Locked Nucleic Acid Oligonucleotides Facilitate RNA•LNA-RNA Triple-Helix Formation and Reduce MALAT1 Levels
Abstract
1. Introduction
 ) [48], respectively. (C) Chemical structures of U•A-U and U•M-U base triples with Hoogsteen and Watson–Crick base pairing denoted with dashed lines. The major-groove triple helix shows the Hoogsteen strand in blue and Watson–Crick strands in purple and green. (D) Chemical structure of ribose for RNA, LNA and phosphorothioate (PS)-LNA backbone. (E) Sequences of oligonucleotides R15, L15 and PS-L15. A + sign inside a square bracket [+] indicates locked nucleic acid sequence, whereas the asterisk (*) denotes phosphorothioate backbone. The colors purple, dark purple and light purple represent the R15, L15 and PS-L15 oligos, respectively.
) [48], respectively. (C) Chemical structures of U•A-U and U•M-U base triples with Hoogsteen and Watson–Crick base pairing denoted with dashed lines. The major-groove triple helix shows the Hoogsteen strand in blue and Watson–Crick strands in purple and green. (D) Chemical structure of ribose for RNA, LNA and phosphorothioate (PS)-LNA backbone. (E) Sequences of oligonucleotides R15, L15 and PS-L15. A + sign inside a square bracket [+] indicates locked nucleic acid sequence, whereas the asterisk (*) denotes phosphorothioate backbone. The colors purple, dark purple and light purple represent the R15, L15 and PS-L15 oligos, respectively.
 ) [48], respectively. (C) Chemical structures of U•A-U and U•M-U base triples with Hoogsteen and Watson–Crick base pairing denoted with dashed lines. The major-groove triple helix shows the Hoogsteen strand in blue and Watson–Crick strands in purple and green. (D) Chemical structure of ribose for RNA, LNA and phosphorothioate (PS)-LNA backbone. (E) Sequences of oligonucleotides R15, L15 and PS-L15. A + sign inside a square bracket [+] indicates locked nucleic acid sequence, whereas the asterisk (*) denotes phosphorothioate backbone. The colors purple, dark purple and light purple represent the R15, L15 and PS-L15 oligos, respectively.
) [48], respectively. (C) Chemical structures of U•A-U and U•M-U base triples with Hoogsteen and Watson–Crick base pairing denoted with dashed lines. The major-groove triple helix shows the Hoogsteen strand in blue and Watson–Crick strands in purple and green. (D) Chemical structure of ribose for RNA, LNA and phosphorothioate (PS)-LNA backbone. (E) Sequences of oligonucleotides R15, L15 and PS-L15. A + sign inside a square bracket [+] indicates locked nucleic acid sequence, whereas the asterisk (*) denotes phosphorothioate backbone. The colors purple, dark purple and light purple represent the R15, L15 and PS-L15 oligos, respectively.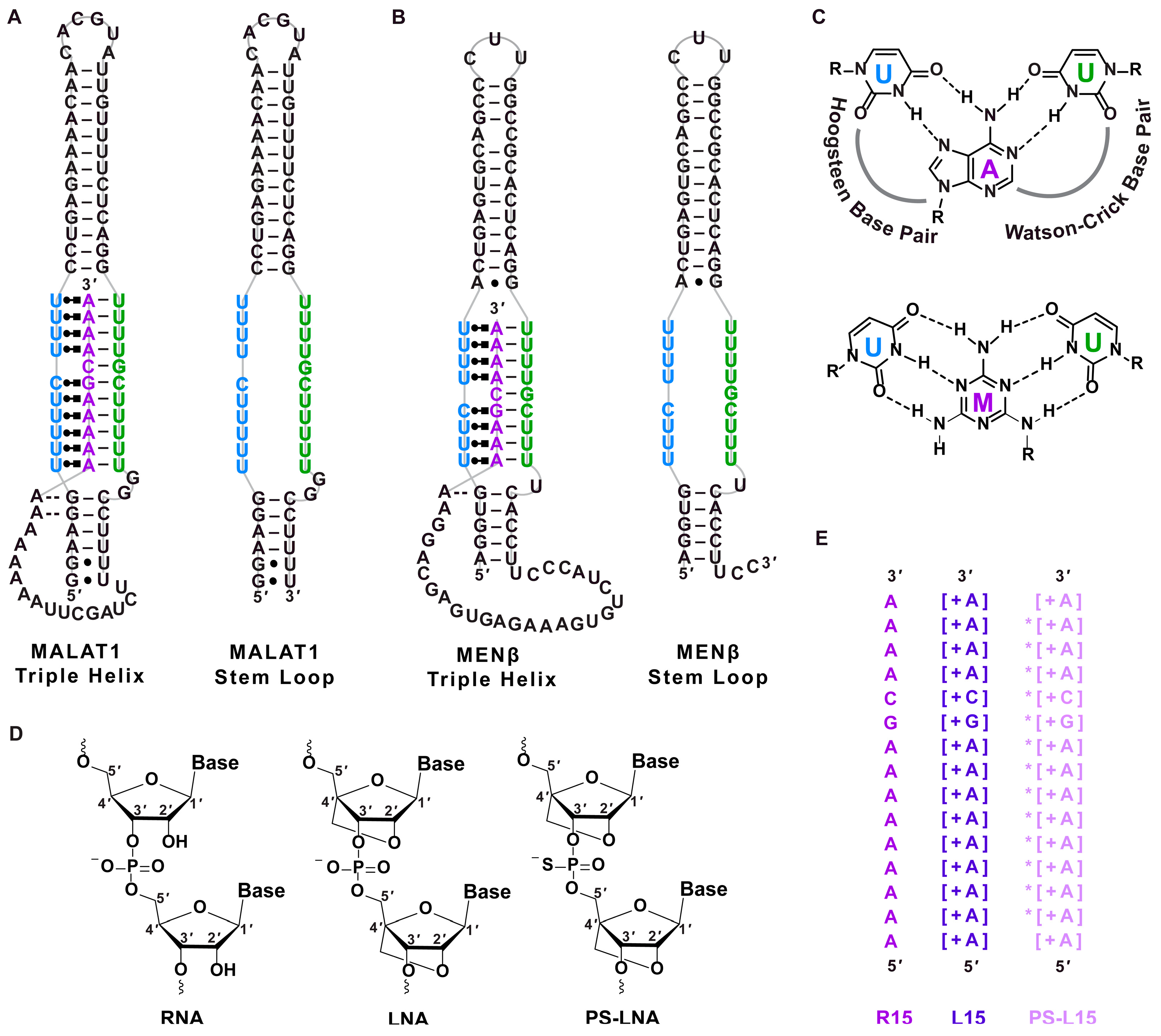
2. Results
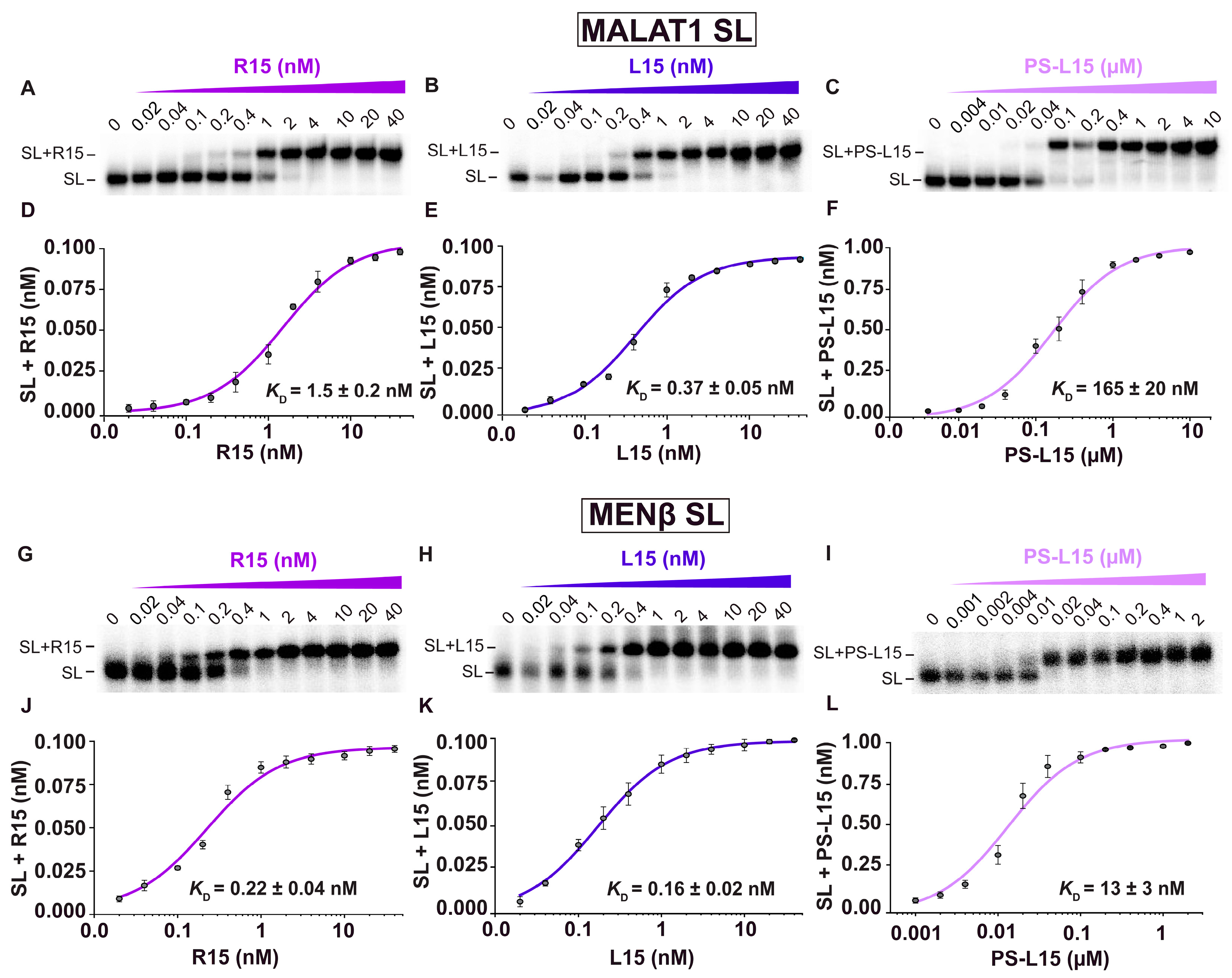
2.1. LNA Oligonucleotides Bind to Both MALAT1 and MENβ SLs with Sub-Nanomolar to Nanomolar Dissociation Constants
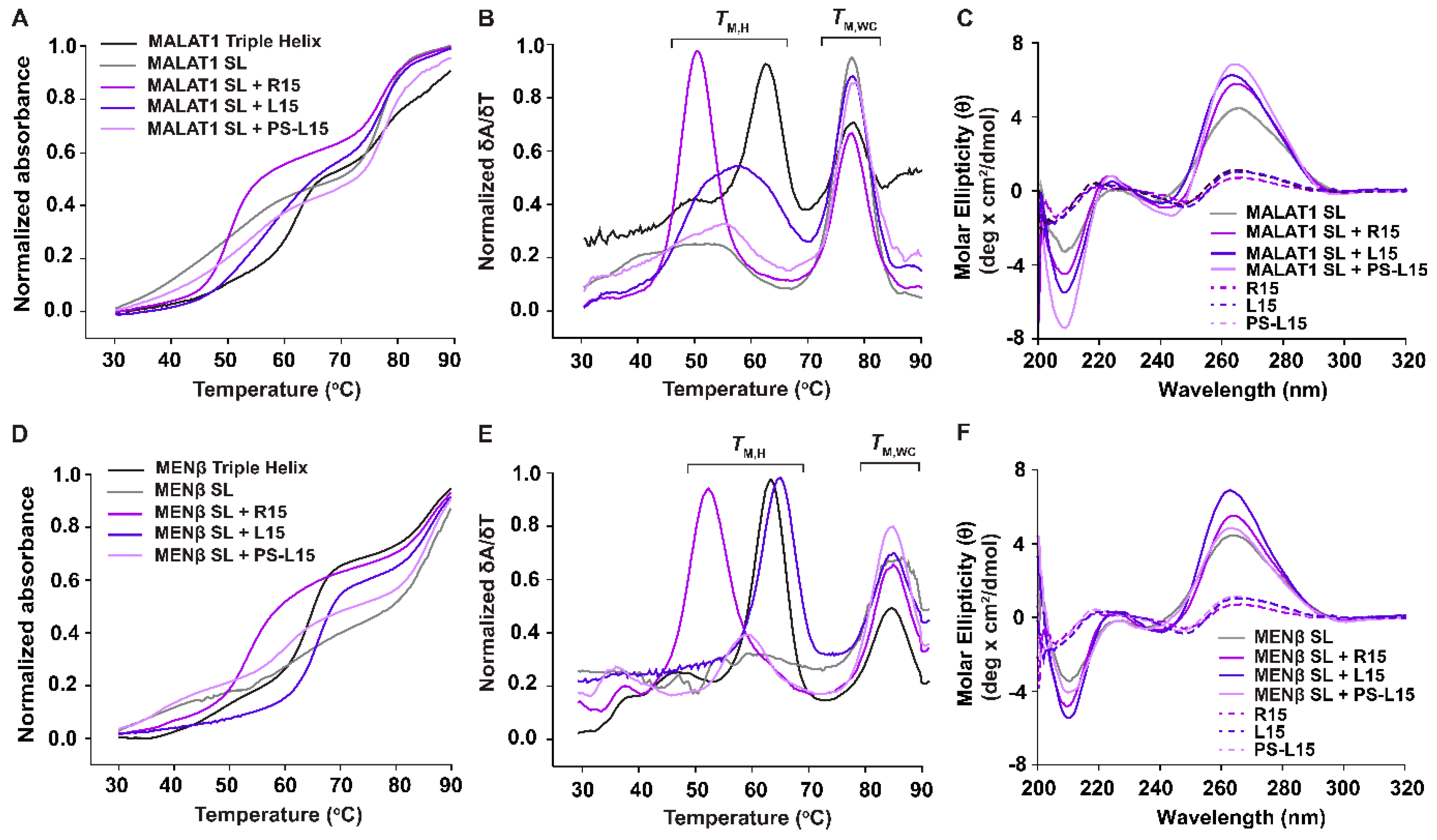
2.2. L15 and PS-L15 Interact with MALAT1 and MENβ SLs via RNA•LNA-RNA Triple-Helix Formation
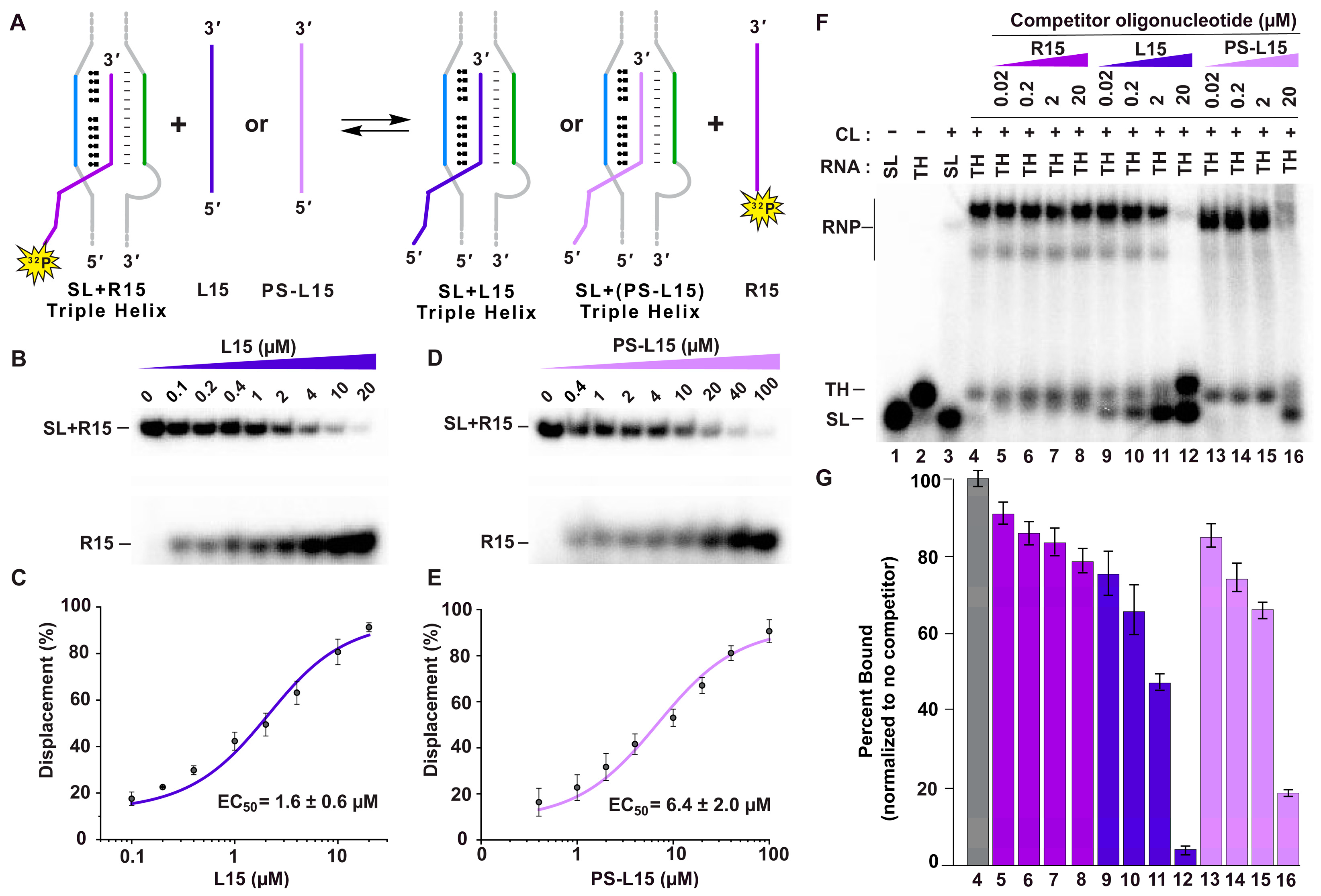
2.3. LNAs Displace the A-Rich Tract and METTL16 from the MALAT1 Triple Helix
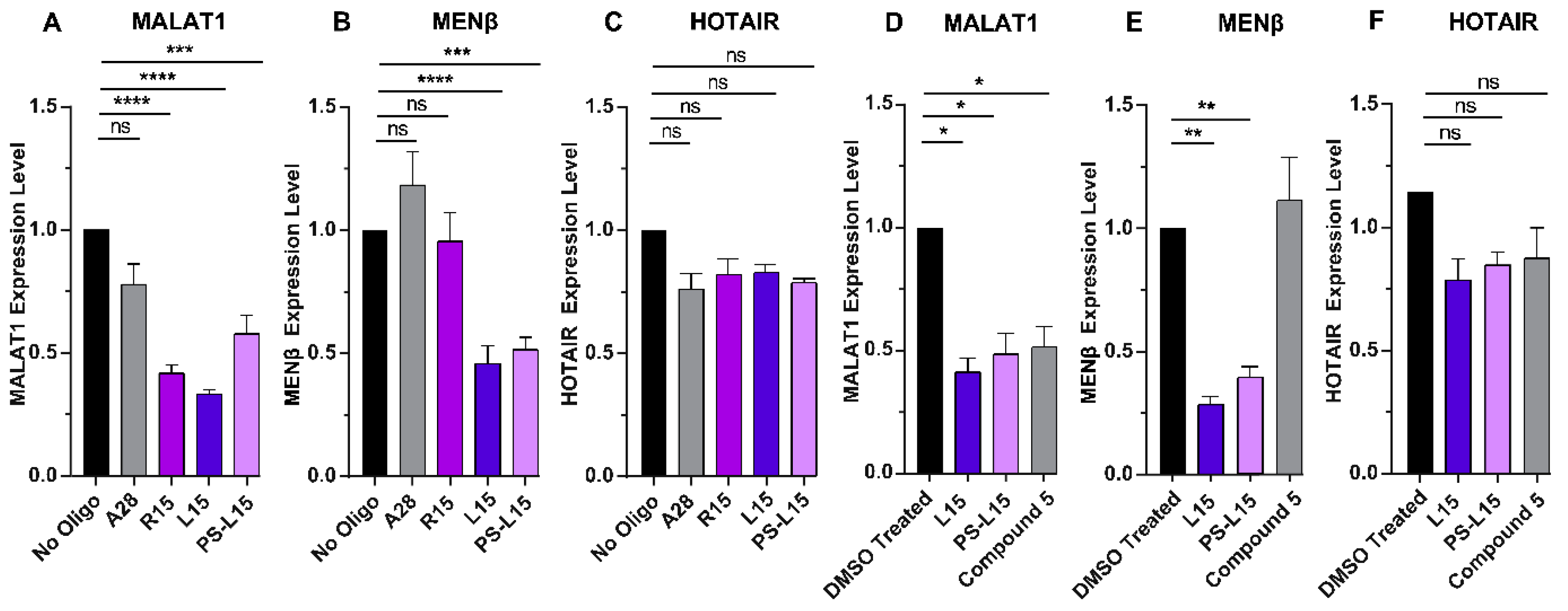
2.4. LNA Oligonucleotides Reduce MALAT1 and MENβ Levels in HCT116 Cells
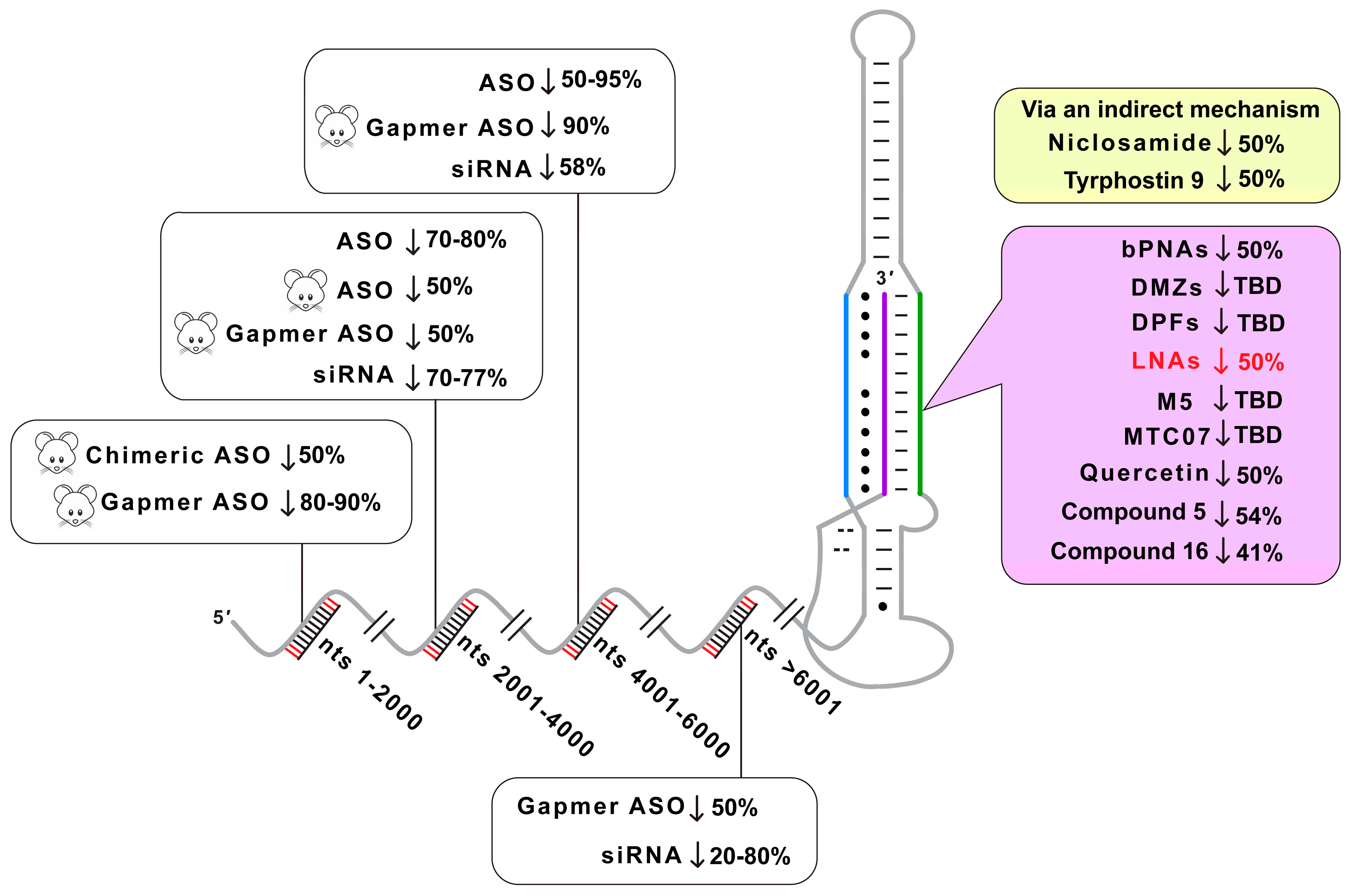
3. Discussion
4. Materials and Methods
4.1. RNA and Oligonucleotide Preparation
4.2. Electrophoretic Mobility Shift Assays
4.3. UV Thermal Denaturation Assay
