The Postbiotic Butyrate Mitigates Gut Mucosal Disruption Caused by Acute Ethanol Exposure
Abstract
1. Introduction
2. Results
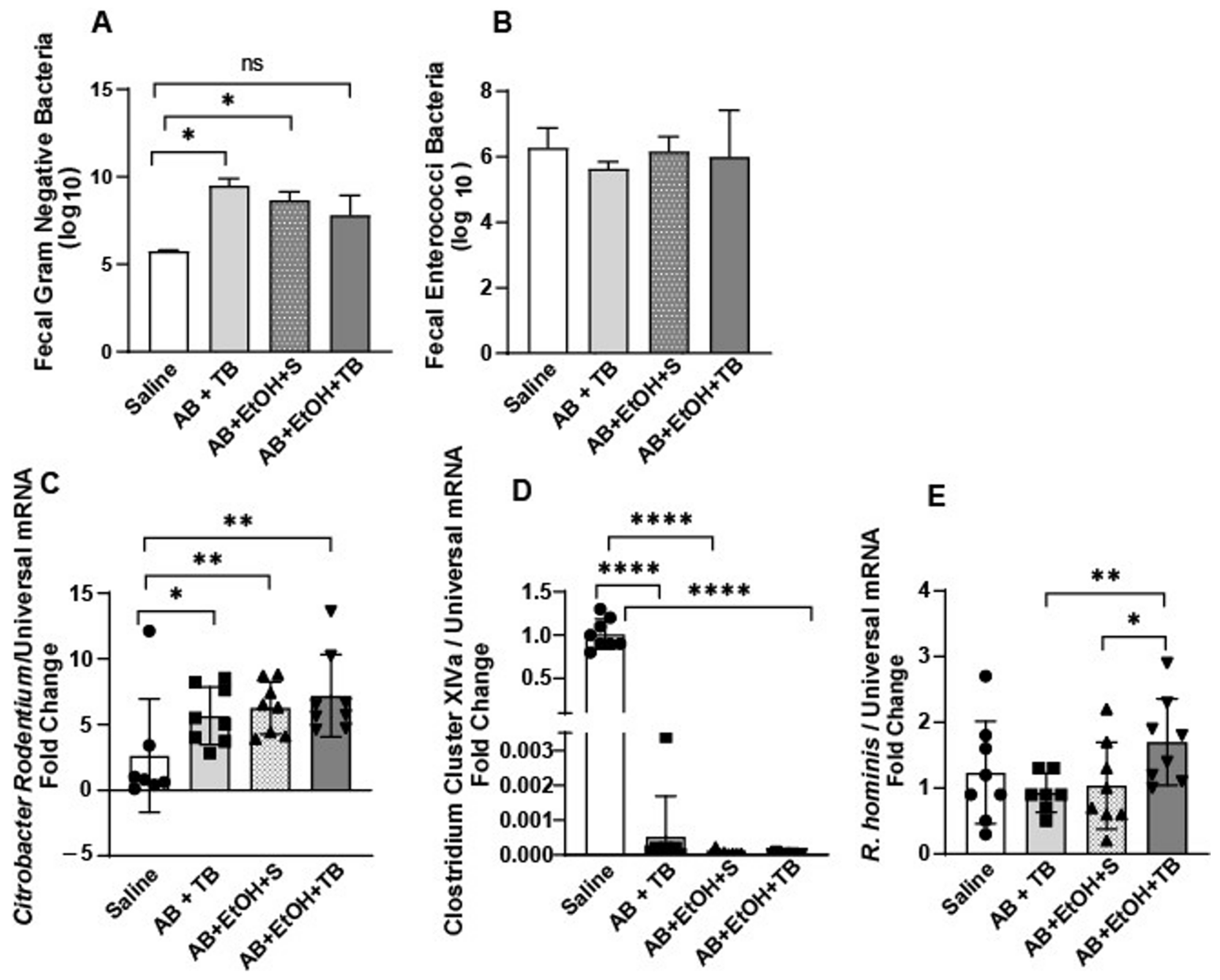
2.1. Tributyrin Supplementation Mitigated Acute Antibiotic- and Ethanol-Induced Gut Microbial Disturbances
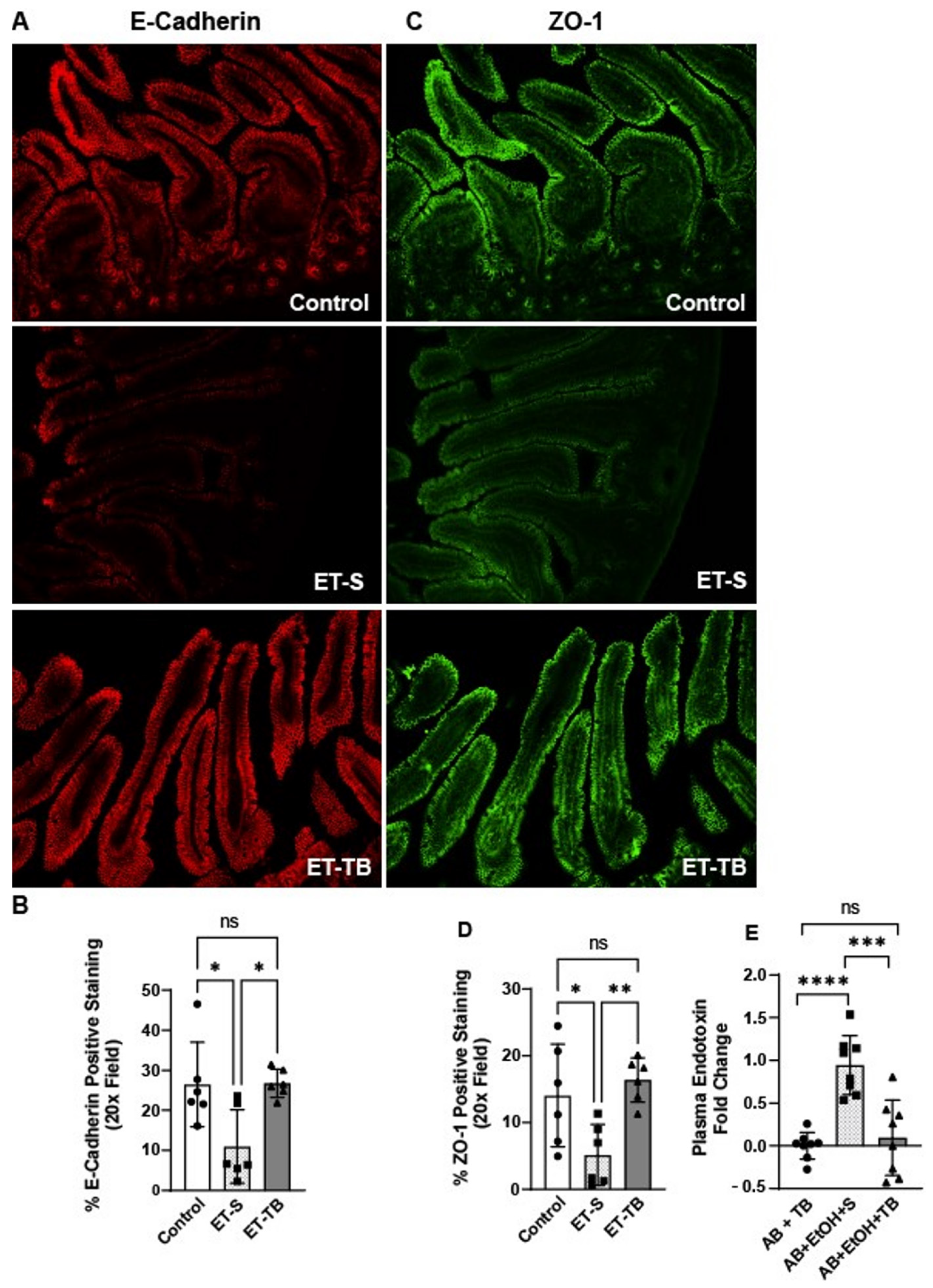
2.2. Tributyrin Supplementation Protected Small Intestinal Epithelial Integrity
2.3. Tributyrin Supplementation Attenuates Small Intestinal Microvascular Endothelium Activation
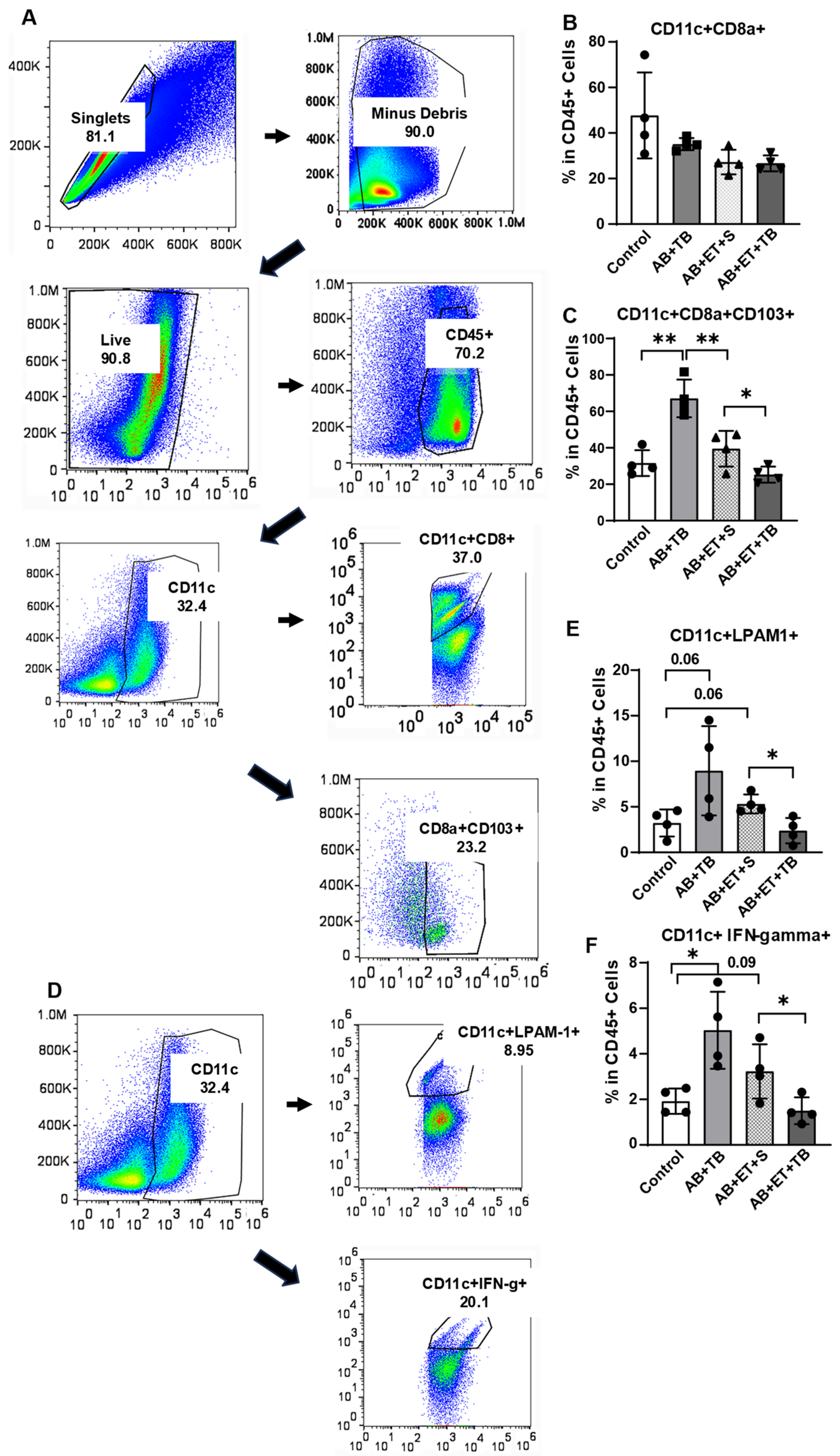
2.4. Tributyrin Supplementation during Acute Antibiotic and Ethanol Exposure Maintained a Tolerogenic Response in Lamina Propria Dendritic Cells
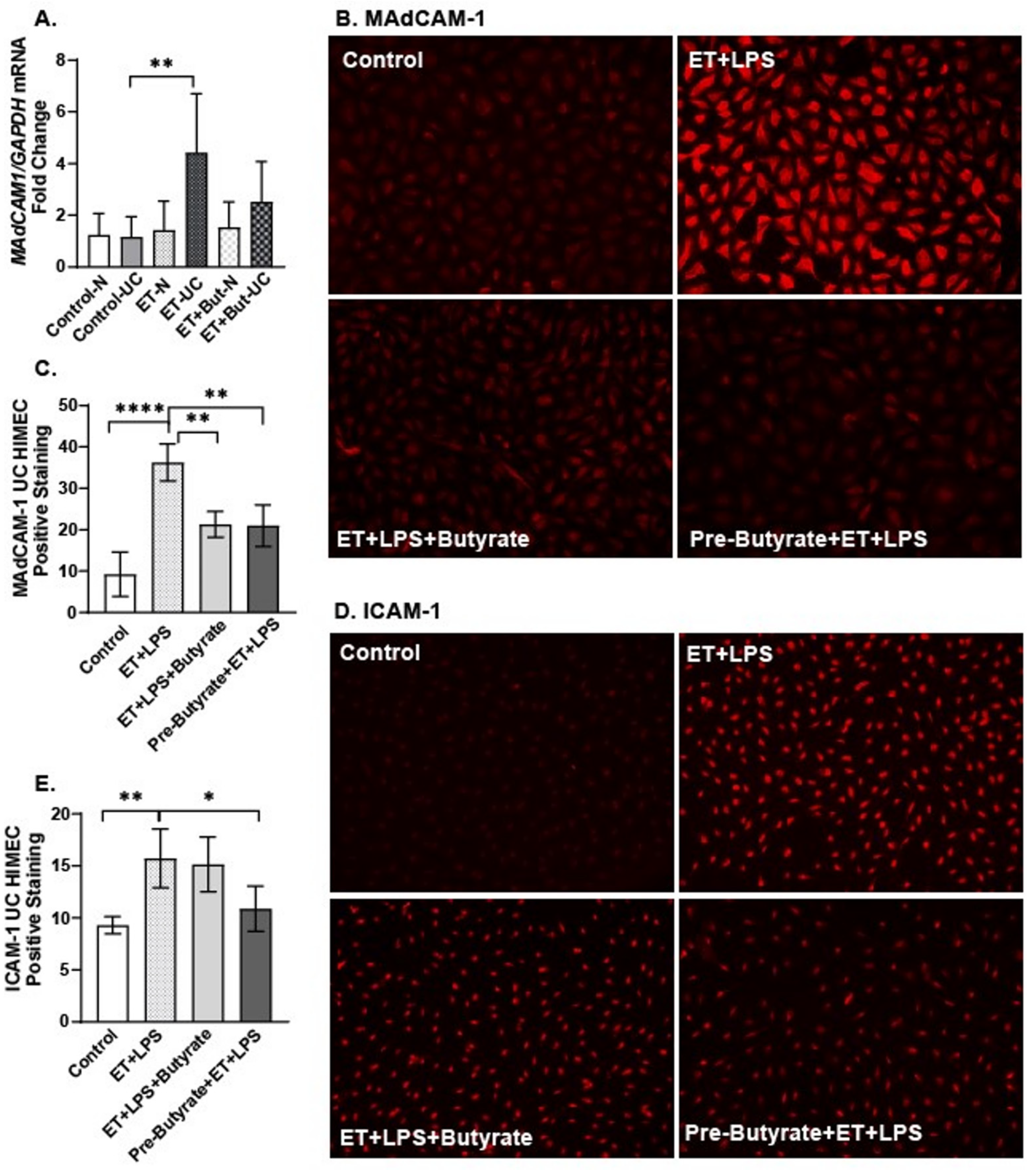
2.5. Sodium Butyrate Attenuated the Direct Effects of Ethanol and LPS on HIMEC Activation
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Mouse Models
4.3. Ethanol Exposure and Tributyrin Supplementation
4.4. Fecal Plating
4.5. Fecal qRT-PCR
4.6. Tissue qRT-PCR
4.7. Endotoxin Assay
4.8. Lamina Propria Immune Cell Isolation and Activation
4.9. Flow Cytometry
4.10. Immunohistochemistry (IHC)
4.11. Human Intestinal Microvascular Endothelial Cells (HIMECs) Culturing
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hou, K.; Wu, Z.X.; Chen, X.Y.; Wang, J.Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Stärkel, P.; Leclercq, S.; de Timary, P.; Schnabl, B. Intestinal dysbiosis and permeability: The yin and yang in alcohol dependence and alcoholic liver disease. Clin. Sci. 2018, 132, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Behara, R.; Swanson, G.R.; Forsyth, C.B.; Voigt, R.M.; Keshavarzian, A. Alcohol and the intestine. Biomolecules 2015, 5, 2573–2588. [Google Scholar] [CrossRef] [PubMed]
