Nicotine Motivated Behavior in C. elegans
Abstract
1. Introduction
2. Results
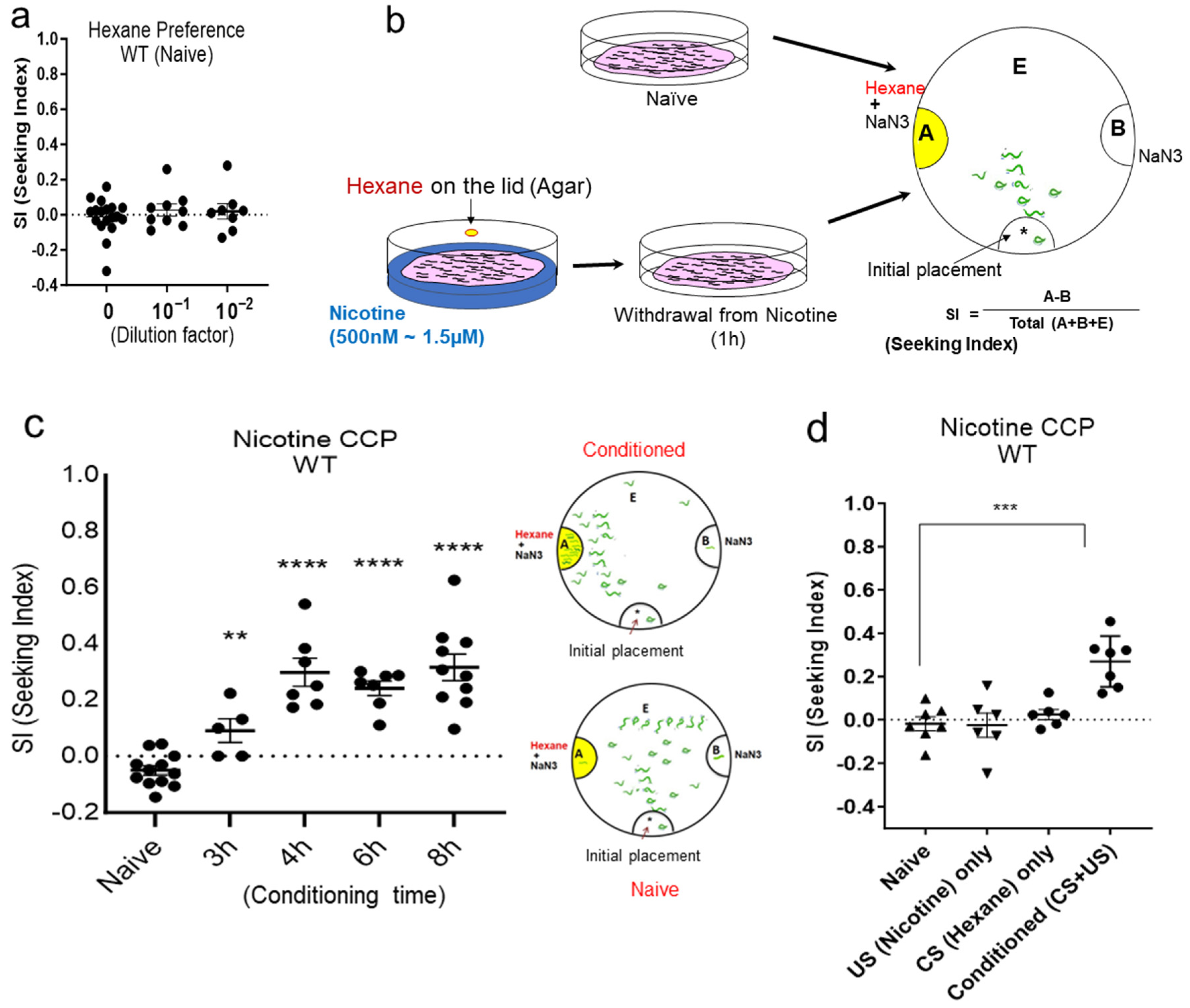
2.1. Establishment of CCP (Conditioned Cue Preference)
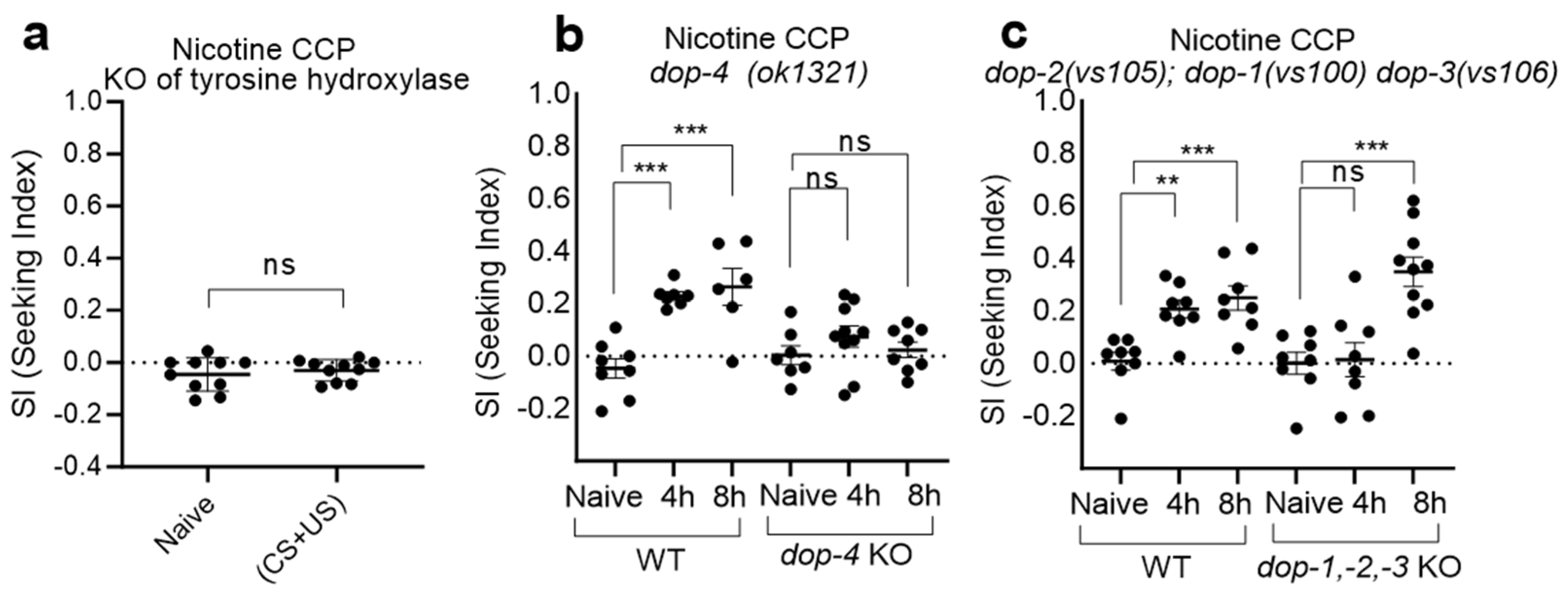
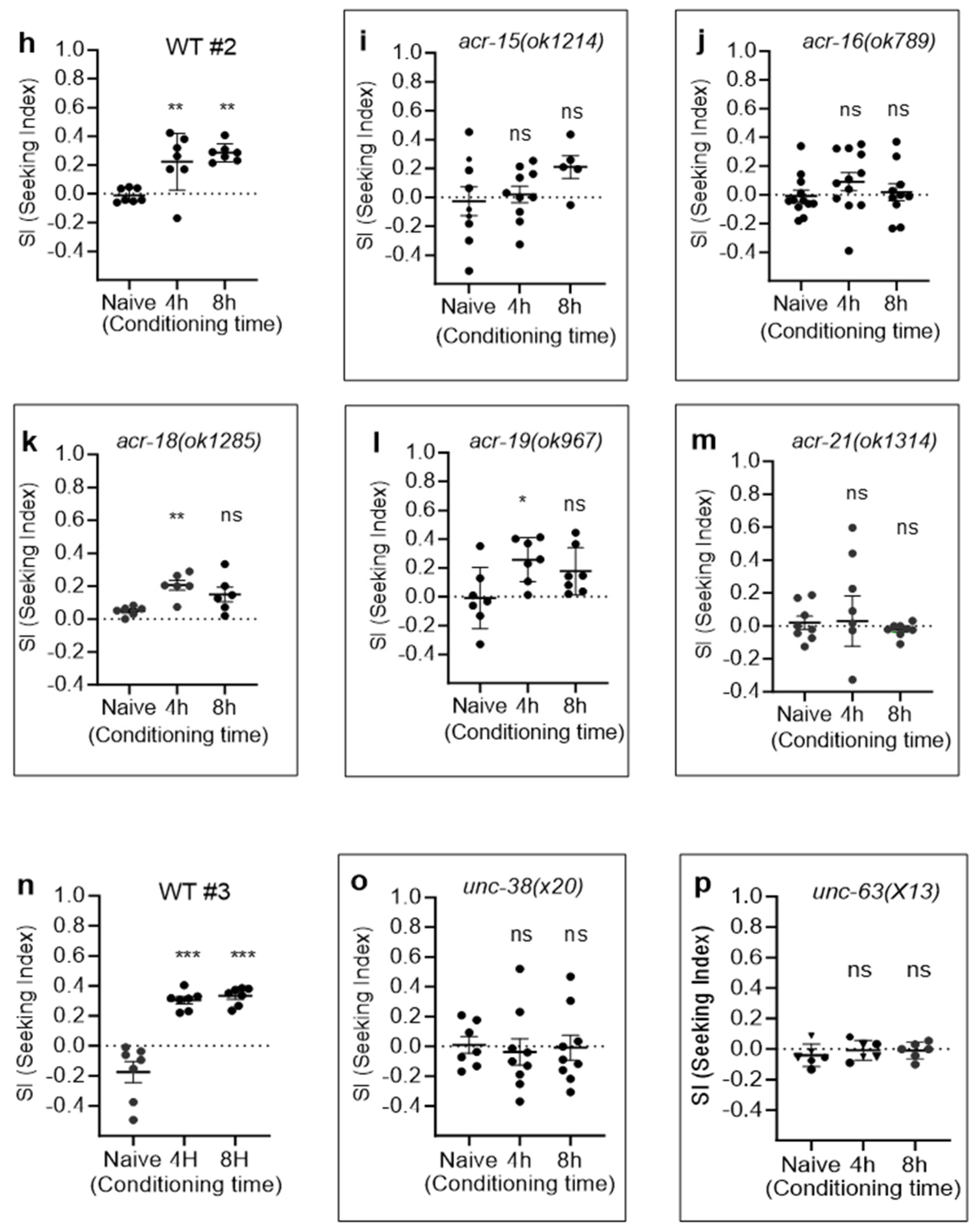
2.2. CCP via nAChRs
2.3. Orthogonal Test for Nicotine Preference
3. Discussion
4. Materials and Methods
4.1. Behavioral Assay
4.1.1. Nicotine Conditioning
4.1.2. Chemotaxis to CS
4.2. Statistical Analysis
4.3. Sequence Alignment
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. World Health Organization (WHO) Report on the Global Tobacco Epidemic 2017—Monitoring Tobacco Use and Prevention Policies; World Health Organization: Geneva, Switzerland, 2017. [Google Scholar]
- Koopmans, J.R.; Slutske, W.S.; Heath, A.C.; Neale, M.C.; Boomsma, D.I. The genetics of smoking initiation and quantity smoked in Dutch adolescent and young adult twins. Behav. Genet. 1999, 29, 383–393. [Google Scholar] [CrossRef]
- Stallings, M.C.; Hewitt, J.K.; Beresford, T.; Heath, A.C.; Eaves, L.J. A twin study of drinking and smoking onset and latencies from first use to regular use. Behav. Genet. 1999, 29, 409–421. [Google Scholar] [CrossRef]
- Heath, A.C.; Kirk, K.M.; Meyer, J.M.; Martin, N.G. Genetic and social determinants of initiation and age at onset of smoking in Australian twins. Behav. Genet. 1999, 29, 395–407. [Google Scholar] [CrossRef]
- Vink, J.M.; Willemsen, G.; Boomsma, D.I. Heritability of smoking initiation and nicotine dependence. Behav. Genet. 2005, 35, 397–406. [Google Scholar] [CrossRef]
- Hall, W.D.; Gartner, C.E.; Carter, A. The genetics of nicotine addiction liability: Ethical and social policy implications. Addiction 2008, 103, 350–359. [Google Scholar] [CrossRef]
- Hancock, D.B.; Reginsson, G.W.; Gaddis, N.C.; Chen, X.; Saccone, N.L.; Lutz, S.M.; Qaiser, B.; Sherva, R.; Steinberg, S.; Zink, F.; et al. Genome-wide meta-analysis reveals common splice site acceptor variant in CHRNA4 associated with nicotine dependence. Transl. Psychiatry 2015, 5, e651. [Google Scholar] [CrossRef]
- Gorwood, P.; Le Strat, Y.; Ramoz, N. Genetics of addictive behavior: The example of nicotine dependence. Dialogues Clin. Neurosci. 2017, 19, 237–245. [Google Scholar] [CrossRef]
- Gelernter, J.; Kranzler, H.R.; Sherva, R.; Almasy, L.; Herman, A.I.; Koesterer, R.; Zhao, H.; Farrer, L.A. Genome-wide association study of nicotine dependence in American populations: Identification of novel risk loci in both African-Americans and European-Americans. Biol. Psychiatry 2015, 77, 493–503. [Google Scholar] [CrossRef]
- Gelernter, J.; Kranzler, H.R.; Sherva, R.; Koesterer, R.; Almasy, L.; Zhao, H.; Farrer, L.A. Genome-wide association study of opioid dependence: Multiple associations mapped to calcium and potassium pathways. Biol. Psychiatry 2014, 76, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Gelernter, J.; Sherva, R.; Koesterer, R.; Almasy, L.; Zhao, H.; Kranzler, H.R.; Farrer, L. Genome-wide association study of cocaine dependence and related traits: FAM53B identified as a risk gene. Mol. Psychiatry 2014, 19, 717–723. [Google Scholar] [CrossRef]
