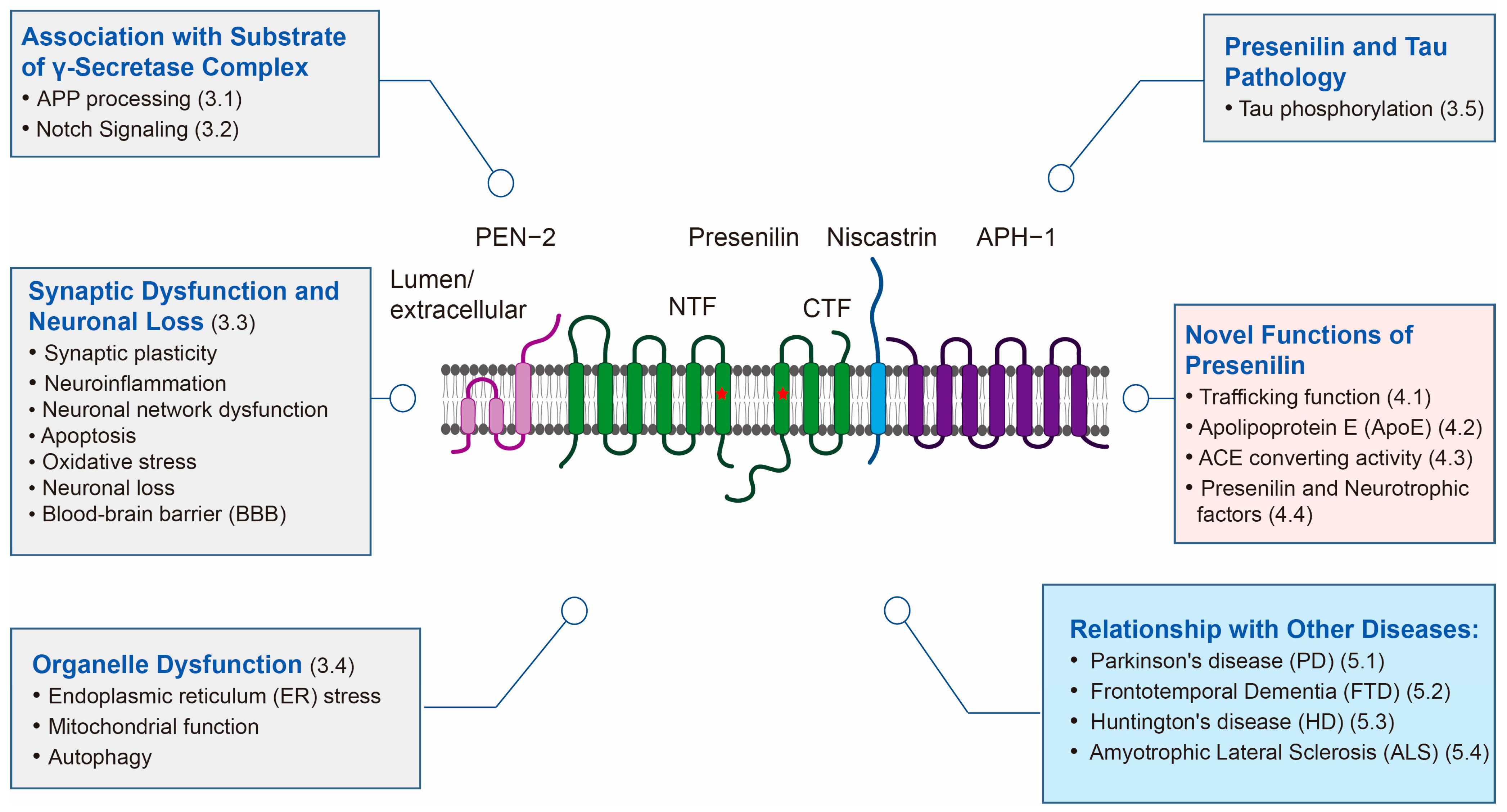
2. Structure
3. Association with Alzheimer’s Disease
3.1. Presenilin and APP Processing
3.2. Notch Signaling and Other Substrates of Presenilin
3.3. Presenilin and Synaptic Dysfunction and Neuronal Loss
3.4. Presenilin and Organelle Dysfunction
3.5. Presenilin and Tau Pathology
4. Novel Functions of Presenilin
4.1. Presenilin and Trafficking Function
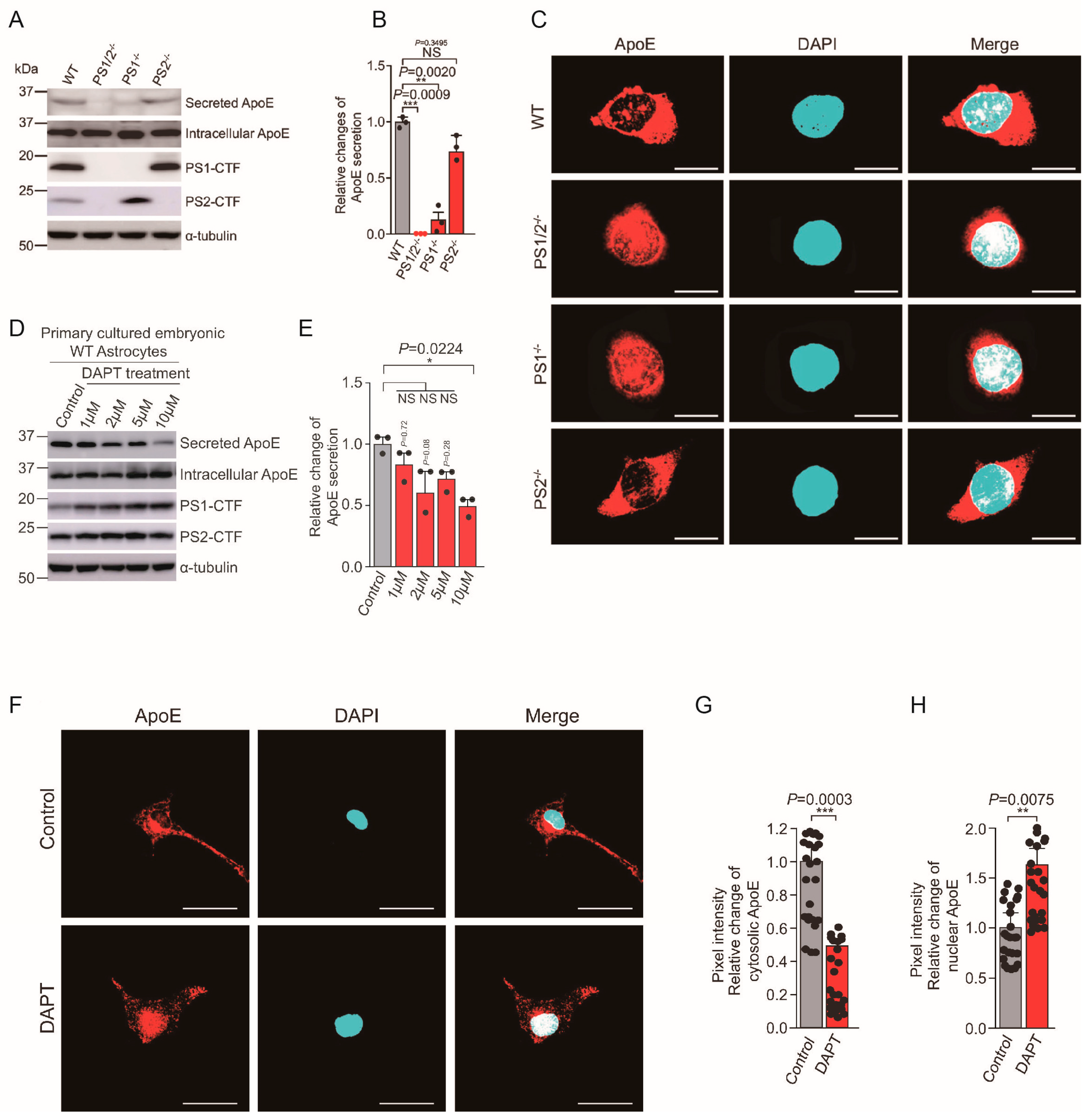
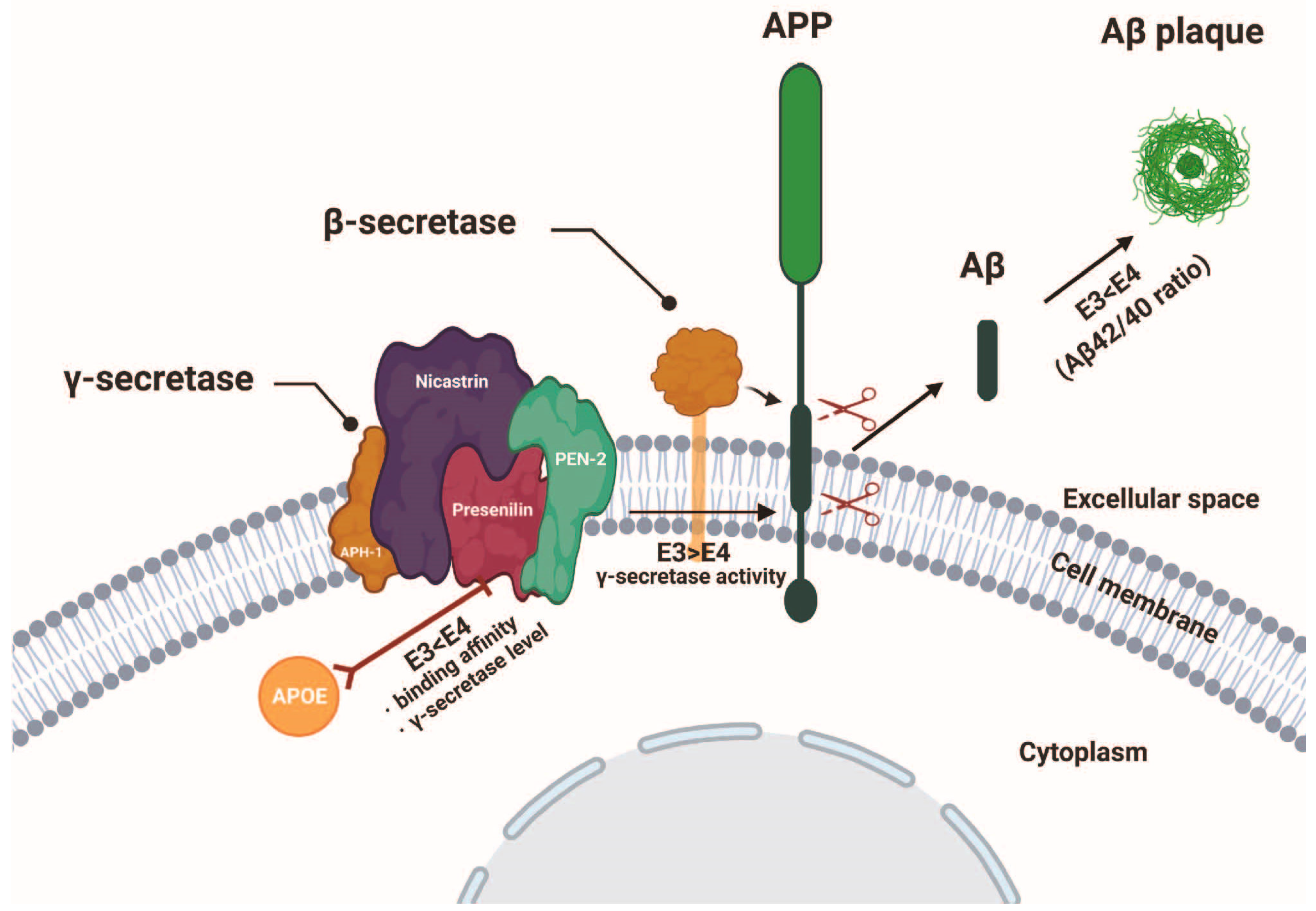
4.2. Presenilin and ApoE
4.3. Presenilin and Aβ42-to-Aβ40-Converting Activity of Angiotensin-Converting Enzyme (ACE)
4.4. Presenilin and Neurotrophic Factors
5. Relationship with Other Diseases
5.1. Presenilin and Parkinson’s Disease (PD)
5.2. Presenilin and Frontotemporal Dementia (FTD)
5.3. Presenilin and Huntington’s Disease (HD)
5.4. Presenilin and Amyotrophic Lateral Sclerosis (ALS)
6. Treatment and Research Progress
7. Conclusions
8. Method
Author Contributions
Funding
Conflicts of Interest
References
- De Strooper, B.; Iwatsubo, T.; Wolfe, M.S. Presenilins and γ-secretase: Structure, function, and role in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2012, 2, a006304. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.-Y. γ-Secretase in Alzheimer’s disease. Exp. Mol. Med. 2022, 54, 433–446. [Google Scholar] [CrossRef] [PubMed]
- Bertram, L.; Tanzi, R.E. The genetic epidemiology of neurodegenerative disease. J. Clin. Investig. 2005, 115, 1449–1457. [Google Scholar] [CrossRef]
- Vetrivel, K.S.; Zhang, Y.-W.; Xu, H.; Thinakaran, G. Pathological and physiological functions of presenilins. Mol. Neurodegener. 2006, 1, 4. [Google Scholar] [CrossRef] [PubMed]
- Seeger, M.; Nordstedt, C.; Petanceska, S.; Kovacs, D.M.; Gouras, G.K.; Hahne, S.; Fraser, P.; Levesque, L.; Czernik, A.J.; George-Hyslop, P.S.; et al. Evidence for phosphorylation and oligomeric assembly of presenilin 1. Proc. Natl. Acad. Sci. USA 1997, 94, 5090–5094. [Google Scholar] [CrossRef]
- Ahn, K.; Shelton, C.C.; Tian, Y.; Zhang, X.; Gilchrist, M.L.; Sisodia, S.S.; Li, Y.M. Activation and intrinsic gamma-secretase activity of presenilin 1. Proc. Natl. Acad. Sci. USA 2010, 107, 21435–21440. [Google Scholar] [CrossRef]
- Takasugi, N.; Tomita, T.; Hayashi, I.; Tsuruoka, M.; Niimura, M.; Takahashi, Y.; Thinakaran, G.; Iwatsubo, T. The role of presenilin cofactors in the gamma-secretase complex. Nature 2003, 422, 438–441. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Bai, X.C.; Ma, D.; Xie, T.; Yan, C.; Sun, L.; Yang, G.; Zhao, Y.; Zhou, R.; Scheres, S.H.W.; et al. Three-dimensional structure of human γ-secretase. Nature 2014, 512, 166–170. [Google Scholar] [CrossRef]
- LaVoie, M.J.; Fraering, P.C.; Ostaszewski, B.L.; Ye, W.; Kimberly, W.T.; Wolfe, M.S.; Selkoe, D.J. Assembly of the γ-Secretase Complex Involves Early Formation of an Intermediate Subcomplex of APH-1 and Nicastrin. J. Biol. Chem. 2003, 278, 37213–37222. [Google Scholar] [CrossRef]
- Escamilla-Ayala, A.; Wouters, R.; Sannerud, R.; Annaert, W. Contribution of the Presenilins in the cell biology, structure and function of γ-secretase. Semin. Cell Dev. Biol. 2020, 105, 12–26. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Gutiérrez, L.; Bammens, L.; Benilova, I.; Vandersteen, A.; Benurwar, M.; Borgers, M.; Lismont, S.; Zhou, L.; Van Cleynenbreugel, S.; Esselmann, H.; et al. The mechanism of γ-Secretase dysfunction in familial Alzheimer disease. EMBO J. 2012, 31, 2261–2274. [Google Scholar] [CrossRef]
- Bateman, R.J.; Xiong, C.; Benzinger, T.L.; Fagan, A.M.; Goate, A.; Fox, N.C.; Marcus, D.S.; Cairns, N.J.; Xie, X.; Blazey, T.M.; et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med. 2012, 367, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Zhou, R.; Yang, G.; Shi, Y. Analysis of 138 pathogenic mutations in presenilin-1 on the in vitro production of Abeta42 and Abeta40 peptides by gamma-secretase. Proc. Natl. Acad. Sci. USA 2017, 114, E476–E485. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Yang, G.; Shi, Y. Dominant negative effect of the loss-of-function gamma-secretase mutants on the wild-type enzyme through heterooligomerization. Proc. Natl. Acad. Sci. USA 2017, 114, 12731–12736. [Google Scholar] [CrossRef]