- Cromer, W.E.; Mathhis, J.M.; Granger, D.N.; Chaitanya, G.V.; Alexander, J.S. Role of the endothelium in inflammatory bowel diseases. Biologicals 2005, 33, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, K.E.B.; Lærke, H.N.; Hedemann, M.S.; Nielsen, T.S.; Ingerslev, A.K.; Nielsen, D.S.G.; Theil, P.K.; Purup, S.; Hald, S.; Schioldan, A.G.; et al. Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients 2018, 10, 1499. [Google Scholar] [CrossRef]
- Smirnova, E.; Puri, P.; Muthiah, M.D.; Daitya, K.; Brown, R.; Chalasani, N.; Liangpunsakul, S.; Shah, V.H.; Gelow, K.; Siddiqui, M.S.; et al. Fecal microbiome distinguishes alcohol consumption from alcoholic hepatitis but does not discriminate disease severity. Hepatology 2020, 71, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Xie, G.; Zhong, W.; Zheng, X.; Li, Q.; Qiu, Y.; Li, H.; Chen, H.; Zhou, Z.; Jia, W. Chronic ethanol consumption alters mammalian gastrointestinal content metabolites. J. Proteome Res. 2013, 12, 3297–3306. [Google Scholar] [CrossRef] [PubMed]
- Roychowdhury, S.; Glueck, B.; Han, Y.; Mohammad, M.; Cresci, G. A Designer Synbiotic Attenuates Chronic-Binge Ethanol-Induced Gut-Liver Injury in Mice. Nutrients 2019, 11, 97. [Google Scholar] [CrossRef]
- Agace, W.W.; McCoy, K.D. Regionalized development and maintenance of the intestinal adaptive immune landscape. Immunity 2017, 46, 532–548. [Google Scholar] [CrossRef]
- Ramos, G.P.; Kane, S. Alcohol use in patients with Inflammatory Bowel Disease. Gastroenterol. Hepatol. 2021, 17, 221–225. [Google Scholar]
- Tarnawski, A.S.; Sarfeh, I.J.; Stachura, J.; Hajduczek, A.X.H.; Dabros, W.; Gergely, H. Microvascular abnormalities of the portal hypertensive gastric mucosa. Hepatology 1988, 8, 1488–1494. [Google Scholar] [CrossRef]