- Gelernter, J.; Kranzler, H.R.; Sherva, R.; Almasy, L.; Koesterer, R.; Smith, A.H.; Anton, R.; Preuss, U.W.; Ridinger, M.; Rujescu, D.; et al. Genome-wide association study of alcohol dependence:significant findings in African- and European-Americans including novel risk loci. Mol. Psychiatry 2014, 19, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.J.; Xu, X.; Mukherjee, S.; Willis, J.; Hayes, J. Successes of genome-wide association studies. Cell 2010, 142, 350–351, author reply 353–355. [Google Scholar] [CrossRef]
- Hirschhorn, J.N. Genomewide association studies--illuminating biologic pathways. N. Engl. J. Med. 2009, 360, 1699–1701. [Google Scholar] [CrossRef]
- White, J.G.; Southgate, E.; Thomson, J.N.; Brenner, S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos. Trans. R. Soc. London Ser. B Biol. Sci. 1986, 314, 340. [Google Scholar] [CrossRef]
- Varshney, L.R.; Chen, B.L.; Paniagua, E.; Hall, D.H.; Chklovskii, D.B. Structural properties of the Caenorhabditis elegans neuronal network. PLoS Comput. Biol. 2011, 7, e1001066. [Google Scholar] [CrossRef]
- Jarrell, T.A.; Wang, Y.; Bloniarz, A.E.; Brittin, C.A.; Xu, M.; Thomson, J.N.; Albertson, D.G.; Hall, D.H.; Emmons, S.W. The connectome of a decision-making neural network. Science 2012, 337, 437–444. [Google Scholar] [CrossRef] [PubMed]
- Jee, C.; Lee, J.; Lim, J.P.; Parry, D.; Messing, R.O.; McIntire, S.L. SEB-3, a CRF receptor-like GPCR, regulates locomotor activity states, stress responses and ethanol tolerance in Caenorhabditis elegans. Genes Brain Behav. 2013, 12, 250–262. [Google Scholar] [CrossRef]
- Bierut, L.J. Genetic vulnerability and susceptibility to substance dependence. Neuron 2011, 69, 618–627. [Google Scholar] [CrossRef]
- Davies, A.G.; Pierce-Shimomura, J.T.; Kim, H.; VanHoven, M.K.; Thiele, T.R.; Bonci, A.; Bargmann, C.I.; McIntire, S.L. A central role of the BK potassium channel in behavioral responses to ethanol in C. elegans. Cell 2003, 115, 655–666. [Google Scholar] [CrossRef]
- Davies, A.G.; Bettinger, J.C.; Thiele, T.R.; Judy, M.E.; McIntire, S.L. Natural variation in the npr-1 gene modifies ethanol responses of wild strains of C. elegans. Neuron 2004, 42, 731–743. [Google Scholar] [CrossRef]
- Lee, J.; Jee, C.; McIntire, S.L. Ethanol preference in C. elegans. Genes Brain Behav. 2009, 8, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Feng, Z.; Li, W.; Ward, A.; Piggott, B.J.; Larkspur, E.R.; Sternberg, P.W.; Xu, X.Z. A C. elegans model of nicotine-dependent behavior: Regulation by TRP-family channels. Cell 2006, 127, 621–633. [Google Scholar] [CrossRef] [PubMed]
- Ward, A.; Walker, V.J.; Feng, Z.; Xu, X.Z. Cocaine modulates locomotion behavior in C. elegans. PLoS ONE 2009, 4, e5946. [Google Scholar] [CrossRef] [PubMed]