4.4. Circular Dichroism Spectroscopy
4.5. A-Tract Displacement Assay
4.6. Preparation of Native HCT116 Protein Lysate
4.7. Competitive EMSA
4.8. Transfection of HCT116 Cells and RT-qPCR
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chen, Y.; Li, Z.; Chen, X.; Zhang, S. Long Non-Coding RNAs: From Disease Code to Drug Role. Acta Pharm. Sin. B 2021, 11, 340–354. [Google Scholar] [CrossRef] [PubMed]
- Winkle, M.; El-Daly, S.M.; Fabbri, M.; Calin, G.A. Noncoding RNA Therapeutics—Challenges and Potential Solutions. Nat. Rev. Drug Discov. 2021, 20, 629–651. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, G.K.; Khullar, N.; Sidhu, I.S.; Navik, U.S.; Reddy, A.P.; Reddy, P.H.; Bhatti, J.S. Emerging Role of Non-coding RNA in Health and Disease. Metab. Brain Dis. 2021, 36, 1119–1134. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Fu, J.; Zhu, L.; Chen, Y.; Liu, B. Designing Strategies of Small-Molecule Compounds for Modulating Non-Coding RNAs in Cancer Therapy. J. Hematol. Oncol. 2022, 15, 14. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Naganuma, T.; Shioi, G.; Hirose, T. Paraspeckles Are Subpopulation-Specific Nuclear Bodies That Are Not Essential in Mice. J. Cell Biol. 2011, 193, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Ip, J.Y.; Shioi, G.; Tripathi, V.; Zong, X.; Hirose, T.; Prasanth, K.V. Malat1 Is Not an Essential Component of Nuclear Speckles in Mice. RNA 2012, 18, 1487–1499. [Google Scholar] [CrossRef]
- Eißmann, M.; Gutschner, T.; Hämmerle, M.; Günther, S.; Caudron-Herger, M.; Groß, M.; Schirmacher, P.; Rippe, K.; Braun, T.; Zörnig, M.; et al. Loss of the Abundant Nuclear Non-Coding RNA MALAT1 Is Compatible with Life and Development. RNA Biol. 2012, 9, 1076–1087. [Google Scholar] [CrossRef]
- Zhang, B.; Arun, G.; Mao, Y.S.; Lazar, Z.; Hung, G.; Bhattacharjee, G.; Xiao, X.; Booth, C.J.; Wu, J.; Zhang, C.; et al. The lncRNA Malat1 Is Dispensable for Mouse Development but Its Transcription Plays a Cis-Regulatory Role in the Adult. Cell Rep. 2012, 2, 111–123. [Google Scholar] [CrossRef]
- Dong, P.; Xiong, Y.; Yue, J.; Hanley, S.J.B.; Kobayashi, N.; Todo, Y.; Watari, H. Long Non-Coding RNA NEAT1: A Novel Target for Diagnosis and Therapy in Human Tumors. Front. Genet. 2018, 9, 471. [Google Scholar] [CrossRef]
- Goyal, B.; Yadav, S.R.M.; Awasthee, N.; Gupta, S.; Kunnumakkara, A.B.; Gupta, S.C. Diagnostic, Prognostic, and Therapeutic Significance of Long Non-Coding RNA MALAT1 in Cancer. Biochim. Biophys. Acta Rev. Cancer 2021, 1875, 188502. [Google Scholar] [CrossRef]
- Arun, G.; Diermeier, S.; Akerman, M.; Chang, K.-C.; Wilkinson, J.E.; Hearn, S.; Kim, Y.; MacLeod, A.R.; Krainer, A.R.; Norton, L.; et al. Differentiation of Mammary Tumors and Reduction in Metastasis upon Malat1 lncRNA Loss. Genes Dev. 2016, 30, 34–51. [Google Scholar] [CrossRef]
- Zhu, N.; Ahmed, M.; Li, Y.; Liao, J.C.; Wong, P.K. Long Noncoding RNA MALAT1 Is Dynamically Regulated in Leader Cells during Collective Cancer Invasion. Proc. Natl. Acad. Sci. USA 2023, 120, e2305410120. [Google Scholar] [CrossRef]
- Wilusz, J.E.; Freier, S.M.; Spector, D.L. 3′ End Processing of a Long Nuclear-Retained Non-Coding RNA Yields a tRNA-like Cytoplasmic RNA. Cell 2008, 135, 919–932. [Google Scholar] [CrossRef]
- Sunwoo, H.; Dinger, M.E.; Wilusz, J.E.; Amaral, P.P.; Mattick, J.S.; Spector, D.L. MEN Epsilon/Beta Nuclear-Retained Non-Coding RNAs Are up-Regulated upon Muscle Differentiation and Are Essential Components of Paraspeckles. Genome Res. 2009, 19, 347–359. [Google Scholar] [CrossRef]