- Haapasalo, A.; Kovacs, D.M. The many substrates of presenilin/γ-secretase. J. Alzheimer’s Dis. 2011, 25, 3–28. [Google Scholar] [CrossRef]
- Pamrén, A.; Wanngren, J.; Tjernberg, L.O.; Winblad, B.; Bhat, R.; Näslund, J.; Karlström, H. Mutations in nicastrin protein differentially affect amyloid beta-peptide production and Notch protein processing. J. Biol. Chem. 2011, 286, 31153–31158. [Google Scholar] [CrossRef]
- Zhou, R.; Yang, G.; Guo, X.; Zhou, Q.; Lei, J.; Shi, Y. Recognition of the amyloid precursor protein by human γ-secretase. Science 2019, 363, eaaw0930. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, V.; Trecroci, F.; La Russa, A.; Cittadella, R.; Liguori, M.; Spadafora, P.; Caracciolo, M.; Di Palma, G.; Colica, C.; Gambardella, A.; et al. Presenilin enhancer-2 gene: Identification of a novel promoter mutation in a patient with early-onset familial Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Sala Frigerio, C.; Piscopo, P.; Calabrese, E.; Crestini, A.; Malvezzi Campeggi, L.; Civita di Fava, R.; Fogliarino, S.; Albani, D.; Marcon, G.; Cherchi, R.; et al. PEN-2 gene mutation in a familial Alzheimer’s disease case. J. Neurol. 2005, 252, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.; Lin, W.; Long, Y.; Yang, Y.; Zhang, H.; Wu, K.; Chu, Q. Notch signaling pathway: Architecture, disease, and therapeutics. Signal Transduct. Target. Ther. 2022, 7, 95. [Google Scholar] [CrossRef] [PubMed]
- Jorissen, E.; De Strooper, B. Gamma-secretase and the intramembrane proteolysis of Notch. Curr. Top. Dev. Biol. 2010, 92, 201–230. [Google Scholar]
- Kopan, R.; Ilagan, M.X. The canonical Notch signaling pathway: Unfolding the activation mechanism. Cell 2009, 137, 216–233. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, M.S.; Xia, W.; Ostaszewski, B.L.; Diehl, T.S.; Kimberly, W.T.; Selkoe, D.J. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and gamma-secretase activity. Nature 1999, 398, 513–517. [Google Scholar] [CrossRef] [PubMed]
- Haass, C.; Selkoe, D.J. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid beta-peptide. Nat. Rev. Mol. Cell Biol. 2007, 8, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Kuperstein, I.; Broersen, K.; Benilova, I.; Rozenski, J.; Jonckheere, W.; Debulpaep, M.; Vandersteen, A.; Segers-Nolten, I.; Van Der Werf, K.; Subramaniam, V.; et al. Neurotoxicity of Alzheimer’s disease Aβ peptides is induced by small changes in the Aβ42 to Aβ40 ratio. EMBO J. 2010, 29, 3408–3420. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Jung, K.M.; Huang, Y.Z.; Bennett, L.B.; Lee, J.S.; Mei, L.; Kim, T.W. Presenilin-dependent gamma-secretase-like intramembrane cleavage of ErbB4. J. Biol. Chem. 2002, 277, 6318–6323. [Google Scholar] [CrossRef] [PubMed]
- Lammich, S.; Okochi, M.; Takeda, M.; Kaether, C.; Capell, A.; Zimmer, A.-K.; Edbauer, D.; Walter, J.; Steiner, H.; Haass, C. Presenilin-dependent Intramembrane Proteolysis of CD44 Leads to the Liberation of Its Intracellular Domain and the Secretion of an Aβ-like Peptide. J. Biol. Chem. 2002, 277, 44754–44759. [Google Scholar] [CrossRef] [PubMed]
- Uemura, K.; Kitagawa, N.; Kohno, R.; Kuzuya, A.; Kageyama, T.; Chonabayashi, K.; Shibasaki, H.; Shimohama, S. Presenilin 1 is involved in maturation and trafficking of N-cadherin to the plasma membrane. J. Neurosci. Res. 2003, 74, 184–191. [Google Scholar] [CrossRef]
- Parisiadou, L.; Fassa, A.; Fotinopoulou, A.; Bethani, I.; Efthimiopoulos, S. Presenilin 1 and cadherins: Stabilization of cell-cell adhesion and proteolysis-dependent regulation of transcription. Neurodegener. Dis. 2004, 1, 184–191. [Google Scholar] [CrossRef]
