Bafilomycin A1 Molecular Effect on ATPase Activity of Subcellular Fraction of Human Colorectal Cancer and Rat Liver
Abstract
1. Introduction
2. Results
2.1. Bafilomycin A1 Effect on ATPase Activity in Subcellular Fraction of Human Colon Mucosal Tissue Samples and Colorectal Cancer Tissue Samples
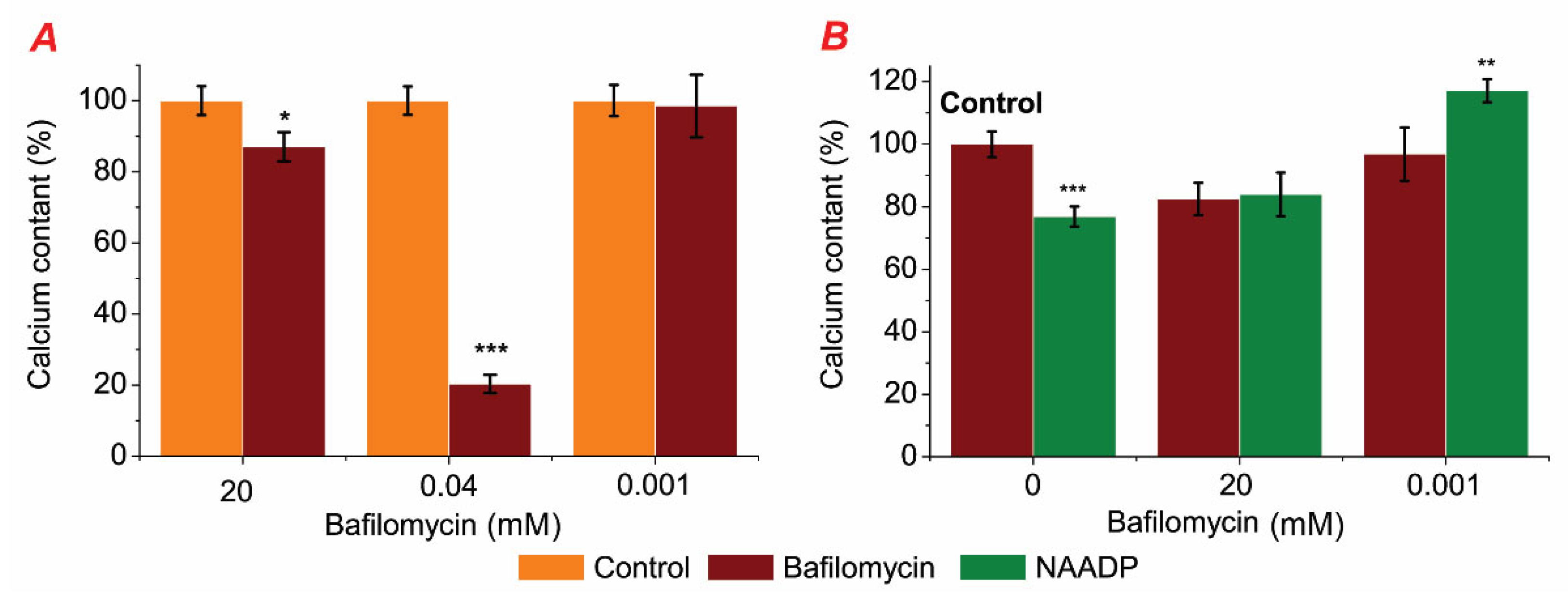
2.2. Modulation of ATPase Activity by Bafilomycin A1 and Its Impact on NAADP-Induced Effects in Subcellular Rat Liver Fractions
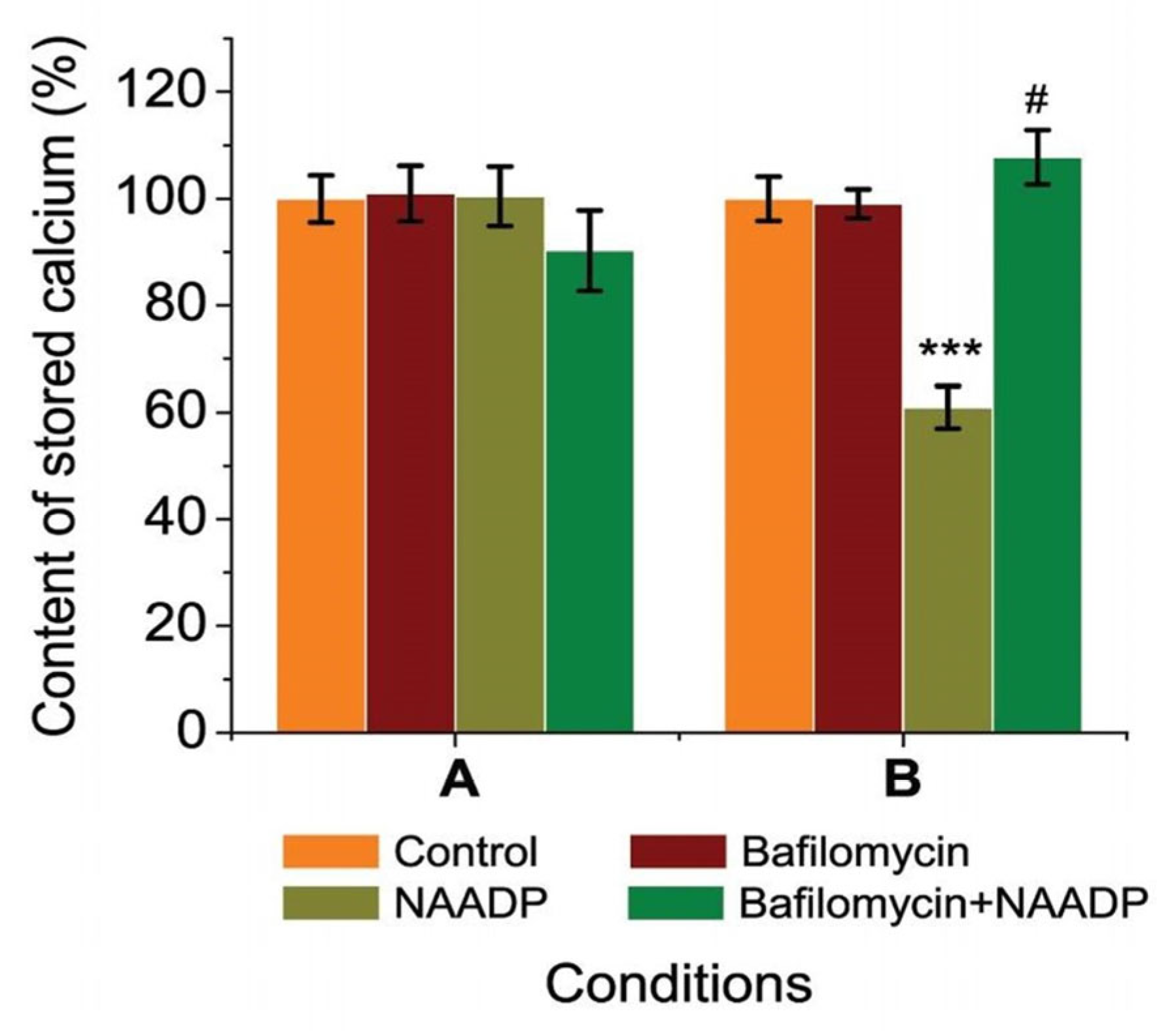
2.3. Bafilomycin Affected Stored Calcium Content and Prevented NAADP-Induced Changes of Stored Calcium in Rat Hepatocytes
3. Discussion
4. Materials and Methods
4.1. Ethical Standards and Characteristics of Patients
4.2. Rat Hepatocytes Isolation
4.3. Chlortetracycline Chemiluminescent Imaging as a Quantitative Measure of Stored Calcium in Rat Hepatocytes
4.4. Assay of ATPase Activity in Subcellular Post-Mitochondrial Fraction of Rat Liver and Human Samples of Colon Mucosa
4.5. Specific ATPase Activity Calculation
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dröse, S.; Altendorf, K. Bafilomycins and concanamycins as inhibitors of V-ATPases and P-ATPases. J. Exp. Biol. 1997, 200, 1–8. [Google Scholar] [CrossRef]
- Sun, J.; Li, Y.; Yang, X.; Dong, W.; Yang, J.; Hu, Q.; Zhang, C.; Fang, H.; Liu, A. Growth differentiation factor 11 accelerates liver senescence through the inhibition of autophagy. Aging Cell 2022, 21, e13532. [Google Scholar] [CrossRef] [PubMed]
- Park, H.-S.; Song, J.-W.; Park, J.-H.; Lim, B.-K.; Moon, O.-S.; Son, H.-Y.; Lee, J.-H.; Gao, B.; Won, Y.-S.; Kwon, H.-J. TXNIP/VDUP1 attenuates steatohepatitis via autophagy and fatty acid oxidation. Autophagy 2021, 17, 2549–2564. [Google Scholar] [CrossRef]
- Lee, D.H.; Park, J.S.; Lee, Y.S.; Han, J.; Lee, D.-K.; Kwon, S.W.; Han, D.H.; Lee, Y.-H.; Bae, S.H. SQSTM1/p62 activates NFE2L2/NRF2 via ULK1-mediated autophagic KEAP1 degradation and protects mouse liver from lipotoxicity. Autophagy 2020, 16, 1949–1973. [Google Scholar] [CrossRef]
- Lu, X.; Chen, L.; Chen, Y.; Shao, Q.; Qin, W. Bafilomycin A1 inhibits the growth and metastatic potential of the BEL-7402 liver cancer and HO-8910 ovarian cancer cell lines and induces alterations in their microRNA expression. Exp. Ther. Med. 2015, 10, 1829–1834. [Google Scholar] [CrossRef]
- Wang, R.; Wang, J.; Hassan, A.; Lee, C.-H.; Xie, X.-S.; Li, X. Molecular basis of V-ATPase inhibition by bafilomycin A1. Nat. Commun. 2021, 12, 1782. [Google Scholar] [CrossRef] [PubMed]