- Wilusz, J.E.; JnBaptiste, C.K.; Lu, L.Y.; Kuhn, C.-D.; Joshua-Tor, L.; Sharp, P.A. A Triple Helix Stabilizes the 3′ Ends of Long Noncoding RNAs That Lack Poly(A) Tails. Genes Dev. 2012, 26, 2392. [Google Scholar] [CrossRef]
- Brown, J.A.; Valenstein, M.L.; Yario, T.A.; Tycowski, K.T.; Steitz, J.A. Formation of Triple-Helical Structures by the 3′-End Sequences of MALAT1 and MENβ Noncoding RNAs. Proc. Natl. Acad. Sci. USA 2012, 109, 19202–19207. [Google Scholar] [CrossRef]
- Brown, J.A.; Bulkley, D.; Wang, J.; Valenstein, M.L.; Yario, T.A.; Steitz, T.A.; Steitz, J.A. Structural Insights into the Stabilization of MALAT1 Noncoding RNA by a Bipartite Triple Helix. Nat. Struct. Mol. Biol. 2014, 21, 633–640. [Google Scholar] [CrossRef]
- Brown, J.A.; Kinzig, C.G.; DeGregorio, S.J.; Steitz, J.A. Methyltransferase-like Protein 16 Binds the 3′-Terminal Triple Helix of MALAT1 Long Noncoding RNA. Proc. Natl. Acad. Sci. USA 2016, 113, 14013–14018. [Google Scholar] [CrossRef]
- Warda, A.S.; Kretschmer, J.; Hackert, P.; Lenz, C.; Urlaub, H.; Höbartner, C.; Sloan, K.E.; Bohnsack, M.T. Human METTL16 Is a N6-Methyladenosine (m6A) Methyltransferase That Targets Pre-mRNAs and Various Non-Coding RNAs. EMBO Rep. 2017, 18, 2004–2014. [Google Scholar] [CrossRef]
- Breger, K.; Brown, J.A. Elucidating the Kinetic Mechanism of Human METTL16. Biochemistry 2023, 62, 494–506. [Google Scholar] [CrossRef]
- Zablowsky, N.; Farack, L.; Rofall, S.; Kramer, J.; Meyer, H.; Nguyen, D.; Ulrich, A.K.C.; Bader, B.; Steigemann, P. High Throughput FISH Screening Identifies Small Molecules That Modulate Oncogenic lncRNA MALAT1 via GSK3B and hnRNPs. Non-Coding RNA 2023, 9, 2. [Google Scholar] [CrossRef]
- Donlic, A.; Morgan, B.S.; Xu, J.L.; Liu, A.; Roble, C.; Hargrove, A.E. Discovery of Small Molecule Ligands for MALAT1 by Tuning an RNA-Binding Scaffold. Angew. Chem. Int. Ed. Engl. 2018, 57, 13242–13247. [Google Scholar] [CrossRef]
- Abulwerdi, F.A.; Xu, W.; Ageeli, A.A.; Yonkunas, M.J.; Arun, G.; Nam, H.; Schneekloth, J.S., Jr.; Dayie, T.K.; Spector, D.; Baird, N.; et al. Selective Small-Molecule Targeting of a Triple Helix Encoded by the Long Noncoding RNA, MALAT1. ACS Chem. Biol. 2019, 14, 223–235. [Google Scholar] [CrossRef]
- Donlic, A.; Zafferani, M.; Padroni, G.; Puri, M.; Hargrove, A.E. Regulation of MALAT1 Triple Helix Stability and in Vitro Degradation by Diphenylfurans. Nucleic Acids Res. 2020, 48, 7653–7664. [Google Scholar] [CrossRef]
- François-Moutal, L.; Miranda, V.G.; Mollasalehi, N.; Gokhale, V.; Khanna, M. In Silico Targeting of the Long Noncoding RNA MALAT1. ACS Med. Chem. Lett. 2021, 12, 915–921. [Google Scholar] [CrossRef]
- Zafferani, M.; Martyr, J.G.; Muralidharan, D.; Montalvan, N.I.; Cai, Z.; Hargrove, A.E. Multiassay Profiling of a Focused Small Molecule Library Reveals Predictive Bidirectional Modulation of the lncRNA MALAT1 Triplex Stability In Vitro. ACS Chem. Biol. 2022, 17, 2437–2447. [Google Scholar] [CrossRef]
- Rakheja, I.; Ansari, A.H.; Ray, A.; Chandra Joshi, D.; Maiti, S. Small Molecule Quercetin Binds MALAT1 Triplex and Modulates Its Cellular Function. Mol. Ther. Nucleic Acids 2022, 30, 241–256. [Google Scholar] [CrossRef]
- Bagnolini, G.; Luu, T.B.; Hargrove, A.E. Recognizing the Power of Machine Learning and Other Computational Methods to Accelerate Progress in Small Molecule Targeting of RNA. RNA 2023, 29, 473–488. [Google Scholar] [CrossRef]
