Caenorhabditis elegans as a Screening Model for Probiotics with Properties against Metabolic Syndrome
Abstract
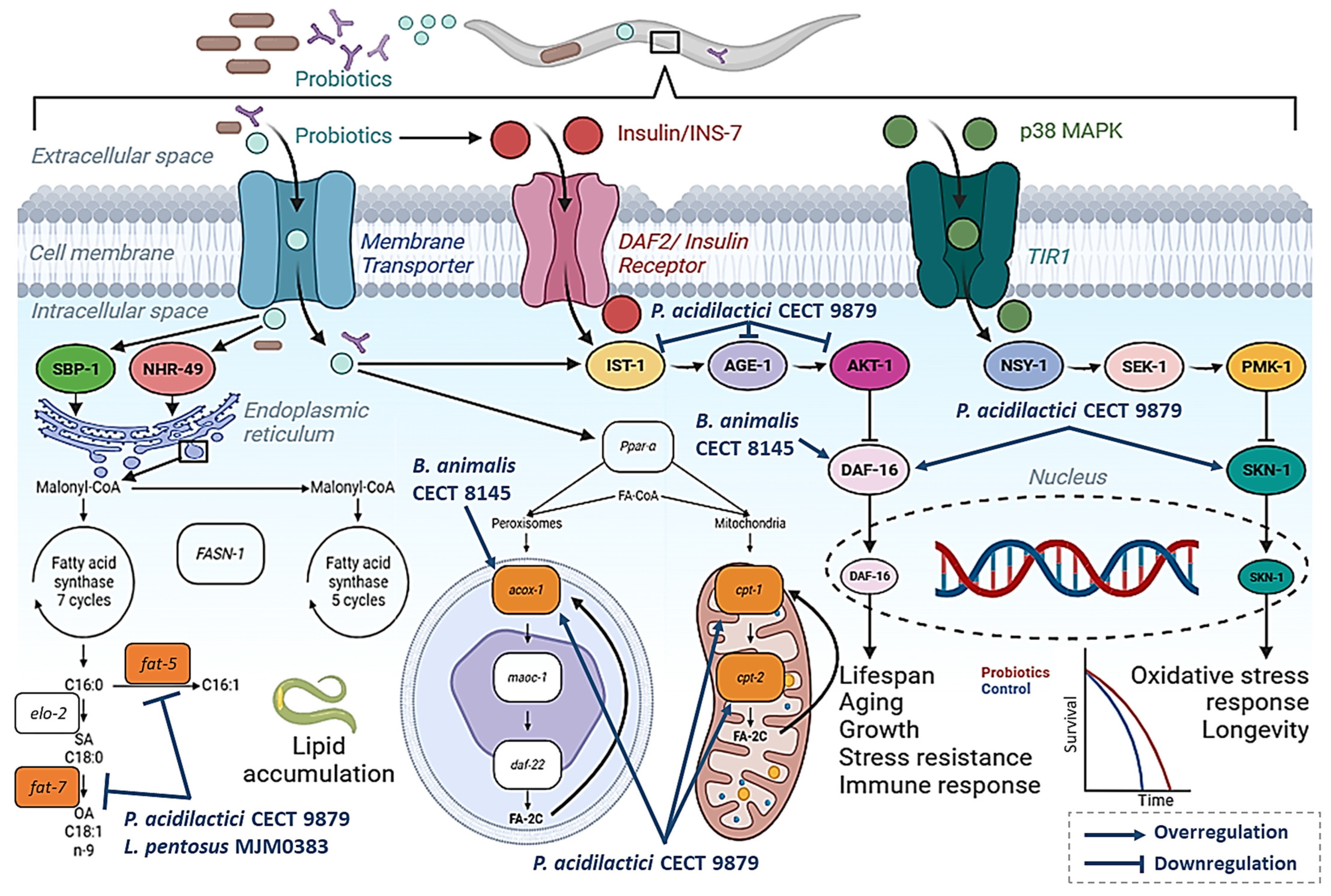
1. Introduction
2. Probiotics with Lipid-Reducing Activity in C. elegans
2.1. Bifidobacterium Strains with Anti-Obesity Properties in C. elegans
2.2. Pediococcus Acidilactici Strains with Anti-Obesity Properties in C. elegans
2.3. Other Lactic Acid Bacteria with Anti-Obesity Properties in C. elegans
3. Probiotics Counteracting the Effect of High Glucose Exposure in C. elegans
4. Probiotics with Anti-Inflammatory Properties in C. elegans
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wolters, M.; Ahrens, J.; Romaní-Pérez, M.; Watkins, C.; Sanz, Y.; Benítez-Páez, A.; Stanton, C.; Günther, K. Dietary Fat, the Gut Microbiota, and Metabolic Health—A Systematic Review Conducted within the MyNewGut Project. Clin. Nutr. 2019, 38, 2504–2520. [Google Scholar] [CrossRef] [PubMed]
- Lv, L.X.; Fang, D.Q.; Shi, D.; Chen, D.Y.; Yan, R.; Zhu, Y.X.; Chen, Y.F.; Shao, L.; Guo, F.F.; Wu, W.R.; et al. Alterations and Correlations of the Gut Microbiome, Metabolism and Immunity in Patients with Primary Biliary Cirrhosis. Environ. Microbiol. 2016, 18, 2272–2286. [Google Scholar] [CrossRef] [PubMed]
- Brial, F.; Le Lay, A.; Dumas, M.E.; Gauguier, D. Implication of Gut Microbiota Metabolites in Cardiovascular and Metabolic Diseases. Cell. Mol. Life Sci. 2018, 75, 3977–3990. [Google Scholar] [CrossRef] [PubMed]
- Tavassol, Z.H.; Ejtahed, H.S.; Atlasi, R.; Saghafian, F.; Khalagi, K.; Hasani-Ranjbar, S.; Siadat, S.D.; Nabipour, I.; Ostovar, A.; Larijani, B. Alteration in Gut Microbiota Composition of Older Adults Is Associated with Obesity and Its Indices: A Systematic Review. J. Nutr. Health Aging 2023, 27, 817–823. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.; Rivera, L.; Furness, J.B.; Angus, P.W. The Role of the Gut Microbiota in NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Jiang, W.; Huang, W.; Lin, Y.; Chan, F.K.L.; Ng, S.C. Gut Microbiota in Patients with Obesity and Metabolic Disorders—A Systematic Review. Genes Nutr. 2022, 17, 2. [Google Scholar] [CrossRef]
- Xiao, Y.; Niu, Y.; Mao, M.; Lin, H.; Wang, B.; Wu, E.; Zhao, H.; Li, S. Correlation Analysis between Type 2 Diabetes and Core Gut Microbiota. Nan Fang Yi Ke Da Xue Xue Bao 2021, 41, 358–369. [Google Scholar] [CrossRef]
- Iatcu, C.O.; Steen, A.; Covasa, M. Gut Microbiota and Complications of Type-2 Diabetes. Nutrients 2022, 14, 166. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, N.; Tan, H.Y.; Li, S.; Zhang, C.; Feng, Y. Function of Akkermansia Muciniphila in Obesity: Interactions With Lipid Metabolism, Immune Response and Gut Systems. Front. Microbiol. 2020, 11, 219. [Google Scholar] [CrossRef]
- Antony, M.A.; Chowdhury, A.; Edem, D.; Raj, R.; Nain, P.; Joglekar, M.; Verma, V.; Kant, R. Gut Microbiome Supplementation as Therapy for Metabolic Syndrome. World J. Diabetes 2023, 14, 1502–1513. [Google Scholar] [CrossRef]