- Carvelli, L.; Matthies, D.S.; Galli, A. Molecular mechanisms of amphetamine actions in Caenorhabditis elegans. Mol. Pharmacol. 2010, 78, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Waggoner, L.E.; Dickinson, K.A.; Poole, D.S.; Tabuse, Y.; Miwa, J.; Schafer, W.R. Long-term nicotine adaptation in Caenorhabditis elegans involves PKC-dependent changes in nicotinic receptor abundance. J. Neurosci. Off. J. Soc. Neurosci. 2000, 20, 8802–8811. [Google Scholar] [CrossRef] [PubMed]
- Rauthan, M.; Gong, J.; Liu, J.; Li, Z.; Wescott, S.A.; Liu, J.; Xu, X.Z. MicroRNA Regulation of nAChR Expression and Nicotine-Dependent Behavior in C. elegans. Cell Rep. 2017, 21, 1434–1441. [Google Scholar] [CrossRef]
- Changeux, J.P. Nicotine addiction and nicotinic receptors: Lessons from genetically modified mice. Nat. Rev. Neurosci. 2010, 11, 389–401. [Google Scholar] [CrossRef]
- Sellings, L.; Pereira, S.; Qian, C.; Dixon-McDougall, T.; Nowak, C.; Zhao, B.; Tyndale, R.F.; van der Kooy, D. Nicotine-motivated behavior in Caenorhabditis elegans requires the nicotinic acetylcholine receptor subunits acr-5 and acr-15. Eur. J. Neurosci. 2013, 37, 743–756. [Google Scholar] [CrossRef]
- Campusano, J.M.; Su, H.; Jiang, S.A.; Sicaeros, B.; O’Dowd, D.K. nAChR-mediated calcium responses and plasticity in Drosophila Kenyon cells. Dev. Neurobiol. 2007, 67, 1520–1532. [Google Scholar] [CrossRef]
- Vijayaraghavan, S.; Pugh, P.C.; Zhang, Z.W.; Rathouz, M.M.; Berg, D.K. Nicotinic receptors that bind alpha-bungarotoxin on neurons raise intracellular free Ca2+. Neuron 1992, 8, 353–362. [Google Scholar] [CrossRef]
- Sharma, G.; Grybko, M.; Vijayaraghavan, S. Action potential-independent and nicotinic receptor-mediated concerted release of multiple quanta at hippocampal CA3-mossy fiber synapses. J. Neurosci. 2008, 28, 2563–2575. [Google Scholar] [CrossRef]
- Fudala, P.J.; Teoh, K.W.; Iwamoto, E.T. Pharmacologic characterization of nicotine-induced conditioned place preference. Pharmacol. Biochem. Behav. 1985, 22, 237–241. [Google Scholar] [CrossRef]
- Spyraki, C.; Fibiger, H.C.; Phillips, A.G. Dopaminergic substrates of amphetamine-induced place preference conditioning. Brain Res. 1982, 253, 185–193. [Google Scholar] [CrossRef]
- Kruszewska, A.; Romandini, S.; Samanin, R. Different effects of zimelidine on the reinforcing properties of d-amphetamine and morphine on conditioned place preference in rats. Eur. J. Pharmacol. 1986, 125, 283–286. [Google Scholar] [CrossRef]
- De Biasi, M.; Dani, J.A. Reward, addiction, withdrawal to nicotine. Annu. Rev. Neurosci. 2011, 34, 105–130. [Google Scholar] [CrossRef]
- Wilar, G.; Shinoda, Y.; Sasaoka, T.; Fukunaga, K. Crucial Role of Dopamine D2 Receptor Signaling in Nicotine-Induced Conditioned Place Preference. Mol. Neurobiol. 2019, 56, 7911–7928. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Yu, Y.; Li, Y.; Wang, Y.; Wang, D. Dopamine Receptors Antagonistically Regulate Behavioral Choice between Conflicting Alternatives in C. elegans. PLoS ONE 2014, 9, e115985. [Google Scholar] [CrossRef] [PubMed]
