Microbiota–Gastric Cancer Interactions and the Potential Influence of Nutritional Therapies
Abstract
1. Introduction
2. Gastric Microbiota and Gastric Carcinogenesis
2.1. Mutual Relationships between Chronic Gastric Inflammatory Response to H. pylori and Gastric Microbiota Dysbiosis
2.2. Close Association between Gastric Microbiota Dysbiosis and GC
2.3. Potential Implications of Other Specific Gastric Bacteria in GC
2.3.1. Fusobacterium nucleatum
2.3.2. Lactobacillaceae Family
2.3.3. Nitrate Reductase Bacteria
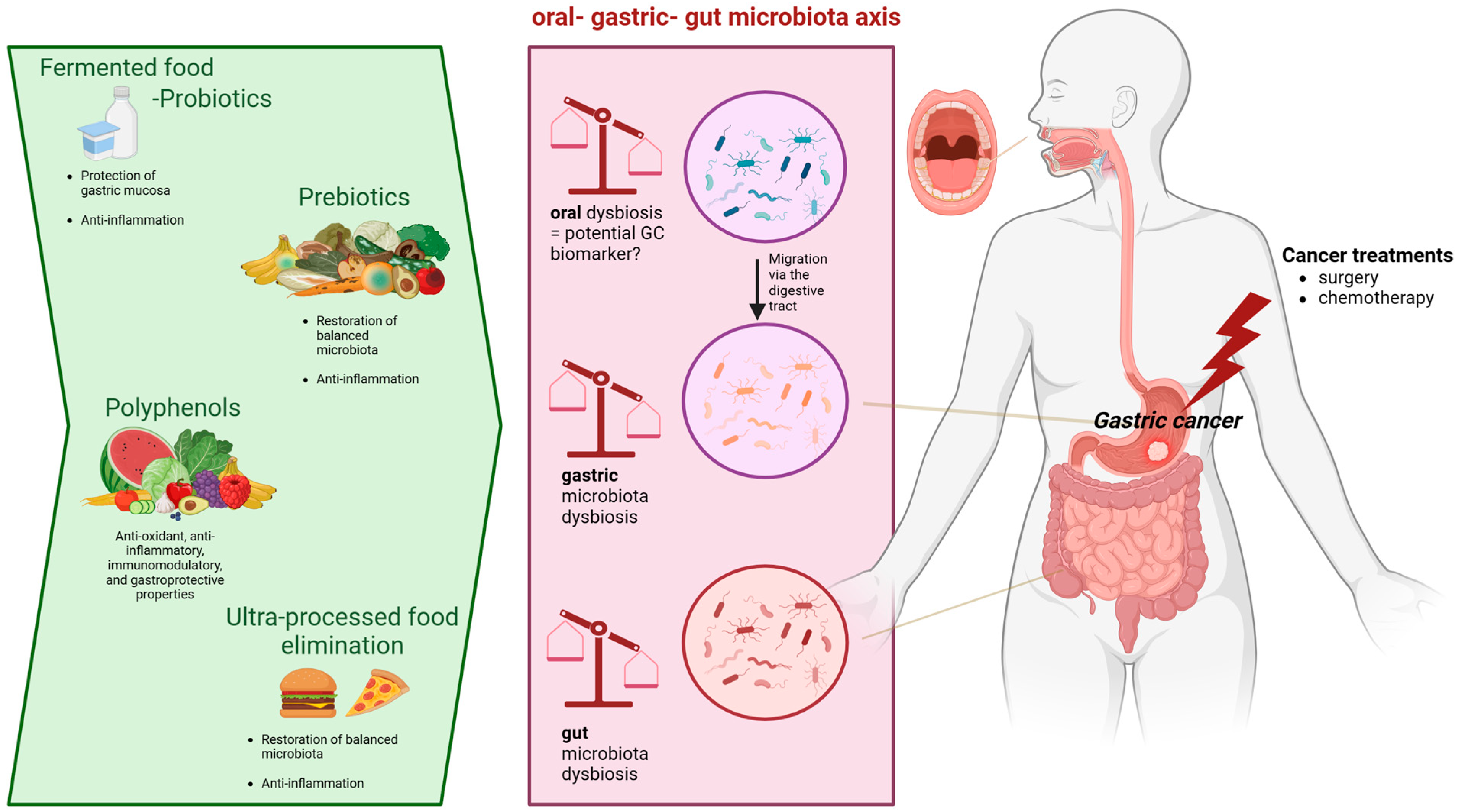
3. Oral Microbiota and GC
4. Gut Microbiota and GC
5. Diet: A Potential Therapy to Restore Microbiota Dysbiosis Associated with GC?
5.1. Role of SCFAs
5.2. Prebiotics
5.3. Polyphenols
5.4. Fermented Foods and Probiotics
5.5. Dietary Patterns
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018, GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2020, 70, 313. [Google Scholar] [CrossRef] [PubMed]
- Petryszyn, P.; Chapelle, N.; Matysiak-Budnik, T. GC: Where are we heading? Dig. Dis. 2020, 38, 280–285. [Google Scholar] [CrossRef] [PubMed]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nikšić, M.; Hood, M. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): Analysis of individual records for 37,513,025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- Binato, R.; Santos, E.C.; Boroni, M.; Demachki, S.; Assumpção, P.; Abdelhay, E. A common molecular signature of intestinal-type gastric carcinoma indicates processes related to gastric carcinogenesis. Oncotarget 2018, 9, 7359–7371. [Google Scholar] [CrossRef]
- Yu, G.; Torres, J.; Hu, N.; Medrano-Guzman, R.; Herrera-Goepfert, R.; Humphrys, M.S.; Wang, L.; Wang, C.; Ding, T.; Ravel, J.; et al. Molecular Characterization of the Human Stomach Microbiota in GC Patients. Front. Cell. Infect. Microbiol. 2017, 7, 302. [Google Scholar] [CrossRef] [PubMed]
- de Martel, C.; Georges, D.; Bray, F.; Ferlay, J.; Clifford, G.M. Global burden of cancer attributable to infections in 2018, a worldwide incidence analysis. Lancet Glob. Health 2020, 8, e180–e190. [Google Scholar] [CrossRef] [PubMed]
- Caselli, E.; Fabbri, C.; D’accolti, M.; Soffritti, I.; Bassi, C.; Mazzacane, S.; Franchi, M. Defining the oral microbiome by whole-genome sequencing and resistome analysis: The complexity of the healthy picture. BMC Microbiol. 2020, 20, 120. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-Y.; Hwang, B.-O.; Lim, M.; Ok, S.-H.; Lee, S.-K.; Chun, K.-S.; Park, K.-K.; Hu, Y.; Chung, W.-Y.; Song, N.-Y. Oral-gut microbiome axis in gastrointestinal disease and cancer. Cancers 2021, 13, 2124. [Google Scholar] [CrossRef]
- Chen, C.; Chen, L.; Lin, L.; Jin, D.; Du, Y.; Lyu, J. Research progress on gut microbiota in patients with gastric cancer, esophageal cancer, and small intestine cancer. Appl. Microbiol. Biotechnol. 2021, 105, 4415–4425. [Google Scholar] [CrossRef]
- Muscaritoli, M.; Arends, J.; Bachmann, P.; Baracos, V.; Barthelemy, N.; Bertz, H.; Bozzetti, F.; Hütterer, E.; Isenring, E.; Kaasa, S.; et al. ESPEN practical guideline: Clinical Nutrition in cancer. Clin. Nutr. 2021, 40, 2898–2913. [Google Scholar] [CrossRef]
- Llorca, L.; Pérez-Pérez, G.; Urruzuno, P.; Martinez, M.J.; Iizumi, T.; Gao, Z.; Sohn, J.; Chung, J.; Cox, L.; Simón-Soro, A.; et al. Characterization of the Gastric Microbiota in a Pediatric Population According to Helicobacter pylori Status. Pediatr. Infect. Dis. J. 2017, 36, 173–178. [Google Scholar] [CrossRef]
- Toh, J.W.T.; Wilson, R.B. Pathways of gastric carcinogenesis, Helicobacter pylori virulence and interactions with antioxidant systems, vitamin C and phytochemicals. Int. J. Mol. Sci. 2020, 21, 6451. [Google Scholar] [CrossRef]