- Crudele, L.; Gadaleta, R.M.; Cariello, M.; Moschetta, A. Gut Microbiota in the Pathogenesis and Therapeutic Approaches of Diabetes. EBioMedicine 2023, 97, 104821. [Google Scholar] [CrossRef] [PubMed]
- Turroni, S.; Liu, F.; Wang, X.; Zhao, X.; Zhong, X.; Liu, X.; Gao, X. Therapeutic and Improving Function of Lactobacilli in the Prevention and Treatment of Cardiovascular-Related Diseases: A Novel Perspective from Gut Microbiota. Front. Nutr. 2021, 1, 693412. [Google Scholar] [CrossRef]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document. The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Tonucci, L.B.; Olbrich dos Santos, K.M.; Licursi de Oliveira, L.; Rocha Ribeiro, S.M.; Duarte Martino, H.S. Clinical Application of Probiotics in Type 2 Diabetes Mellitus: A Randomized, Double-Blind, Placebo-Controlled Study. Clin. Nutr. 2017, 36, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Toshimitsu, T. Development of a Lactic Acid Bacteria Strain That Suppresses Chronic Inflammation and Improves Glucose and Lipid Metabolism. Biosci. Microbiota Food Health 2023, 42, 3–7. [Google Scholar] [CrossRef]
- Zanni, E.; Laudenzi, C.; Schifano, E.; Palleschi, C.; Perozzi, G.; Uccelletti, D.; Devirgiliis, C. Impact of a Complex Food Microbiota on Energy Metabolism in the Model Organism Caenorhabditis elegans. BioMed Res. Int. 2015, 2015, 621709. [Google Scholar] [CrossRef]
- Pedret, A.; Valls, R.M.; Calderón-Pérez, L.; Llauradó, E.; Companys, J.; Pla-Pagà, L.; Moragas, A.; Martín-Luján, F.; Ortega, Y.; Giralt, M.; et al. Effects of Daily Consumption of the Probiotic Bifidobacterium animalis subsp. lactis CECT 8145 on Anthropometric Adiposity Biomarkers in Abdominally Obese Subjects: A Randomized Controlled Trial. Int. J. Obes. 2019, 43, 1863–1868. [Google Scholar] [CrossRef]
- Chakravarty, B. The Evolving Role of the Caenorhabditis elegans Model as a Tool to Advance Studies in Nutrition and Health. Nutr. Res. 2022, 106, 47–59. [Google Scholar] [CrossRef]
- Shen, P.; Yue, Y.; Zheng, J.; Park, Y. Caenorhabditis elegans: A Convenient In Vivo Model for Assessing the Impact of Food Bioactive Compounds on Obesity, Aging, and Alzheimer’s Disease. Annu. Rev. Food Sci. Technol. 2018, 9, 1–22. [Google Scholar] [CrossRef]
- Shen, P.; Yue, Y.; Park, Y. A Living Model for Obesity and Aging Research: Caenorhabditis elegans. Crit. Rev. Food Sci. Nutr. 2018, 58, 741–754. [Google Scholar] [CrossRef]
- Lemieux, G.A.; Ashrafi, K. Insights and Challenges in Using C. elegans for Investigation of Fat Metabolism. Crit. Rev. Biochem. Mol. Biol. 2015, 50, 69–84. [Google Scholar] [CrossRef] [PubMed]
- Lemieux, G.A.; Liu, J.; Mayer, N.; Bainton, R.J.; Ashrafi, K.; Werb, Z. A Whole-Organism Screen Identifies New Regulators of Fat Storage. Nat. Chem. Biol. 2011, 7, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Martorell, P.; Llopis, S.; González, N.; Chenoll, E.; López-Carreras, N.; Aleixandre, A.; Chen, Y.; Karoly, E.D.; Ramón, D.; Genovés, S. Probiotic Strain Bifidobacterium animalis subsp. lactis CECT 8145 Reduces Fat Content and Modulates Lipid Metabolism and Antioxidant Response in Caenorhabditis elegans. J. Agric. Food Chem. 2016, 64, 3462–3472. [Google Scholar] [CrossRef] [PubMed]