- Futai, M.; Sun-Wada, G.-H.; Wada, Y.; Matsumoto, N.; Nakanishi-Matsui, M. Vacuolar-type ATPase: A proton pump to lysosomal trafficking. Proc. Jpn. Acad. Ser. B 2019, 95, 261–277. [Google Scholar] [CrossRef] [PubMed]
- Jeger, J.L. Endosomes, lysosomes, and the role of endosomal and lysosomal biogenesis in cancer development. Mol. Biol. Rep. 2020, 47, 9801–9810. [Google Scholar] [CrossRef]
- Mauvezin, C.; Neufeld, T.P. Bafilomycin A1 disrupts autophagic flux by inhibiting both V-ATPase-dependent acidification and Ca-P60A/SERCA-dependent autophagosome-lysosome fusion. Autophagy 2015, 11, 1437–1438. [Google Scholar] [CrossRef]
- Wu, Y.C.; Wu, W.K.K.; Li, Y.; Yu, L.; Li, Z.J.; Wong, C.C.M.; Li, H.T.; Sung, J.J.Y.; Cho, C.H. Inhibition of macroautophagy by bafilomycin A1 lowers proliferation and induces apoptosis in colon cancer cells. Biochem. Biophys. Res. Commun. 2009, 382, 451–456. [Google Scholar] [CrossRef]
- Yan, Y.; Jiang, K.; Liu, P.; Zhang, X.; Dong, X.; Gao, J.; Liu, Q.; Barr, M.P.; Zhang, Q.; Hou, X.; et al. Bafilomycin A1 induces caspase-independent cell death in hepatocellular carcinoma cells via targeting of autophagy and MAPK pathways. Sci. Rep. 2016, 6, 37052. [Google Scholar] [CrossRef]
- Li, F.; Hu, Y.; Hu, Y.; Zhou, R.; Mao, Z. Bafilomycin A1 Induces Caspase-Dependent Apoptosis and Inhibits Autophagy Flux in Diffuse Large B Cell Lymphoma. Med. Pharmacol. 2021, 2021070520. [Google Scholar] [CrossRef]
- Hreniukh, V.; Bychkova, S.; Kulachkovsky, O.; Babsky, A. Effect of bafilomycin and NAADP on membrane-associated ATPases and respiration of isolated mitochondria of the murine Nemeth-Kellner lymphoma: Mitochondria and ATPase activities in lymphoma. Cell Biochem. Funct. 2016, 34, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Bychkova, S.V.; Stasyshyn, A.R.; Bychkov, M.A. The role of bafilomycin as a therapeutic agent in the modulation of endo-lysosomal store of rat hepatocytes. Med. Perspekt. 2022, 27, 22–26. [Google Scholar] [CrossRef]
- Morgan, A.J. Ca2+ dialogue between acidic vesicles and ER. Biochem. Soc. Trans. 2016, 44, 546–553. [Google Scholar] [CrossRef]
- Faris, P.; Pellavio, G.; Ferulli, F.; Di Nezza, F.; Shekha, M.; Lim, D.; Maestri, M.; Guerra, G.; Ambrosone, L.; Pedrazzoli, P.; et al. Nicotinic Acid Adenine Dinucleotide Phosphate (NAADP) Induces Intracellular Ca2+ Release through the Two-Pore Channel TPC1 in Metastatic Colorectal Cancer Cells. Cancers 2019, 11, 542. [Google Scholar] [CrossRef]
