CB2 Cannabinoid Receptor as a Potential Target in Myocardial Infarction: Exploration of Molecular Pathogenesis and Therapeutic Strategies
Abstract
1. Introduction
2. CB2 Receptor in the Cardiovascular System
3. Functional Differences and Rivalry between the CB2 Receptor and the CB1 Receptor in Myocardial Injury
4. CB2 Receptor-Dependent Molecular Mechanisms in MI
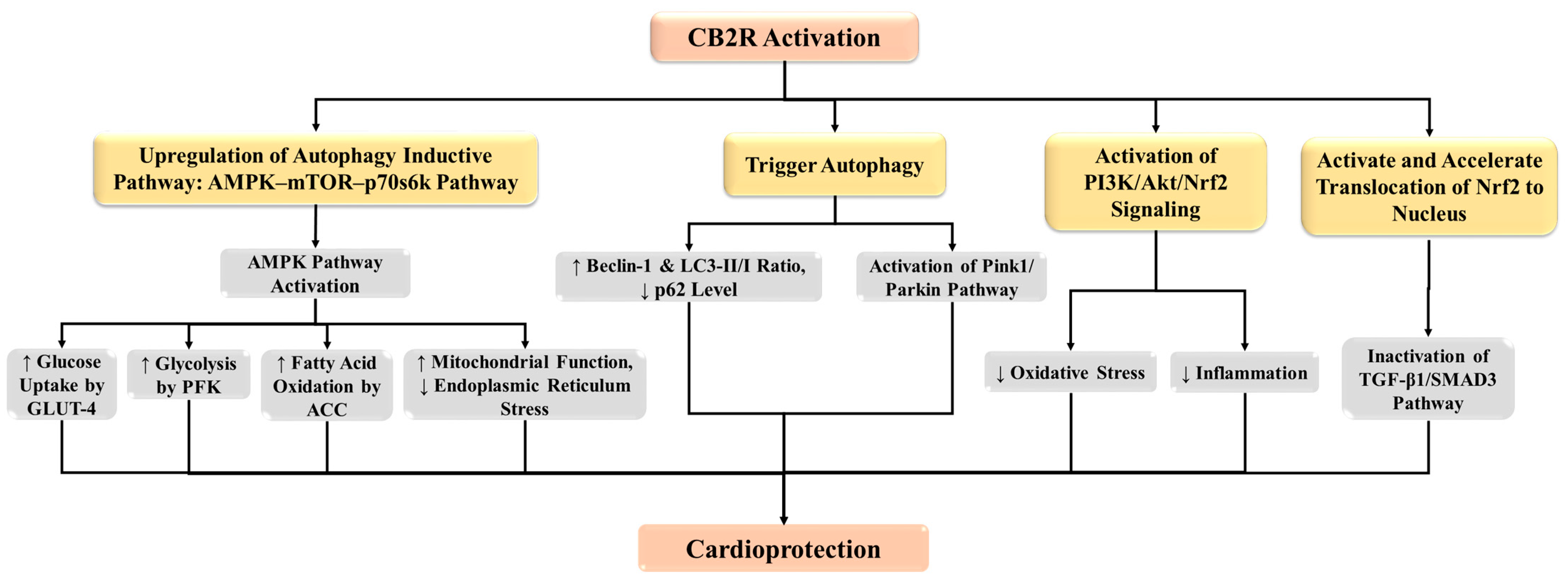
4.1. CB2 Receptor Protects against MI through the Induction of Autophagy
4.2. CB2 Receptor Protects against Myocardial Fibrosis in MI via Modulation of Transforming Growth Factor Beta (TGF-β)/Small Mother against Decapentaplegic Homolog 3 (Smad3) Superfamilies Signaling Pathway
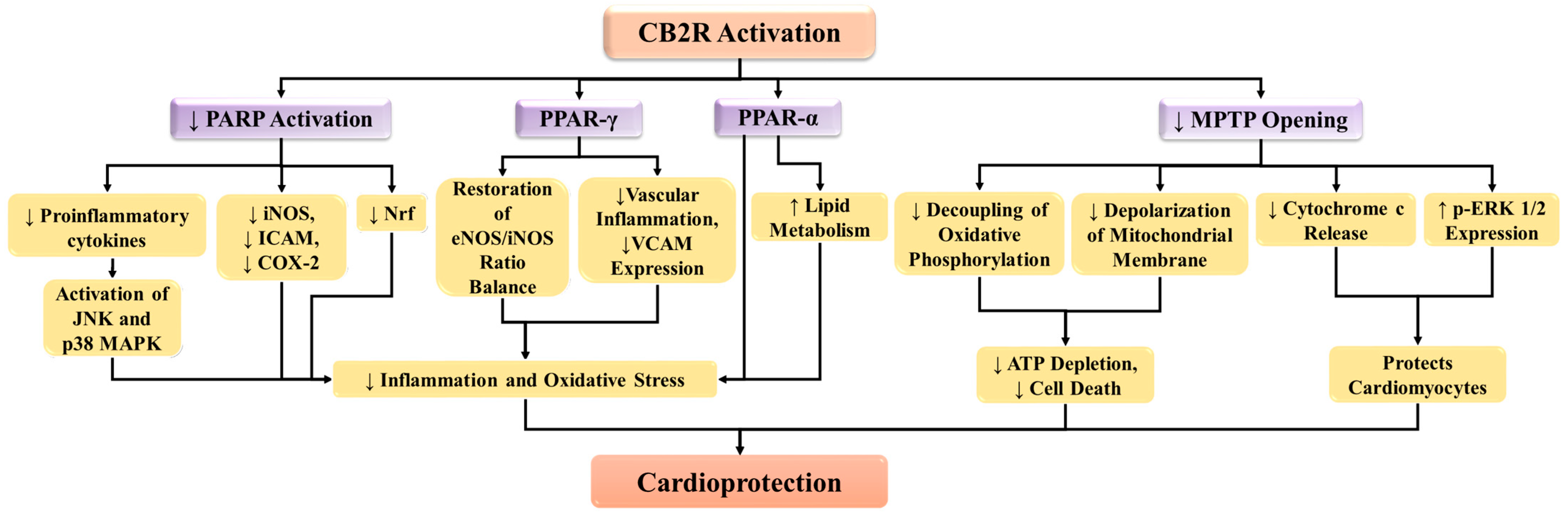
4.3. CB2 Receptor Suppresses Poly (ADP-Ribose) Polymerase-1 (PARP-1) Activity in MI
4.4. CB2 Receptor Modulates the Activity of Peroxisome Proliferator-Activated Receptors (PPAR)
4.5. CB2 Receptor Inhibits Mitochondrial Permeability Transition Pore (MPTP) in MI
5. CB2 Receptor Suppresses Oxidative Stress in MI
6. CB2 Receptor Suppresses Inflammatory Response in MI
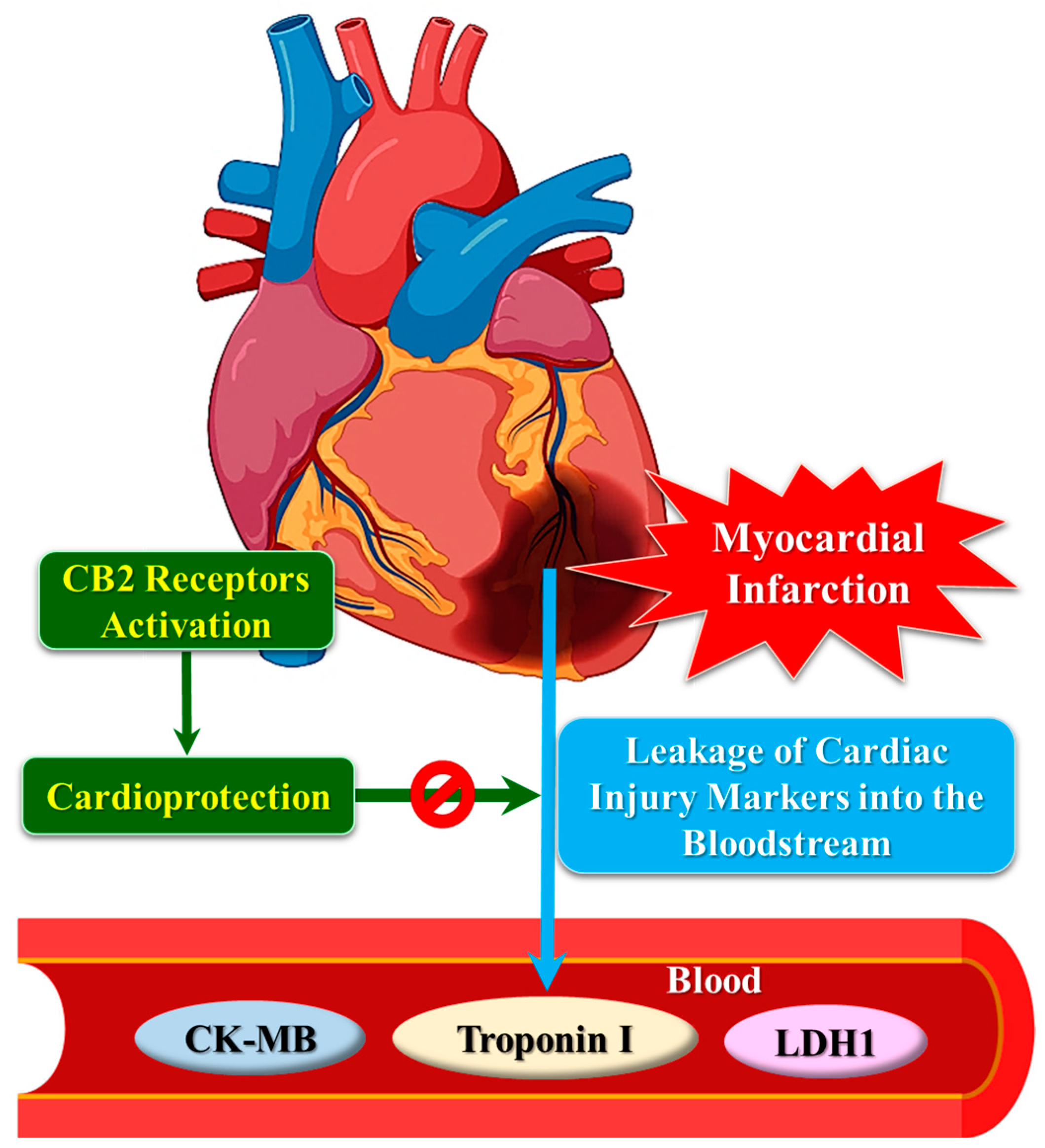
7. CB2 Receptor and Cardiac Injury Markers
8. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mechanic, O.J.; Gavin, M.; Grossman, S.A. Acute Myocardial Infarction. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar]
- Mozaffarian, S.; Etemad, K.; Aghaali, M.; Khodakarim, S.; Sotoodeh Ghorbani, S.; Hashemi Nazari, S.S. Short and Long-Term Survival Rates Following Myocardial Infarction and Its Predictive Factors: A Study Using National Registry Data. J. Tehran Heart Cent. 2021, 16, 68–74. [Google Scholar] [CrossRef]
- Smolina, K.; Wright, F.L.; Rayner, M.; Goldacre, M.J. Long-Term Survival and Recurrence After Acute Myocardial Infarction in England, 2004 to 2010. Circ. Cardiovasc. Qual. Outcomes 2012, 5, 532–540. [Google Scholar] [CrossRef]
- Woollard, K.J.; Geissmann, F. Monocytes in Atherosclerosis: Subsets and Functions. Nat. Rev. Cardiol. 2010, 7, 77–86. [Google Scholar] [CrossRef]
- Santos-Gallego, C.G.; Picatoste, B.; Badimón, J.J. Pathophysiology of Acute Coronary Syndrome. Curr. Atheroscler. Rep. 2014, 16, 401. [Google Scholar] [CrossRef]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Poznyak, A.V.; Sukhorukov, V.N.; Surkova, R.; Orekhov, N.A.; Orekhov, A.N. Glycation of LDL: AGEs, Impact on Lipoprotein Function, and Involvement in Atherosclerosis. Front. Cardiovasc. Med. 2023, 10, 1094188. [Google Scholar] [CrossRef]
- Frangogiannis, N.G. The Inflammatory Response in Myocardial Injury, Repair and Remodeling. Nat. Rev. Cardiol. 2014, 11, 255–265. [Google Scholar] [CrossRef]