- Yavorov-Dayliev, D.; Milagro, F.I.; Ayo, J.; Oneca, M.; Aranaz, P. Pediococcus acidilactici CECT9879 (PA1c) Counteracts the Effect of a High-Glucose Exposure in C. elegans by Affecting the Insulin Signaling Pathway (IIS). Int. J. Mol. Sci. 2022, 23, 2689. [Google Scholar] [CrossRef] [PubMed]
- Poupet, C.; Chassard, C.; Nivoliez, A.; Bornes, S. Caenorhabditis elegans, a Host to Investigate the Probiotic Properties of Beneficial Microorganisms. Front. Nutr. 2020, 7, 135. [Google Scholar] [CrossRef] [PubMed]
- Komura, T.; Ikeda, T.; Yasui, C.; Saeki, S.; Nishikawa, Y. Mechanism Underlying Prolongevity Induced by Bifidobacteria in Caenorhabditis elegans. Biogerontology 2013, 14, 73–87. [Google Scholar] [CrossRef] [PubMed]
- Leñini, C.; Rodriguez Ayala, F.; Goñi, A.J.; Rateni, L.; Nakamura, A.; Grau, R.R. Probiotic Properties of Bacillus Subtilis DG101 Isolated from the Traditional Japanese Fermented Food Nattō. Front. Microbiol. 2023, 14, 3480. [Google Scholar] [CrossRef] [PubMed]
- Giron, M.; Thomas, M.; Jarzaguet, M.; Mayeur, C.; Ferrere, G.; Noordine, M.L.; Bornes, S.; Dardevet, D.; Chassard, C.; Savary-Auzeloux, I. Lacticaseibacillus Casei CNCM I-5663 Supplementation Maintained Muscle Mass in a Model of Frail Rodents. Front. Nutr. 2022, 9, 8798. [Google Scholar] [CrossRef]
- Wiciński, M.; Gębalski, J.; Gołębiewski, J.; Malinowski, B. Probiotics for the Treatment of Overweight and Obesity in Humans—A Review of Clinical Trials. Microorganisms 2020, 8, 1148. [Google Scholar] [CrossRef]
- Marquez, A.; Andrada, E.; Russo, M.; Bolondi, M.L.; Fabersani, E.; Medina, R.; Gauffin-Cano, P. Characterization of Autochthonous Lactobacilli from Goat Dairy Products with Probiotic Potential for Metabolic Diseases. Heliyon 2022, 8, E10462. [Google Scholar] [CrossRef]
- Silva, Á.; Gonzalez, N.; Terrén, A.; García, A.; Martinez-Blanch, J.F.; Illescas, V.; Morales, J.; Maroto, M.; Genovés, S.; Ramón, D.; et al. An Infant Milk Formula Supplemented with Heat-Treated Probiotic Bifidobacterium animalis subsp. lactis CECT 8145, Reduces Fat Deposition in C. elegans and Augments Acetate and Lactate in a Fermented Infant Slurry. Foods 2020, 9, 652. [Google Scholar] [CrossRef] [PubMed]
- Balaguer, F.; Enrique, M.; Llopis, S.; Barrena, M.; Navarro, V.; Álvarez, B.; Chenoll, E.; Ramón, D.; Tortajada, M.; Martorell, P. Lipoteichoic Acid from Bifidobacterium animalis subsp. lactis BPL1: A Novel Postbiotic That Reduces Fat Deposition via IGF-1 Pathway. Microb. Biotechnol. 2022, 15, 805–816. [Google Scholar] [CrossRef] [PubMed]
- Barathikannan, K.; Chelliah, R.; Elahi, F.; Tyagi, A.; Selvakumar, V.; Agastian, P.; Arasu, M.V.; Oh, D.H. Anti-Obesity Efficacy of Pediococcus acidilactici MNL5 in Canorhabditis elegans Gut Model. Int. J. Mol. Sci. 2022, 23, 1276. [Google Scholar] [CrossRef] [PubMed]