- Ardiel, E.L.; Giles, A.C.; Yu, A.J.; Lindsay, T.H.; Lockery, S.R.; Rankin, C.H. Dopamine receptor DOP-4 modulates habituation to repetitive photoactivation of a C. elegans polymodal nociceptor. Learn. Mem. 2016, 23, 495–503. [Google Scholar] [CrossRef] [PubMed]
- Sugiura, M.; Fuke, S.; Suo, S.; Sasagawa, N.; Van Tol, H.H.; Ishiura, S. Characterization of a novel D2-like dopamine receptor with a truncated splice variant and a D1-like dopamine receptor unique to invertebrates from Caenorhabditis elegans. J. Neurochem. 2005, 94, 1146–1157. [Google Scholar] [CrossRef] [PubMed]
- Wes, P.D.; Bargmann, C.I. C. elegans odour discrimination requires asymmetric diversity in olfactory neurons. Nature 2001, 410, 698–701. [Google Scholar] [CrossRef] [PubMed]
- Hukema, R.K.; Rademakers, S.; Jansen, G. Gustatory plasticity in C. elegans involves integration of negative cues and NaCl taste mediated by serotonin, dopamine, and glutamate. Learn. Mem. 2008, 15, 829–836. [Google Scholar] [CrossRef]
- Bargmann, C.I.; Hartwieg, E.; Horvitz, H.R. Odorant-selective genes and neurons mediate olfaction in C. elegans. Cell 1993, 74, 515–527. [Google Scholar] [CrossRef]
- Hilliard, M.A.; Bergamasco, C.; Arbucci, S.; Plasterk, R.H.; Bazzicalupo, P. Worms taste bitter: ASH neurons, QUI-1, GPA-3 and ODR-3 mediate quinine avoidance in Caenorhabditis elegans. EMBO J. 2004, 23, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Colbert, H.A.; Bargmann, C.I. The C. elegans gene odr-7 encodes an olfactory-specific member of the nuclear receptor superfamily. Cell 1994, 79, 971–980. [Google Scholar] [CrossRef]
- Bargmann, C.I.; Horvitz, H.R. Chemosensory neurons with overlapping functions direct chemotaxis to multiple chemicals in C. elegans. Neuron 1991, 7, 729–742. [Google Scholar] [CrossRef] [PubMed]
- Larsch, J.; Flavell, S.W.; Liu, Q.; Gordus, A.; Albrecht, D.R.; Bargmann, C.I. A Circuit for Gradient Climbing in C. elegans Chemotaxis. Cell Rep. 2015, 12, 1748–1760. [Google Scholar] [CrossRef] [PubMed]
- Chelur, D.S.; Chalfie, M. Targeted cell killing by reconstituted caspases. Proc. Natl. Acad. Sci. USA 2007, 104, 2283–2288. [Google Scholar] [CrossRef]
- Beverly, M.; Anbil, S.; Sengupta, P. Degeneracy and neuromodulation among thermosensory neurons contribute to robust thermosensory behaviors in Caenorhabditis elegans. J. Neurosci. Off. J. Soc. Neurosci. 2011, 31, 11718–11727. [Google Scholar] [CrossRef]
- Albuquerque, E.X.; Alkondon, M.; Pereira, E.F.; Castro, N.G.; Schrattenholz, A.; Barbosa, C.T.; Bonfante-Cabarcas, R.; Aracava, Y.; Eisenberg, H.M.; Maelicke, A. Properties of neuronal nicotinic acetylcholine receptors: Pharmacological characterization and modulation of synaptic function. J. Pharmacol. Exp. Ther. 1997, 280, 1117–1136. [Google Scholar] [PubMed]