- Kushkevych, I.; Bychkov, M.; Bychkova, S.; Gajdács, M.; Merza, R.; Vítězová, M. ATPase Activity of the Subcellular Fractions of Colorectal Cancer Samples under the Action of Nicotinic Acid Adenine Dinucleotide Phosphate. Biomedicines 2021, 9, 1805. [Google Scholar] [CrossRef]
- Bychkova, S.V.; Chorna, T.I. NAADP-sensitive Ca2+ stores in permeabilized rat hepatocytes. Ukr. Biochem. J. 2014, 86, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Sitsel, A.; Benoy, V.; Sepúlveda, M.R.; Vangheluwe, P. Primary Active Ca2+ Transport Systems in Health and Disease. Cold Spring Harb. Perspect. Biol. 2020, 12, a035113. [Google Scholar] [CrossRef] [PubMed]
- Peters, A.A.; Milevskiy, M.J.G.; Lee, W.C.; Curry, M.C.; Smart, C.E.; Saunus, J.M.; Reid, L.; da Silva, L.; Marcial, D.L.; Dray, E.; et al. The calcium pump plasma membrane Ca2+-ATPase 2 (PMCA2) regulates breast cancer cell proliferation and sensitivity to doxorubicin. Sci. Rep. 2016, 6, 25505. [Google Scholar] [CrossRef] [PubMed]
- Aung, C.S.; Ye, W.; Plowman, G.; Peters, A.A.; Monteith, G.R.; Roberts-Thomson, S.J. Plasma membrane calcium ATPase 4 and the remodeling of calcium homeostasis in human colon cancer cells. Carcinogenesis 2009, 30, 1962–1969. [Google Scholar] [CrossRef]
- Aung, C.S.; Kruger, W.A.; Poronnik, P.; Roberts-Thomson, S.J.; Monteith, G.R. Plasma membrane Ca2+-ATPase expression during colon cancer cell line differentiation. Biochem. Biophys. Res. Commun. 2007, 355, 932–936. [Google Scholar] [CrossRef] [PubMed]
- Bychkova, S.; Hreniuh, V. Activity of ATPases in postmitichondtial fraction of lymphoma NK/Ly cells under bafilomicine and NAADP presence. Biol. Stud. 2015, 9, 31–38. [Google Scholar] [CrossRef]
- Papp, B.; Launay, S.; Gélébart, P.; Arbabian, A.; Enyedi, A.; Brouland, J.-P.; Carosella, E.D.; Adle-Biassette, H. Endoplasmic Reticulum Calcium Pumps and Tumor Cell Differentiation. Int. J. Mol. Sci. 2020, 21, 3351. [Google Scholar] [CrossRef] [PubMed]
- Chung, F.-Y.; Lin, S.-R.; Lu, C.-Y.; Yeh, C.-S.; Chen, F.-M.; Hsieh, J.-S.; Huang, T.-J.; Wang, J.-Y. Sarco/Endoplasmic Reticulum Calcium-ATPase 2 Expression as a Tumor Marker in Colorectal Cancer. Am. J. Surg. Pathol. 2006, 30, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.I.; Gonçalves-de-Albuquerque, C.F.; de Moraes, B.P.T.; Garcia, D.G.; Burth, P. Na/K-ATPase: Their role in cell adhesion and migration in cancer. Biochimie 2021, 185, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Lee, S.-Y.; Kim, S.; Choi, I.; Kim, S.-H.; Shum, D.; Heo, J.; Kim, A.-R.; Kim, K.M.; Seo, H.R. Inhibitors of Na+/K+ ATPase exhibit antitumor effects on multicellular tumor spheroids of hepatocellular carcinoma. Sci. Rep. 2020, 10, 5318. [Google Scholar] [CrossRef]