- Yavorov-Dayliev, D.; Milagro, F.I.; Ayo, J.; Oneca, M.; Goyache, I.; López-Yoldi, M.; Aranaz, P. Glucose-Lowering Effects of a Synbiotic Combination Containing Pediococcus acidilactici in C. elegans and Mice. Diabetologia 2023, 66, 2117–2138. [Google Scholar] [CrossRef] [PubMed]
- Daliri, E.B.M.; Kim, Y.; Do, Y.; Chelliah, R.; Oh, D.H. In Vitro and In Vivo Cholesterol Reducing Ability and Safety of Probiotic Candidates Isolated from Korean Fermented Soya Beans. Probiotics Antimicrob. Proteins 2022, 14, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Gu, M.; Werlinger, P.; Cho, J.H.; Jang, N.; Choi, S.S.; Suh, J.W.; Cheng, J. Lactobacillus Pentosus MJM60383 Inhibits Lipid Accumulation in Caenorhabditis elegans Induced by Enterobacter Cloacae and Glucose. Int. J. Mol. Sci. 2022, 24, 280. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Tategaki, A.; Hamada, K.; Kishida, H.; Hosoe, K.; Morikawa, H.; Nakagawa, K. Effects of Pediococcus acidilactici R037 on Serum Triglyceride Levels in Mice and Rats after Oral Administration. J. Nutr. Sci. Vitaminol. 2018, 64, 41–47. [Google Scholar] [CrossRef]
- Mizoguchi, T.; Kasahara, K.; Yamashita, T.; Sasaki, N.; Yodoi, K.; Matsumoto, T.; Emoto, T.; Hayashi, T.; Kitano, N.; Yoshida, N.; et al. Oral Administration of the Lactic Acid Bacterium Pediococcus acidilactici Attenuates Atherosclerosis in Mice by Inducing Tolerogenic Dendritic Cells. Heart Vessel. 2017, 32, 768–776. [Google Scholar] [CrossRef]
- Cabello-Olmo, M.; Oneca, M.; Pajares, M.J.; Jiménez, M.; Ayo, J.; Encío, I.J.; Barajas, M.; Araña, M. Antidiabetic Effects of Pediococcus acidilactici PA1c on HFD-Induced Mice. Nutrients 2022, 14, 692. [Google Scholar] [CrossRef]
- Yavorov-Dayliev, D.; Milagro, F.; López-Yoldi, M.; Clemente, I.; Riezu-Boj, J.I.; Ayo, J.; Oneca, M.; Aranaz, P. Pediococcus acidilactici (PA1c®) Alleviates Obesity-Related Dyslipidemia and Inflammation in Wistar Rats by Activating Beta-Oxidation and Modulating the Gut Microbiota. Food Funct. 2023, 14, 10855–10867. [Google Scholar] [CrossRef]
- Tissenbaum, H.A. DAF-16/FOXO: Many Paths To a Single Fork(Head) in The Road. Antioxid. Redox Signal. 2010, 14, 623–634. [Google Scholar] [CrossRef]
- Tissenbaum, H.A. DAF-16: FOXO in the Context of C. elegans. In Current Topics in Developmental Biology; Elsevier: Amsterdam, The Netherlands, 2018. [Google Scholar]
- Peng, H.; Wei, Z.; Luo, H.; Yang, Y.; Wu, Z.; Gan, L.; Yang, X. Inhibition of Fat Accumulation by Hesperidin in Caenorhabditis elegans. J. Agric. Food Chem. 2016, 64, 5207–5214. [Google Scholar] [CrossRef] [PubMed]
- Brock, T.J.; Browse, J.; Watts, J.L. Genetic Regulation of Unsaturated Fatty Acid Composition in C. elegans. PLoS Genet. 2006, 2, e108. [Google Scholar] [CrossRef]
- Wu, Z.; Xiao, Y.; Zhou, F.; Chen, J.; Chen, X.; Hou, A.; Wang, Y.; Li, Z. Pasteurized Akkermansia Muciniphila Reduces Fat Accumulation via Nhr-49-Mediated Nuclear Hormone Signaling Pathway in Caenorhabditis elegans. Molecules 2022, 27, 6159. [Google Scholar] [CrossRef] [PubMed]