- Güner, G.; Lichtenthaler, S.F. The substrate repertoire of γ-secretase/presenilin. Semin. Cell Dev. Biol. 2020, 105, 27–42. [Google Scholar] [CrossRef]
- Fukumori, A.; Feilen, L.P.; Steiner, H. Substrate recruitment by γ-secretase. Semin. Cell Dev. Biol. 2020, 105, 54–63. [Google Scholar] [CrossRef]
- Hitzenberger, M.; Götz, A.; Menig, S.; Brunschweiger, B.; Zacharias, M.; Scharnagl, C. The dynamics of γ-secretase and its substrates. Semin. Cell Dev. Biol. 2020, 105, 86–101. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, B.; Beglopoulos, V.; Wines-Samuelson, M.; Zhang, D.; Dragatsis, I.; Südhof, T.C.; Shen, J. Presenilins are essential for regulating neurotransmitter release. Nature 2009, 460, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Cheung, K.H.; Shineman, D.; Müller, M.; Cárdenas, C.; Mei, L.; Yang, J.; Tomita, T.; Iwatsubo, T.; Lee, V.M.; Foskett, J.K. Mechanism of Ca2+ disruption in Alzheimer’s disease by presenilin regulation of InsP3 receptor channel gating. Neuron 2008, 58, 871–883. [Google Scholar] [CrossRef]
- Saura, C.A.; Choi, S.Y.; Beglopoulos, V.; Malkani, S.; Zhang, D.; Shankaranarayana Rao, B.S.; Chattarji, S.; Kelleher, R.J., 3rd; Kandel, E.R.; Duff, K.; et al. Loss of presenilin function causes impairments of memory and synaptic plasticity followed by age-dependent neurodegeneration. Neuron 2004, 42, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Golenbock, D.T.; Latz, E. Innate immunity in Alzheimer’s disease. Nat. Immunol. 2015, 16, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Chan, S.L.; Mattson, M.P. Adverse effect of a presenilin-1 mutation in microglia results in enhanced nitric oxide and inflammatory cytokine responses to immune challenge in the brain. Neuromol. Med. 2002, 2, 29–45. [Google Scholar]
- Palop, J.J.; Chin, J.; Roberson, E.D.; Wang, J.; Thwin, M.T.; Bien-Ly, N.; Yoo, J.; Ho, K.O.; Yu, G.Q.; Kreitzer, A.; et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of Alzheimer’s disease. Neuron 2007, 55, 697–711. [Google Scholar] [CrossRef] [PubMed]
- Wirths, O.; Zampar, S. Neuron Loss in Alzheimer’s Disease: Translation in Transgenic Mouse Models. Int. J. Mol. Sci. 2020, 21, 8144. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.N.; Guo, Q.; Holtsberg, F.W.; Bruce-Keller, A.J.; Mattson, M.P. Increased Sensitivity to Mitochondrial Toxin-Induced Apoptosis in Neural Cells Expressing Mutant Presenilin-1 Is Linked to Perturbed Calcium Homeostasis and Enhanced Oxyradical Production. J. Neurosci. 1998, 18, 4439–4450. [Google Scholar] [CrossRef] [PubMed]
- Mohmmad Abdul, H.; Sultana, R.; Keller, J.N.; St Clair, D.K.; Markesbery, W.R.; Butterfield, D.A. Mutations in amyloid precursor protein and presenilin-1 genes increase the basal oxidative stress in murine neuronal cells and lead to increased sensitivity to oxidative stress mediated by amyloid β-peptide (1–42), H2O2 and kainic acid: Implications for Alzheimer’s disease. J. Neurochem. 2006, 96, 1322–1335. [Google Scholar] [PubMed]
- Erekat, N.S. Apoptosis and its therapeutic implications in neurodegenerative diseases. Clin. Anat. 2022, 35, 65–78. [Google Scholar] [CrossRef] [PubMed]
- Li, L.-H.; Tu, Q.-Y.; Deng, X.-H.; Xia, J.; Hou, D.-R.; Guo, K.; Zi, X.-H. Mutant presenilin2 promotes apoptosis through the p53/miR-34a axis in neuronal cells. Brain Res. 2017, 1662, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Tamagno, E.; Parola, M.; Bardini, P.; Piccini, A.; Borghi, R.; Guglielmotto, M.; Santoro, G.; Davit, A.; Danni, O.; Smith, M.A.; et al. β-Site APP cleaving enzyme up-regulation induced by 4-hydroxynonenal is mediated by stress-activated protein kinases pathways. J. Neurochem. 2005, 92, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Oda, A.; Tamaoka, A.; Araki, W. Oxidative stress up-regulates presenilin 1 in lipid rafts in neuronal cells. J. Neurosci. Res. 2010, 88, 1137–1145. [Google Scholar] [CrossRef]