- Li, Q.; Liu, J.; Gong, Y.; Yuan, Y. Serum VacA antibody is associated with risks of peptic ulcer and gastric cancer: A meta-analysis. Microb. Pathog. 2016, 99, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Liatsos, C.; Papaefthymiou, A.; Kyriakos, N.; Galanopoulos, M.; Doulberis, M.; Giakoumis, M.; Petridou, E.; Mavrogiannis, C.; Rokkas, T.; Kountouras, J. Helicobacter pylori, gastric microbiota and GC relationship: Unrolling the tangle. World J. Gastrointest. Oncol. 2022, 14, 959. [Google Scholar] [CrossRef]
- Pereira-Marques, J.; Ferreira, R.M.; Pinto-Ribeiro, I.; Figueiredo, C. Helicobacter pylori Infection, the Gastric Microbiome and GC. Adv. Exp. Med. Biol. 2019, 1149, 195–210. [Google Scholar]
- Guo, Y.; Zhang, Y.; Gerhard, M.; Gao, J.J.; Mejias-Luque, R.; Zhang, L.; Vieth, M.; Ma, J.-L.; Bajbouj, M.; Suchanek, S.; et al. Effect of Helicobacter pylori on gastrointestinal microbiota: A population-based study in Linqu, a high-risk area of GC. Gut 2020, 69, 1598–1607. [Google Scholar] [CrossRef] [PubMed]
- Vogtmann, E.; Goedert, J.J. Epidemiologic studies of the human microbiome and cancer. Br. J. Cancer 2016, 114, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Aviles-Jimenez, F.; Vazquez-Jimenez, F.; Medrano-Guzman, R.; Mantilla, A.; Torres, J. Stomach microbiota composition varies between patients with non-atrophic gastritis and patients with intestinal type of gastric cancer. Sci. Rep. 2014, 4, 4202. [Google Scholar] [CrossRef] [PubMed]
- Coker, O.O.; Dai, Z.; Nie, Y.; Zhao, G.; Cao, L.; Nakatsu, G.; Wu, W.K.; Wong, S.H.; Chen, Z.; Sung, J.J.Y.; et al. Mucosal microbiome dysbiosis in gastric carcinogenesis. Gut 2018, 67, 1024–1032. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.M.; Pereira-Marques, J.; Pinto-Ribeiro, I.; Costa, J.L.; Carneiro, F.; Machado, J.C.; Figueiredo, C. Gastric microbial community profiling reveals a dysbiotic cancer-associated microbiota. Gut 2018, 67, 226–236. [Google Scholar] [CrossRef]
- Gunathilake, M.N.; Lee, J.; Choi, I.J.; Kim, Y.I.; Ahn, Y.; Park, C.; Kim, J. Association between the relative abundance of gastric microbiota and the risk of GC: A case-control study. Sci. Rep. 2019, 9, 13589. [Google Scholar] [CrossRef]
- Gantuya, B.; El Serag, H.B.; Matsumoto, T.; Ajami, N.J.; Uchida, T.; Oyuntsetseg, K.; Bolor, D.; Yamaoka, Y. Gastric mucosal microbiota in a Mongolian population with GC and precursor conditions. Aliment. Pharmacol. Ther. 2020, 51, 770–780. [Google Scholar] [CrossRef] [PubMed]
- Rajilic-Stojanovic, M.; Figueiredo, C.; Smet, A.; Hansen, R.; Kupcinskas, J.; Rokkas, T.; Andersen, L.; Machado, J.C.; Ianiro, G.; Gasbarrini, A.; et al. Systematic review: Gastric microbiota in health and disease. Aliment. Pharmacol. Ther. 2020, 51, 582–602. [Google Scholar] [CrossRef] [PubMed]
- Brennan, C.A.; Garrett, W.S. Gut microbiota, inflammation, and colorectal cancer. Annu. Rev. Microbiol. 2016, 70, 395–411. [Google Scholar] [CrossRef] [PubMed]
- Kostic, A.D.; Chun, E.; Robertson, L.; Glickman, J.N.; Gallini, C.A.; Michaud, M.; Clancy, T.E.; Chung, D.C.; Lochhead, P.; Hold, G.L.; et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 2013, 14, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Rubinstein, M.R.; Wang, X.; Liu, W.; Hao, Y.; Cai, G.; Han, Y.W. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 2013, 14, 195–206. [Google Scholar] [CrossRef] [PubMed]