- Ellulu, M.S.; Patimah, I.; Khaza’ai, H.; Rahmat, A.; Abed, Y. Obesity & Inflammation: The Linking Mechanism & the Complications. Arch. Med. Sci. 2017, 13, 851–863. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Inflammation 2010: New Adventures of an Old Flame. Cell 2010, 140, 771–776. [Google Scholar] [CrossRef] [PubMed]
- Roselli, M.; Schifano, E.; Guantario, B.; Zinno, P.; Uccelletti, D.; Devirgiliis, C. Caenorhabditis elegans and Probiotics Interactions from a Prolongevity Perspective. Int. J. Mol. Sci. 2019, 20, 5020. [Google Scholar] [CrossRef]
- Huang, X.; Pan, W.; Kim, W.; White, A.; Li, S.; Li, H.; Lee, K.; Fuchs, B.B.; Zeng, K.; Mylonakis, E. Caenorhabditis elegans Mounts a P38 MAPK Pathway-Mediated Defence to Cutibacterium Acnes Infection. Cell. Microbiol. 2020, 22, e13234. [Google Scholar] [CrossRef]
- JebaMercy, G.; Vigneshwari, L.; Balamurugan, K. A MAP Kinase Pathway in Caenorhabditis elegans Is Required for Defense against Infection by Opportunistic Proteus Species. Microbes Infect. 2013, 15, 550–568. [Google Scholar] [CrossRef]
- Xu, A.; Shi, G.; Liu, F.; Ge, B. Caenorhabditis elegans Mom-4 Is Required for the Activation of the P38 MAPK Signaling Pathway in the Response to Pseudomonas Aeruginosa Infection. Protein Cell 2013, 4, 53. [Google Scholar] [CrossRef]
- Xiao, Y.; Liu, F.; Li, S.; Jiang, N.; Yu, C.; Zhu, X.; Qin, Y.; Hui, J.; Meng, L.; Song, C.; et al. Metformin Promotes Innate Immunity through a Conserved PMK-1/P38 MAPK Pathway. Virulence 2020, 11, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Hu, R.; Zhang, Y.; Qian, W.; Leng, Y.; Long, Y.; Liu, X.; Li, J.; Wan, X.; Wei, X. Pediococcus acidilactici Promotes the Longevity of C. elegans by Regulating the Insulin/IGF-1 and JNK/MAPK Signaling, Fat Accumulation and Chloride Ion. Front. Nutr. 2022, 9, 821685. [Google Scholar] [CrossRef] [PubMed]
- Kwon, G.; Lee, J.; Lim, Y.H. Dairy Propionibacterium Extends the Mean Lifespan of Caenorhabditis elegans via Activation of the Innate Immune System. Sci. Rep. 2016, 6, 31713. [Google Scholar] [CrossRef] [PubMed]
- Park, M.R.; Ryu, S.; Maburutse, B.E.; Oh, N.S.; Kim, S.H.; Oh, S.; Jeong, S.Y.; Jeong, D.Y.; Oh, S.; Kim, Y. Probiotic Lactobacillus Fermentum Strain JDFM216 Stimulates the Longevity and Immune Response of Caenorhabditis elegans through a Nuclear Hormone Receptor. Sci. Rep. 2018, 8, 7441. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Choi, H.J.; Yun, B.; Lee, J.; Yoo, J.; Yang, H.J.; Jeong, D.Y.; Kim, Y.; Oh, S. Bacillus Amyloliquefaciens SCGB1 Alleviates Dextran Sulfate Sodium-Induced Colitis in Mice Through Immune Regulation. J. Med. Food 2021, 24, 709–719. [Google Scholar] [CrossRef] [PubMed]
- Komura, T.; Takemoto, A.; Kosaka, H.; Suzuki, T.; Nishikawa, Y. Prolonged Lifespan, Improved Perception, and Enhanced Host Defense of Caenorhabditis elegans by Lactococcus cremoris subsp. cremoris. Microbiol. Spectr. 2022, 10, e00454-21. [Google Scholar] [CrossRef]
- Nakagawa, H.; Shiozaki, T.; Kobatake, E.; Hosoya, T.; Moriya, T.; Sakai, F.; Taru, H.; Miyazaki, T. Effects and Mechanisms of Prolongevity Induced by Lactobacillus gasseri SBT2055 in Caenorhabditis elegans. Aging Cell 2016, 15, 227–236. [Google Scholar] [CrossRef]