- Zou, K.; Islam, S.; Sun, Y.; Gao, Y.; Nakamura, T.; Komano, H.; Tomita, T.; Michikawa, M. Presenilin Deficiency Increases Susceptibility to Oxidative Damage in Fibroblasts. Front. Aging Neurosci. 2022, 14, 902525. [Google Scholar] [CrossRef] [PubMed]
- Raut, S.; Patel, R.; Al-Ahmad, A.J. Presence of a mutation in PSEN1 or PSEN2 gene is associated with an impaired brain endothelial cell phenotype in vitro. Fluids Barriers CNS 2021, 18, 3. [Google Scholar] [CrossRef] [PubMed]
- Montagne, A.; Zhao, Z.; Zlokovic, B.V. Alzheimer’s disease: A matter of blood–brain barrier dysfunction? J. Exp. Med. 2017, 214, 3151–3169. [Google Scholar] [CrossRef]
- Brandl, S.; Reindl, M. Blood–Brain Barrier Breakdown in Neuroinflammation: Current In Vitro Models. Int. J. Mol. Sci. 2023, 24, 12699. [Google Scholar] [CrossRef] [PubMed]
- Supnet, C.; Bezprozvanny, I. Presenilins function in ER calcium leak and Alzheimer’s disease pathogenesis. Cell Calcium 2011, 50, 303–309. [Google Scholar] [CrossRef]
- Stutzmann, G.E.; Smith, I.; Caccamo, A.; Oddo, S.; Laferla, F.M.; Parker, I. Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer’s disease mice. J. Neurosci. 2006, 26, 5180–5189. [Google Scholar] [CrossRef] [PubMed]
- Zampese, E.; Fasolato, C.; Kipanyula, M.J.; Bortolozzi, M.; Pozzan, T.; Pizzo, P. Presenilin 2 modulates endoplasmic reticulum (ER)-mitochondria interactions and Ca2+ cross-talk. Proc. Natl. Acad. Sci. USA 2011, 108, 2777–2782. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Zeh, H.J.; Lotze, M.T.; Tang, D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ. 2011, 18, 571–580. [Google Scholar] [CrossRef] [PubMed]
- Shipton, O.A.; Leitz, J.R.; Dworzak, J.; Acton, C.E.; Tunbridge, E.M.; Denk, F.; Dawson, H.N.; Vitek, M.P.; Wade-Martins, R.; Paulsen, O.; et al. Tau protein is required for amyloid {beta}-induced impairment of hippocampal long-term potentiation. J. Neurosci. 2011, 31, 1688–1692. [Google Scholar] [CrossRef]
- Boutajangout, A.; Frangione, B.; Brion, J.-P.; Wisniewski, T.; Sigurdsson, E.M. O4-01–05: Presenilin 1 mutation promotes Tau phosphorylation and aggregation in a novel Alzheimer’s disease mouse model. Alzheimer’s Dement. 2008, 4, T185. [Google Scholar] [CrossRef]
- Yang, X.; Yang, Y.; Liu, J.; Li, G.; Yang, E. Increased Phosphorylation of Tau and Synaptic Protein Loss in the Aged Transgenic Mice Expressing Familiar Alzheimer’s Disease-Linked Presenilin 1 Mutation. Neurochem. Res. 2012, 37, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, L.; Tomaino, C.; Anfossi, M.; Gallo, M.; Geracitano, S.; Costanzo, A.; Colao, R.; Puccio, G.; Frangipane, F.; Curcio, S.A.; et al. Novel PSEN1 and PGRN mutations in early-onset familial frontotemporal dementia. Neurobiol. Aging 2009, 30, 1825–1833. [Google Scholar] [CrossRef]
- Soto-Faguás, C.M.; Sanchez-Molina, P.; Saura, C.A. Loss of presenilin function enhances tau phosphorylation and aggregation in mice. Acta Neuropathol. Commun. 2021, 9, 162. [Google Scholar] [CrossRef]
- Zou, K.; Hosono, T.; Nakamura, T.; Shiraishi, H.; Maeda, T.; Komano, H.; Yanagisawa, K.; Michikawa, M. Novel Role of Presenilins in Maturation and Transport of Integrin β1. Biochemistry 2008, 47, 3370–3378. [Google Scholar] [CrossRef] [PubMed]
- Uemura, K.; Kuzuya, A.; Shimohama, S. Protein trafficking and Alzheimer’s disease. Curr. Alzheimer Res. 2004, 1, 1–10. [Google Scholar] [CrossRef]