- Zhang, F.; Xia, M.; Li, P.-L. Lysosome-dependent Ca2+ release response to Fas activation in coronary arterial myocytes through NAADP: Evidence from CD38 gene knockouts. Am. J. Physiol. Cell Physiol. 2010, 298, C1209–C1216. [Google Scholar] [CrossRef]
- Chemaly, E.R.; Troncone, L.; Lebeche, D. SERCA control of cell death and survival. Cell Calcium 2018, 69, 46–61. [Google Scholar] [CrossRef]
- Rüschoff, J.H.; Brandenburger, T.; Strehler, E.E.; Filoteo, A.G.; Heinmöller, E.; Aumüller, G.; Wilhelm, B. Plasma Membrane Calcium ATPase Expression in Human Colon Multistep Carcinogenesis. Cancer Investig. 2012, 30, 251–257. [Google Scholar] [CrossRef]
- Rüschoff, J.; Hanna, W.; Bilous, M.; Hofmann, M.; Osamura, R.Y.; Penault-Llorca, F.; van de Vijver, M.; Viale, G. HER2 testing in gastric cancer: A practical approach. Mod. Pathol. 2012, 25, 637–650. [Google Scholar] [CrossRef]
- Brouland, J.-P.; Gélébart, P.; Kovàcs, T.; Enouf, J.; Grossmann, J.; Papp, B. The Loss of Sarco/Endoplasmic Reticulum Calcium Transport ATPase 3 Expression Is an Early Event during the Multistep Process of Colon Carcinogenesis. Am. J. Pathol. 2005, 167, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Ribiczey, P.; Tordai, A.; Andrikovics, H.; Filoteo, A.G.; Penniston, J.T.; Enouf, J.; Enyedi, Á.; Papp, B.; Kovács, T. Isoform-specific up-regulation of plasma membrane Ca2+ ATPase expression during colon and gastric cancer cell differentiation. Cell Calcium 2007, 42, 590–605. [Google Scholar] [CrossRef]
- Varga, K.; Hollósi, A.; Pászty, K.; Hegedűs, L.; Szakács, G.; Tímár, J.; Papp, B.; Enyedi, Á.; Padányi, R. Expression of calcium pumps is differentially regulated by histone deacetylase inhibitors and estrogen receptor alpha in breast cancer cells. BMC Cancer 2018, 18, 1029. [Google Scholar] [CrossRef]
- Faris, P.; Casali, C.; Negri, S.; Iengo, L.; Biggiogera, M.; Maione, A.S.; Moccia, F. Nicotinic Acid Adenine Dinucleotide Phosphate Induces Intracellular Ca2+ Signalling and Stimulates Proliferation in Human Cardiac Mesenchymal Stromal Cells. Front. Cell Dev. Biol. 2022, 10, 874043. [Google Scholar] [CrossRef]
- Fameli, N.; Ogunbayo, O.A.; van Breemen, C.; Evans, A.M. Cytoplasmic nanojunctions between lysosomes and sarcoplasmic reticulum are required for specific calcium signaling. F1000Research 2014, 3, 93. [Google Scholar] [CrossRef]
