Two-Photon and Multiphoton Microscopy in Anterior Segment Diseases of the Eye
Abstract
1. Introduction
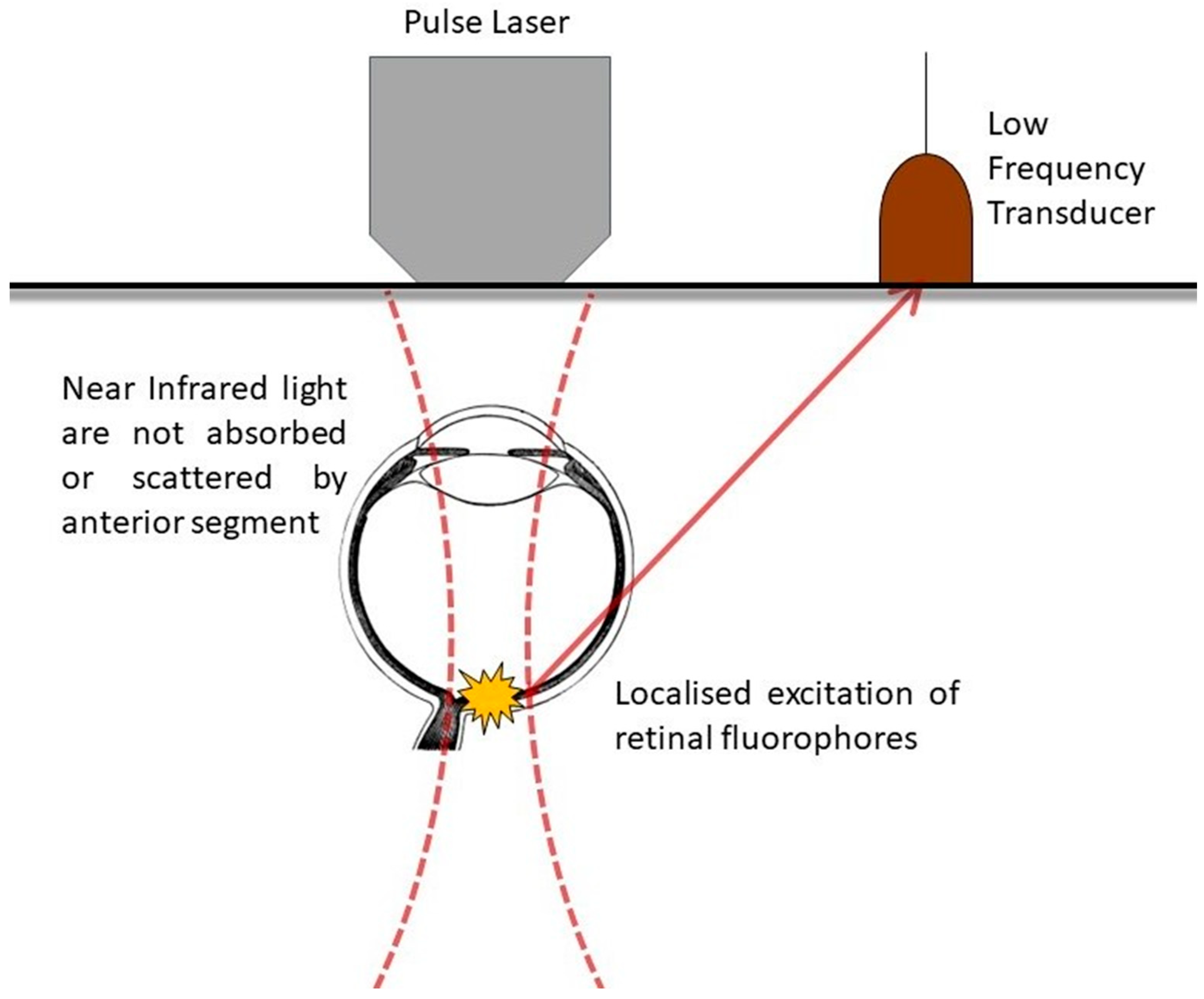
2. Applied Physics
3. History of Use in the Eye
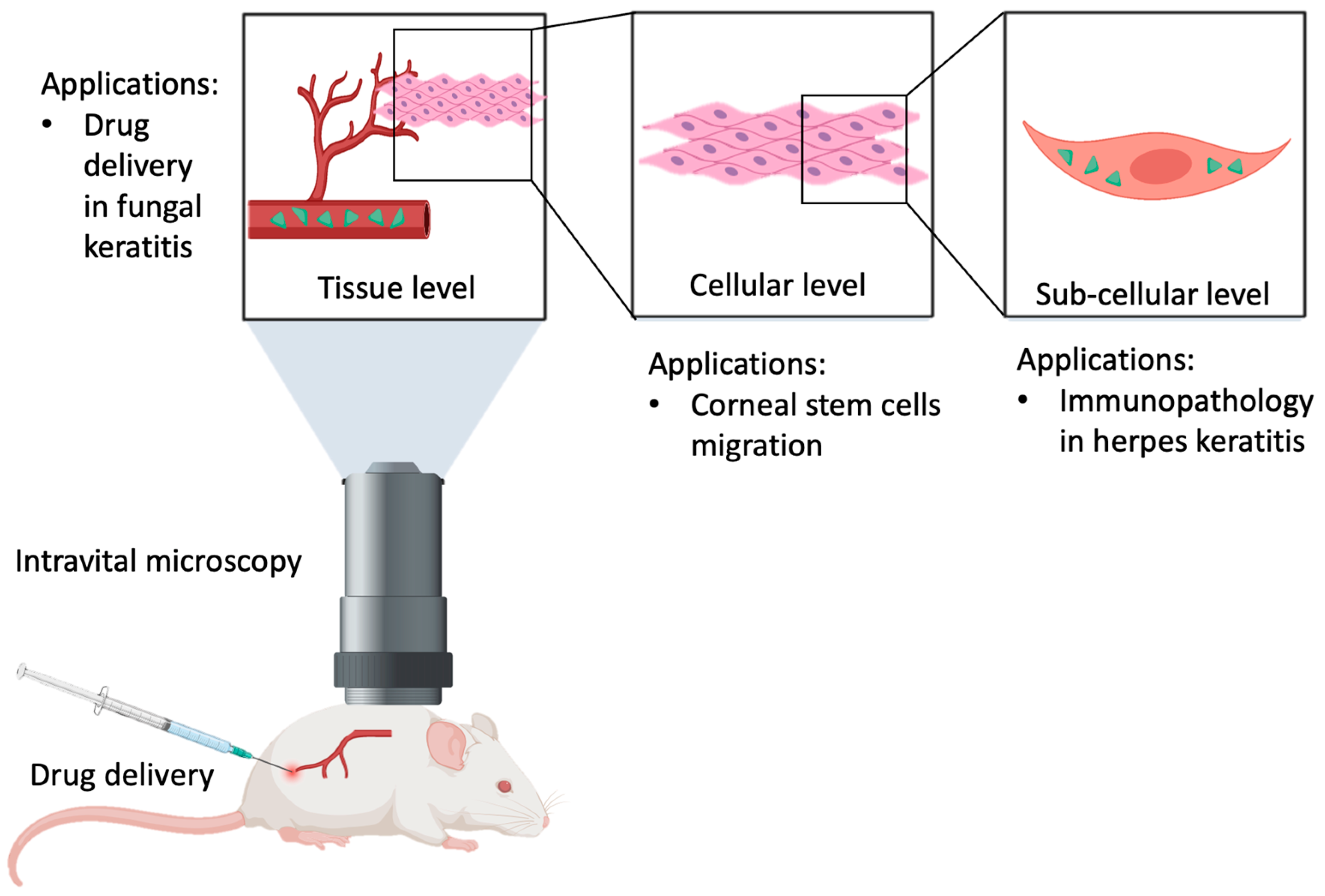
4. Methodology
5. Corneal Immunology
6. Ocular Surface Disease
7. Stem Cell Biology
8. Ocular Drug Delivery
9. Limitations and Future Directions
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Denk, W.; Strickler, J.H.; Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 1990, 248, 73–76. [Google Scholar] [CrossRef]
- Palczewska, G.; Maeda, T.; Imanishi, Y.; Sun, W.; Chen, Y.; Williams, D.R.; Piston, D.W.; Maeda, A.; Palczewski, K. Noninvasive multiphoton fluorescence microscopy resolves retinol and retinal condensation products in mouse eyes. Nat. Med. 2010, 16, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Guo, H.; Horng, H.; Liu, Y.; Li, H.; Daneshpajouhnejad, P.; Rosenberg, A.; Albanese, C.; Ranjit, S.; Andrews, P.M.; et al. Morphological and functional characteristics of aging kidneys based on two-photon microscopy in vivo. J. Biophotonics 2020, 13, e201900246. [Google Scholar] [CrossRef] [PubMed]
- Erickson-Bhatt, S.J.; Boppart, S.A. 7—Biophotonics for assessing breast cancer. In Biophotonics for Medical Applications; Meglinski, I., Ed.; Woodhead Publishing: Cambridge, UK, 2015; pp. 175–214. [Google Scholar]
- Boguslawski, J.; Palczewska, G.; Tomczewski, S.; Milkiewicz, J.; Kasprzycki, P.; Stachowiak, D.; Komar, K.; Marzejon, M.J.; Sikorski, B.L.; Hudzikowski, A.; et al. In vivo imaging of the human eye using a 2-photon-excited fluorescence scanning laser ophthalmoscope. J. Clin. Investig. 2022, 132, e154218. [Google Scholar] [CrossRef] [PubMed]
- Söderberg, P.G.; Talebizadeh, N.; Yu, Z.; Galichanin, K. Does infrared or ultraviolet light damage the lens? Eye 2016, 30, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Piston, D.W. Imaging living cells and tissues by two-photon excitation microscopy. Trends Cell Biol. 1999, 9, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Imanishi, Y.; Lodowski, K.H.; Koutalos, Y. Two-photon microscopy: Shedding light on the chemistry of vision. Biochemistry 2007, 46, 9674–9684. [Google Scholar] [CrossRef]
- Xu, C.; Zipfel, W.; Shear, J.B.; Williams, R.M.; Webb, W.W. Multiphoton fluorescence excitation: New spectral windows for biological nonlinear microscopy. Proc. Natl. Acad. Sci. USA 1996, 93, 10763–10768. [Google Scholar] [CrossRef] [PubMed]