- Heiss, C.; Rodriguez-Mateos, A.; Kelm, M. Central Role of eNOS in the Maintenance of Endothelial Homeostasis. Antioxid. Redox Signal. 2015, 22, 1230–1242. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Harrison, D.G. Endothelial Dysfunction in Cardiovascular Diseases: The Role of Oxidant Stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Liu, Z.; Lu, H.; Zhang, W.; Mi, Q.; Li, X.; Tang, Y.; Chen, Q.; Ferro, A.; Ji, Y. Pyridoxine Inhibits Endothelial NOS Uncoupling Induced by Oxidized Low-Density Lipoprotein via the PKCα Signalling Pathway in Human Umbilical Vein Endothelial Cells. Br. J. Pharmacol. 2012, 165, 754–764. [Google Scholar] [CrossRef] [PubMed]
- Asada, Y.; Yamashita, A.; Sato, Y.; Hatakeyama, K. Pathophysiology of Atherothrombosis: Mechanisms of Thrombus Formation on Disrupted Atherosclerotic Plaques. Pathol. Int. 2020, 70, 309–322. [Google Scholar] [CrossRef]
- Huang, H.; Koelle, P.; Fendler, M.; Schröttle, A.; Czihal, M.; Hoffmann, U.; Conrad, M.; Kuhlencordt, P.J. Induction of Inducible Nitric Oxide Synthase (iNOS) Expression by oxLDL Inhibits Macrophage Derived Foam Cell Migration. Atherosclerosis 2014, 235, 213–222. [Google Scholar] [CrossRef]
- Navarro-Yepes, J.; Burns, M.; Anandhan, A.; Khalimonchuk, O.; del Razo, L.M.; Quintanilla-Vega, B.; Pappa, A.; Panayiotidis, M.I.; Franco, R. Oxidative Stress, Redox Signaling, and Autophagy: Cell Death Versus Survival. Antioxid. Redox Signal. 2014, 21, 66–85. [Google Scholar] [CrossRef]
- Kent, A.C.; El Baradie, K.B.Y.; Hamrick, M.W. Targeting the Mitochondrial Permeability Transition Pore to Prevent Age-Associated Cell Damage and Neurodegeneration. Oxid. Med. Cell. Longev. 2021, 2021, 6626484. [Google Scholar] [CrossRef]
- Cipriani, A.; D’Amico, G.; Brunello, G.; Perazzolo Marra, M.; Migliore, F.; Cacciavillani, L.; Tarantini, G.; Bauce, B.; Iliceto, S.; Corrado, D.; et al. The Electrocardiographic “Triangular QRS-ST-T Waveform” Pattern in Patients with ST-Segment Elevation Myocardial Infarction: Incidence, Pathophysiology and Clinical Implications. J. Electrocardiol. 2018, 51, 8–14. [Google Scholar] [CrossRef]
- Aydin, S.; Ugur, K.; Aydin, S.; Sahin, İ.; Yardim, M. Biomarkers in Acute Myocardial Infarction: Current Perspectives. Vasc. Health Risk Manag. 2019, 15, 1–10. [Google Scholar] [CrossRef]
- 2-Arachidonoylglycerol Mobilizes Myeloid Cells and Worsens Heart Function after Acute Myocardial Infarction | Cardiovascular Research | Oxford Academic. Available online: https://academic.oup.com/cardiovascres/article/115/3/602/5115994 (accessed on 8 October 2023).
- Ranieri, R.; Laezza, C.; Bifulco, M.; Marasco, D.; Malfitano, A.M. Endocannabinoid System in Neurological Disorders. Recent Pat. CNS Drug Discov. Discontin. 2015, 10, 90–112. [Google Scholar] [CrossRef] [PubMed]
- Steffens, S.; Pacher, P. The Activated Endocannabinoid System in Atherosclerosis: Driving Force or Protective Mechanism? Curr. Drug Targets 2015, 16, 334–341. [Google Scholar] [CrossRef] [PubMed]
- The Therapeutic Potential of Targeting the Peripheral Endocannabinoid/CB1 Receptor System—European Journal of Internal Medicine. Available online: https://www.ejinme.com/article/S0953-6205(18)30009-8/fulltext (accessed on 8 October 2023).