- Nie, S.; Wang, A.; Yuan, Y. Comparison of clinicopathological parameters, prognosis, micro-ecological environment and metabolic function of Gastric Cancer with or without Fusobacterium sp. Infect. J. Cancer 2021, 12, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.Y.; Tung, S.Y.; Pan, H.Y.; Chang, T.S.; Wei, K.L.; Chen, W.M.; Deng, Y.-F.; Lu, C.-K.; Lai, Y.-H.; Wu, C.-S.; et al. Fusobacterium nucleatum colonization is associated with decreased survival of Helicobacter pylori-positive gastric cancer patients. World J. Gastroenterol. 2021, 27, 7311–7323. [Google Scholar] [CrossRef]
- Hsieh, Y.Y.; Tung, S.Y.; Pan, H.Y.; Yen, C.W.; Xu, H.W.; Lin, Y.J.; Deng, Y.-F.; Hsu, W.-T.; Wu, C.-S.; Li, C. Increased abundance of clostridium and fusobacterium in gastric microbiota of patients with GC in Taiwan. Sci. Rep. 2018, 8, 158. [Google Scholar] [CrossRef]
- San-Millán, I.; Brooks, G.A. Reexamining cancer metabolism: Lactate production for carcinogenesis could be the purpose and explanation of the Warburg Effect. Carcinogenesis 2017, 38, 119–133. [Google Scholar] [CrossRef]
- Voss, D.M.; Spina, R.; Carter, D.L.; Lim, K.S.; Jeffery, C.J.; Bar, E.E. Disruption of the monocarboxylate transporter-4-basigin interaction inhibits the hypoxic response, proliferation, and tumor progression. Sci. Rep. 2017, 7, 4292. [Google Scholar] [CrossRef]
- Brand, A.; Singer, K.; Koehl, G.E.; Kolitzus, M.; Schoenhammer, G.; Thiel, A.; Matos, C.; Bruss, C.; Klobuch, S.; Peter, K.; et al. LDHA-Associated Lactic Acid Production Blunts Tumor Immunosurveillance by T and NK Cells. Cell Metab. 2016, 24, 657–671. [Google Scholar] [CrossRef]
- Castaño-Rodríguez, N.; Goh, K.L.; Fock, K.M.; Mitchell, H.M.; Kaakoush, N.O. Dysbiosis of the microbiome in gastric carcinogenesis. Sci. Rep. 2017, 7, 15957. [Google Scholar] [CrossRef]
- Lertpiriyapong, K.; Whary, M.T.; Muthupalani, S.; Lofgren, J.L.; Gamazon, E.R.; Feng, Y.; Ge, Z.; Wang, T.C.; Fox, J.G. Gastric colonisation with a restricted commensal microbiota replicates the promotion of neoplastic lesions by diverse intestinal microbiota in the Helicobacter pylori INS-GAS mouse model of gastric carcinogenesis. Gut 2014, 63, 54–63. [Google Scholar] [CrossRef]
- Chen, Y.X.; He, L.L.; Xiang, X.P.; Shen, J.; Qi, H.Y. O6-methylguanine DNA methyltransferase is upregulated in malignant transformation of gastric epithelial cells via its gene promoter DNA hypomethylation. World J. Gastrointest. Oncol. 2022, 14, 664–677. [Google Scholar] [CrossRef]
- Thorell, K.; Bengtsson-Palme, J.; Liu, O.H.; Palacios Gonzales, R.V.; Nookaew, I.; Rabeneck, L.; Paszat, L.; Graham, D.Y.; Nielsen, J.; Lundin, S.B.; et al. In Vivo Analysis of the Viable Microbiota and Helicobacter pylori Transcriptome in Gastric Infection and Early Stages of Carcinogenesis. Infect. Immun. 2017, 85, e00031-17. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Zhou, J.; Xin, Y.; Geng, C.; Tian, Z.; Yu, X.; Dong, Q. Bacterial overgrowth and diversification of microbiota in GC. Eur. J. Gastroenterol. Hepatol. 2016, 28, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Huang, X.; Tong, D.; Jiang, C.; Zhu, X.; Wei, Z.; Jin, C. Relationships among microbiota, gastric cancer, and immunotherapy. Front. Microbiol. 2022, 13, 987763. [Google Scholar] [CrossRef] [PubMed]