- Dajas-Bailador, F.A.; Mogg, A.J.; Wonnacott, S. Intracellular Ca2+ signals evoked by stimulation of nicotinic acetylcholine receptors in SH-SY5Y cells: Contribution of voltage-operated Ca2+ channels and Ca2+ stores. J. Neurochem. 2002, 81, 606–614. [Google Scholar] [CrossRef]
- Dajas-Bailador, F.; Wonnacott, S. Nicotinic acetylcholine receptors and the regulation of neuronal signalling. Trends Pharmacol. Sci. 2004, 25, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Polli, J.R.; Dobbins, D.L.; Kobet, R.A.; Farwell, M.A.; Zhang, B.; Lee, M.-H.; Pan, X. Drug-dependent behaviors and nicotinic acetylcholine receptor expressions in Caenorhabditis elegans following chronic nicotine exposure. NeuroToxicology 2015, 47, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Brejc, K.; van Dijk, W.J.; Klaassen, R.V.; Schuurmans, M.; van Der Oost, J.; Smit, A.B.; Sixma, T.K. Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature 2001, 411, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Sharma, G.; Vijayaraghavan, S. Nicotinic receptors containing the alpha7 subunit: A model for rational drug design. Curr. Med. Chem. 2008, 15, 2921–2932. [Google Scholar] [CrossRef] [PubMed]
- Hammarlund, M.; Hobert, O.; Miller, D.M., 3rd; Sestan, N. The CeNGEN Project: The Complete Gene Expression Map of an Entire Nervous System. Neuron 2018, 99, 430–433. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, B.; Wang, Z.W. GABAergic motor neurons bias locomotor decision-making in C. elegans. Nat. Commun. 2020, 11, 5076. [Google Scholar] [CrossRef] [PubMed]
- Macosko, E.Z.; Pokala, N.; Feinberg, E.H.; Chalasani, S.H.; Butcher, R.A.; Clardy, J.; Bargmann, C.I. A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans. Nature 2009, 458, 1171–1175. [Google Scholar] [CrossRef] [PubMed]
- Fleming, J.T.; Squire, M.D.; Barnes, T.M.; Tornoe, C.; Matsuda, K.; Ahnn, J.; Fire, A.; Sulston, J.E.; Barnard, E.A.; Sattelle, D.B.; et al. Caenorhabditis elegans levamisole resistance genes lev-1, unc-29, and unc-38 encode functional nicotinic acetylcholine receptor subunits. J. Neurosci. 1997, 17, 5843–5857. [Google Scholar] [CrossRef]
- Culetto, E.; Baylis, H.A.; Richmond, J.E.; Jones, A.K.; Fleming, J.T.; Squire, M.D.; Lewis, J.A.; Sattelle, D.B. The Caenorhabditis elegans unc-63 gene encodes a levamisole-sensitive nicotinic acetylcholine receptor alpha subunit. J. Biol. Chem. 2004, 279, 42476–42483. [Google Scholar] [CrossRef]
- Adkins, A.E.; Hack, L.M.; Bigdeli, T.B.; Williamson, V.S.; McMichael, G.O.; Mamdani, M.; Edwards, A.C.; Aliev, F.; Chan, R.F.; Bhandari, P.; et al. Genomewide Association Study of Alcohol Dependence Identifies Risk Loci Altering Ethanol-Response Behaviors in Model Organisms. Alcohol. Clin. Exp. Res. 2017, 41, 911–928. [Google Scholar] [CrossRef]
- Yin, X.; Bizon, C.; Tilson, J.; Lin, Y.; Gizer, I.R.; Ehlers, C.L.; Wilhelmsen, K.C. Genome-wide meta-analysis identifies a novel susceptibility signal at CACNA2D3 for nicotine dependence. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2017, 174, 557–567. [Google Scholar] [CrossRef]