- Palczewski, K.; Kiser, P.D. Shedding new light on the generation of the visual chromophore. Proc. Natl. Acad. Sci. USA 2020, 117, 19629–19638. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Fishkin, N.; Zhou, J.; Cai, B.; Jang, Y.P.; Krane, S.; Itagaki, Y.; Nakanishi, K. A2E, a byproduct of the visual cycle. Vision Res. 2003, 43, 2983–2990. [Google Scholar] [CrossRef]
- Feshki, M.; Martel, S.; De Koninck, Y.; Gosselin, B. Improving flat fluorescence microscopy in scattering tissue through deep learning strategies. Opt. Express 2023, 31, 23008–23026. [Google Scholar] [CrossRef] [PubMed]
- Three-Photon Imaging: How It Works. Available online: https://www.scientifica.uk.com/learning-zone/three-photon-imaging-how-it-works (accessed on 17 January 2024).
- Hegde, K.R.; Ray, K.; Szmacinski, H.; Sorto, S.; Puche, A.C.; Lengyel, I.; Thompson, R.B. Two-Photon Excited Fluorescence Lifetime Imaging of Tetracycline-Labeled Retinal Calcification. Sensors 2023, 23, 6626. [Google Scholar] [CrossRef] [PubMed]
- Emmerich, K.; White, D.T.; Kambhampati, S.P.; Casado, G.L.; Fu, T.-M.; Chunawala, Z.; Sahoo, A.; Nimmagadda, S.; Krishnan, N.; Saxena, M.T.; et al. Nanoparticle-based targeting of microglia improves the neural regeneration enhancing effects of immunosuppression in the zebrafish retina. Commun. Biol. 2023, 6, 534. [Google Scholar] [CrossRef] [PubMed]
- Fang, V.; Haynes, M.E.; Hayashi, V.; Arias, E.; Lavine, J.A.; Sullivan, D.P.; Muller, W.A. Methods for Imaging Inflammation and Transendothelial Migration in Vivo and ex Vivo. Curr. Protoc. 2023, 3, e739. [Google Scholar] [CrossRef] [PubMed]
- Paidi, S.K.; Zhang, Q.; Yang, Y.; Xia, C.H.; Ji, N.; Gong, X. Adaptive Optical Two-Photon Fluorescence Microscopy Probes Cellular Organization of Ocular Lenses In Vivo. Investig. Ophthalmol. Vis. Sci. 2023, 64, 20. [Google Scholar] [CrossRef] [PubMed]
- Helmchen, F.; Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2005, 2, 932–940. [Google Scholar] [CrossRef]
- Xu, C.; Webb, W.W. Measurement of two-photon excitation cross sections of molecular fluorophores with data from 690 to 1050 nm. J. Opt. Soc. Am. B JOSAB 1996, 13, 481–491. [Google Scholar] [CrossRef]
- Patterson, G.H.; Piston, D.W. Photobleaching in two-photon excitation microscopy. Biophys. J. 2000, 78, 2159–2162. [Google Scholar] [CrossRef]
- Kawano, H.; Nabekawa, Y.; Suda, A.; Oishi, Y.; Mizuno, H.; Miyawaki, A.; Midorikawa, K. Attenuation of photobleaching in two-photon excitation fluorescence from green fluorescent protein with shaped excitation pulses. Biochem. Biophys. Res. Commun. 2003, 311, 592–596. [Google Scholar] [CrossRef]