- Dicksved, J.; Lindberg, M.; Rosenquist, M.; Enroth, H.; Jansson, J.K.; Engstrand, L. Molecular characterization of the stomach microbiota in patients with gastric cancer and in controls. J. Med. Microbiol. 2009, 58 Pt 4, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Han, S.; Chen, Y.; Ji, Z. Variations of Tongue Coating Microbiota in Patients with Gastric Cancer. Biomed. Res. Int. 2015, 2015, 173729. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.H.; Li, X.L.; Yin, J.; Li, Y.H.; Hou, B.X.; Zhang, Z. A screening method for gastric cancer by oral microbiome detection. Oncol. Rep. 2018, 39, 2217–2224. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Gao, X.; Wu, L.; Yan, B.; Wang, Z.; Zhang, X.; Peng, L.; Yu, J.; Sun, G.; Yang, Y. Salivary microbiota for GC prediction: An exploratory study. Front. Cell. Infect. Microbiol. 2021, 11, 640309. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Xu, S.; Xiang, C.; Cao, Q.; Li, Q.; Huang, J.; Shi, L.; Zhang, J.; Zhan, Z. Tongue coating microbiota community and risk effect on GC. J. Cancer 2018, 9, 4039–4048. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Long, J.; Wang, C.; Blot, W.; Pei, Z.; Shu, X.; Wu, F.; Rothman, N.; Wu, J.; Lan, Q.; et al. Prospective study of oral microbiome and GC risk among Asian, African American and European American populations. Int. J. Cancer 2022, 150, 916–927. [Google Scholar] [CrossRef]
- Nomura, R.; Kadota, T.; Ogaya, Y.; Matayoshi, S.; Iwashita, N.; Okawa, R.; Nakano, K. Contribution of streptococcus mutans to Helicobacter pylori colonisation in oral cavity and gastric tissue. Sci. Rep. 2020, 10, 12540. [Google Scholar] [CrossRef]
- Chen, C.C.; Liou, J.M.; Lee, Y.C.; Hong, T.C.; El-Omar, E.M.; Wu, M.S. The interplay between helicobacter pylori and gastrointestinal microbiota. Gut Microbes 2021, 13, 1909459. [Google Scholar] [CrossRef]
- Hu, Y.L.; Pang, W.; Huang, Y.; Zhang, Y.; Zhang, C.J. The Gastric Microbiome Is Perturbed in Advanced Gastric Adenocarcinoma Identified Through Shotgun Metagenomics. Front. Cell. Infect. Microbiol. 2018, 8, 433. [Google Scholar] [CrossRef]
- Barra, W.F.; Sarquis, D.P.; Khayat, A.S.; Khayat, B.C.M.; Demachki, S.; Anaissi, A.K.M.; Ishak, G.; Santos, N.P.C.; dos Santos, S.E.B.; Burbano, R.R.; et al. Gastric Cancer Microbiome. Pathobiology 2021, 88, 156–169. [Google Scholar] [CrossRef]
- Wang, D.; Li, Y.; Zhong, H.; Ding, Q.; Lin, Y.; Tang, S.; Zong, Y.; Wang, Q.; Zhang, X.; Yang, H.; et al. Alterations in the human gut microbiome associated with Helicobacter pylori infection. FEBS Open Bio 2019, 9, 1552–1560. [Google Scholar] [CrossRef]
- Liang, W.; Yang, Y.; Wang, H.; Wang, H.; Yu, X.; Lu, Y.; Shen, S.; Teng, L. Gut microbiota shifts in patients with GC in perioperative period. Medicine 2019, 98, e16626. [Google Scholar] [CrossRef]
- Sarhadi, V.; Mathew, B.; Kokkola, A.; Karla, T.; Tikkanen, M.; Rautelin, H.; Lahti, L.; Puolakkainen, P.; Knuutila, S. Gut microbiota of patients with different subtypes of GC and gastrointestinal stromal tumors. Gut Pathog. 2021, 13, 11. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.B.; Pan, S.Y.; Jin, P.; Deng, J.W.; Xue, J.H.; Ma, X.Y.; Xie, Y.-H.; Cao, H.; Liu, Q.; Xie, W.-F.; et al. Fecal Signatures of Streptococcus anginosus and Streptococcus constellatus for Noninvasive Screening and Early Warning of GC. Gastroenterology 2022, 162, 1933–1947.E18. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.-B.; Qiu, H.-Y. Effects of Artesunate on chondrocyte proliferation, apoptosis and autophagy through the PI3K/AKT/mTOR signaling pathway in rat models with rheumatoid arthritis. Biomed. Pharmacother. 2018, 102, 1209–1220. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Ran, X.; Li, B.; Li, Y.; He, D.; Huang, B.; Fu, S.; Liu, J.; Wang, W. Sodium butyrate inhibits inflammation and maintains epithelium barrier integrity in a TNBS-induced inflammatory bowel disease mice model. eBioMedicine 2018, 30, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.L.; Lee, W.; Chung, S.H.; Yu, B.M.; Lee, Y.C.; Hong, J. Metabolic alterations of short-chain fatty acids and TCA cycle intermediates in human plasma from patients with gastric cancer. Life Sci. 2022, 309, 121010. [Google Scholar] [CrossRef] [PubMed]