- Rathod, S.S.; Agrawal, Y.O.; Nakhate, K.T.; Meeran, M.F.N.; Ojha, S.; Goyal, S.N. Neuroinflammation in the Central Nervous System: Exploring the Evolving Influence of Endocannabinoid System. Biomedicines 2023, 11, 2642. [Google Scholar] [CrossRef] [PubMed]
- Kendall, D.A.; Yudowski, G.A. Cannabinoid Receptors in the Central Nervous System: Their Signaling and Roles in Disease. Front. Cell. Neurosci. 2017, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Kaschina, E. Cannabinoid CB1/CB2 Receptors in the Heart: Expression, Regulation, and Function. In Cannabinoids in Health and Disease; IntechOpen: London, UK, 2016; ISBN 978-953-51-2430-6. [Google Scholar]
- Vrechi, T.A.; Crunfli, F.; Costa, A.P.; Torrão, A.S. Cannabinoid Receptor Type 1 Agonist ACEA Protects Neurons from Death and Attenuates Endoplasmic Reticulum Stress-Related Apoptotic Pathway Signaling. Neurotox. Res. 2018, 33, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Hytti, M.; Andjelic, S.; Josifovska, N.; Piippo, N.; Korhonen, E.; Hawlina, M.; Kaarniranta, K.; Nevalainen, T.J.; Petrovski, G.; Parkkari, T.; et al. CB2 Receptor Activation Causes an ERK1/2-Dependent Inflammatory Response in Human RPE Cells. Sci. Rep. 2017, 7, 16169. [Google Scholar] [CrossRef] [PubMed]
- Braun, M.; Khan, Z.T.; Khan, M.B.; Kumar, M.; Ward, A.; Achyut, B.R.; Arbab, A.S.; Hess, D.C.; Hoda, M.N.; Baban, B.; et al. Selective Activation of Cannabinoid Receptor-2 Reduces Neuroinflammation after Traumatic Brain Injury via Alternative Macrophage Polarization. Brain. Behav. Immun. 2018, 68, 224–237. [Google Scholar] [CrossRef]
- Li, X.; Han, D.; Tian, Z.; Gao, B.; Fan, M.; Li, C.; Li, X.; Wang, Y.; Ma, S.; Cao, F. Activation of Cannabinoid Receptor Type II by AM1241 Ameliorates Myocardial Fibrosis via Nrf2-Mediated Inhibition of TGF-Β1/Smad3 Pathway in Myocardial Infarction Mice. Cell. Physiol. Biochem. 2016, 39, 1521–1536. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Li, X.; Fan, W.-S.; Chen, J.-W.; Gou, T.-T.; Su, T.; Fan, M.-M.; Xu, M.-Q.; Wang, Y.-B.; Ma, S.; et al. Activation of Cannabinoid Receptor Type II by AM1241 Protects Adipose-Derived Mesenchymal Stem Cells from Oxidative Damage and Enhances Their Therapeutic Efficacy in Myocardial Infarction Mice via Stat3 Activation. Oncotarget 2017, 8, 64853–64866. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Duan, Y.H.; Ji, Y.Y.; Wang, Z.L.; Wu, Y.R.; Gunosewoyo, H.; Xie, X.; Chen, J.; Yang, F.; Li, J.; et al. Amidoalkylindoles as Potent and Selective Cannabinoid Type 2 Receptor Agonists with in Vivo Efficacy in a Mouse Model of Multiple Sclerosis. J. Med. Chemistry 2017, 60, 7067–7083. Available online: https://pubs.acs.org/doi/10.1021/acs.jmedchem.7b00724 (accessed on 8 October 2023). [CrossRef]
- Ho, W.S.V.; Kelly, M.E.M. Chapter Ten—Cannabinoids in the Cardiovascular System. In Advances in Pharmacology; Kendall, D., Alexander, S.P.H., Eds.; Cannabinoid Pharmacology; Academic Press: Cambridge, MA, USA, 2017; Volume 80, pp. 329–366. [Google Scholar]
- Bravo-Ferrer, I.; Cuartero, M.I.; Zarruk, J.G.; Pradillo, J.M.; Hurtado, O.; Romera, V.G.; Díaz-Alonso, J.; García-Segura, J.M.; Guzmán, M.; Lizasoain, I.; et al. Cannabinoid Type-2 Receptor Drives Neurogenesis and Improves Functional Outcome after Stroke. Stroke 2017, 48, 204–212. [Google Scholar] [CrossRef]
- Li, L.; Tao, Y.; Tang, J.; Chen, Q.; Yang, Y.; Feng, Z.; Chen, Y.; Yang, L.; Yang, Y.; Zhu, G.; et al. A Cannabinoid Receptor 2 Agonist Prevents Thrombin-Induced Blood–Brain Barrier Damage via the Inhibition of Microglial Activation and Matrix Metalloproteinase Expression in Rats. Transl. Stroke Res. 2015, 6, 467–477. [Google Scholar] [CrossRef]
- Prospects for the Use of Cannabinoid Receptor Ligands for the Treatment of Metabolic Syndrome and Atherosclerosis: Analysis of Experimental and Clinical Data—Maslov—Annals of the Russian Academy of Medical Sciences. Available online: https://vestnikramn.spr-journal.ru/jour/article/view/779 (accessed on 8 October 2023).
- Maslov, L.N.; Khaliulin, I.; Zhang, Y.; Krylatov, A.V.; Naryzhnaya, N.V.; Mechoulam, R.; De Petrocellis, L.; Downey, J.M. Prospects for Creation of Cardioprotective Drugs Based on Cannabinoid Receptor Agonists. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 262–272. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, S.; Wang, Q.; Hu, W.; Wang, D.; Li, X.; Su, T.; Qin, X.; Zhang, X.; Ma, K.; et al. Effects of Cannabinoid Receptor Type 2 on Endogenous Myocardial Regeneration by Activating Cardiac Progenitor Cells in Mouse Infarcted Heart. Sci. China Life Sci. 2014, 57, 201–208. [Google Scholar] [CrossRef]
- Wang, P.-F.; Jiang, L.-S.; Bu, J.; Huang, X.-J.; Song, W.; Du, Y.-P.; He, B. Cannabinoid-2 Receptor Activation Protects against Infarct and Ischemia-Reperfusion Heart Injury. J. Cardiovasc. Pharmacol. 2012, 59, 301–307. [Google Scholar] [CrossRef]
- Pawar, H.D.; Mahajan, U.B.; Nakhate, K.T.; Agrawal, Y.O.; Patil, C.R.; Meeran, M.F.N.; Sharma, C.; Ojha, S.; Goyal, S.N. Curcumin Protects Diabetic Mice against Isoproterenol-Induced Myocardial Infarction by Modulating CB2 Cannabinoid Receptors. Life 2022, 12, 624. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, Y.; Chen, S.; Xu, Z.; Zhang, B.; Liu, A.; He, Q.; Zhan, J. Activation of Cannabinoid Receptors 2 Alleviates Myocardial Damage in Cecal Ligation and Puncture-Induced Sepsis by Inhibiting Pyroptosis. Immunol. Lett. 2023, 264, 17–24. [Google Scholar] [CrossRef]
- Munro, S.; Thomas, K.L.; Abu-Shaar, M. Molecular Characterization of a Peripheral Receptor for Cannabinoids. Nature 1993, 365, 61–65. [Google Scholar] [CrossRef]
- Galiègue, S.; Mary, S.; Marchand, J.; Dussossoy, D.; Carrière, D.; Carayon, P.; Bouaboula, M.; Shire, D.; Le Fur, G.; Casellas, P. Expression of Central and Peripheral Cannabinoid Receptors in Human Immune Tissues and Leukocyte Subpopulations. Eur. J. Biochem. 1995, 232, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Woodcock, E.A.; Matkovich, S.J. Cardiomyocytes Structure, Function and Associated Pathologies. Int. J. Biochem. Cell Biol. 2005, 37, 1746–1751. [Google Scholar] [CrossRef]
- Steffens, S.; Pacher, P. Targeting Cannabinoid Receptor CB2 in Cardiovascular Disorders: Promises and Controversies. Br. J. Pharmacol. 2012, 167, 313–323. [Google Scholar] [CrossRef]
- Lépicier, P.; Lagneux, C.; Sirois, M.G.; Lamontagne, D. Endothelial CB1-Receptors Limit Infarct Size through NO Formation in Rat Isolated Hearts. Life Sci. 2007, 81, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Dewald, O.; Duerr, G.D. The Role for the Endocannabinoid System in Cardioprotection and Myocardial Adaptation. In Cannabinoids in Health and Disease; Meccariello, R., Chianese, R., Eds.; IntechOpen: London, UK, 2016; ISBN 978-953-51-2429-0. [Google Scholar]