- Di Vincenzo, F.; Del Gaudio, A.; Petito, V.; Lopetuso, L.R.; Scaldaferri, F. Gut Microbiota, Intestinal Permeability, and Systemic Inflammation: A Narrative Review. Intern. Emerg. Med. 2023, 1–19. [Google Scholar] [CrossRef]
- Ahmadi, S.; Wang, S.; Nagpal, R.; Wang, B.; Jain, S.; Razazan, A.; Mishra, S.P.; Zhu, X.; Wang, Z.; Kavanagh, K.; et al. A Human-Origin Probiotic Cocktail Ameliorates Aging-Related Leaky Gut and Inflammation via Modulating the Microbiota/Taurine/Tight Junction Axis. JCI Insight 2020, 5, e132055. [Google Scholar] [CrossRef]
- Le, T.A.N.; Selvaraj, B.; Lee, J.W.; Kang, K. Measuring the Effects of Bacteria and Chemicals on the Intestinal Permeability of Caenorhabditis elegans. J. Vis. Exp. 2019, 2019, e60419. [Google Scholar] [CrossRef]
- Brenner, S. The Genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef]
- Houthoofd, K.; Johnson, T.E.; Vanfleteren, J.R. Dietary Restriction in the Nematode Caenorhabditis elegans. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Huayta, J.; Crapster, J.P.; San-Miguel, A. Endogenous DAF-16 Spatiotemporal Activity Quantitatively Predicts Lifespan Extension Induced by Dietary Restriction. Commun. Biol. 2023, 6, 203. [Google Scholar] [CrossRef] [PubMed]
- Alonzo-De la Rosa, C.M.; Miard, S.; Taubert, S.; Picard, F. Methods to Extract and Study the Biological Effects of Murine Gut Microbiota Using Caenorhabditis elegans as a Screening Host. PLoS ONE 2023, 18, e0281887. [Google Scholar] [CrossRef] [PubMed]
| Probiotic Strain | Food Sources and Culture Conditions | Main Findings | Mechanisms (Signaling Pathways Involved) | Reference |
|---|---|---|---|---|
| Bifidobacterium animalis subsp. lactis CECT 8145 | ||||
| Active form of Bifidobacterium animalis subsp. lactis CECT 8145 (probiotic) | E. coli OP50 strain or B. animalis subsp. lactis CECT 8145; No dose specification 20 °C; Three days until young adults. | ↓ Fat content (Nile red and TG quantification) ↑ Resistance to acute oxidative stress ↑ worm survival | Downregulation of positive regulators of growth rate and the xenobiotic metabolism. Up-regulation of metabolic pathways for energy production. ↑ Lipid glycosylation ↑ acox-1 | [23] |
| Heat-treated Bifidobacterium animalis subsp. lactis CECT 8145 | NGM surface previously seeded with E. coli OP50; Worms were incubated for 3 days at 20 °C. | ↓ Fat content (Nile red and TG quantification) ↑ SCFAs production: acetate, lactic acids | NF-κB | [31] |
| Lipoteichoic acid from Bifidobacterium animalis subsp. lactis BPL1 and LTA metabolite | Escherichia coli OP50 strain NGM and glucose-NGM medium; B. animalis and HI-B. animalis (108 cells/plate) were added to the NGM surface. Lipoteichoic acid (LTA) as bioactive compound (50 to 0.1 µg mL−1). | ↓ Fat accumulation by probiotic and LTA, also in NGM+ glucose | No effect on fat reduction on daf-2 or daf-16 mutants. Not dependent on skn-1 shown in mutants | [32] |
| Pediococcus acidilactici | ||||