- Uhl, G.R.; Liu, Q.R.; Drgon, T.; Johnson, C.; Walther, D.; Rose, J.E.; David, S.P.; Niaura, R.; Lerman, C. Molecular genetics of successful smoking cessation: Convergent genome-wide association study results. Arch. Gen. Psychiatry 2008, 65, 683–693. [Google Scholar] [CrossRef]
- Davies, A.; Hendrich, J.; Van Minh, A.T.; Wratten, J.; Douglas, L.; Dolphin, A.C. Functional biology of the alpha(2)delta subunits of voltage-gated calcium channels. Trends Pharmacol. Sci. 2007, 28, 220–228. [Google Scholar] [CrossRef]
- Lainé, V.; Frøkjær-Jensen, C.; Couchoux, H.; Jospin, M. The alpha1 subunit EGL-19, the alpha2/delta subunit UNC-36, and the beta subunit CCB-1 underlie voltage-dependent calcium currents in Caenorhabditis elegans striated muscle. J. Biol. Chem. 2011, 286, 36180–36187. [Google Scholar] [CrossRef]
- Liu, M.; Jiang, Y.; Wedow, R.; Li, Y.; Brazel, D.M.; Chen, F.; Datta, G.; Davila-Velderrain, J.; McGuire, D.; Tian, C.; et al. Association studies of up to 1.2 million individuals yield new insights into the genetic etiology of tobacco and alcohol use. Nat. Genet. 2019, 51, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Hills, T.; Brockie, P.J.; Maricq, A.V. Dopamine and glutamate control area-restricted search behavior in Caenorhabditis elegans. J. Neurosci. 2004, 24, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Sawin, E.R.; Ranganathan, R.; Horvitz, H.R. C. elegans locomotory rate is modulated by the environment through a dopaminergic pathway and by experience through a serotonergic pathway. Neuron 2000, 26, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Albin, R.L.; Young, A.B.; Penney, J.B. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989, 12, 366–375. [Google Scholar] [CrossRef]
- Redgrave, P.; Rodriguez, M.; Smith, Y.; Rodriguez-Oroz, M.C.; Lehericy, S.; Bergman, H.; Agid, Y.; DeLong, M.R.; Obeso, J.A. Goal-directed and habitual control in the basal ganglia: Implications for Parkinson’s disease. Nat. Rev. Neurosci. 2010, 11, 760–772. [Google Scholar] [CrossRef] [PubMed]
- Olds, J.; Milner, P. Positive reinforcement produced by electrical stimulation of septal area and other regions of rat brain. J. Comp. Physiol. Psychol. 1954, 47, 419–427. [Google Scholar] [CrossRef]
- Peciña, S.; Berridge, K.C. Dopamine or opioid stimulation of nucleus accumbens similarly amplify cue-triggered ‘wanting’ for reward: Entire core and medial shell mapped as substrates for PIT enhancement. Eur. J. Neurosci. 2013, 37, 1529–1540. [Google Scholar] [CrossRef]
- Everitt, B.J.; Belin, D.; Economidou, D.; Pelloux, Y.; Dalley, J.W.; Robbins, T.W. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 3125–3135. [Google Scholar] [CrossRef]
- Chase, D.L.; Pepper, J.S.; Koelle, M.R. Mechanism of extrasynaptic dopamine signaling in Caenorhabditis elegans. Nat. Neurosci. 2004, 7, 1096–1103. [Google Scholar] [CrossRef]