- González, C.; Herradón, E.; Abalo, R.; Vera, G.; Pérez-Nievas, B.G.; Leza, J.C.; Martín, M.I.; López-Miranda, V. Cannabinoid/Agonist WIN 55,212-2 Reduces Cardiac Ischaemia–Reperfusion Injury in Zucker Diabetic Fatty Rats: Role of CB2 Receptors and iNOS/eNOS. Diabetes Metab. Res. Rev. 2011, 27, 331–340. [Google Scholar] [CrossRef]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Arif, M.; Varga, Z.V.; Mátyás, C.; Paloczi, J.; Lehocki, A.; Haskó, G.; Pacher, P. Cannabinoid Receptor 2 Activation Alleviates Diabetes-Induced Cardiac Dysfunction, Inflammation, Oxidative Stress, and Fibrosis. GeroScience 2022, 44, 1727–1741. [Google Scholar] [CrossRef] [PubMed]
- Weis, F.; Beiras-Fernandez, A.; Sodian, R.; Kaczmarek, I.; Reichart, B.; Beiras, A.; Schelling, G.; Kreth, S. Substantially Altered Expression Pattern of Cannabinoid Receptor 2 and Activated Endocannabinoid System in Patients with Severe Heart Failure. J. Mol. Cell. Cardiol. 2010, 48, 1187–1193. [Google Scholar] [CrossRef]
- Duerr, G.D.; Heinemann, J.C.; Suchan, G.; Kolobara, E.; Wenzel, D.; Geisen, C.; Matthey, M.; Passe-Tietjen, K.; Mahmud, W.; Ghanem, A.; et al. The Endocannabinoid-CB2 Receptor Axis Protects the Ischemic Heart at the Early Stage of Cardiomyopathy. Basic Res. Cardiol. 2014, 109, 425. [Google Scholar] [CrossRef]
- Matyas, C.; Erdelyi, K.; Trojnar, E.; Zhao, S.; Varga, Z.V.; Paloczi, J.; Mukhopadhyay, P.; Nemeth, B.T.; Haskó, G.; Cinar, R.; et al. Interplay of Liver-Heart Inflammatory Axis and Cannabinoid 2 Receptor Signalling in an Experimental Model of Hepatic Cardiomyopathy. Hepatol. Baltim. Md 2020, 71, 1391–1407. [Google Scholar] [CrossRef]
- Shmist, Y.A.; Goncharov, I.; Eichler, M.; Shneyvays, V.; Isaac, A.; Vogel, Z.; Shainberg, A. Delta-9-Tetrahydrocannabinol Protects Cardiac Cells from Hypoxia via CB2 Receptor Activation and Nitric Oxide Production. Mol. Cell. Biochem. 2006, 283, 75–83. [Google Scholar] [CrossRef]
- Banaszkiewicz, M.; Tarwacka, P.; Krzywonos-Zawadzka, A.; Olejnik, A.; Laprairie, R.; Noszczyk-Nowak, A.; Sawicki, G.; Bil-Lula, I. Δ9-Tetrahydrocannabinol (Δ9-THC) Improves Ischemia/Reperfusion Heart Dysfunction and Might Serve as a Cardioprotective Agent in the Future Treatment. Front. Biosci.-Landmark 2022, 27, 114. [Google Scholar] [CrossRef]
- Sandoo, A.; van Zanten, J.J.C.S.V.; Metsios, G.S.; Carroll, D.; Kitas, G.D. The Endothelium and Its Role in Regulating Vascular Tone. Open Cardiovasc. Med. J. 2010, 4, 302–312. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Zhou, Y.; Nabavi, S.M.; Sahebkar, A.; Little, P.J.; Xu, S.; Weng, J.; Ge, J. Mechanisms of Oxidized LDL-Mediated Endothelial Dysfunction and Its Consequences for the Development of Atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 925923. [Google Scholar] [CrossRef]
- Hadi, H.A.; Carr, C.S.; Al Suwaidi, J. Endothelial Dysfunction: Cardiovascular Risk Factors, Therapy, and Outcome. Vasc. Health Risk Manag. 2005, 1, 183–198. [Google Scholar]
- Herrera-Zelada, N.; Zuñiga-Cuevas, U.; Ramirez-Reyes, A.; Lavandero, S.; Riquelme, J.A. Targeting the Endothelium to Achieve Cardioprotection. Front. Pharmacol. 2021, 12, 636134. [Google Scholar] [CrossRef] [PubMed]
- Bullock, T.A.; Galpayage Dona, K.N.U.; Hale, J.F.; Morales, P.; Jagerovic, N.; Andrews, A.M.; Ramirez, S.H. Activation of CB2R by Synthetic CB2R Agonist, PM289, Improves Brain Endothelial Barrier Properties, Decreases Inflammatory Response and Enhances Endothelial Repair. Neuroimmune Pharmacol. Ther. 2023, 2, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiù, V.; Lanuti, M.; Catanzaro, G.; Fezza, F.; Rapino, C.; Maccarrone, M. Detailed Characterization of the Endocannabinoid System in Human Macrophages and Foam Cells, and Anti-Inflammatory Role of Type-2 Cannabinoid Receptor. Atherosclerosis 2014, 233, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, Ż.; Niezgoda, M.; Łebkowski, W.; Filipek, A.; Domian, N.; Kasacka, I. Sex Differences in Distribution of Cannabinoid Receptors (CB1 and CB2), S100A6 and CacyBP/SIP in Human Ageing Hearts. Biol. Sex Differ. 2018, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Lagneux, C.; Lamontagne, D. Involvement of Cannabinoids in the Cardioprotection Induced by Lipopolysaccharide. Br. J. Pharmacol. 2001, 132, 793–796. [Google Scholar] [CrossRef]
- Joyeux, M.; Arnaud, C.; Godin-Ribuot, D.; Demenge, P.; Lamontagne, D.; Ribuot, C. Endocannabinoids Are Implicated in the Infarct Size-Reducing Effect Conferred by Heat Stress Preconditioning in Isolated Rat Hearts. Cardiovasc. Res. 2002, 55, 619–625. [Google Scholar] [CrossRef] [PubMed]
- Lépicier, P.; Bouchard, J.-F.; Lagneux, C.; Lamontagne, D. Endocannabinoids Protect the Rat Isolated Heart against Ischaemia. Br. J. Pharmacol. 2003, 139, 805. [Google Scholar] [CrossRef]
- Defer, N.; Wan, J.; Souktani, R.; Escoubet, B.; Perier, M.; Caramelle, P.; Manin, S.; Deveaux, V.; Bourin, M.-C.; Zimmer, A.; et al. The Cannabinoid Receptor Type 2 Promotes Cardiac Myocyte and Fibroblast Survival and Protects against Ischemia/Reperfusion-Induced Cardiomyopathy. FASEB J. 2009, 23, 2120–2130. [Google Scholar] [CrossRef]
- Wagner, J.A.; Abesser, M.; Harvey-White, J.; Ertl, G. 2-Arachidonylglycerol Acting on CB1 Cannabinoid Receptors Mediates Delayed Cardioprotection Induced by Nitric Oxide in Rat Isolated Hearts. J. Cardiovasc. Pharmacol. 2006, 47, 650. [Google Scholar] [CrossRef]
- Li, L.; Dong, X.; Tu, C.; Li, X.; Peng, Z.; Zhou, Y.; Zhang, D.; Jiang, J.; Burke, A.; Zhao, Z.; et al. Opposite Effects of Cannabinoid CB1 and CB2 Receptors on Antipsychotic Clozapine-Induced Cardiotoxicity. Br. J. Pharmacol. 2019, 176, 890–905. [Google Scholar] [CrossRef]
- Li, X.; Peng, Z.; Zhou, Y.; Wang, J.; Lin, X.; Dong, X.; Liu, X.; Jiang, J.; Jiang, Y.; Li, L. Quetiapine Induces Myocardial Necroptotic Cell Death through Bidirectional Regulation of Cannabinoid Receptors. Toxicol. Lett. 2019, 313, 77–90. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, D.; Dong, X.; Zhu, R.; Ye, Y.; Li, L.; Jiang, Y. Pharmacological Activation of CB2 Receptor Protects against Ethanol-Induced Myocardial Injury Related to RIP1/RIP3/MLKL-Mediated Necroptosis. Mol. Cell. Biochem. 2020, 474, 1–14. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Bátkai, S.; Rajesh, M.; Czifra, N.; Harvey-White, J.; Haskó, G.; Zsengeller, Z.; Gerard, N.P.; Liaudet, L.; Kunos, G.; et al. Pharmacological Inhibition of CB1Cannabinoid Receptor Protects Against Doxorubicin-Induced Cardiotoxicity. J. Am. Coll. Cardiol. 2007, 50, 528–536. [Google Scholar] [CrossRef]