| Pediococcus acidilactici MNL5 | E. coli OP50 or P. acidilactici MNL5. | ↑ nematode lifespan and median survival ↓ Fat content vs. NGM OP50 + glucose (Nile red and oil red) | ↓ fat-4 fat-5 and fat-6 Glucose upregulated de novo fatty acid synthesis | [33] |
| Pediococcus acidilactici CECT 9879 (p1Ac) | Worms were grown from L1 to L4 at 20 °C; Probiotic dose: 5 × 106 CFU/mL; NGM and high-glucose NGM (10 mM) previously seeded with E. coli OP50 as normal nematode diet. | ↓ Fat content (Nile red and oil red) Normal worm development ↓ oxidative stress (ROS) ↓ aging (lipofuscin) ↑ nematode lifespan and median survival | IIS signaling pathway: pA1c inhibits the high-glucose-induced nuclear translocation of daf-16 ↓ fasn-1, fat-5, fat-7, and mdt-15 gene expression ↑ acox-1, daf-22, maoc-1, and cpt-2 gene expression ↑ skn-1 and nhr-49 gene expression | [24] |
| Pediococcus acidilactici CECT 9879 (p1Ac) combined with prebiotics | Worms were grown from L1 to L4 at 20 °C; Probiotic dose: 5 × 106 CFU/mL; 0.5 µg/mL of PC; 50 µg/mL of BGC NGM and high-glucose NGM (10 mM) previously seeded with E. coli OP50 as normal nematode diet. | ↓ Fat content (Nile red and oil red) Normal worm development ↓ oxidative stress (ROS) ↓ aging (lipofuscin) ↑ nematode lifespan and median survival | pA1c inhibits the high-glucose-induced nuclear translocation of daf-16 ↓ expression of fatty acid biosynthesis genes: fat-5 ↑ expression of β-oxidation genes: acox-1 and cpt-2 | [34] |
| Other Lactic Acid Bacteria (LAB) | ||||
| LAB strains from Korean Fermented Soya Beans: Pediococcus acidilatici SDL1402 P. acidilactici SDL1406 Weisella cibaria SCCB2306 Lactobacillus rhamnosus JDFM6 | E. coli OP50 or 50 µL of LAB; (8 Log CFU/mL). | ↓ Cholesterol accumulation irrespective to the order of treatment ↑ worm survival | [35] | |
| Lactobacillus delbrueckii subsp. indicus CRL1447 combined with mixes of Limosilactobacillus fermentum CRL1446, Lactiplantibacillus paraplantarum CRL1449, and CRL1472 strains | E. coli OP50 (control group) or a combination of E. coli OP50 and each lactobacilli strain in a ratio of 25:75; 20 °C; L1 to L4/adult. | ↓ TG content | [30] | |
| Lactobacillus pentosus MJM60383 | E. coli OP50 or E. cloacae 20 °C; Synchronized L1 worms were fed with OP50, or E. cloacae; NGM plate supplemented with 100 mM glucose. | ↓ Fat content (Nile red and oil content) ↓ ratio of C18:1∆9/C18:0 | ↑ acs-2 and nhr-49 genes, enhancing fatty acid β-oxidation ↓ fat6 and fat7 and tub1 | [36] |
| Probiotic Strain | Food Source and Culture Conditions | Main Findings | Mechanisms (Signaling Pathways Involved) | Reference |
|---|---|---|---|---|
| Probiotic cocktail containing five Lactobacillus and five Enterococcus strains isolated from healthy infants | E. coli OP50 with or without taurine; Supplementation of synchronized worms from L1 stage; (proof of concept of the probiotic bile hydrolase activity). | ↓ leaky gut (smurf assay) ↑ motility ↑ worm survival | Not described in C. elegans. | [60] |