- Nouri, Z.; Choi, S.W.; Choi, I.J.; Ryu, K.W.; Woo, S.M.; Park, S.J.; Lee, W.J.; Choi, W.; Jung, Y.-S.; Myung, S.-K.; et al. Exploring Connections between Oral Microbiota, Short-Chain Fatty Acids, and Specific Cancer Types: A Study of Oral Cancer, Head and Neck Cancer, Pancreatic Cancer, and Gastric Cancer. Cancers 2023, 15, 2898. [Google Scholar] [CrossRef] [PubMed]
- Yonezawa, H.; Osaki, T.; Hanawa, T.; Kurata, S.; Zaman, C.; Woo, T.D.H.; Takahashi, M.; Matsubara, S.; Kawakami, H.; Ochiai, K.; et al. Destructive effects of butyrate on the cell envelope of Helicobacter pylori. J. Med. Microbiol. 2012, 61, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; He, P.; Liu, Y.; Qi, M.; Dong, W. Combining sodium butyrate with cisplatin increases the apoptosis of gastric cancer in vivo and in vitro via the mitochondrial apoptosis pathway. Front. Pharmacol. 2021, 2, 708093. [Google Scholar] [CrossRef]
- Sanders, M.E.; Merenstein, D.J.; Reid, G.; Gibson, G.R.; Rastall, R.A. Probiotics and prebiotics in intestinal health and disease: From biology to the clinic. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 605–616. [Google Scholar] [CrossRef]
- Hansen, S.L.; Purup, S.; Christensen, L.P. Bioactivity of falcarinol and the influence of processing and storage on its content in carrots (Daucus carota L.). J. Sci. Food Agric. 2003, 83, 1010–1017. [Google Scholar] [CrossRef]
- Young, J.F.; Duthie, S.J.; Milne, L.; Christensen, L.P.; Duthie, G.G.; Bestwick, C.S. Biphasic effect of falcarinol on CaCo-2 cell proliferation, DNA damage, and apoptosis. J. Agric. Food Chem. 2007, 55, 618–623. [Google Scholar] [CrossRef]
- Zaini, R.G.; Brandt, K.; Clench, M.R.; Le Maitre, C.L. Effects of bioactive compounds from carrots (Daucus carota L.), polyacetylenes, beta-carotene and lutein on human lymphoid leukaemia cells. Anticancer Agents Med. Chem. 2012, 12, 640–652. [Google Scholar] [CrossRef]
- Tarifa, M.C.; Piqueras, C.M.; Genovese, D.B.; Brugnoni, L.I. Microencapsulation of Lactobacillus casei and Lactobacillus rhamnosus in pectin and pectin-inulin microgel particles: Effect on bacterial survival under storage conditions. Int. J. Biol. Macromol. 2021, 179, 457–465. [Google Scholar] [CrossRef]
- Weimann, A.; Braga, M.; Harsanyi, L.; Laviano, A.; Ljungqvist, O.; Soeters, P.; Jauch, K.; Kemen, M.; Hiesmayr, J.; Horbach, T.; et al. ESPEN guidelines on enteral nutrition: Surgery including organ transplantation. Clin. Nutr. 2006, 25, 224–244. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, Y.; Huang, Y.; Cui, Y.; Xia, L.; Rao, Z.; Zhou, Y.; Wu, X. Effects of fiber and probiotics on diarrhea associated with enteral nutrition in gastric cancer patients: A prospective randomized and controlled trial. Medicine 2017, 96, e8418. [Google Scholar] [CrossRef]
- Bloemen, J.G.; Schreinemacher, M.H.; de Bruine, A.P.; Buurman, W.A.; Bouvy, N.D.; Dejong, C.H. Butyrate enemas improve intestinal anastomotic strength in a rat model. Dis. Colon. Rectum. 2010, 53, 1069–1075. [Google Scholar] [CrossRef]
- Larrosa, M.; Luceri, C.; Vivoli, E.; Pagliuca, C.; Lodovici, M.; Moneti, G.; Dolara, P. Polyphenol metabolites from colonic microbiota exert anti-inflammatory activity on different inflammation models. Mol. Nutr. Food Res. 2009, 53, 1044–1054. [Google Scholar] [CrossRef] [PubMed]