- Rajesh, M.; Bátkai, S.; Kechrid, M.; Mukhopadhyay, P.; Lee, W.-S.; Horváth, B.; Holovac, E.; Cinar, R.; Liaudet, L.; Mackie, K.; et al. Cannabinoid 1 Receptor Promotes Cardiac Dysfunction, Oxidative Stress, Inflammation, and Fibrosis in Diabetic Cardiomyopathy. Diabetes 2012, 61, 716–727. [Google Scholar] [CrossRef]
- Duerr, G.D.; Feißt, A.; Halbach, K.; Verfuerth, L.; Gestrich, C.; Wenzel, D.; Zimmer, A.; Breuer, J.; Dewald, O. CB2-Deficiency Is Associated with a Stronger Hypertrophy and Remodeling of the Right Ventricle in a Murine Model of Left Pulmonary Artery Occlusion. Life Sci. 2018, 215, 96–105. [Google Scholar] [CrossRef]
- Lin, C.-Y.; Hsu, Y.-J.; Hsu, S.-C.; Chen, Y.; Lee, H.-S.; Lin, S.-H.; Huang, S.-M.; Tsai, C.-S.; Shih, C.-C. CB1 Cannabinoid Receptor Antagonist Attenuates Left Ventricular Hypertrophy and Akt-Mediated Cardiac Fibrosis in Experimental Uremia. J. Mol. Cell. Cardiol. 2015, 85, 249–261. [Google Scholar] [CrossRef] [PubMed]
- Slavic, S.; Lauer, D.; Sommerfeld, M.; Kemnitz, U.R.; Grzesiak, A.; Trappiel, M.; Thöne-Reineke, C.; Baulmann, J.; Paulis, L.; Kappert, K.; et al. Cannabinoid Receptor 1 Inhibition Improves Cardiac Function and Remodelling after Myocardial Infarction and in Experimental Metabolic Syndrome. J. Mol. Med. 2013, 91, 811–823. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Fan, Q.; Guo, J.; Galli, G.; Du, G.; Wang, X.; Xiao, W. Ginkgolide K Protects the Heart against Endoplasmic Reticulum Stress Injury by Activating the Inositol-Requiring Enzyme 1α/X Box-Binding Protein-1 Pathway: Ginkgolide K Protects the Heart. Br. J. Pharmacol. 2016, 173, 2402–2418. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.-Z.; Ke, P.; Xu, Z.-Q.; Wei, W.; Cheng, M.-H.; Han, B.-Z.; Chen, X.-W.; Su, D.-F.; Liu, C. Autophagy Plays an Important Role in Anti-Inflammatory Mechanisms Stimulated by Alpha7 Nicotinic Acetylcholine Receptor. Front. Immunol. 2017, 8, 553. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zhang, K.; Hu, P. The Role of Autophagy in Acute Myocardial Infarction. Front. Pharmacol. 2019, 10, 551. [Google Scholar] [CrossRef]
- Wang, P.; Shao, B.-Z.; Deng, Z.; Chen, S.; Yue, Z.; Miao, C.-Y. Autophagy in Ischemic Stroke. Prog. Neurobiol. 2018, 163–164, 98–117. [Google Scholar] [CrossRef]
- Li, X.; Wang, M.-H.; Qin, C.; Fan, W.-H.; Tian, D.-S.; Liu, J.-L. Fingolimod Suppresses Neuronal Autophagy through the mTOR/p70S6K Pathway and Alleviates Ischemic Brain Damage in Mice. PLoS ONE 2017, 12, e0188748. [Google Scholar] [CrossRef]
- Wu, X.; Zheng, D.; Qin, Y.; Liu, Z.; Zhang, G.; Zhu, X.; Zeng, L.; Liang, Z. Nobiletin Attenuates Adverse Cardiac Remodeling after Acute Myocardial Infarction in Rats via Restoring Autophagy Flux. Biochem. Biophys. Res. Commun. 2017, 492, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Sala-Mercado, J.A.; Wider, J.; Reddy Undyala, V.V.; Jahania, S.; Yoo, W.; Mentzer, R.M.; Gottlieb, R.A.; Przyklenk, K. Profound Cardioprotection With Chloramphenicol Succinate in the Swine Model of Myocardial Ischemia-Reperfusion Injury. Circulation 2010, 122, S179–S184. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Hu, P.; Lin, J.; Xia, W.; Zhang, R. Activating Cannabinoid Receptor 2 Protects against Diabetic Cardiomyopathy through Autophagy Induction. Front. Pharmacol. 2018, 9, 1292. [Google Scholar] [CrossRef]
- Denaës, T.; Lodder, J.; Chobert, M.-N.; Ruiz, I.; Pawlotsky, J.-M.; Lotersztajn, S.; Teixeira-Clerc, F. The Cannabinoid Receptor 2 Protects against Alcoholic Liver Disease via a Macrophage Autophagy-Dependent Pathway. Sci. Rep. 2016, 6, 28806. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.; Yang, Y.; Shao, Y.; Wu, M.; Sun, Y. Activation of Cannabinoid Receptor Type 2-Induced Osteogenic Differentiation Involves Autophagy Induction and P62-Mediated Nrf2 Deactivation. Cell Commun. Signal. 2020, 18, 9. [Google Scholar] [CrossRef]
- Liu, W.; Chen, C.; Gu, X.; Zhang, L.; Mao, X.; Chen, Z.; Tao, L. AM1241 Alleviates Myocardial Ischemia-Reperfusion Injury in Rats by Enhancing Pink1/Parkin-Mediated Autophagy. Life Sci. 2021, 272, 119228. [Google Scholar] [CrossRef]
- Hu, Y.; Tao, Y.; Hu, J. Cannabinoid Receptor 2 Deletion Deteriorates Myocardial Infarction through the Down-Regulation of AMPK-mTOR-p70S6K Signaling-Mediated Autophagy. Biosci. Rep. 2019, 39, BSR20180650. [Google Scholar] [CrossRef]
- Qi, D.; Young, L.H. AMPK: Energy Sensor and Survival Mechanism in the Ischemic Heart. Trends Endocrinol. Metab. TEM 2015, 26, 422–429. [Google Scholar] [CrossRef]
- Lu, Y.; Lee, D.I.; Roy Chowdhury, S.; Lu, P.; Kamboj, A.; Anderson, C.M.; Fernyhough, P.; Anderson, H.D. Activation of Cannabinoid Receptors Attenuates Endothelin-1–Induced Mitochondrial Dysfunction in Rat Ventricular Myocytes. J. Cardiovasc. Pharmacol. 2020, 75, 54–63. [Google Scholar] [CrossRef]
- Lu, Y.; Akinwumi, B.C.; Shao, Z.; Anderson, H.D. Ligand Activation of Cannabinoid Receptors Attenuates Hypertrophy of Neonatal Rat Cardiomyocytes. J. Cardiovasc. Pharmacol. 2014, 64, 420–430. [Google Scholar] [CrossRef]
- Arad, M.; Seidman, C.E.; Seidman, J.G. AMP-Activated Protein Kinase in the Heart. Circ. Res. 2007, 100, 474–488. [Google Scholar] [CrossRef]
- Terai, K.; Hiramoto, Y.; Masaki, M.; Sugiyama, S.; Kuroda, T.; Hori, M.; Kawase, I.; Hirota, H. AMP-Activated Protein Kinase Protects Cardiomyocytes against Hypoxic Injury through Attenuation of Endoplasmic Reticulum Stress. Mol. Cell. Biol. 2005, 25, 9554–9575. [Google Scholar] [CrossRef]
- González, A.; Schelbert, E.B.; Díez, J.; Butler, J. Myocardial Interstitial Fibrosis in Heart Failure. J. Am. Coll. Cardiol. 2018, 71, 1696–1706. [Google Scholar] [CrossRef]
- Feng, Y.; Bao, Y.; Ding, J.; Li, H.; Liu, W.; Wang, X.; Guan, H.; Chen, Z. MicroRNA-130a Attenuates Cardiac Fibrosis after Myocardial Infarction through TGF-β/Smad Signaling by Directly Targeting TGF-β Receptor 1. Bioengineered 2022, 13, 5779–5791. [Google Scholar] [CrossRef]
- Du, W.; Liang, H.; Gao, X.; Li, X.; Zhang, Y.; Pan, Z.; Li, C.; Wang, Y.; Liu, Y.; Yuan, W. MicroRNA-328, a Potential Anti-Fibrotic Target in Cardiac Interstitial Fibrosis. Cell. Physiol. Biochem. 2016, 39, 827–836. [Google Scholar] [PubMed]
- Ruiz-Ortega, M.; Rodríguez-Vita, J.; Sanchez-Lopez, E.; Carvajal, G.; Egido, J. TGF-β Signaling in Vascular Fibrosis. Cardiovasc. Res. 2007, 74, 196–206. [Google Scholar] [CrossRef]
- TGF-β Type I Receptor Kinase Inhibitor EW-7197 Suppresses Cholestatic Liver Fibrosis by Inhibiting HIF1α-Induced Epithelial Mesenchymal Transition | Cellular Physiology and Biochemistry | Karger Publishers. Available online: https://karger.com/cpb/article/38/2/571/72552 (accessed on 28 December 2023).