- Ronco, V.; Potenza, D.M.; Denti, F.; Vullo, S.; Gagliano, G.; Tognolina, M.; Guerra, G.; Pinton, P.; Genazzani, A.A.; Mapelli, L.; et al. A novel Ca2+-mediated cross-talk between endoplasmic reticulum and acidic organelles: Implications for NAADP-dependent Ca2+ signalling. Cell Calcium 2015, 57, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Shimamoto, C.; Ito, S.; Daikoku, E.; Nakahari, T. HCO3−-dependent transient acidification induced by ionomycin in rat submandibular acinar cells. J. Physiol. Sci. 2010, 60, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-M.; Lee, J.Y.; Park, C.-K.; Kim, Y.H. The Role of TRP Channels and PMCA in Brain Disorders: Intracellular Calcium and pH Homeostasis. Front. Cell Dev. Biol. 2021, 9, 584388. [Google Scholar] [CrossRef]
- Kushkevych, I.; Fafula, R.; Parák, T.; Bartoš, M. Activity of Na+/K+-activated Mg2+-dependent ATP-hydrolase in the cell-free extracts of the sulfate-reducing bacteria Desulfovibrio piger Vib-7 and Desulfomicrobium sp. Rod-9. Acta Vet. Brno 2015, 84, 3–12. [Google Scholar] [CrossRef]
- Kosterin, S.O.; Veklich, T.O.; Pryluts’kyĭ, I.I.; Borysko, P.O. Kinetic interpretation of the original pH-dependence of enzymatic activity of “basal” Mg(2+)-ATPase of the smooth muscle sarcolemma. Ukr. Biokhim. Zh. (1999) 2005, 77, 37–45. [Google Scholar] [PubMed]
- Sakai, H.; Suzuki, T.; Maeda, M.; Takahashi, Y.; Horikawa, N.; Minamimura, T.; Tsukada, K.; Takeguchi, N. Up-regulation of Na+, K+-ATPase α3-isoform and down-regulation of the α1-isoform in human colorectal cancer. FEBS Lett. 2004, 563, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Khajah, M.A.; Mathew, P.M.; Luqmani, Y.A. Na+/K+ ATPase activity promotes invasion of endocrine resistant breast cancer cells. PLoS ONE 2018, 13, e0193779. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Shiozaki, A.; Kosuga, T.; Shimizu, H.; Kudou, M.; Ohashi, T.; Arita, T.; Konishi, H.; Komatsu, S.; Kubota, T.; et al. The expression of the alpha1 subunit of Na+/K+-ATPase is related to tumor development and clinical outcomes in gastric cancer. Gastric Cancer 2021, 24, 1278–1292. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Yu, H.-Q.; Xiong, H.-J.; Zhang, Y.-J.; Lin, X.-T.; Zhang, J.; Wu, W.; Wang, T.; Liu, X.-Y.; Xie, C.-M. Elevated Sodium Pump α3 Subunit Expression Promotes Colorectal Liver Metastasis via the p53-PTEN/IGFBP3-AKT-mTOR Axis. Front. Oncol. 2021, 11, 743824. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.M.; Lew, V.L. The effect of intracellular calcium on the sodium pump of human red cells. J. Physiol. 1983, 343, 455–493. [Google Scholar] [CrossRef] [PubMed]