- Hamrah, P.; Huq, S.O.; Liu, Y.; Zhang, Q.; Dana, M.R. Corneal immunity is mediated by heterogeneous population of antigen-presenting cells. J. Leukoc. Biol. 2003, 74, 172–178. [Google Scholar] [CrossRef]
- Brissette-Storkus, C.S.; Reynolds, S.M.; Lepisto, A.J.; Hendricks, R.L. Identification of a novel macrophage population in the normal mouse corneal stroma. Investig. Ophthalmol. Vis. Sci. 2002, 43, 2264–2271. [Google Scholar]
- Liesegang, T.J. Classification of herpes simplex virus keratitis and anterior uveitis. Cornea 1999, 18, 127–143. [Google Scholar] [CrossRef] [PubMed]
- Rajasagi, N.K.; Rouse, B.T. The Role of T Cells in Herpes Stromal Keratitis. Front. Immunol. 2019, 10, 512. [Google Scholar] [CrossRef]
- Conrady, C.D.; Zheng, M.; Stone, D.U.; Carr, D.J.J. CD8+ T cells suppress viral replication in the cornea but contribute to VEGF-C-induced lymphatic vessel genesis. J. Immunol. 2012, 189, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Downie, L.E.; Zhang, X.; Wu, M.; Karunaratne, S.; Loi, J.K.; Senthil, K.; Arshad, S.; Bertram, K.; Cunningham, A.L.; Carnt, N.; et al. Redefining the human corneal immune compartment using dynamic intravital imaging. Proc. Natl. Acad. Sci. USA 2023, 120, e2217795120. [Google Scholar] [CrossRef]
- Loi, J.K.; Alexandre, Y.O.; Senthil, K.; Schienstock, D.; Sandford, S.; Devi, S.; Christo, S.N.; Mackay, L.K.; Chinnery, H.R.; Osborne, P.B.; et al. Corneal tissue-resident memory T cells form a unique immune compartment at the ocular surface. Cell Rep. 2022, 39, 110852. [Google Scholar] [CrossRef]
- Jamali, A.; Hu, K.; Sendra, V.G.; Blanco, T.; Lopez, M.J.; Ortiz, G.; Qazi, Y.; Zheng, L.; Turhan, A.; Harris, D.L.; et al. Characterization of Resident Corneal Plasmacytoid Dendritic Cells and Their Pivotal Role in Herpes Simplex Keratitis. Cell Rep. 2020, 32, 108099. [Google Scholar] [CrossRef] [PubMed]
- Knoop, K.A.; Newberry, R.D. Goblet cells: Multifaceted players in immunity at mucosal surfaces. Mucosal Immunol. 2018, 11, 1551–1557. [Google Scholar] [CrossRef] [PubMed]
- McCauley, H.A.; Guasch, G. Three cheers for the goblet cell: Maintaining homeostasis in mucosal epithelia. Trends Mol. Med. 2015, 21, 492–503. [Google Scholar] [CrossRef]
- Ma, J.; Rubin, B.K.; Voynow, J.A. Mucins, Mucus, and Goblet Cells. Chest 2018, 154, 169–176. [Google Scholar] [CrossRef]