- Wang, W.; Moerman-Herzog, A.M.; Slaton, A.; Barger, S.W. Presenilin 1 mutations influence processing and trafficking of the ApoE receptor apoER2. Neurobiol. Aging 2017, 49, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Naruse, S.; Thinakaran, G.; Luo, J.J.; Kusiak, J.W.; Tomita, T.; Iwatsubo, T.; Qian, X.; Ginty, D.D.; Price, D.L.; Borchelt, D.R.; et al. Effects of PS1 deficiency on membrane protein trafficking in neurons. Neuron 1998, 21, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Esselens, C.; Oorschot, V.; Baert, V.; Raemaekers, T.; Spittaels, K.; Serneels, L.; Zheng, H.; Saftig, P.; De Strooper, B.; Klumperman, J.; et al. Presenilin 1 mediates the turnover of telencephalin in hippocampal neurons via an autophagic degradative pathway. J. Cell Biol. 2004, 166, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Repetto, E.; Yoon, I.S.; Zheng, H.; Kang, D.E. Presenilin 1 regulates epidermal growth factor receptor turnover and signaling in the endosomal-lysosomal pathway. J. Biol. Chem. 2007, 282, 31504–31516. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Huang, T.; Jiang, L.-L.; Tan, Z.; Zhang, M.; Cheng, I.H.-J.; Wang, X.; Bu, G.; Zhang, Y.-W.; et al. Intracellular trafficking of TREM2 is regulated by presenilin 1. Exp. Mol. Med. 2017, 49, e405. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.; Sun, Y.; Gao, Y.; Nakamura, T.; Noorani, A.A.; Li, T.; Wong, P.C.; Kimura, N.; Matsubara, E.; Kasuga, K.; et al. Presenilin Is Essential for ApoE Secretion, a Novel Role of Presenilin Involved in Alzheimer’s Disease Pathogenesis. J. Neurosci. 2022, 42, 1574–1586. [Google Scholar] [CrossRef]
- Sun, Y.; Islam, S.; Gao, Y.; Nakamura, T.; Zou, K.; Michikawa, M. Apolipoprotein E4 inhibits γ-secretase activity via binding to the γ-secretase complex. J. Neurochem. 2023, 164, 858–874. [Google Scholar] [CrossRef] [PubMed]
- Zou, K.; Yamaguchi, H.; Akatsu, H.; Sakamoto, T.; Ko, M.; Mizoguchi, K.; Gong, J.S.; Yu, W.; Yamamoto, T.; Kosaka, K.; et al. Angiotensin-converting enzyme converts amyloid beta-protein 1-42 (Abeta(1-42)) to Abeta(1-40), and its inhibition enhances brain Abeta deposition. J. Neurosci. 2007, 27, 8628–8635. [Google Scholar] [CrossRef]
- Liu, S.; Ando, F.; Fujita, Y.; Liu, J.; Maeda, T.; Shen, X.; Kikuchi, K.; Matsumoto, A.; Yokomori, M.; Tanabe-Fujimura, C.; et al. A clinical dose of angiotensin-converting enzyme (ACE) inhibitor and heterozygous ACE deletion exacerbate Alzheimer’s disease pathology in mice. J. Biol. Chem. 2019, 294, 9760–9770. [Google Scholar] [CrossRef]
- Zou, K.; Liu, J.; Watanabe, A.; Hiraga, S.; Liu, S.; Tanabe, C.; Maeda, T.; Terayama, Y.; Takahashi, S.; Michikawa, M.; et al. Aβ43 is the earliest-depositing Aβ species in APP transgenic mouse brain and is converted to Aβ41 by two active domains of ACE. Am. J. Pathol. 2013, 182, 2322–2331. [Google Scholar] [CrossRef]
- Gao, Y.; Sun, Y.; Islam, S.; Nakamura, T.; Tomita, T.; Zou, K.; Michikawa, M. Presenilin 1 deficiency impairs Aβ42-to-Aβ40- and angiotensin-converting activities of ACE. Front. Aging Neurosci. 2023, 15, 1098034. [Google Scholar] [CrossRef] [PubMed]
- Colucci-D’Amato, L.; Speranza, L.; Volpicelli, F. Neurotrophic Factor BDNF, Physiological Functions and Therapeutic Potential in Depression, Neurodegeneration and Brain Cancer. Int. J. Mol. Sci. 2020, 21, 7777. [Google Scholar] [CrossRef]
- Wang, Z.H.; Xiang, J.; Liu, X.; Yu, S.P.; Manfredsson, F.P.; Sandoval, I.M.; Wu, S.; Wang, J.Z.; Ye, K. Deficiency in BDNF/TrkB Neurotrophic Activity Stimulates δ-Secretase by Upregulating C/EBPβ in Alzheimer’s Disease. Cell Rep. 2019, 28, 655–669.e5. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl. Neurodegener. 2022, 11, 4. [Google Scholar] [CrossRef]
- Barthet, G.; Dunys, J.; Shao, Z.; Xuan, Z.; Ren, Y.; Xu, J.; Arbez, N.; Mauger, G.; Bruban, J.; Georgakopoulos, A.; et al. Presenilin mediates neuroprotective functions of ephrinB and brain-derived neurotrophic factor and regulates ligand-induced internalization and metabolism of EphB2 and TrkB receptors. Neurobiol. Aging 2013, 34, 499–510. [Google Scholar] [CrossRef] [PubMed]
- Elliott, E.; Atlas, R.; Lange, A.; Ginzburg, I. Brain-derived neurotrophic factor induces a rapid dephosphorylation of tau protein through a PI-3 Kinase signalling mechanism. Eur. J. Neurosci. 2005, 22, 1081–1089. [Google Scholar] [CrossRef]