- Arai, K.; Shimaya, A.; Hiratani, N.; Ohkuma, S. Purification and characterization of lysosomal H(+)-ATPase. An anion-sensitive v-type H(+)-ATPase from rat liver lysosomes. J. Biol. Chem. 1993, 268, 5649–5660. [Google Scholar] [CrossRef] [PubMed]
- Bychkova, S. Influence of NAADP and bafilomycine A1 on activity of ATPase in liver postmitochondrial fraction. Biol. Stud. 2015, 9, 31–40. [Google Scholar] [CrossRef]
- Hohmann, J.; Kowalewski, H.; Vogel, M.; Zimmerman, H. Isolation of a Ca2+ or Mg2+-activated ATPase (ecto-ATPase) from bovine brain synaptic membranes. Biochim. Biophys. Acta BBA Biomembr. 1993, 1152, 146–154. [Google Scholar] [CrossRef]
- Konno, Y.; Kato, K.; Dairaku, N.; Koike, T.; Iijima, K.; Imatani, A.; Sekine, H.; Ohara, S.; Shimosegawa, T. Expression of Mg2+-dependent, HCO3−-stimulated adenine triphosphatase in the human duodenum. J. Gastroenterol. Hepatol. 2004, 19, 18–23. [Google Scholar] [CrossRef]
- Wang, H.; Gilles-Baillien, M. Ca2+-ATPase and Mg2+-ATPase activities distinct from alkaline phosphatase in rat jejunal brush-border membranes. Arch. Int. Physiol. Biochim. Biophys. 1993, 101, 387–393. [Google Scholar] [CrossRef]
- Jacob, J.; Chandran, D.; Sasidharan, R.; Kuruvila, L.; Madhusudan, U.K.; Rao, N.L.; Banerjee, D. Chlortetracycline, a fluorescent probe for pH of calcium stores in cells. Curr. Sci. 2003, 2003, 671–674. [Google Scholar]
- Colacurcio, D.J.; Nixon, R.A. Disorders of lysosomal acidification—The emerging role of v-ATPase in aging and neurodegenerative disease. Ageing Res. Rev. 2016, 32, 75–88. [Google Scholar] [CrossRef]
- Kim, H.-K.; Lee, G.-H.; Bhattarai, K.R.; Lee, M.-S.; Back, S.H.; Kim, H.-R.; Chae, H.-J. TMBIM6 (transmembrane BAX inhibitor motif containing 6) enhances autophagy through regulation of lysosomal calcium. Autophagy 2021, 17, 761–778. [Google Scholar] [CrossRef] [PubMed]
- Fitzpatrick, J.S.; Hagenston, A.M.; Hertle, D.N.; Gipson, K.E.; Bertetto-D’Angelo, L.; Yeckel, M.F. Inositol-1,4,5-trisphosphate receptor-mediated Ca2+ waves in pyramidal neuron dendrites propagate through hot spots and cold spots: Ca2+ waves propagate through hot and cold spots. J. Physiol. 2009, 587, 1439–1459. [Google Scholar] [CrossRef]
- Giacomello, M.; Drago, I.; Bortolozzi, M.; Scorzeto, M.; Gianelle, A.; Pizzo, P.; Pozzan, T. Ca2+ Hot Spots on the Mitochondrial Surface Are Generated by Ca2+ Mobilization from Stores, but Not by Activation of Store-Operated Ca2+ Channels. Mol. Cell 2010, 38, 280–290. [Google Scholar] [CrossRef]