- Ko, B.Y.; Xiao, Y.; Barbosa, F.L.; de Paiva, C.S.; Pflugfelder, S.C. Goblet cell loss abrogates ocular surface immune tolerance. JCI Insight 2018, 3, e98222. [Google Scholar] [CrossRef]
- McDole, J.R.; Wheeler, L.W.; McDonald, K.G.; Wang, B.; Konjufca, V.; Knoop, K.A.; Newberry, R.D.; Miller, M.J. Goblet cells deliver luminal antigen to CD103+ dendritic cells in the small intestine. Nature 2012, 483, 345–349. [Google Scholar] [CrossRef]
- Knoop, K.A.; McDonald, K.G.; McCrate, S.; McDole, J.R.; Newberry, R.D. Microbial sensing by goblet cells controls immune surveillance of luminal antigens in the colon. Mucosal Immunol. 2015, 8, 198–210. [Google Scholar] [CrossRef]
- Tang, M.; Mei, J.; Sun, M.; Ma, K.; Zhao, A.; Fu, X. An optimized method to visualize the goblet cell-associated antigen passages and identify goblet cells in the intestine, conjunctiva, and airway. Immunobiology 2022, 227, 152260. [Google Scholar] [CrossRef]
- Jamali, A.; Harris, D.L.; Blanco, T.; Lopez, M.J.; Hamrah, P. Resident plasmacytoid dendritic cells patrol vessels in the naïve limbus and conjunctiva. Ocul. Surf. 2020, 18, 277–285. [Google Scholar] [CrossRef]
- Zhan, Y.; Carrington, E.M.; Ko, H.J.; Vikstrom, I.B.; Oon, S.; Zhang, J.-G.; Vremec, D.; Brady, J.L.; Bouillet, P.; Wu, L.; et al. Bcl-2 antagonists kill plasmacytoid dendritic cells from lupus-prone mice and dampen interferon-α production. Arthritis Rheumatol. 2015, 67, 797–808. [Google Scholar] [CrossRef]
- Sisirak, V.; Ganguly, D.; Lewis, K.L.; Couillault, C.; Tanaka, L.; Bolland, S.; D’agati, V.; Elkon, K.B.; Reizis, B. Genetic evidence for the role of plasmacytoid dendritic cells in systemic lupus erythematosus. J. Exp. Med. 2014, 211, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Skrzeczynska-Moncznik, J.; Wlodarczyk, A.; Zabieglo, K.; Kapinska-Mrowiecka, M.; Marewicz, E.; Dubin, A.; Potempa, J.; Cichy, J. Secretory leukocyte proteinase inhibitor-competent DNA deposits are potent stimulators of plasmacytoid dendritic cells: Implication for psoriasis. J. Immunol. 2012, 189, 1611–1617. [Google Scholar] [CrossRef] [PubMed]
- Gschwandtner, M.; Mommert, S.; Köther, B.; Werfel, T.; Gutzmer, R. The histamine H4 receptor is highly expressed on plasmacytoid dendritic cells in psoriasis and histamine regulates their cytokine production and migration. J. Investig. Dermatol. 2011, 131, 1668–1676. [Google Scholar] [CrossRef] [PubMed]
- Aung, L.L.; Fitzgerald-Bocarsly, P.; Dhib-Jalbut, S.; Balashov, K. Plasmacytoid dendritic cells in multiple sclerosis: Chemokine and chemokine receptor modulation by interferon-beta. J. Neuroimmunol. 2010, 226, 158–164. [Google Scholar] [CrossRef]