- Rocca, R.; Polerà, N.; Juli, G.; Grillone, K.; Maruca, A.; Martino, M.T.D.; Artese, A.; Amato, J.; Pagano, B.; Randazzo, A.; et al. Hit Identification of Novel Small Molecules Interfering with MALAT1 Triplex by a Structure-Based Virtual Screening. Arch. Pharm. 2023, 356, e2300134. [Google Scholar] [CrossRef]
- An, H.; Elvers, K.T.; Gillespie, J.A.; Jones, K.; Atack, J.R.; Grubisha, O.; Shelkovnikova, T.A. A Toolkit for the Identification of NEAT1_2/Paraspeckle Modulators. Nucleic Acids Res. 2022, 50, e119. [Google Scholar] [CrossRef]
- Amodio, N.; Stamato, M.A.; Juli, G.; Morelli, E.; Fulciniti, M.; Manzoni, M.; Taiana, E.; Agnelli, L.; Cantafio, M.E.G.; Romeo, E.; et al. Drugging the lncRNA MALAT1 via LNA gapmeR ASO Inhibits Gene Expression of Proteasome Subunits and Triggers Anti-Multiple Myeloma Activity. Leukemia 2018, 32, 1948–1957. [Google Scholar] [CrossRef]
- Gong, N.; Teng, X.; Li, J.; Liang, X.-J. Antisense Oligonucleotide-Conjugated Nanostructure-Targeting lncRNA MALAT1 Inhibits Cancer Metastasis. ACS Appl. Mater. Interfaces 2019, 11, 37–42. [Google Scholar] [CrossRef]
- Gomes, C.P.; Nóbrega-Pereira, S.; Domingues-Silva, B.; Rebelo, K.; Alves-Vale, C.; Marinho, S.P.; Carvalho, T.; Dias, S.; Bernardes de Jesus, B. An Antisense Transcript Mediates MALAT1 Response in Human Breast Cancer. BMC Cancer 2019, 19, 771. [Google Scholar] [CrossRef]
- Shin, M.; Chan, I.L.; Cao, Y.; Gruntman, A.M.; Lee, J.; Sousa, J.; Rodríguez, T.C.; Echeverria, D.; Devi, G.; Debacker, A.J.; et al. Intratracheally Administered LNA Gapmer Antisense Oligonucleotides Induce Robust Gene Silencing in Mouse Lung Fibroblasts. Nucleic Acids Res. 2022, 50, 8418–8430. [Google Scholar] [CrossRef]
- Tano, K.; Mizuno, R.; Okada, T.; Rakwal, R.; Shibato, J.; Masuo, Y.; Ijiri, K.; Akimitsu, N. MALAT-1 Enhances Cell Motility of Lung Adenocarcinoma Cells by Influencing the Expression of Motility-Related Genes. FEBS Lett. 2010, 584, 4575–4580. [Google Scholar] [CrossRef]
- Kim, S.-S.; Harford, J.B.; Moghe, M.; Rait, A.; Pirollo, K.F.; Chang, E.H. Targeted Nanocomplex Carrying siRNA against MALAT1 Sensitizes Glioblastoma to Temozolomide. Nucleic Acids Res. 2018, 46, 1424–1440. [Google Scholar] [CrossRef]
- Tan, S.; Chen, J. Si-MALAT1 Attenuates Thymic Cancer Cell Proliferation and Promotes Apoptosis via the miR-145-5p/HMGA2 Pathway. Oncol. Lett. 2021, 22, 585. [Google Scholar] [CrossRef]
- Gutschner, T.; Hämmerle, M.; Eißmann, M.; Hsu, J.; Kim, Y.; Hung, G.; Revenko, A.; Arun, G.; Stentrup, M.; Groß, M.; et al. The Noncoding RNA MALAT1 Is a Critical Regulator of the Metastasis Phenotype of Lung Cancer Cells. Cancer Res. 2013, 73, 1180–1189. [Google Scholar] [CrossRef]
- Duschmalé, J.; Hansen, H.F.; Duschmalé, M.; Koller, E.; Albaek, N.; Møller, M.R.; Jensen, K.; Koch, T.; Wengel, J.; Bleicher, K. In Vitro and in Vivo Properties of Therapeutic Oligonucleotides Containing Non-Chiral 3′ and 5′ Thiophosphate Linkages. Nucleic Acids Res. 2020, 48, 63–74. [Google Scholar] [CrossRef]
- Nanni, S.; Aiello, A.; Salis, C.; Re, A.; Cencioni, C.; Bacci, L.; Pierconti, F.; Pinto, F.; Ripoli, C.; Ostano, P.; et al. Metabolic Reprogramming by Malat1 Depletion in Prostate Cancer. Cancers 2021, 13, 15. [Google Scholar] [CrossRef]