- Xu, M.; Chen, G.; Fu, W.; Liao, M.; Frank, J.A.; Bower, K.A.; Fang, S.; Zhang, Z.; Shi, X.; Luo, J. Ethanol disrupts vascular endothelial barrier: Implication in cancer metastasis. Toxicol. Sci. 2012, 127, 42–53. [Google Scholar] [CrossRef]
- Edelman, M.J.; Bauer, K.; Khanwani, S.; Tait, N.; Trepel, J.; Karp, J.; Nemieboka, N.; Van Echo, D. Clinical and pharmacologic study of tributyrin: An oral butyrate prodrug. Cancer Chemother. Pharmacol. 2003, 51, 439–444. [Google Scholar] [CrossRef]
- Grenda, T.; Grenda, A.; Domaradzki, P.; Krawczyk, P.; Kwiatek, K. Probiotic potential of Clostridium spp. Advantages and doubts. Curr. Issues Mol. Biol. 2022, 44, 3118–3130. [Google Scholar] [CrossRef]
- Patterson, A.M.; Mulder, I.E.; Travis, A.J.; Lan, A.; Cerf-Bensussan, N.; Gaboriau-Routhiau, V.; Garden, K.; Logan, E.; Delday, M.I.; Coutts, A.G.P.; et al. Human gut symbiont Roseburia hominis promotes and regulates innate immunity. Front. Immunol. 2017, 8, 1166. [Google Scholar] [CrossRef]
- Cresci, G.A.; Glueck, B.; McMullen, M.R.; Xin, W.; Allende, D.; Nagy, L.E. Prophylactic tributyrin treatment mitigates chronic-binge ethanol-induced intestinal barrier and liver injury. J. Gastroenterol. Hepatol. 2017, 32, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Andre, P.; Laugerette, F.; Feart, C. Metabolic endotoxemia: A potential underlying mechanism of the relationship between dietary fat intake and risk for congnitive impairments in humans? Nutrients 2019, 11, 1887. [Google Scholar] [CrossRef] [PubMed]