- Chong, C.M.; Ke, M.; Tan, Y.; Huang, Z.; Zhang, K.; Ai, N.; Ge, W.; Qin, D.; Lu, J.H.; Su, H. Presenilin 1 deficiency suppresses autophagy in human neural stem cells through reducing γ-secretase-independent ERK/CREB signaling. Cell Death Dis. 2018, 9, 879. [Google Scholar] [CrossRef] [PubMed]
- Meng, C.; He, Z.; Xing, D. Low-level laser therapy rescues dendrite atrophy via upregulating BDNF expression: Implications for Alzheimer’s disease. J. Neurosci. 2013, 33, 13505–13517. [Google Scholar] [CrossRef] [PubMed]
- Boonen, R.A.; van Tijn, P.; Zivkovic, D. Wnt signaling in Alzheimer’s disease: Up or down, that is the question. Ageing Res. Rev. 2009, 8, 71–82. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, C.L.; Tong, M.M.; Zhao, Z.; Chen, S.Q. Both Wnt/β-catenin and ERK5 signaling pathways are involved in BDNF-induced differentiation of pluripotent stem cells into neural stem cells. Neurosci. Lett. 2019, 708, 134345. [Google Scholar] [CrossRef]
- Dong, X.F.; Wang, T.; Li, T.; Jiao, J.J.; Qu, X.S.; Qi, J.S.; Yang, W. [Expressions of synaptophysin and BDNF/Trk-B in cerebellum of APPswe/PS1dE9 transgenic mice]. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2017, 33, 488–492. [Google Scholar]
- Al Rahim, M.; Yoon, Y.; Dimovasili, C.; Shao, Z.; Huang, Q.; Zhang, E.; Kezunovic, N.; Chen, L.; Schaffner, A.; Huntley, G.W.; et al. Presenilin1 familial Alzheimer disease mutants inactivate EFNB1- and BDNF-dependent neuroprotection against excitotoxicity by affecting neuroprotective complexes of N-methyl-d-aspartate receptor. Brain Commun. 2020, 2, fcaa100. [Google Scholar] [CrossRef]
- Nicoletti, V.G.; Pajer, K.; Calcagno, D.; Pajenda, G.; Nógrádi, A. The Role of Metals in the Neuroregenerative Action of BDNF, GDNF, NGF and Other Neurotrophic Factors. Biomolecules 2022, 12, 1015. [Google Scholar] [CrossRef] [PubMed]
- Marlin, M.C.; Li, G. Chapter Six—Biogenesis and Function of the NGF/TrkA Signaling Endosome. In International Review of Cell and Molecular Biology; Jeon, K.W., Ed.; Academic Press: Cambridge, MA, USA, 2015; pp. 239–257. [Google Scholar]
- Dehvari, N.; Cedazo-Minguez, A.; Isacsson, O.; Nilsson, T.; Winblad, B.; Karlström, H.; Benedikz, E.; Cowburn, R.F. Presenilin dependence of phospholipase C and protein kinase C signaling. J. Neurochem. 2007, 102, 848–857. [Google Scholar] [CrossRef]
- Barabás, K.; Kobolák, J.; Godó, S.; Kovács, T.; Ernszt, D.; Kecskés, M.; Varga, C.; Jánosi, T.Z.; Fujiwara, T.; Kusumi, A.; et al. Live-Cell Imaging of Single Neurotrophin Receptor Molecules on Human Neurons in Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 13260. [Google Scholar] [CrossRef]
- Chartier-Harlin, M.C.; Kachergus, J.; Roumier, C.; Mouroux, V.; Douay, X.; Lincoln, S.; Levecque, C.; Larvor, L.; Andrieux, J.; Hulihan, M.; et al. Alpha-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet 2004, 364, 1167–1169. [Google Scholar] [CrossRef]
- Lathia, J.D.; Mattson, M.P.; Cheng, A. Notch: From neural development to neurological disorders. J. Neurochem. 2008, 107, 1471–1481. [Google Scholar] [CrossRef]
- Gandhi, S.; Abramov, A.Y. Mechanism of oxidative stress in neurodegeneration. Oxidative Med. Cell. Longev. 2012, 2012, 428010. [Google Scholar] [CrossRef]
- Yang, Y.; Bagyinszky, E.; An, S.S.A. Presenilin-1 (PSEN1) Mutations: Clinical Phenotypes beyond Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 8417. [Google Scholar]
- Gatto, E.M.; Rojas, G.J.; Nemirovsky, S.I.; Da Prat, G.; Persi, G.; Cesarini, M.; Etcheverry, J.L.; Rojas, N.G.; Parisi, V.; Cordoba, M.; et al. A novel mutation in PSEN1 (p.Arg41Ser) in an Argentinian woman with early onset Parkinsonism. Park. Relat. Disord. 2020, 77, 21–25. [Google Scholar] [CrossRef]