- Wildenberg, M.E.; van Helden-Meeuwsen, C.G.; van de Merwe, J.P.; Drexhage, H.A.; Versnel, M.A. Systemic increase in type I interferon activity in Sjögren’s syndrome: A putative role for plasmacytoid dendritic cells. Eur. J. Immunol. 2008, 38, 2024–2033. [Google Scholar] [CrossRef]
- Stern, M.E.; Schaumburg, C.S.; Pflugfelder, S.C. Dry eye as a mucosal autoimmune disease. Int. Rev. Immunol. 2013, 32, 19–41. [Google Scholar] [CrossRef]
- Le, A.; Saverin, M.; Hand, A.R. Distribution of dendritic cells in normal human salivary glands. Acta Histochem. Cytochem. 2011, 44, 165–173. [Google Scholar] [CrossRef][Green Version]
- Gottenberg, J.E.; Cagnard, N.; Lucchesi, C.; Letourneur, F.; Mistou, S.; Lazure, T.; Jacques, S.; Ba, N.; Ittah, M.; Lepajolec, C.; et al. Activation of IFN pathways and plasmacytoid dendritic cell recruitment in target organs of primary Sjögren’s syndrome. Proc. Natl. Acad. Sci. USA 2006, 103, 2770–2775. [Google Scholar] [CrossRef]
- Park, C.Y.; Marando, C.M.; Liao, J.A.; Lee, J.K.; Kwon, J.; Chuck, R.S. Details of the Collagen and Elastin Architecture in the Human Limbal Conjunctiva, Tenon’s Capsule and Sclera Revealed by Two-Photon Excited Fluorescence Microscopy. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5602–5610. [Google Scholar] [CrossRef] [PubMed]
- Kojima, S.; Inoue, T.; Kikuta, J.; Furuya, M.; Koga, A.; Fujimoto, T.; Ueta, M.; Kinoshita, S.; Ishii, M.; Hidenobu, T. Visualization of Intravital Immune Cell Dynamics After Conjunctival Surgery Using Multiphoton Microscopy. Investig. Ophthalmol. Vis. Sci. 2016, 57, 1207–1212. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yap, Z.L.; Seet, L.F.; Chu, S.W.; Toh, L.Z.; Ibrahim, F.I.; Wong, T.T. Effect of valproic acid on functional bleb morphology in a rabbit model of minimally invasive surgery. Br. J. Ophthalmol. 2022, 106, 1028–1036. [Google Scholar] [CrossRef] [PubMed]
- West, J.D.; Dorà, N.J.; Collinson, J.M. Evaluating alternative stem cell hypotheses for adult corneal epithelial maintenance. World J. Stem Cells 2015, 7, 281–299. [Google Scholar] [CrossRef]
- Farrelly, O.; Suzuki-Horiuchi, Y.; Brewster, M.; Kuri, P.; Huang, S.; Rice, G.; Bae, H.; Xu, J.; Dentchev, T.; Lee, V.; et al. Two-photon live imaging of single corneal stem cells reveals compartmentalized organization of the limbal niche. Cell Stem Cell 2021, 28, 1233–1247.e4. [Google Scholar] [CrossRef]