- He, T.; Bai, X.; Yang, L.; Fan, L.; Li, Y.; Su, L.; Gao, J.; Han, S.; Hu, D. Loureirin B Inhibits Hypertrophic Scar Formation via Inhibition of the TGF-Β1-ERK/JNK Pathway. Cell. Physiol. Biochem. 2015, 37, 666–676. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Luque, J.; Ros, J.; Fernández-Varo, G.; Tugues, S.; Morales-Ruiz, M.; Alvarez, C.E.; Friedman, S.L.; Arroyo, V.; Jiménez, W. Regression of Fibrosis after Chronic Stimulation of Cannabinoid CB2 Receptor in Cirrhotic Rats. J. Pharmacol. Exp. Ther. 2008, 324, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Akhmetshina, A.; Dees, C.; Busch, N.; Beer, J.; Sarter, K.; Zwerina, J.; Zimmer, A.; Distler, O.; Schett, G.; Distler, J.H.W. The Cannabinoid Receptor CB2 Exerts Antifibrotic Effects in Experimental Dermal Fibrosis. Arthritis Rheum. 2009, 60, 1129–1136. [Google Scholar] [CrossRef] [PubMed]
- Dibba, P.; Li, A.; Cholankeril, G.; Iqbal, U.; Gadiparthi, C.; Khan, M.A.; Kim, D.; Ahmed, A. Mechanistic Potential and Therapeutic Implications of Cannabinoids in Nonalcoholic Fatty Liver Disease. Medicines 2018, 5, 47. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, P.; Rajesh, M.; Pan, H.; Patel, V.; Mukhopadhyay, B.; Bátkai, S.; Gao, B.; Haskó, G.; Pacher, P. Cannabinoid-2 Receptor Limits Inflammation, Oxidative/Nitrosative Stress, and Cell Death in Nephropathy. Free Radic. Biol. Med. 2010, 48, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, M.; Pan, H.; Mukhopadhyay, P.; Bátkai, S.; Osei-Hyiaman, D.; Haskó, G.; Liaudet, L.; Gao, B.; Pacher, P. Cannabinoid-2 Receptor Agonist HU-308 Protects against Hepatic Ischemia/Reperfusion Injury by Attenuating Oxidative Stress, Inflammatory Response, and Apoptosis. J. Leukoc. Biol. 2007, 82, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Dong, S.; Li, T.; Yang, F.; Yu, X.; Wu, J.; Zhong, X.; Zhao, Y.; Wang, L.; Xu, C.; et al. Exogenous Hydrogen Sulfide Attenuates Cardiac Fibrosis Through Reactive Oxygen Species Signal Pathways in Experimental Diabetes Mellitus Models. Cell. Physiol. Biochem. 2015, 36, 917–929. [Google Scholar] [CrossRef] [PubMed]
- NOX2-Induced Myocardial Fibrosis and Diastolic Dysfunction: Role of the Endothelium—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/24681139/ (accessed on 28 December 2023).
- Palazuelos, J.; Ortega, Z.; Díaz-Alonso, J.; Guzmán, M.; Galve-Roperh, I. CB2 Cannabinoid Receptors Promote Neural Progenitor Cell Proliferation via mTORC1 Signaling. J. Biol. Chem. 2012, 287, 1198–1209. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Sun, W.; Zhang, Z.; Zheng, Y. The Role of Nrf2-Mediated Pathway in Cardiac Remodeling and Heart Failure. Oxid. Med. Cell. Longev. 2014, 2014, 260429. [Google Scholar] [CrossRef]
- Yeh, Y.-H.; Kuo, C.-T.; Chang, G.-J.; Chen, Y.-H.; Lai, Y.-J.; Cheng, M.-L.; Chen, W.-J. Rosuvastatin Suppresses Atrial Tachycardia-Induced Cellular Remodeling via Akt/Nrf2/Heme Oxygenase-1 Pathway. J. Mol. Cell. Cardiol. 2015, 82, 84–92. [Google Scholar] [CrossRef]
- Jagtap, P.; Soriano, F.G.; Virág, L.; Liaudet, L.; Mabley, J.; Szabó, É.; Haskó, G.; Marton, A.; Lorigados, C.B.; Gallyas Jr, F. Novel Phenanthridinone Inhibitors of Poly (Adenosine 5′-Diphosphate-Ribose) Synthetase: Potent Cytoprotective and Antishock Agents. Crit. Care Med. 2002, 30, 1071–1082. [Google Scholar] [CrossRef]
- Schreiber, V.; Dantzer, F.; Ame, J.-C.; De Murcia, G. Poly (ADP-Ribose): Novel Functions for an Old Molecule. Nat. Rev. Mol. Cell Biol. 2006, 7, 517–528. [Google Scholar] [CrossRef]
- Virag, L. Structure and Function of Poly (ADP-Ribose) Polymerase-1: Role in Oxidative Stress-Related Pathologies. Curr. Vasc. Pharmacol. 2005, 3, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Szabó, C. Poly (ADP-Ribose) Polymerase Activation by Reactive Nitrogen Species—Relevance for the Pathogenesis of Inflammation. Nitric Oxide 2006, 14, 169–179. [Google Scholar] [CrossRef] [PubMed]
- Oliver, F.J. Resistance to Endotoxic Shock as a Consequence of Defective NF-Kappa B Activation in Poly (ADP-Ribose) Polymerase-1 Deficient Mice. EMBO J. 1999, 18, 4446–4454. [Google Scholar] [CrossRef]
- Ha, H.C.; Hester, L.D.; Snyder, S.H. Poly(ADP-Ribose) Polymerase-1 Dependence of Stress-Induced Transcription Factors and Associated Gene Expression in Glia. Proc. Natl. Acad. Sci. USA 2002, 99, 3270–3275. [Google Scholar] [CrossRef]
- Zingarelli, B.; Hake, P.W.; O’Connor, M.; Denenberg, A.; Wong, H.R.; Kong, S.; Aronow, B.J. Differential Regulation of Activator Protein-1 and Heat Shock Factor-1 in Myocardial Ischemia and Reperfusion Injury: Role of Poly(ADP-Ribose) Polymerase-1. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1408–H1415. [Google Scholar] [CrossRef]
- Bátkai, S.; Osei-Hyiaman, D.; Pan, H.; El-Assal, O.; Rajesh, M.; Mukhopadhyay, P.; Hong, F.; Harvey-White, J.; Jafri, A.; Haskó, G. Cannabinoid-2 Receptor Mediates Protection against Hepatic Ischemia/Reperfusion Injury. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2007, 21, 1788. [Google Scholar] [CrossRef]
- Bátkai, S.; Rajesh, M.; Mukhopadhyay, P.; Haskó, G.; Liaudet, L.; Cravatt, B.F.; Csiszár, A.; Ungvári, Z.; Pacher, P. Decreased Age-Related Cardiac Dysfunction, Myocardial Nitrative Stress, Inflammatory Gene Expression, and Apoptosis in Mice Lacking Fatty Acid Amide Hydrolase. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H909–H918. [Google Scholar] [CrossRef]
- Vendel, E.; de Lange, E.C. Functions of the CB 1 and CB 2 Receptors in Neuroprotection at the Level of the Blood–Brain Barrier. Neuromolecular Med. 2014, 16, 620–642. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, M.; Mukhopadhyay, P.; Bátkai, S.; Haskó, G.; Liaudet, L.; Huffman, J.W.; Csiszar, A.; Ungvari, Z.; Mackie, K.; Chatterjee, S.; et al. CB 2 -Receptor Stimulation Attenuates TNF-α-Induced Human Endothelial Cell Activation, Transendothelial Migration of Monocytes, and Monocyte-Endothelial Adhesion. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H2210–H2218. [Google Scholar] [CrossRef] [PubMed]
- Turcotte, C.; Blanchet, M.-R.; Laviolette, M.; Flamand, N. The CB2 Receptor and Its Role as a Regulator of Inflammation. Cell. Mol. Life Sci. 2016, 73, 4449–4470. [Google Scholar] [CrossRef] [PubMed]
- Montecucco, F.; Lenglet, S.; Braunersreuther, V.; Burger, F.; Pelli, G.; Bertolotto, M.; Mach, F.; Steffens, S. CB(2) Cannabinoid Receptor Activation Is Cardioprotective in a Mouse Model of Ischemia/Reperfusion. J. Mol. Cell. Cardiol. 2009, 46, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Rastaldo, R.; Pagliaro, P.; Cappello, S.; Penna, C.; Mancardi, D.; Westerhof, N.; Losano, G. Nitric Oxide and Cardiac Function. Life Sci. 2007, 81, 779–793. [Google Scholar] [CrossRef] [PubMed]