- Iwashita, S.; Shoji, T.; Koizumi, M. Evaluating the Knockdown Activity of MALAT1 ENA Gapmers In Vitro. Methods Mol. Biol. 2020, 2176, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, T.; Hirakawa, Y.; Yamairi, F.; Kurita, T.; Murahashi, K.; Nishimura, H.; Iwazaki, N.; Yasuhara, H.; Tateoka, T.; Ohta, T.; et al. Altered Biodistribution and Hepatic Safety Profile of a Gapmer Antisense Oligonucleotide Bearing Guanidine-Bridged Nucleic Acids. Nucleic Acid Ther. 2022, 32, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Debacker, A.J.; Sharma, V.K.; Meda Krishnamurthy, P.; O’Reilly, D.; Greenhill, R.; Watts, J.K. Next-Generation Peptide Nucleic Acid Chimeras Exhibit High Affinity and Potent Gene Silencing. Biochemistry 2019, 58, 582–589. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Hu, Y.; Zhao, J.-J. Repression of Multiple Myeloma Cell Growth In Vivo by Single-Wall Carbon Nanotube (SWCNT)-Delivered MALAT1 Antisense Oligos. J. Vis. Exp. 2018, 142, e58598. [Google Scholar] [CrossRef]
- Kusznir, E.-A.; Hau, J.-C.; Portmann, M.; Reinhart, A.-G.; Falivene, F.; Bastien, J.; Worm, J.; Ross, A.; Lauer, M.; Ringler, P.; et al. Propensities of Fatty Acid-Modified ASOs: Self-Assembly vs Albumin Binding. Bioconjug. Chem. 2023, 34, 866–879. [Google Scholar] [CrossRef]
- Tanaka, K.; Okuda, T.; Kasahara, Y.; Obika, S. Base-Modified Aptamers Obtained by Cell-Internalization SELEX Facilitate Cellular Uptake of an Antisense Oligonucleotide. Mol. Ther. Nucleic Acids 2021, 23, 440–449. [Google Scholar] [CrossRef]
- Miao, S.; Bhunia, D.; Devari, S.; Liang, Y.; Munyaradzi, O.; Rundell, S.; Bong, D. Bifacial PNAs Destabilize MALAT1 by 3′ A-Tail Displacement from the U-Rich Internal Loop. ACS Chem. Biol. 2021, 16, 1600–1609. [Google Scholar] [CrossRef]
- Leontis, N.B.; Westhof, E. Geometric Nomenclature and Classification of RNA Base Pairs. RNA 2001, 7, 499–512. [Google Scholar] [CrossRef]
- Obika, S.; Nanbu, D.; Hari, Y.; Morio, K.; In, Y.; Ishida, T.; Imanishi, T. Synthesis of 2′-O,4′-C-Methyleneuridine and -Cytidine. Novel Bicyclic Nucleosides Having a Fixed C3, -Endo Sugar Puckering. Tetrahedron Lett. 1997, 38, 8735–8738. [Google Scholar] [CrossRef]
- Sørensen, J.J.; Nielsen, J.T.; Petersen, M. Solution Structure of a dsDNA:LNA Triplex. Nucleic Acids Res. 2004, 32, 6078–6085. [Google Scholar] [CrossRef]
- Pande, V.; Nilsson, L. Insights into Structure, Dynamics and Hydration of Locked Nucleic Acid (LNA) Strand-Based Duplexes from Molecular Dynamics Simulations. Nucleic Acids Res. 2008, 36, 1508–1516. [Google Scholar] [CrossRef]
- Kumar, R.; Singh, S.K.; Koshkin, A.A.; Rajwanshi, V.K.; Meldgaard, M.; Wengel, J. The First Analogues of LNA (Locked Nucleic Acids): Phosphorothioate-LNA and 2′-Thio-LNA. Bioorg. Med. Chem. Lett. 1998, 8, 2219–2222. [Google Scholar] [CrossRef]
- Obika, S.; Nanbu, D.; Hari, Y.; Andoh, J.; Morio, K.; Doi, T.; Imanishi, T. Stability and Structural Features of the Duplexes Containing Nucleoside Analogues with a Fixed N-Type Conformation, 2′-O,4′-C-Methyleneribonucleosides. Tetrahedron Lett. 1998, 39, 5401–5404. [Google Scholar] [CrossRef]
- Koshkin, A.A.; Singh, S.K.; Nielsen, P.; Rajwanshi, V.K.; Kumar, R.; Meldgaard, M.; Olsen, C.E.; Wengel, J. LNA (Locked Nucleic Acids): Synthesis of the Adenine, Cytosine, Guanine, 5-Methylcytosine, Thymine and Uracil Bicyclonucleoside Monomers, Oligomerisation, and Unprecedented Nucleic Acid Recognition. Tetrahedron 1998, 54, 3607–3630. [Google Scholar] [CrossRef]
- Torigoe, H.; Hari, Y.; Sekiguchi, M.; Obika, S.; Imanishi, T. 2′-O,4′-C-Methylene Bridged Nucleic Acid Modification Promotes Pyrimidine Motif Triplex DNA Formation at Physiological pH: THERMODYNAMIC AND KINETIC STUDIES. J. Biol. Chem. 2001, 276, 2354–2360. [Google Scholar] [CrossRef]