- Said, D.G.; Otri, M.; Miri, A.; Kailasanathan, A.; Khatib, T.; Dua, H.S. The challenge of fungal keratitis. Br. J. Ophthalmol. 2011, 95, 1623–1624. [Google Scholar] [CrossRef]
- Sharma, N.; Bagga, B.; Singhal, D.; Nagpal, R.; Kate, A.; Saluja, G.; Maharana, P.K. Fungal keratitis: A review of clinical presentations, treatment strategies and outcomes. Ocul. Surf. 2022, 24, 22–30. [Google Scholar] [CrossRef]
- Albadr, A.A.; Tekko, I.A.; Vora, L.K.; Ali, A.A.; Laverty, G.; Donnelly, R.F.; Thakur, R.R.S. Rapidly dissolving microneedle patch of amphotericin B for intracorneal fungal infections. Drug Deliv. Transl. Res. 2022, 12, 931–943. [Google Scholar] [CrossRef]
- Abe, T.; Fujimori, T. Reporter mouse lines for fluorescence imaging. Dev. Growth Differ. 2013, 55, 390–405. [Google Scholar] [CrossRef]
- Ávila, F.J.; Gambín, A.; Artal, P.; Bueno, J.M. In vivo two-photon microscopy of the human eye. Sci. Rep. 2019, 9, 10121. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y. Two-Photon Microscopy (TPM) and Fluorescence Lifetime Imaging Microscopy (FLIM) of Retinal Pigment Epithelium (RPE) of Mice In Vivo. Methods Mol. Biol. 2018, 1753, 73–88. [Google Scholar] [PubMed]
- Köllner, M.; Wolfrum, J. How many photons are necessary for fluorescence-lifetime measurements? Chem. Phys. Lett. 1992, 200, 199–204. [Google Scholar] [CrossRef]
- Lee, J.; Ingle, A.; Chacko, J.V.; Eliceiri, K.W.; Gupta, M. CASPI: Collaborative photon processing for active single-photon imaging. Nat. Commun. 2023, 14, 3158. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, M.; Chong, S.Z.; Goh, Y.Y.; Tong, L. Two-Photon and Multiphoton Microscopy in Anterior Segment Diseases of the Eye. Int. J. Mol. Sci. 2024, 25, 1670. https://doi.org/10.3390/ijms25031670
Hong M, Chong SZ, Goh YY, Tong L. Two-Photon and Multiphoton Microscopy in Anterior Segment Diseases of the Eye. International Journal of Molecular Sciences. 2024; 25(3):1670. https://doi.org/10.3390/ijms25031670
Chicago/Turabian StyleHong, Merrelynn, Shu Zhen Chong, Yun Yao Goh, and Louis Tong. 2024. "Two-Photon and Multiphoton Microscopy in Anterior Segment Diseases of the Eye" International Journal of Molecular Sciences 25, no. 3: 1670. https://doi.org/10.3390/ijms25031670
APA StyleHong, M., Chong, S. Z., Goh, Y. Y., & Tong, L. (2024). Two-Photon and Multiphoton Microscopy in Anterior Segment Diseases of the Eye. International Journal of Molecular Sciences, 25(3), 1670. https://doi.org/10.3390/ijms25031670