- Seddon, M.; Shah, A.M.; Casadei, B. Cardiomyocytes as Effectors of Nitric Oxide Signalling. Cardiovasc. Res. 2007, 75, 315–326. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Zhang, W.; Xue, J.; Wu, Y.Z.; Xu, W.; Liang, X.; Chen, T.; Kishimoto, C.; Yuan, Z. WIN55212-2 Ameliorates Atherosclerosis Associated with Suppression of pro-Inflammatory Responses in ApoE-Knockout Mice. Eur. J. Pharmacol. 2010, 649, 285–292. [Google Scholar] [CrossRef]
- Tyagi, S.; Gupta, P.; Saini, A.S.; Kaushal, C.; Sharma, S. The Peroxisome Proliferator-Activated Receptor: A Family of Nuclear Receptors Role in Various Diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236–240. [Google Scholar] [CrossRef]
- Yue, T.; Bao, W.; Jucker, B.M.; Gu, J.; Romanic, A.M.; Brown, P.J.; Cui, J.; Thudium, D.T.; Boyce, R.; Burns-Kurtis, C.L.; et al. Activation of Peroxisome Proliferator-Activated Receptor-Alpha Protects the Heart from Ischemia/Reperfusion Injury. Circulation 2003, 108, 2393–2399. [Google Scholar] [CrossRef]
- Hamblin, M.; Chang, L.; Fan, Y.; Zhang, J.; Chen, Y.E. PPARs and the Cardiovascular System. Antioxid. Redox Signal. 2009, 11, 1415–1452. [Google Scholar] [CrossRef]
- Sambandam, N.; Morabito, D.; Wagg, C.; Finck, B.N.; Kelly, D.P.; Lopaschuk, G.D. Chronic Activation of PPARalpha Is Detrimental to Cardiac Recovery after Ischemia. Am. J. Physiol. Heart Circ. Physiol. 2006, 290, H87–H95. [Google Scholar] [CrossRef]
- Zhong, C.-B.; Chen, X.; Zhou, X.-Y.; Wang, X.-B. The Role of Peroxisome Proliferator-Activated Receptor γ in Mediating Cardioprotection Against Ischemia/Reperfusion Injury. J. Cardiovasc. Pharmacol. Ther. 2018, 23, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Irrera, N.; D’Ascola, A.; Pallio, G.; Bitto, A.; Mazzon, E.; Mannino, F.; Squadrito, V.; Arcoraci, V.; Minutoli, L.; Campo, G.M.; et al. β-Caryophyllene Mitigates Collagen Antibody Induced Arthritis (CAIA) in Mice Through a Cross-Talk between CB2 and PPAR-γ Receptors. Biomolecules 2019, 9, 326. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.Y.; Kuo, Y.H.; Chang, Y.L.; Teng, C.M.; Wang, E.C.; Ishikawa, T.; Chen, I.S. Anti-Platelet Aggregation and Chemical Constituents from the Rhizome of Gynura Japonica. Planta Med. 2003, 69, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Baldissera, M.D.; Souza, C.F.; Grando, T.H.; Stefani, L.M.; Monteiro, S.G. β-Caryophyllene Reduces Atherogenic Index and Coronary Risk Index in Hypercholesterolemic Rats: The Involvement of Cardiac Oxidative Damage. Chem. Biol. Interact. 2017, 270, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Dong, Z.; Liu, S. β-Caryophyllene Ameliorates the Alzheimer-like Phenotype in APP/PS1 Mice through CB2 Receptor Activation and the PPARγ Pathway. Pharmacology 2014, 94, 1–12. [Google Scholar] [CrossRef]
- Bento, A.F.; Marcon, R.; Dutra, R.C.; Claudino, R.F.; Cola, M.; Leite, D.F.P.; Calixto, J.B. β-Caryophyllene Inhibits Dextran Sulfate Sodium-Induced Colitis in Mice through CB2 Receptor Activation and PPARγ Pathway. Am. J. Pathol. 2011, 178, 1153–1166. [Google Scholar] [CrossRef] [PubMed]
- Youssef, D.A.; El-Fayoumi, H.M.; Mahmoud, M.F. Beta-Caryophyllene Protects against Diet-Induced Dyslipidemia and Vascular Inflammation in Rats: Involvement of CB2 and PPAR-γ Receptors. Chem. Biol. Interact. 2019, 297, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Hunter, D.R.; Haworth, R.A.; Southard, J.H. Relationship between Configuration, Function, and Permeability in Calcium-Treated Mitochondria. J. Biol. Chem. 1976, 251, 5069–5077. [Google Scholar] [CrossRef] [PubMed]
- Saelens, X.; Festjens, N.; Walle, L.V.; van Gurp, M.; van Loo, G.; Vandenabeele, P. Toxic Proteins Released from Mitochondria in Cell Death. Oncogene 2004, 23, 2861–2874. [Google Scholar] [CrossRef] [PubMed]
- Perrelli, M.-G.; Pagliaro, P.; Penna, C. Ischemia/Reperfusion Injury and Cardioprotective Mechanisms: Role of Mitochondria and Reactive Oxygen Species. World J. Cardiol. 2011, 3, 186–200. [Google Scholar] [CrossRef] [PubMed]
- Hausenloy, D.J.; Yellon, D.M. The Mitochondrial Permeability Transition Pore: Its Fundamental Role in Mediating Cell Death during Ischaemia and Reperfusion. J. Mol. Cell. Cardiol. 2003, 35, 339–341. [Google Scholar] [CrossRef] [PubMed]
- Pacher, P.; Haskó, G. Endocannabinoids and Cannabinoid Receptors in Ischaemia–Reperfusion Injury and Preconditioning. Br. J. Pharmacol. 2008, 153, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Lépicier, P.; Bibeau-Poirier, A.; Lagneux, C.; Servant, M.J.; Lamontagne, D. Signaling Pathways Involved in the Cardioprotective Effects of Cannabinoids. J. Pharmacol. Sci. 2006, 102, 155–166. [Google Scholar] [CrossRef]
- Li, Q.; Guo, H.; Maslov, L.N.; Qiao, X.; Zhou, J.; Zhang, Y. Mitochondrial Permeability Transition Pore Plays a Role in the Cardioprotection of CB2 Receptor against Ischemia–Reperfusion Injury. Can. J. Physiol. Pharmacol. 2014, 92, 205–214. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive Oxygen Species (ROS) Homeostasis and Redox Regulation in Cellular Signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed]
- Kukielka, G.L.; Smith, C.W.; Manning, A.M.; Youker, K.A.; Michael, L.H.; Entman, M.L. Induction of Interleukin-6 Synthesis in the Myocardium. Circulation 1995, 92, 1866–1875. [Google Scholar] [CrossRef]
- von Knethen, A.; Callsen, D.; Brüne, B. Superoxide Attenuates Macrophage Apoptosis by NF-κB and AP-1 Activation That Promotes Cyclooxygenase-2 Expression1. J. Immunol. 1999, 163, 2858–2866. [Google Scholar] [CrossRef]
- Waypa, G.B.; Marks, J.D.; Mack, M.M.; Boriboun, C.; Mungai, P.T.; Schumacker, P.T. Mitochondrial Reactive Oxygen Species Trigger Calcium Increases During Hypoxia in Pulmonary Arterial Myocytes. Circ. Res. 2002, 91, 719–726. [Google Scholar] [CrossRef]
- Scarabelli, T.; Stephanou, A.; Rayment, N.; Pasini, E.; Comini, L.; Curello, S.; Ferrari, R.; Knight, R.; Latchman, D. Apoptosis of Endothelial Cells Precedes Myocyte Cell Apoptosis in Ischemia/Reperfusion Injury. Circulation 2001, 104, 253–256. [Google Scholar] [CrossRef]
- Karimian Azari, E.; Kerrigan, A.; O’Connor, A. Naturally Occurring Cannabinoids and Their Role in Modulation of Cardiovascular Health. J. Diet. Suppl. 2020, 17, 625–650. [Google Scholar] [CrossRef]
- Krijnen, P.a.J.; Meischl, C.; Hack, C.E.; Meijer, C.J.L.M.; Visser, C.A.; Roos, D.; Niessen, H.W.M. Increased Nox2 Expression in Human Cardiomyocytes after Acute Myocardial Infarction. J. Clin. Pathol. 2003, 56, 194–199. [Google Scholar] [CrossRef]
- Xu, D.; Xu, C.; Xue, X.; Xu, Y.; Zhao, J.; Huang, T.; Wang, Z.; Zhao, Q.; Zhou, Z.; Huang, Y.; et al. Activation of Cannabinoid Receptor 2 Attenuates Angiotensin II-Induced Atrial Fibrillation via a Potential NOX/CaMKII Mechanism. Front. Cardiovasc. Med. 2022, 9, 968014. [Google Scholar] [CrossRef]
- Libby, P. Current Concepts of the Pathogenesis of the Acute Coronary Syndromes. Circulation 2001, 104, 365–372. [Google Scholar] [CrossRef]
- Antioxidants | Free Full-Text | Glutathione: Antioxidant Properties Dedicated to Nanotechnologies. Available online: https://www.mdpi.com/2076-3921/7/5/62 (accessed on 30 December 2023).