- Xu, Y.; Cao, L.; Zhao, X.; Yao, Y.; Liu, Q.; Zhang, B.; Wang, Y.; Mao, Y.; Ma, Y.; Ma, J.Z.; et al. Prediction of Smoking Behavior From Single Nucleotide Polymorphisms With Machine Learning Approaches. Front. Psychiatry 2020, 11, 416. [Google Scholar] [CrossRef]
- Liedtke, W.; Tobin, D.M.; Bargmann, C.I.; Friedman, J.M. Mammalian TRPV4 (VR-OAC) directs behavioral responses to osmotic and mechanical stimuli in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2003, 100 (Suppl. S2), 14531–14536. [Google Scholar] [CrossRef]
- Young, A.T.; Ly, K.N.; Wilson, C.; Lehnert, K.; Snell, R.G.; Reid, S.J.; Jacobsen, J.C. Modelling brain dopamine-serotonin vesicular transport disease in Caenorhabditis elegans. Dis. Model. Mech. 2018, 11, dmm035709. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Stoveken, H.M.; Zucca, S.; Dao, M.; Orlandi, C.; Song, C.; Masuho, I.; Johnston, C.; Opperman, K.J.; Giles, A.C.; et al. Genetic behavioral screen identifies an orphan anti-opioid system. Science 2019, 365, 1267–1273. [Google Scholar] [CrossRef] [PubMed]
- Katsura, M.; Mohri, Y.; Shuto, K.; Hai-Du, Y.; Amano, T.; Tsujimura, A.; Sasa, M.; Ohkuma, S. Up-regulation of L-type voltage-dependent calcium channels after long term exposure to nicotine in cerebral cortical neurons. J. Biol. Chem. 2002, 277, 7979–7988. [Google Scholar] [CrossRef] [PubMed]
- Michalak, A.; Biala, G. Calcium homeostasis and protein kinase/phosphatase balance participate in nicotine-induced memory improvement in passive avoidance task in mice. Behav. Brain Res. 2017, 317, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Risher, W.C.; Eroglu, C. Emerging roles for α2δ subunits in calcium channel function and synaptic connectivity. Curr. Opin. Neurobiol. 2020, 63, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Tong, X.J.; López-Soto, E.J.; Li, L.; Liu, H.; Nedelcu, D.; Lipscombe, D.; Hu, Z.; Kaplan, J.M. Retrograde Synaptic Inhibition Is Mediated by α-Neurexin Binding to the α2δ Subunits of N-Type Calcium Channels. Neuron 2017, 95, 326–340.e325. [Google Scholar] [CrossRef]
- Chen, J.; Li, L.; Chen, S.R.; Chen, H.; Xie, J.D.; Sirrieh, R.E.; MacLean, D.M.; Zhang, Y.; Zhou, M.H.; Jayaraman, V.; et al. The α2δ-1-NMDA Receptor Complex Is Critically Involved in Neuropathic Pain Development and Gabapentin Therapeutic Actions. Cell Rep. 2018, 22, 2307–2321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.X.; Gadotti, V.M.; Souza, I.A.; Chen, L.; Zamponi, G.W. BK Potassium Channels Suppress Cavα2δ Subunit Function to Reduce Inflammatory and Neuropathic Pain. Cell Rep. 2018, 22, 1956–1964. [Google Scholar] [CrossRef] [PubMed]
- Kadurin, I.; Rothwell, S.W.; Lana, B.; Nieto-Rostro, M.; Dolphin, A.C. LRP1 influences trafficking of N-type calcium channels via interaction with the auxiliary α(2)δ-1 subunit. Sci. Rep. 2017, 7, 43802. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Chen, S.R.; Chen, H.; Pan, H.L. α2δ-1-Bound N-Methyl-D-aspartate Receptors Mediate Morphine-induced Hyperalgesia and Analgesic Tolerance by Potentiating Glutamatergic Input in Rodents. Anesthesiology 2019, 130, 804–819. [Google Scholar] [CrossRef] [PubMed]