- Privratsky, J.R.; Paddock, C.M.; Florey, O.; Newman, D.K.; Muller, W.A.; Newman, P.J. Relative contribution of PECAM-1 adhesion and signaling to the maintenance of vascular integrity. J. Cell Sci. 2011, 124, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Pusztaszeri, M.P.; Seelentag, W.; Bosman, F.T. Immunohistochemical expression of endothelial markers CD31, CD34, von Willebrand factor, and Fli-1 in normal human tissues. J. Histochem. Cytochem. 2006, 54, 385–395. [Google Scholar] [CrossRef] [PubMed]
- Gorfu, G.; Rivera-Nieves, J.; Ley, K. Role of β7 integrins in intestinal lymphocyte homing and retention. Curr. Mol. Med. 2009, 9, 836–850. [Google Scholar] [CrossRef]
- Hansen, I.S.; Krabbendam, L.; Bernink, J.H.; Loayza-Puch, F.; Hoepel, W.; van Burgsteden, J.A.; Kuijper, E.C.; Buskens, C.J.; Bemelman, W.A.; Zaat, S.A.J.; et al. FcαRI co-stimulation converts human intestinal CD103+ dendritic cells into pro-inflammatory cells through glycolytic reprogramming. Nat. Commun. 2018, 9, 863. [Google Scholar] [CrossRef] [PubMed]
- Cresci, G.; Bush, K.; Nagy, L. Tributyrin supplementation protects mice from acute ethanol-induced gut injury. Alcoholism: Clinical and Experimental Research. Alcohol. Clin. Exp. Res. 2014, 38, 1489–1501. [Google Scholar] [CrossRef]
- Cresci, G.; Nagy, L.E.; Ganapathy, V. Lactobacillus GG and Tributyrin Supplementation Reduce Antibiotic-Induced Intestinal Injury. JPEN J. Parenter. Enter. Nutr. 2013, 37, 763–774. [Google Scholar] [CrossRef]
- Kaisar, M.M.M.; Pelgrom, L.R.; van der Ham, A.J.; Yazdanbakhsh, M.; Everts, B. Butyrte conditions human dendritic cells to prime type 1 regulatory T cells via both histone deacetylase inhibition and G protein-coupled receptor 109A signaling. Front. Immunol. 2017, 8, 1429. [Google Scholar] [CrossRef] [PubMed]