- Cueva, C.; Gil-Sánchez, I.; Ayuda-Durán, B.; González-Manzano, S.; González-Paramás, A.M.; Santos-Buelga, C.; Bartolomé, B.; Moreno-Arribas, M.V. An Integrated View of the Effects of Wine Polyphenols and Their Relevant Metabolites on Gut and Host Health. Molecules 2017, 22, 99. [Google Scholar] [CrossRef] [PubMed]
- Ma, G.; Yanting, C. Polyphenol supplementation benefits human health via gut microbiota: A systematic review via meta-analysis. J. Funct. Foods 2020, 66, 103829. [Google Scholar] [CrossRef]
- Afzal, M.; Safer, A.M.; Menon, M. Green Tea Polyphenols and Their Potential Role in Health and Disease. Inflammopharmacology 2015, 23, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.Y.; Zhao, S.; Lv, G.J.; Ma, X.J.; Zhang, J.B. Mechanisms of Resveratrol in the Prevention and Treatment of Gastrointestinal Cancer. World J. Clin. Cases 2020, 8, 2425–2437. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Anand, P.; Aggarwal, B.B. Curcumin Inhibits Proliferation, Invasion, Angiogenesis and Metastasis of Different Cancers through Interaction with Multiple Cell Signaling Proteins. Cancer Lett. 2008, 269, 199–225. [Google Scholar] [CrossRef]
- Davoodvandi, A.; Shabani Varkani, M.; Clark, C.C.T.; Jafarnejad, S. Quercetin as an Anticancer Agent: Focus on Esophageal Cancer. J. Food Biochem. 2020, 44, e13374. [Google Scholar] [CrossRef]
- Chiu, H.F.; Venkatakrishnan, K.; Golovinskaia, O.; Wang, C.K. Gastroprotective Effects of Polyphenols against Various Gastro-Intestinal Disorders: A Mini-Review with Special Focus on Clinical Evidence. Molecules 2021, 26, 2090. [Google Scholar] [CrossRef]
- Kim, S.; Kim, W.; Kim, D.H.; Jang, J.H.; Kim, S.J.; Park, S.A.; Hahn, H.; Han, B.W.; Na, H.-K.; Chun, K.-S.; et al. Resveratrol suppresses gastric cancer cell proliferation and survival through inhibition of PIM-1 kinase activity. Arch. Biochem. Biophys. 2020, 689, 108413. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, Q.; Chen, Z.; Ni, H.; Xia, L.; Zhao, Q.; Chen, Z.; Chen, P. Resveratrol suppresses the invasion and migration of human gastric cancer cells via inhibition of malat1-mediated epithelial-to-mesenchymal transition. Exp. Ther. Med. 2019, 17, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Zhang, R.; Hu, Y.; Li, W.; Wang, M.; Liang, Z.; Sun, Z.; Ji, R.; Xu, W.; Qian, H. Gastric-cancer-derived mesenchymal stem cells: A promising target for resveratrol in the suppression of gas- tric cancer metastasis. Hum. Cell 2020, 33, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.H.; Xiao, Y.; Li, X.Q.; Fan, L.; Zhou, C.C.; Cheng, L.; Jiang, Z.-D.; Wang, G.-H. Resveratrol counteracts hypoxia-induced gastric cancer invasion and EMT through hedge- hog pathway suppression. Anti-Cancer Agents Med. Chem. 2020, 20, 1105–1114. [Google Scholar] [CrossRef]
- Ren, M.; Zhou, X.; Gu, M.; Jiao, W.; Yu, M.; Wang, Y.; Liu, S.; Yang, J.; Ji, F. Resveratrol synergizes with cis- platin in antineoplastic effects against AGS gastric cancer cells by inducing endoplasmic reticulum stress-mediated apoptosis and G2/M phase arrest. Oncol. Rep. 2020, 44, 1605–1615. [Google Scholar] [CrossRef] [PubMed]
- Pilotte, L.; Larrieu, P.; Stroobant, V.; Colau, D.; Dolusic, E.; Frédérick, R.; De Plaen, E.; Uyttenhove, C.; Wouters, J.; Masereel, B.; et al. Reversal of tumoral immune resistance by inhibition of tryptophan 2, 3-dioxygenase. Proc. Natl. Acad. Sci. USA 2012, 109, 2497–2502. [Google Scholar] [CrossRef] [PubMed]
- Hull, M.A. Biospecimen Collection for: Prebiotic Effect of Eicosapentaenoic Acid Treatment for Colorectal Cancer Liver Metastases. 2021; Report No.: NCT04682665. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT04682665 (accessed on 1 December 2023).