- Risher, M.L.; Sexton, H.G.; Risher, W.C.; Wilson, W.A.; Fleming, R.L.; Madison, R.D.; Moore, S.D.; Eroglu, C.; Swartzwelder, H.S. Adolescent Intermittent Alcohol Exposure: Dysregulation of Thrombospondins and Synapse Formation are Associated with Decreased Neuronal Density in the Adult Hippocampus. Alcohol. Clin. Exp. Res. 2015, 39, 2403–2413. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Underwood, R.S.; Greenwald, I.; Shaye, D.D. OrthoList 2: A New Comparative Genomic Analysis of Human and Caenorhabditis elegans Genes. Genetics 2018, 210, 445–461. [Google Scholar] [CrossRef]
- Caylor, R.C.; Jin, Y.; Ackley, B.D. The Caenorhabditis elegans voltage-gated calcium channel subunits UNC-2 and UNC-36 and the calcium-dependent kinase UNC-43/CaMKII regulate neuromuscular junction morphology. Neural Dev. 2013, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.; Douglas, L.; Hendrich, J.; Wratten, J.; Tran Van Minh, A.; Foucault, I.; Koch, D.; Pratt, W.S.; Saibil, H.R.; Dolphin, A.C. The calcium channel alpha2delta-2 subunit partitions with CaV2.1 into lipid rafts in cerebellum: Implications for localization and function. J. Neurosci. 2006, 26, 8748–8757. [Google Scholar] [CrossRef]
- Brenner, S. The genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Kim, K.; Kim, R.; Sengupta, P. The HMX/NKX homeodomain protein MLS-2 specifies the identity of the AWC sensory neuron type via regulation of the ceh-36 Otx gene in C. elegans. Development 2010, 137, 963–974. [Google Scholar] [CrossRef] [PubMed]
- Colbert, H.A.; Bargmann, C.I. Odorant-specific adaptation pathways generate olfactory plasticity in C. elegans. Neuron 1995, 14, 803–812. [Google Scholar] [CrossRef] [PubMed]
- Gish, W.; States, D.J. Identification of protein coding regions by database similarity search. Nat. Genet. 1993, 3, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, J.S.; Agarwala, R. COBALT: Constraint-based alignment tool for multiple protein sequences. Bioinformatics 2007, 23, 1073–1079. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salim, C.; Batsaikhan, E.; Kan, A.K.; Chen, H.; Jee, C. Nicotine Motivated Behavior in C. elegans. Int. J. Mol. Sci. 2024, 25, 1634. https://doi.org/10.3390/ijms25031634
Salim C, Batsaikhan E, Kan AK, Chen H, Jee C. Nicotine Motivated Behavior in C. elegans. International Journal of Molecular Sciences. 2024; 25(3):1634. https://doi.org/10.3390/ijms25031634
Chicago/Turabian StyleSalim, Chinnu, Enkhzul Batsaikhan, Ann Ke Kan, Hao Chen, and Changhoon Jee. 2024. "Nicotine Motivated Behavior in C. elegans" International Journal of Molecular Sciences 25, no. 3: 1634. https://doi.org/10.3390/ijms25031634
APA StyleSalim, C., Batsaikhan, E., Kan, A. K., Chen, H., & Jee, C. (2024). Nicotine Motivated Behavior in C. elegans. International Journal of Molecular Sciences, 25(3), 1634. https://doi.org/10.3390/ijms25031634