- Checler, F.; Goiran, T.; Alves da Costa, C. Presenilins at the crossroad of a functional interplay between PARK2/PARKIN and PINK1 to control mitophagy: Implication for neurodegenerative diseases. Autophagy 2017, 13, 2004–2005. [Google Scholar] [CrossRef]
- Winslow, A.R.; Moussaud, S.; Zhu, L.; Post, K.L.; Dickson, D.W.; Berezovska, O.; McLean, P.J. Convergence of pathology in dementia with Lewy bodies and Alzheimer’s disease: A role for the novel interaction of alpha-synuclein and presenilin 1 in disease. Brain 2014, 137, 1958–1970. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Blauwendraat, C.; Wilke, C.; Jansen, I.E.; Schulte, C.; Simón-Sánchez, J.; Metzger, F.G.; Bender, B.; Gasser, T.; Maetzler, W.; Rizzu, P.; et al. Pilot whole-exome sequencing of a German early-onset Alzheimer’s disease cohort reveals a substantial frequency of PSEN2 variants. Neurobiol. Aging 2016, 37, 208.e11–208.e17. [Google Scholar] [CrossRef] [PubMed]
- Mendez, M.F.; McMurtray, A. Frontotemporal dementia-like phenotypes associated with presenilin-1 mutations. Am. J. Alzheimer’s Dis. Other Dement. 2006, 21, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Hutton, M. Presenilin mutations associated with fronto-temporal dementia. Ann. Neurol. 2004, 55, 604–606. [Google Scholar] [CrossRef]
- Evin, G.; Smith, M.J.; Tziotis, A.; McLean, C.; Canterford, L.; Sharples, R.A.; Cappai, R.; Weidemann, A.; Beyreuther, K.; Cotton, R.G.; et al. Alternative transcripts of presenilin-1 associated with frontotemporal dementia. Neuroreport 2002, 13, 917–921. [Google Scholar] [CrossRef]
- Raemaekers, T.; Esselens, C.; Annaert, W. Presenilin 1: More than just γ-secretase. Biochem. Soc. Trans. 2005, 33, 559–562. [Google Scholar] [CrossRef]
- Roizin, L.; Stellar, S.; Willson, N.; Whittier, J.; Liu, J.C. Electron microscope and enzyme studies in cerebral biopsies of Huntington’s chorea. Trans. Am. Neurol. Assoc. 1974, 99, 240–243. [Google Scholar] [PubMed]
- Kegel, K.B.; Kim, M.; Sapp, E.; McIntyre, C.; Castaño, J.G.; Aronin, N.; DiFiglia, M. Huntingtin expression stimulates endosomal-lysosomal activity, endosome tubulation, and autophagy. J. Neurosci. 2000, 20, 7268–7278. [Google Scholar] [CrossRef]
- Rideout, H.J.; Lang-Rollin, I.; Stefanis, L. Involvement of macroautophagy in the dissolution of neuronal inclusions. Int. J. Biochem. Cell Biol. 2004, 36, 2551–2562. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Yu, W.H.; Kumar, A.; Lee, S.; Mohan, P.S.; Peterhoff, C.M.; Wolfe, D.M.; Martinez-Vicente, M.; Massey, A.C.; Sovak, G.; et al. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell 2010, 141, 1146–1158. [Google Scholar] [CrossRef] [PubMed]
- Neely, K.M.; Green, K.N.; LaFerla, F.M. Presenilin is necessary for efficient proteolysis through the autophagy-lysosome system in a γ-secretase-independent manner. J. Neurosci. 2011, 31, 2781–2791. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Costa, J.F.; Payá-Montes, M.; Martínez-Molina, M.; Jaijo, T.; Szymanski, J.; Mazón, M.; Sopena-Novales, P.; Pérez-Tur, J.; Sevilla, T. Presenilin-1 Mutations Are a Cause of Primary Lateral Sclerosis-Like Syndrome. Front. Mol. Neurosci. 2021, 14, 721047. [Google Scholar] [CrossRef] [PubMed]
- Muyderman, H.; Chen, T. Mitochondrial dysfunction in amyotrophic lateral sclerosis—A valid pharmacological target? Br. J. Pharmacol. 2014, 171, 2191–2205. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, X.; Huo, Z.; Chen, Y.; Liu, J.; Zhao, Z.; Meng, F.; Su, Q.; Bao, W.; Zhang, L.; et al. The Impact of Mitochondrial Dysfunction in Amyotrophic Lateral Sclerosis. Cells 2022, 11, 2049. [Google Scholar] [CrossRef] [PubMed]
- Sarasija, S.; Laboy, J.T.; Ashkavand, Z.; Bonner, J.; Tang, Y.; Norman, K.R. Presenilin mutations deregulate mitochondrial Ca2+ homeostasis and metabolic activity causing neurodegeneration in Caenorhabditis elegans. eLife 2018, 7, e33052. [Google Scholar] [CrossRef] [PubMed]