- Stagg, A.J. Intestinal dendritic cells in health and gut inflammation. Front. Immunol. 2018, 9, 2883. [Google Scholar] [CrossRef]
- Siddiqui, M.T.; Cresci, G.A.M. The Immunomodulatory Functions of Butyrate. J. Inflamm. Res. 2021, 14, 6025–6041. [Google Scholar] [CrossRef] [PubMed]
- Jowett, S.L.; Seal, C.J.; Pearce, M.S.; Phillips, E.; Gregory, W.; Barton, J.R.; Welfare, M.R. Influence of dietary factors on the clinical course of ulcerative colitis: A prospective cohort study. Gut 2004, 53, 1479–1484. [Google Scholar] [CrossRef]
- Cannon, A.R.; Kuprys, P.V.; Cobb, A.N.; Ding, X.; Kothari, A.N.; Kuo, P.C.; Eberhardt, J.M.; Hammer, A.M.; Morris, N.L.; Li, X.; et al. Alcohol enhances symptoms and propensity for infection in inflammatory bowel disease patients and a murine model of DSS-induced colitis. J. Leukoc. Biol. 2018, 104, 543–555. [Google Scholar] [CrossRef]
- Sands, B.E.; Peyrin-Biroulet, L.; Loftus, E.V., Jr.; Danese, S.; Colombel, J.-F.; Törüner, M.; Jonaitis, L.; Abhyankar, B.; Chen, J.; Rogers, R.; et al. Vedolizumab versus adalimumab for moderate-to-severe ulcerative colitis. N. Engl. J. Med. 2019, 381, 1215–1226. [Google Scholar] [CrossRef]
- Haberman, Y.; Karns, R.; Dexheimer, P.J.; Schirmer, M.; Somekh, J.; Jurickova, I.; Braun, T.; Novak, E.; Bauman, L.; Collins, M.H.; et al. Ulcerative colitis mucosal transcriptomes reveal mitochondriopathy and personalized mechanism underlying disease severity and treatment response. Nat. Commun. 2019, 10, 38. [Google Scholar] [CrossRef]
- Boix-Amoros, A.; Monaco, H.; Sambataro, E.; Clemente, J.C. Novel technologies to characterize and engineer the microbiome in inflammatory bowel disease. Gut Microbes 2022, 14, 2107866. [Google Scholar] [CrossRef]