- Brunet, E.; Alberti, P.; Perrouault, L.; Babu, R.; Wengel, J.; Giovannangeli, C. Exploring Cellular Activity of Locked Nucleic Acid-Modified Triplex-Forming Oligonucleotides and Defining Its Molecular Basis. J. Biol. Chem. 2005, 280, 20076–20085. [Google Scholar] [CrossRef]
- Szabat, M.; Kierzek, E.; Kierzek, R. Modified RNA Triplexes: Thermodynamics, Structure and Biological Potential. Sci. Rep. 2018, 8, 13023. [Google Scholar] [CrossRef]
- Ruszkowska, A.; Ruszkowski, M.; Hulewicz, J.P.; Dauter, Z.; Brown, J.A. Molecular Structure of a U•A-U-Rich RNA Triple Helix with 11 Consecutive Base Triples. Nucleic Acids Res. 2020, 48, 3304–3314. [Google Scholar] [CrossRef]
- Abels, J.A.; Moreno-Herrero, F.; van der Heijden, T.; Dekker, C.; Dekker, N.H. Single-Molecule Measurements of the Persistence Length of Double-Stranded RNA. Biophys. J. 2005, 88, 2737–2744. [Google Scholar] [CrossRef]
- Bao, L.; Zhang, X.; Jin, L.; Tan, Z.-J. Flexibility of Nucleic Acids: From DNA to RNA. Chin. Phys. B 2015, 25, 018703. [Google Scholar] [CrossRef]
- Herrero-Galán, E.; Fuentes-Perez, M.E.; Carrasco, C.; Valpuesta, J.M.; Carrascosa, J.L.; Moreno-Herrero, F.; Arias-Gonzalez, J.R. Mechanical Identities of RNA and DNA Double Helices Unveiled at the Single-Molecule Level. J. Am. Chem. Soc. 2013, 135, 122–131. [Google Scholar] [CrossRef]
- Mitton-Fry, R.M.; DeGregorio, S.J.; Wang, J.; Steitz, T.A.; Steitz, J.A. Poly(A) Tail Recognition by a Viral RNA Element through Assembly of a Triple Helix. Science 2010, 330, 1244–1247. [Google Scholar] [CrossRef]
- Torabi, S.-F.; Vaidya, A.T.; Tycowski, K.T.; DeGregorio, S.J.; Wang, J.; Shu, M.-D.; Steitz, T.A.; Steitz, J.A. RNA Stabilization by a Poly(A) Tail 3′-End Binding Pocket and Other Modes of Poly(A)-RNA Interaction. Science 2021, 371, eabe6523. [Google Scholar] [CrossRef]
- Ageeli, A.A.; McGovern-Gooch, K.R.; Kaminska, M.M.; Baird, N.J. Finely Tuned Conformational Dynamics Regulate the Protective Function of the lncRNA MALAT1 Triple Helix. Nucleic Acids Res. 2019, 47, 1468–1481. [Google Scholar] [CrossRef]
- Kurreck, J.; Wyszko, E.; Gillen, C.; Erdmann, V.A. Design of Antisense Oligonucleotides Stabilized by Locked Nucleic Acids. Nucleic Acids Res. 2002, 30, 1911–1918. [Google Scholar] [CrossRef]
- Kierzek, E.; Ciesielska, A.; Pasternak, K.; Mathews, D.H.; Turner, D.H.; Kierzek, R. The Influence of Locked Nucleic Acid Residues on the Thermodynamic Properties of 2′-O-Methyl RNA/RNA Heteroduplexes. Nucleic Acids Res. 2005, 33, 5082–5093. [Google Scholar] [CrossRef]
- Koizumi, M.; Morita, K.; Daigo, M.; Tsutsumi, S.; Abe, K.; Obika, S.; Imanishi, T. Triplex Formation with 2′-O,4′-C-Ethylene-Bridged Nucleic Acids (ENA) Having C3′-Endo Conformation at Physiological pH. Nucleic Acids Res. 2003, 31, 3267–3273. [Google Scholar] [CrossRef]
- Gray, D.M.; Hung, S.-H.; Johnson, K.H. [3] Absorption and Circular Dichroism Spectroscopy of Nucleic Acid Duplexes and Triplexes. In Methods in Enzymology; Biochemical Spectroscopy; Academic Press: Cambridge, MA, USA, 1995; Volume 246, pp. 19–34. [Google Scholar]
- Swain, M.; Ageeli, A.A.; Kasprzak, W.K.; Li, M.; Miller, J.T.; Sztuba-Solinska, J.; Schneekloth, J.S.; Koirala, D.; Piccirili, J.; Fraboni, A.J.; et al. Dynamic Bulge Nucleotides in the KSHV PAN ENE Triple Helix Provide a Unique Binding Platform for Small Molecule Ligands. Nucleic Acids Res. 2021, 49, 13179–13193. [Google Scholar] [CrossRef]
- Bohr, H.G.; Shim, I.; Stein, C.; Ørum, H.; Hansen, H.F.; Koch, T. Electronic Structures of LNA Phosphorothioate Oligonucleotides. Mol. Ther. Nucleic Acids 2017, 8, 428–441. [Google Scholar] [CrossRef]