- Korkotian, E.; Meshcheriakova, A.; Segal, M. Presenilin 1 Regulates [Ca2+]i and Mitochondria/ER Interaction in Cultured Rat Hippocampal Neurons. Oxidative Med. Cell. Longev. 2019, 2019, 7284967. [Google Scholar] [CrossRef]
- Sreedharan, J.; Blair, I.P.; Tripathi, V.B.; Hu, X.; Vance, C.; Rogelj, B.; Ackerley, S.; Durnall, J.C.; Williams, K.L.; Buratti, E.; et al. TDP-43 mutations in familial and sporadic amyotrophic lateral sclerosis. Science 2008, 319, 1668–1672. [Google Scholar] [CrossRef]
- Kim, S.H.; Shi, Y.; Hanson, K.A.; Williams, L.M.; Sakasai, R.; Bowler, M.J.; Tibbetts, R.S. Potentiation of amyotrophic lateral sclerosis (ALS)-associated TDP-43 aggregation by the proteasome-targeting factor, ubiquilin 1. J. Biol. Chem. 2009, 284, 8083–8092. [Google Scholar] [CrossRef]
- Couthouis, J.; RAPHael, A.R.; Daneshjou, R.; Gitler, A.D. Targeted exon capture and sequencing in sporadic amyotrophic lateral sclerosis. PLoS Genet. 2014, 10, e1004704. [Google Scholar] [CrossRef][Green Version]
- Shen, L.; Qin, W.; Wu, L.; Zhou, A.; Tang, Y.; Wang, Q.; Jia, L.; Jia, J. Two novel presenilin-1 mutations (I249L and P433S) in early onset Chinese Alzheimer’s pedigrees and their functional characterization. Biochem. Biophys. Res. Commun. 2019, 516, 264–269. [Google Scholar] [CrossRef]
- Sabbagh, M.N.; Hendrix, S.; Harrison, J.E. FDA position statement “Early Alzheimer’s disease: Developing drugs for treatment, Guidance for Industry”. Alzheimer’s Dement. 2019, 5, 13–19. [Google Scholar] [CrossRef]
- Scheff, S.W.; Price, D.A.; Schmitt, F.A.; DeKosky, S.T.; Mufson, E.J. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology 2007, 68, 1501–1508. [Google Scholar] [CrossRef]
- Barman, N.C.; Khan, N.M.; Islam, M.; Nain, Z.; Roy, R.K.; Haque, A.; Barman, S.K. CRISPR-Cas9: A Promising Genome Editing Therapeutic Tool for Alzheimer’s Disease-A Narrative Review. Neurol. Ther. 2020, 9, 419–434. [Google Scholar] [CrossRef]
- Konstantinidis, E.; Molisak, A.; Perrin, F.; Streubel-Gallasch, L.; Fayad, S.; Kim, D.Y.; Petri, K.; Aryee, M.J.; Aguilar, X.; György, B.; et al. CRISPR-Cas9 treatment partially restores amyloid-β 42/40 in human fibroblasts with the Alzheimer’s disease PSEN 1 M146L mutation. Mol. Ther. Nucleic Acids 2022, 28, 450–461. [Google Scholar] [CrossRef]
- Imbimbo, B.P.; Triaca, V.; Imbimbo, C.; Nisticò, R. Investigational treatments for neurodegenerative diseases caused by inheritance of gene mutations: Lessons from recent clinical trials. Neural Regen. Res. 2023, 18, 1679–1683. [Google Scholar] [CrossRef] [PubMed]
- Doody, R.S.; Raman, R.; Farlow, M.; Iwatsubo, T.; Vellas, B.; Joffe, S.; Kieburtz, K.; He, F.; Sun, X.; Thomas, R.G.; et al. A phase 3 trial of semagacestat for treatment of Alzheimer’s disease. N. Engl. J. Med. 2013, 369, 341–350. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Islam, S.; Michikawa, M.; Zou, K. Presenilin: A Multi-Functional Molecule in the Pathogenesis of Alzheimer’s Disease and Other Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 1757. https://doi.org/10.3390/ijms25031757
Sun Y, Islam S, Michikawa M, Zou K. Presenilin: A Multi-Functional Molecule in the Pathogenesis of Alzheimer’s Disease and Other Neurodegenerative Diseases. International Journal of Molecular Sciences. 2024; 25(3):1757. https://doi.org/10.3390/ijms25031757
Chicago/Turabian StyleSun, Yang, Sadequl Islam, Makoto Michikawa, and Kun Zou. 2024. "Presenilin: A Multi-Functional Molecule in the Pathogenesis of Alzheimer’s Disease and Other Neurodegenerative Diseases" International Journal of Molecular Sciences 25, no. 3: 1757. https://doi.org/10.3390/ijms25031757
APA StyleSun, Y., Islam, S., Michikawa, M., & Zou, K. (2024). Presenilin: A Multi-Functional Molecule in the Pathogenesis of Alzheimer’s Disease and Other Neurodegenerative Diseases. International Journal of Molecular Sciences, 25(3), 1757. https://doi.org/10.3390/ijms25031757