- Jamka, M.; Kokot, M.; Kaczmarek, N.; Bermagambetova, S.; Nowak, J.K.; Walkowiak, J. The effect of sodium butyrate enemas compared with placebo on disease activity, endoscopic scores, and histological and inflammatory parameters in inflammatory bowel diseases: A systematic review of randomized controlled trials. Complement. Med. Res. 2021, 28, 344–356. [Google Scholar] [CrossRef]
- Dilke, S.; Segal, J.; Tozer, P.; Vaizey, C.; Wilson, A. Diversion colitis: Aetiology, diagnosis and treatment. A systematic revie. GastroHep 2020, 2, 266–271. [Google Scholar] [CrossRef]
- Recharla, N.; Geesala, R.; Shi, X.Z. Gut microbial metabolite butyrate and its therapeutic role in Inflammatory Bowel Disease: A literature review. Nutrients 2023, 15, 2275. [Google Scholar] [CrossRef]
- Vernia, P.; Monteleone, G.; Grandinetti, G.; Villotti, G.; Di Giulio, E.; Frieri, G.; Marcheggiano, A.; Pallone, F.; Caprilli, R.; Torsoli, A. Combined oral sodium butyrate and mesalazine treatment compared to oral mesalazine alone in ulcerative colitis. Dig. Dis. Sci. 2000, 45, 976–981. [Google Scholar] [CrossRef] [PubMed]
- Vernero, M.; De Blasio, F.; Ribaldone, D.G.; Bugianesi, E.; Pellicano, R.; Saracco, G.M.; Astegiano, M.; Caviglia, G.P. The usefulness of microencapsulated sodium butyrate add-on therapy in maintaining remission in patients with Ulcerative Colitis: A prospective observational study. J. Clin. Med. 2020, 9, 3941. [Google Scholar] [CrossRef] [PubMed]
- Bertola, A.; Mathews, S.; Ki, S.H.; Wang, H.; Gao, B. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat. Protoc. 2013, 8, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Roychowdhury, S.; Cadnum, J.; Glueck, B.; Obrenovich, M.; Donskey, C.; Cresci, G.A.M. Faecalibacterium prausnitzii and a prebiotic protect intestinal health in a mouse model of antibiotic and Clostridium difficile exposure. JPEN J. Parenter. Enteral. Nutr. 2018, 42, 1156–1167. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Sheridan, B.S. Isolating lymphocytes from the mouse small intestinal immune system. JOVE J. Vis. Exp. 2018, 132, 57281. [Google Scholar]
- Binion, D.G.; West, G.A.; Ina, K.; Ziats, N.P.; Emancipator, S.N.; Fiocchi, C. Enhanced leukocyte binding by intestinal microvascular endothelial cells in inflammatory bowel disease. Gastroenterology 1997, 112, 1895–1907. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siddiqui, M.T.; Han, Y.; Shapiro, D.; West, G.; Fiocchi, C.; Cresci, G.A.M. The Postbiotic Butyrate Mitigates Gut Mucosal Disruption Caused by Acute Ethanol Exposure. Int. J. Mol. Sci. 2024, 25, 1665. https://doi.org/10.3390/ijms25031665
Siddiqui MT, Han Y, Shapiro D, West G, Fiocchi C, Cresci GAM. The Postbiotic Butyrate Mitigates Gut Mucosal Disruption Caused by Acute Ethanol Exposure. International Journal of Molecular Sciences. 2024; 25(3):1665. https://doi.org/10.3390/ijms25031665
Chicago/Turabian StyleSiddiqui, Mohamed Tausif, Yingchun Han, David Shapiro, Gail West, Claudio Fiocchi, and Gail A. M. Cresci. 2024. "The Postbiotic Butyrate Mitigates Gut Mucosal Disruption Caused by Acute Ethanol Exposure" International Journal of Molecular Sciences 25, no. 3: 1665. https://doi.org/10.3390/ijms25031665
APA StyleSiddiqui, M. T., Han, Y., Shapiro, D., West, G., Fiocchi, C., & Cresci, G. A. M. (2024). The Postbiotic Butyrate Mitigates Gut Mucosal Disruption Caused by Acute Ethanol Exposure. International Journal of Molecular Sciences, 25(3), 1665. https://doi.org/10.3390/ijms25031665