- Tepikin, A.V. Mitochondrial junctions with cellular organelles: Ca2+ signalling perspective. Pflügers Arch. Eur. J. Physiol. 2018, 470, 1181–1192. [Google Scholar] [CrossRef] [PubMed]
- Hulsurkar, M.M.; Lahiri, S.K.; Karch, J.; Wang, M.C.; Wehrens, X.H.T. Targeting calcium-mediated inter-organellar crosstalk in cardiac diseases. Expert Opin. Ther. Targets 2022, 26, 303–317. [Google Scholar] [CrossRef]
- Calcraft, P.J.; Ruas, M.; Pan, Z.; Cheng, X.; Arredouani, A.; Hao, X.; Tang, J.; Rietdorf, K.; Teboul, L.; Chuang, K.-T.; et al. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 2009, 459, 596–600. [Google Scholar] [CrossRef]
- Mándi, M.; Tóth, B.; Timár, G.; Bak, J. Ca2+ release triggered by NAADP in hepatocyte microsomes. Biochem. J. 2006, 395, 233–238. [Google Scholar] [CrossRef]
- Blaauboer, B.J.; Boobis, A.R.; Castell, J.V.; Coecke, S.; Groothuis, G.M.M.; Guillouzo, A.; Hall, T.J.; Hawksworth, G.M.; Lorenzon, G.; Miltenburger, H.G.; et al. The Practical Applicability of Hepatocyte Cultures in Routine Testing: The Report and Recommendations of ECVAM Workshop 1. Altern. Lab. Anim. 1994, 22, 231–241. [Google Scholar] [CrossRef]
- Ferents, I.M.; Bychkova, S.V.; Bychkov, M.A. Peculiarities of the effects of bile acids on atpase activity of the colon mucosa in patients with overweight and irritable bowel syndrome. Wiad. Lek. 2020, 73, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Bailey, N.T.J. Statistical Methods in Biology, 3rd ed.; Cambridge University Press: Cambridge, UK, 1995; Volume 75, p. 515. 255p. [Google Scholar]




| Number of pts. | Age of pts. | Sex | Stage | Definition |
|---|---|---|---|---|
| 1 | 53 | female | I | The tumor invades the submucosa—no nodes, no metastases |
| 2 | 52 | female | I | The tumor invades the submucosa—no nodes, no metastases |
| 3 | 76 | female | II | The tumor invades through the muscularis propria into the subserosa—no nodes, no metastases |
| 4 | 53 | female | II | The tumor invades through the muscularis propria into the subserosa—no nodes, no metastases |
| 5 | 52 | female | I | The tumor invades the submucosa—no nodes, no metastases |
| 6 | 50 | female | II | The tumor invades through the muscularis propria into the subserosa—no nodes, no metastases |
| 7 | 74 | female | I | The tumor invades the submucosa—no nodes, no metastases |
| 8 | 51 | female | I | The tumor invades the submucosa—no nodes, no metastases |