- Ruszkowska, A.; Ruszkowski, M.; Dauter, Z.; Brown, J.A. Structural Insights into the RNA Methyltransferase Domain of METTL16. Sci. Rep. 2018, 8, 5311. [Google Scholar] [CrossRef]
- Torabi, S.-F.; DeGregorio, S.J.; Steitz, J.A. tRNA-like Leader-Trailer Interaction Promotes 3ʹ-End Maturation of MALAT1. RNA 2021, 27, 1140–1147. [Google Scholar] [CrossRef]
- Tong, Y.; Lee, Y.; Liu, X.; Childs-Disney, J.L.; Suresh, B.M.; Benhamou, R.I.; Yang, C.; Li, W.; Costales, M.G.; Haniff, H.S.; et al. Programming Inactive RNA-Binding Small Molecules into Bioactive Degraders. Nature 2023, 618, 169–179. [Google Scholar] [CrossRef]
- Xu, J.; Wang, G.A.; Gao, L.; Wu, L.; Lei, Q.; Deng, H.; Li, F. Enabling Programmable Dynamic DNA Chemistry Using Small-Molecule DNA Binders. Nat. Commun. 2023, 14, 4248. [Google Scholar] [CrossRef]
- Wu, H.; Wahane, A.; Alhamadani, F.; Zhang, K.; Parikh, R.; Lee, S.; McCabe, E.M.; Rasmussen, T.P.; Bahal, R.; Zhong, X.; et al. Nephrotoxicity of Marketed Antisense Oligonucleotide Drugs. Curr. Opin. Toxicol. 2022, 32, 100373. [Google Scholar] [CrossRef]
- Hagedorn, P.H.; Brown, J.M.; Easton, A.; Pierdomenico, M.; Jones, K.; Olson, R.E.; Mercer, S.E.; Li, D.; Loy, J.; Høg, A.M.; et al. Acute Neurotoxicity of Antisense Oligonucleotides After Intracerebroventricular Injection Into Mouse Brain Can Be Predicted from Sequence Features. Nucleic Acid Ther. 2022, 32, 151–162. [Google Scholar] [CrossRef]
- Kamola, P.J.; Maratou, K.; Wilson, P.A.; Rush, K.; Mullaney, T.; McKevitt, T.; Evans, P.; Ridings, J.; Chowdhury, P.; Roulois, A.; et al. Strategies for In Vivo Screening and Mitigation of Hepatotoxicity Associated with Antisense Drugs. Mol. Ther. Nucleic Acids 2017, 8, 383–394. [Google Scholar] [CrossRef]
- Thakur, S.; Sinhari, A.; Jain, P.; Jadhav, H.R. A Perspective on Oligonucleotide Therapy: Approaches to Patient Customization. Front. Pharmacol. 2022, 13, 1006304. [Google Scholar] [CrossRef]
- Jen, J.; Tang, Y.-A.; Lu, Y.-H.; Lin, C.-C.; Lai, W.-W.; Wang, Y.-C. Oct4 Transcriptionally Regulates the Expression of Long Non-Coding RNAs NEAT1 and MALAT1 to Promote Lung Cancer Progression. Mol. Cancer 2017, 16, 104. [Google Scholar] [CrossRef]
- Arshi, A.; Sharifi, F.S.; Khorramian Ghahfarokhi, M.; Faghih, Z.; Doosti, A.; Ostovari, S.; Mahmoudi Maymand, E.; Ghahramani Seno, M.M. Expression Analysis of MALAT1, GAS5, SRA, and NEAT1 lncRNAs in Breast Cancer Tissues from Young Women and Women over 45 Years of Age. Mol. Ther. Nucleic Acids 2018, 12, 751–757. [Google Scholar] [CrossRef]
- Park, E.-G.; Pyo, S.-J.; Cui, Y.; Yoon, S.-H.; Nam, J.-W. Tumor Immune Microenvironment lncRNAs. Brief. Bioinform. 2022, 23, bbab504. [Google Scholar] [CrossRef]
- Taiana, E.; Favasuli, V.; Ronchetti, D.; Todoerti, K.; Pelizzoni, F.; Manzoni, M.; Barbieri, M.; Fabris, S.; Silvestris, I.; Gallo Cantafio, M.E.; et al. Long Non-Coding RNA NEAT1 Targeting Impairs the DNA Repair Machinery and Triggers Anti-Tumor Activity in Multiple Myeloma. Leukemia 2020, 34, 234–244. [Google Scholar] [CrossRef]
- Naveed, A.; Cooper, J.A.; Li, R.; Hubbard, A.; Chen, J.; Liu, T.; Wilton, S.D.; Fletcher, S.; Fox, A.H. NEAT1 polyA-Modulating Antisense Oligonucleotides Reveal Opposing Functions for Both Long Non-Coding RNA Isoforms in Neuroblastoma. Cell. Mol. Life Sci. CMLS 2021, 78, 2213–2230. [Google Scholar] [CrossRef]