- Stiemsma, L.T.; Nakamura, R.E.; Nguyen, J.G.; Michels, K.B. Does Consumption of Fermented Foods Modify the Human Gut Microbiota? J. Nutr. 2020, 150, 1680–1692. [Google Scholar] [CrossRef]
- Collatuzzo, G.; Negri, E.; Pelucchi, C.; Bonzi, R.; Turati, F.; Rabkin, C.S.; Liao, L.M.; Sinha, R.; Palli, D.; Ferraroni, M.; et al. Yoghurt Intake and Gastric Cancer: A Pooled Analysis of 16 Studies of the StoP Consortium. Nutrients 2023, 15, 1877. [Google Scholar] [CrossRef]
- Vinasco, K.; Mitchell, H.M.; Kaakoush, N.O.; Castaño-Rodríguez, N. Microbial carcinogenesis: Lactic acid bacteria in gastric cancer. Biochim. Biophys. Acta Rev. Cancer 2019, 1872, 188309. [Google Scholar] [CrossRef]
- Erawijantari, P.P.; Mizutani, S.; Shiroma, H.; Shiba, S.; Nakajima, T.; Sakamoto, T.; Saito, Y.; Fukuda, S.; Yachida, S.; Yamada, T. Influence of gastrectomy for gastric cancer treatment on faecal microbiome and metabolome profiles. Gut 2020, 69, 1404–1415. [Google Scholar] [CrossRef]
- Linsalata, M.; Russo, F.; Notarnicola, M.; Guerra, V.; Cavallini, A.; Clemente, C.; Messa, C. Effects of genistein on the polyamine metabolism and cell growth in DLD-1 human colon cancer cells. Nutr. Cancer 2005, 52, 84–93. [Google Scholar] [CrossRef]
- Cao, W.; Zheng, C.; Xu, X.; Jin, R.; Huang, F.; Shi, M.; He, Z.; Luo, Y.; Liu, L.; Liu, Z.; et al. Clostridium butyricum potentially improves inflammation and immunity through alteration of the microbiota and metabolism of gastric cancer patients after gastrectomy. Front. Immunol. 2022, 13, 1076245. [Google Scholar] [CrossRef]
- Zheng, C.; Chen, T.; Wang, Y.; Gao, Y.; Kong, Y.; Liu, Z.; Deng, X. A randomised trial of probiotics to reduce severity of physiological and microbial disorders induced by partial gastrectomy for patients with gastric cancer. J Cancer 2019, 10, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Arai, J.; Niikura, R.; Hayakawa, Y.; Kawahara, T.; Honda, T.; Hasatani, K.; Yoshida, N.; Nishida, T.; Sumiyoshi, T.; Kiyotoki, S.; et al. Use of antibiotics and probiotics reduces the risk of metachronous gastric cancer after endoscopic resection. Biology 2021, 10, 455. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Sun, S.; Gu, J.; Ni, H.; Zhong, K.; Xu, Q.; Zhou, D.; Wang, X.; Gao, L.; Zhu, X. Roux-en-Y reconstruction alleviates radical gastrectomy-induced colitis via down-regulation of the butyrate/NLRP3 signaling pathway. eBioMedicine 2022, 86, 104347. [Google Scholar] [CrossRef] [PubMed]
- Karimi, P.; Islami, F.; Anandasabapathy, S.; Freedman, N.D.; Kamangar, F. Gastric cancer: Descriptive epidemiology, risk factors, screening, and prevention. Cancer Epidemiol. Biomark. Prev. 2014, 23, 700–713. [Google Scholar] [CrossRef] [PubMed]
- Song, P.; Wu, L.; Guan, W. Dietary Nitrates, Nitrites, and Nitrosamines Intake and the Risk of Gastric Cancer: A Meta-Analysis. Nutrients 2015, 7, 9872–9895. [Google Scholar] [CrossRef] [PubMed]
- Jo, H.J.; Kim, J.; Kim, N.; Park, J.H.; Nam, R.H.; Seok, Y.-J.; Kim, Y.; Kim, J.S.; Kim, J.M.; Lee, D.H.; et al. Analysis of gastric microbiota by pyrosequencing: Minor role of bacteria other than Helicobacter pylori in the gastric carcinogenesis. Helicobacter 2016, 21, 364–374. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Gundamaraju, R.; Jha, N.K.; Gupta, P.K.; Dey, A.; Mandal, C.C.; Ford, B.M. Interplay between dysbiosis of gut microbiome, lipid metabolism, and tumorigenesis: Can gut dysbiosis stand as a prognostic marker in cancer? Dis. Markers 2022, 2022, 2941248. [Google Scholar] [CrossRef]
- Thrastardottir, T.O.; Copeland, V.J.; Constantinou, C. The Association Between the Gut Microbiome, Nutritional Habits, Antibiotics, and Gastric Cancer: A Scoping Review. Curr. Nutr. Rep. 2022, 11, 19–38. [Google Scholar] [CrossRef]
- Rinninella, E.; Mele, M.C.; Cintoni, M.; Raoul, P.; Ianiro, G.; Salerno, L.; Pozzo, C.; Bria, E.; Muscaritoli, M.; Molfino, A.; et al. The facts about food after cancer diagnosis: A systematic review of prospective cohort studies. Nutrients 2020, 12, 2345. [Google Scholar] [CrossRef] [PubMed]
- Vernieri, C.; Fucà, G.; Ligorio, F.; Huber, V.; Vingiani, A.; Iannelli, F.; Raimondi, A.; Rinchai, D.; Frigè, G.; Belfiore, A.; et al. Fasting-mimicking diet is safe and reshapes metabolism and antitumor immunity in patients with cancer. Cancer Discov. 2022, 12, 90–107. [Google Scholar] [CrossRef] [PubMed]
- Hur, H. Prospective Clinical Study for Early Recovery after Gastric Cancer Surgery. 2012; Report No.: NCT01642953. Available online: https://classic.clinicaltrials.gov/ct2/show/NCT01642953 (accessed on 1 December 2023).