| 9 | 51 | female | I | The tumor invades the submucosa—no nodes, no metastases |
| 10 | 48 | female | II | The tumor invades through the muscularis propria into the subserosa—no nodes, no metastases |
| 11 | 45 | female | II | The tumor invades through the muscularis propria into the subserosa—no nodes, no metastases |
| 12 | 56 | female | II | The tumor invades through the muscularis propria into the subserosa—no nodes, no metastases |
| 13 | 55 | male | I | The tumor invades the submucosa—no nodes, no metastases |
| 14 | 45 | male | I | The tumor invades the submucosa—no nodes, no metastases |
| 15 | 56 | male | I | The tumor invades the submucosa—no nodes, no metastases |
| 16 | 55 | male | II | The tumor invades through the muscularis propria into the subserosa—no nodes, no metastases |
| 17 | 50 | male | II | The tumor invades through the muscularis propria into the subserosa—no nodes, no metastases |
| 18 | 54 | male | II | The tumor invades through the muscularis propria into the subserosa—no nodes, no metastases |
| 19 | 50 | male | I | The tumor invades the submucosa—no nodes, no metastases |
| 20 | 60 | male | I | The tumor invades the submucosa—no nodes, no metastases |
| M | 54.3 | |||
| m | 1.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bychkova, S.; Bychkov, M.; Dordevic, D.; Vítězová, M.; Rittmann, S.K.-M.R.; Kushkevych, I. Bafilomycin A1 Molecular Effect on ATPase Activity of Subcellular Fraction of Human Colorectal Cancer and Rat Liver. Int. J. Mol. Sci. 2024, 25, 1657. https://doi.org/10.3390/ijms25031657
Bychkova S, Bychkov M, Dordevic D, Vítězová M, Rittmann SK-MR, Kushkevych I. Bafilomycin A1 Molecular Effect on ATPase Activity of Subcellular Fraction of Human Colorectal Cancer and Rat Liver. International Journal of Molecular Sciences. 2024; 25(3):1657. https://doi.org/10.3390/ijms25031657
Chicago/Turabian StyleBychkova, Solomiia, Mykola Bychkov, Dani Dordevic, Monika Vítězová, Simon K.-M. R. Rittmann, and Ivan Kushkevych. 2024. "Bafilomycin A1 Molecular Effect on ATPase Activity of Subcellular Fraction of Human Colorectal Cancer and Rat Liver" International Journal of Molecular Sciences 25, no. 3: 1657. https://doi.org/10.3390/ijms25031657
APA StyleBychkova, S., Bychkov, M., Dordevic, D., Vítězová, M., Rittmann, S. K.-M. R., & Kushkevych, I. (2024). Bafilomycin A1 Molecular Effect on ATPase Activity of Subcellular Fraction of Human Colorectal Cancer and Rat Liver. International Journal of Molecular Sciences, 25(3), 1657. https://doi.org/10.3390/ijms25031657