- Clemson, C.M.; Hutchinson, J.N.; Sara, S.A.; Ensminger, A.W.; Fox, A.H.; Chess, A.; Lawrence, J.B. An Architectural Role for a Nuclear Noncoding RNA: NEAT1 RNA Is Essential for the Structure of Paraspeckles. Mol. Cell 2009, 33, 717–726. [Google Scholar] [CrossRef]
- Weghorst, F.; Torres Marcén, M.; Faridi, G.; Lee, Y.C.G.; Cramer, K.S. Deep Conservation and Unexpected Evolutionary History of Neighboring lncRNAs MALAT1 and NEAT1. J. Mol. Evol. 2024. [Google Scholar] [CrossRef]
- Jarmoskaite, I.; AlSadhan, I.; Vaidyanathan, P.P.; Herschlag, D. How to Measure and Evaluate Binding Affinities. eLife 2020, 9, e57264. [Google Scholar] [CrossRef]
- Brown, J.A.; Kinzig, C.G.; DeGregorio, S.J.; Steitz, J.A. Hoogsteen-Position Pyrimidines Promote the Stability and Function of the MALAT1 RNA Triple Helix. RNA 2016, 22, 743–749. [Google Scholar] [CrossRef]
- Pabon-Martinez, Y.V.; Xu, Y.; Villa, A.; Lundin, K.E.; Geny, S.; Nguyen, C.-H.; Pedersen, E.B.; Jørgensen, P.T.; Wengel, J.; Nilsson, L.; et al. LNA Effects on DNA Binding and Conformation: From Single Strand to Duplex and Triplex Structures. Sci. Rep. 2017, 7, 11043. [Google Scholar] [CrossRef]
- Kunkler, C.N.; Hulewicz, J.P.; Hickman, S.C.; Wang, M.C.; McCown, P.J.; Brown, J.A. Stability of an RNA•DNA-DNA Triple Helix Depends on Base Triplet Composition and Length of the RNA Third Strand. Nucleic Acids Res. 2019, 47, 7213–7222. [Google Scholar] [CrossRef]
- Sebaugh, J.L. Guidelines for Accurate EC50/IC50 Estimation. Pharm. Stat. 2011, 10, 128–134. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
| RNA/LNAs | MALAT1 SL | MENβ SL | ||
|---|---|---|---|---|
| KD,app (nM) | EC50 (μM) | KD,app (nM) | EC50 (μM) | |
| R15 | 1.5 ± 0.2 | - | 0.22 ± 0.04 | - |
| L15 | 0.37 ± 0.05 | 1.6 ± 0.6 | 0.16 ± 0.02 | 4.6 ± 1.0 |
| PS-L15 | 165 ± 20 | 6.4 ± 2.0 | 13 ± 3 | 11 ± 4 |
| RNA/LNAs | MALAT1 RNA | MENβ RNA | ||||||
|---|---|---|---|---|---|---|---|---|
| TM,H | ΔTM,H | TM,WC | ΔTM,WC | TM,H | ΔTM,H | TM,WC | ΔTM,WC | |
| Triple Helix | 62.5 ± 0.3 | - | 77.2 ± 0.2 | - | 64 ± 0.1 | - | 85.5 ± 0.2 | - |
| SL | - | - | 77.5 ± 0.1 | - | - | - | 85.7 ± 0.2 | - |
| SL + R15 | 49.8 ± 0.2 | - | 77.4 ± 0.1 | - | 52.6 ± 0.1 | - | 85.9 ± 0.3 | - |
| SL + L15 | 56.9 ± 0.2 | 7.1 ± 0.2 | 77.1 ± 0.1 | −0.3 ± 0.1 | 65.5 ± 0.5 | 12.9 ± 0.5 | 85.7 ± 0.2 | −0.2 ± 0.2 |
| SL + PS-L15 | 54.6 ± 0.5 | 4.8 ± 0.5 | 77.3 ± 0.1 | −0.1 ± 0.1 | 59.2 ± 0.5 | 6.6 ± 0.5 | 86.0 ± 0.3 | −0.1 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shivakumar, K.M.; Mahendran, G.; Brown, J.A. Locked Nucleic Acid Oligonucleotides Facilitate RNA•LNA-RNA Triple-Helix Formation and Reduce MALAT1 Levels. Int. J. Mol. Sci. 2024, 25, 1630. https://doi.org/10.3390/ijms25031630
Shivakumar KM, Mahendran G, Brown JA. Locked Nucleic Acid Oligonucleotides Facilitate RNA•LNA-RNA Triple-Helix Formation and Reduce MALAT1 Levels. International Journal of Molecular Sciences. 2024; 25(3):1630. https://doi.org/10.3390/ijms25031630
Chicago/Turabian StyleShivakumar, Krishna M., Gowthami Mahendran, and Jessica A. Brown. 2024. "Locked Nucleic Acid Oligonucleotides Facilitate RNA•LNA-RNA Triple-Helix Formation and Reduce MALAT1 Levels" International Journal of Molecular Sciences 25, no. 3: 1630. https://doi.org/10.3390/ijms25031630
APA StyleShivakumar, K. M., Mahendran, G., & Brown, J. A. (2024). Locked Nucleic Acid Oligonucleotides Facilitate RNA•LNA-RNA Triple-Helix Formation and Reduce MALAT1 Levels. International Journal of Molecular Sciences, 25(3), 1630. https://doi.org/10.3390/ijms25031630