| Microbiota Compositional Changes | Mechanisms of Carcinogenesis | References | |
|---|---|---|---|
| Oral microbiota | ↑ Veillonella ↑ Neisseria ↑ Haemophilus ↑ Porphyromonas ↑ Bacillota ↓ Bacteroides | Periodontal infection → gastric chronic inflammation and dysbiosis ↑ N-nitroso compounds | [40,41,42,43] |
| ↑ Streptococcus | ↑ Induction of alcohol to be oxidized to acetaldehyde, a group I human carcinogen | [45] | |
| ↑ Candida albicans, Fusobacterium nucleatum, Porphyromonas gingivalis, and Streptococcus mutans | ↑ Interaction H. pylori → ↑ H. pylori survival in the unsuitable environment of the mouth, fostering H. pylori migration to the stomach and exertion of its carcinogenic role | [45] | |
| Gastric microbiota | ↑ H. pylori ↑ Gastric dysbiosis | Oncoprotein cytotoxin-associated gene A Destabilization of cellular junctions via disruption of the E-cadherin-β-catenin complex Activation of proliferating pathways such as the ERK-MAP kinase cascade Oncogenic transformation of gastric cells via vacuolation of cytotoxin A Promotion of abnormal cell survival Inflammatory effect on the gastric mucosal surface of the stomach with altered production of mucin | [12,13,14,15,16] |
| ↑ Nitrate reductase bacteria | ↑ Nitrate reductase activity ↑ Proinflammatory activity | [36,37] | |
| ↑ Streptococcus, Lactobacillaceae family, and Lactococcus | ↑ N-nitroso compounds ↑ Reactive oxygen species Promotion of DNA damage | [30,31,32,33] | |
| ↓ Lactococcus lactis | ↓ Antiproliferative activity in a human stomach cancer cell line ↑ Cell apoptosis | [21] | |
| ↑ Fusobacterium nucleatum ↓ Bacterial diversity | ↑ NF-κB, IL-1β, IL-6, IL-8, TNF, and FadA | [22,24,25,26,27,28] | |
| Gut microbiota | ↑ Enterobacteriaceae ↓ Bifidobacterium ↓ Bacteroides ↑ Shigella ↑ Clostridium perfringens ↓ Bacterial diversity | ↓ Production of SCFAs and their protective effects on the cell cycle, apoptosis, and immune stimulation, impacting the Akt/mTOR and MEK/ERK signaling pathways ↓ Inhibition of NF-κB | [50,51,52,53] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raoul, P.; Maccauro, V.; Cintoni, M.; Scarpellini, E.; Ianiro, G.; Gasbarrini, A.; Mele, M.C.; Rinninella, E. Microbiota–Gastric Cancer Interactions and the Potential Influence of Nutritional Therapies. Int. J. Mol. Sci. 2024, 25, 1679. https://doi.org/10.3390/ijms25031679
Raoul P, Maccauro V, Cintoni M, Scarpellini E, Ianiro G, Gasbarrini A, Mele MC, Rinninella E. Microbiota–Gastric Cancer Interactions and the Potential Influence of Nutritional Therapies. International Journal of Molecular Sciences. 2024; 25(3):1679. https://doi.org/10.3390/ijms25031679
Chicago/Turabian StyleRaoul, Pauline, Valeria Maccauro, Marco Cintoni, Emidio Scarpellini, Gianluca Ianiro, Antonio Gasbarrini, Maria Cristina Mele, and Emanuele Rinninella. 2024. "Microbiota–Gastric Cancer Interactions and the Potential Influence of Nutritional Therapies" International Journal of Molecular Sciences 25, no. 3: 1679. https://doi.org/10.3390/ijms25031679
APA StyleRaoul, P., Maccauro, V., Cintoni, M., Scarpellini, E., Ianiro, G., Gasbarrini, A., Mele, M. C., & Rinninella, E. (2024). Microbiota–Gastric Cancer Interactions and the Potential Influence of Nutritional Therapies. International Journal of Molecular Sciences, 25(3), 1679. https://doi.org/10.3390/ijms25031679