| Lactobacillus gasseri SBT2055 | E. coli OP50 or Lactobacillus gasseri SBT2055 (live or UV killed); 20 °C; L1 to L4/adult. | ↑ worm survival ↓ aging (lipofuscin) ↑ Oxidative stress response (Paraquat asay) ↑ Mitochondrial function measured by MitoTracker® CMXRos and cyanine dye JC-1 | Skn-1, nsy-1, sek-1, and pmk-1 dependant mechanism for life-extension via p38 MAPK pathway signaling. Independent effects from daf-2 or daf-16. Upregulation of oxidative stress related genes: skn-1, gst-4, sod-1, trx-1 (thioredoxin), clk-1 (mitochondrial polypeptide), hsp16.2 (heat-shock protein), hsp-70, and gcs-1 (an ortholog of γ-glutamyl-cysteine synthetase). | [58] |
| Propionibacterium freudenreichii KCTC 1063 | E. coli OP50 or Propionibacterium freudenreichii KCTC 1063; 25 °C; Assays performed on L4 adults. | ↑ worm survival ↓ aging (lipofuscin) resistance to Salmonella typhimurium | Skn-1 mutants failed to benefit from extended life. Upregulation of p38/MAPKK pathway genes daf-2, pmk-1, sek-1, mek-1, dbl-1, daf-7, sma-3, and daf-12. Upregulation of antimicrobial peptide-related genes lys-7 and lys-8. | [54] |
| Lactobacillus fermentum Strain JDFM216 | E. coli OP50 or Lactobacillus fermentum JDFM216; 25 °C; L1 to L4/adult. | ↑ worm survival ↑ Resistance to food-borne pathogens, including Staphylococcus aureus and E. coli O157:H7 | Upregulation of the NHR and PMK-1 pathway. | [55] |
| Bacillus amyloliquefaciens SCGB1 | Exposure to E. coli O157:H7 or Bacillus amyloliquefaciens SCGB1. | ↑ worm survival upon exposure to pathogen E. coli O157:H7. | Upregulation of pmk-1. | [56] |
| Lactococcus cremoris subsp. cremoris | E. coli OP50 or Lactococcus cremoris subsp. Cremoris; 25 °C; Young adult worms. | ↑ Resistance to Salmonella enterica subsp. enterica serovar Enteritidis or Staphylococcus aureus ↓ aging (lipofuscin) | No beneficial effects on skn-1 lacking mutants. Upregulation of heme oxygenase-1 ho-1, effector of the SKN-1/Nrf2 pathway. | [57] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goyache, I.; Yavorov-Dayliev, D.; Milagro, F.I.; Aranaz, P. Caenorhabditis elegans as a Screening Model for Probiotics with Properties against Metabolic Syndrome. Int. J. Mol. Sci. 2024, 25, 1321. https://doi.org/10.3390/ijms25021321
Goyache I, Yavorov-Dayliev D, Milagro FI, Aranaz P. Caenorhabditis elegans as a Screening Model for Probiotics with Properties against Metabolic Syndrome. International Journal of Molecular Sciences. 2024; 25(2):1321. https://doi.org/10.3390/ijms25021321
Chicago/Turabian StyleGoyache, Ignacio, Deyan Yavorov-Dayliev, Fermín I. Milagro, and Paula Aranaz. 2024. "Caenorhabditis elegans as a Screening Model for Probiotics with Properties against Metabolic Syndrome" International Journal of Molecular Sciences 25, no. 2: 1321. https://doi.org/10.3390/ijms25021321
APA StyleGoyache, I., Yavorov-Dayliev, D., Milagro, F. I., & Aranaz, P. (2024). Caenorhabditis elegans as a Screening Model for Probiotics with Properties against Metabolic Syndrome. International Journal of Molecular Sciences, 25(2), 1321. https://doi.org/10.3390/ijms25021321