- Dhalla, N.S.; Elmoselhi, A.B.; Hata, T.; Makino, N. Status of Myocardial Antioxidants in Ischemia–Reperfusion Injury. Cardiovasc. Res. 2000, 47, 446–456. [Google Scholar] [CrossRef]
- Díaz-Araya, G.; Nettle, D.; Castro, P.; Miranda, F.; Greig, D.; Campos, X.; Chiong, M.; Nazzal, C.; Corbalán, R.; Lavandero, S. Oxidative Stress after Reperfusion with Primary Coronary Angioplasty: Lack of Effect of Glucose-Insulin-Potassium Infusion. Crit. Care Med. 2002, 30, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Noichri, Y.; Chalghoum, A.; Chkioua, L.; Baudin, B.; Ernez, S.; Ferchichi, S.; Miled, A. Low Erythrocyte Catalase Enzyme Activity Is Correlated with High Serum Total Homocysteine Levels in Tunisian Patients with Acute Myocardial Infarction. Diagn. Pathol. 2013, 8, 68. [Google Scholar] [CrossRef] [PubMed]
- Cikim, G.; Canatan, H.; Gursu, M.F.; Gulcu, F.; Baydas, G.; Kilicoglu, A.E. Levels of Zinc and Lipid Peroxidation in Acute Coronary Syndrome. Biol. Trace Elem. Res. 2003, 96, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Holvoet, P.; Collen, D.; Van de Werf, F. Malondialdehyde-Modified LDL as a Marker of Acute Coronary Syndromes. JAMA 1999, 281, 1718–1721. [Google Scholar] [CrossRef] [PubMed]
- Amioka, N.; Miyoshi, T.; Otsuka, H.; Yamada, D.; Takaishi, A.; Ueeda, M.; Hirohata, S.; Ito, H. Serum Malondialdehyde-Modified Low-Density Lipoprotein Levels on Admission Predict Prognosis in Patients with Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. J. Cardiol. 2019, 74, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. The Immune System and Cardiac Repair. Pharmacol. Res. 2008, 58, 88–111. [Google Scholar] [CrossRef] [PubMed]
- Herskowitz, A.; Choi, S.; Ansari, A.A.; Wesselingh, S. Cytokine mRNA Expression in Postischemic/Reperfused Myocardium. Am. J. Pathol. 1995, 146, 419. [Google Scholar]
- Dewald, O.; Ren, G.; Duerr, G.D.; Zoerlein, M.; Klemm, C.; Gersch, C.; Tincey, S.; Michael, L.H.; Entman, M.L.; Frangogiannis, N.G. Of Mice and Dogs: Species-Specific Differences in the Inflammatory Response Following Myocardial Infarction. Am. J. Pathol. 2004, 164, 665–677. [Google Scholar] [CrossRef]
- Frangogiannis, N.G.; Lindsey, M.L.; Michael, L.H.; Youker, K.A.; Bressler, R.B.; Mendoza, L.H.; Spengler, R.N.; Smith, C.W.; Entman, M.L. Resident Cardiac Mast Cells Degranulate and Release Preformed TNF-α, Initiating the Cytokine Cascade in Experimental Canine Myocardial Ischemia/Reperfusion. Circulation 1998, 98, 699–710. [Google Scholar] [CrossRef]
- Maekawa, N.; Wada, H.; Kanda, T.; Niwa, T.; Yamada, Y.; Saito, K.; Fujiwara, H.; Sekikawa, K.; Seishima, M. Improved Myocardial Ischemia/Reperfusion Injury in Mice Lacking Tumor Necrosis Factor-α. J. Am. Coll. Cardiol. 2002, 39, 1229–1235. [Google Scholar] [CrossRef] [PubMed]
- Saxena, A.; Russo, I.; Frangogiannis, N.G. Inflammation as a Therapeutic Target in Myocardial Infarction: Learning from Past Failures to Meet Future Challenges. Transl. Res. 2016, 167, 152–166. [Google Scholar] [CrossRef] [PubMed]
- Lugrin, J.; Parapanov, R.; Rosenblatt-Velin, N.; Rignault-Clerc, S.; Feihl, F.; Waeber, B.; Müller, O.; Vergely, C.; Zeller, M.; Tardivel, A. Cutting Edge: IL-1α Is a Crucial Danger Signal Triggering Acute Myocardial Inflammation during Myocardial Infarction. J. Immunol. 2015, 194, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Kanda, T.; Takahashi, T. Interleukin-6 and Cardiovascular Diseases. Jpn. Heart J. 2004, 45, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, J.C.; Duerr, G.D.; Keppel, K.; Breitbach, M.; Fleischmann, B.K.; Zimmer, A.; Wehner, S.; Welz, A.; Dewald, O. CB2 Receptor-Mediated Effects of pro-Inflammatory Macrophages Influence Survival of Cardiomyocytes. Life Sci. 2015, 138, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Duerr, G.D.; Heinemann, J.C.; Gestrich, C.; Heuft, T.; Klaas, T.; Keppel, K.; Roell, W.; Klein, A.; Zimmer, A.; Velten, M.; et al. Impaired Border Zone Formation and Adverse Remodeling after Reperfused Myocardial Infarction in Cannabinoid CB2 Receptor Deficient Mice. Life Sci. 2015, 138, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, I.; Rotenberg, Z.; Sagie, A.; Fuchs, J.; Sperling, O.; Agmon, J. “Flipped” Lactic Dehydrogenase Pattern in Acute Coronary Insufficiency. Clin. Cardiol. 1986, 9, 597–599. [Google Scholar] [CrossRef]
- Hashiesh, H.M.; Sheikh, A.; Meeran, M.N.; Al, K.A.; Sadek, B.; Adeghate, E.; Ojha, S.K. β-Caryophyllene, a Dietary CB2 Receptor Selective Cannabinoid Mitigates Myocardial Fibrosis in a Mice Model of Diabetic Cardiomyopathy. Endocr. Abstr. 2022, 81. [Google Scholar] [CrossRef]
- Nagori, K.; Pradhan, M.; Nakhate, K.T.; Badwaik, H.R.; Deshmukh, R.; Roy, A.; Sharma, R.; Srivastava, S.P.; Chawla, S.; Jain, V.; et al. In Silico Molecular Docking Analysis of Some Terpenoids against 3CLpro of SARS-CoV-2. Res. J. Pharm. Technol. 2023, 16, 4791–4798. [Google Scholar] [CrossRef]
- Kamdi, S.P.; Badwaik, H.R.; Raval, A.; Ajazuddin; Nakhate, K.T. Ameliorative Potential of Phloridzin in Type 2 Diabetes-Induced Memory Deficits in Rats. Eur. J. Pharmacol. 2021, 913, 174645. [Google Scholar] [CrossRef]
- Nakhate, K.T.; Bharne, A.P.; Verma, V.S.; Aru, D.N.; Kokare, D.M. Plumbagin Ameliorates Memory Dysfunction in Streptozotocin Induced Alzheimer’s Disease via Activation of Nrf2/ARE Pathway and Inhibition of β-Secretase. Biomed. Pharmacother. 2018, 101, 379–390. [Google Scholar] [CrossRef]
- Katrukha, I.A. Human Cardiac Troponin Complex. Structure and Functions. Biochem. Biokhimiia 2013, 78, 1447–1465. [Google Scholar] [CrossRef] [PubMed]
- Jeremias, A.; Gibson, C.M. Narrative Review: Alternative Causes for Elevated Cardiac Troponin Levels When Acute Coronary Syndromes Are Excluded. Ann. Intern. Med. 2005, 142, 786–791. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
More, S.A.; Deore, R.S.; Pawar, H.D.; Sharma, C.; Nakhate, K.T.; Rathod, S.S.; Ojha, S.; Goyal, S.N. CB2 Cannabinoid Receptor as a Potential Target in Myocardial Infarction: Exploration of Molecular Pathogenesis and Therapeutic Strategies. Int. J. Mol. Sci. 2024, 25, 1683. https://doi.org/10.3390/ijms25031683
More SA, Deore RS, Pawar HD, Sharma C, Nakhate KT, Rathod SS, Ojha S, Goyal SN. CB2 Cannabinoid Receptor as a Potential Target in Myocardial Infarction: Exploration of Molecular Pathogenesis and Therapeutic Strategies. International Journal of Molecular Sciences. 2024; 25(3):1683. https://doi.org/10.3390/ijms25031683
Chicago/Turabian StyleMore, Sagar A., Rucha S. Deore, Harshal D. Pawar, Charu Sharma, Kartik T. Nakhate, Sumit S. Rathod, Shreesh Ojha, and Sameer N. Goyal. 2024. "CB2 Cannabinoid Receptor as a Potential Target in Myocardial Infarction: Exploration of Molecular Pathogenesis and Therapeutic Strategies" International Journal of Molecular Sciences 25, no. 3: 1683. https://doi.org/10.3390/ijms25031683
APA StyleMore, S. A., Deore, R. S., Pawar, H. D., Sharma, C., Nakhate, K. T., Rathod, S. S., Ojha, S., & Goyal, S. N. (2024). CB2 Cannabinoid Receptor as a Potential Target in Myocardial Infarction: Exploration of Molecular Pathogenesis and Therapeutic Strategies. International Journal of Molecular Sciences, 25(3), 1683. https://doi.org/10.3390/ijms25031683






