Radiation-Detoxified Form of Endotoxin Effectively Activates Th1 Responses and Attenuates Ragweed-Induced Th2-Type Airway Inflammation in Mice
Abstract
1. Introduction
2. Results
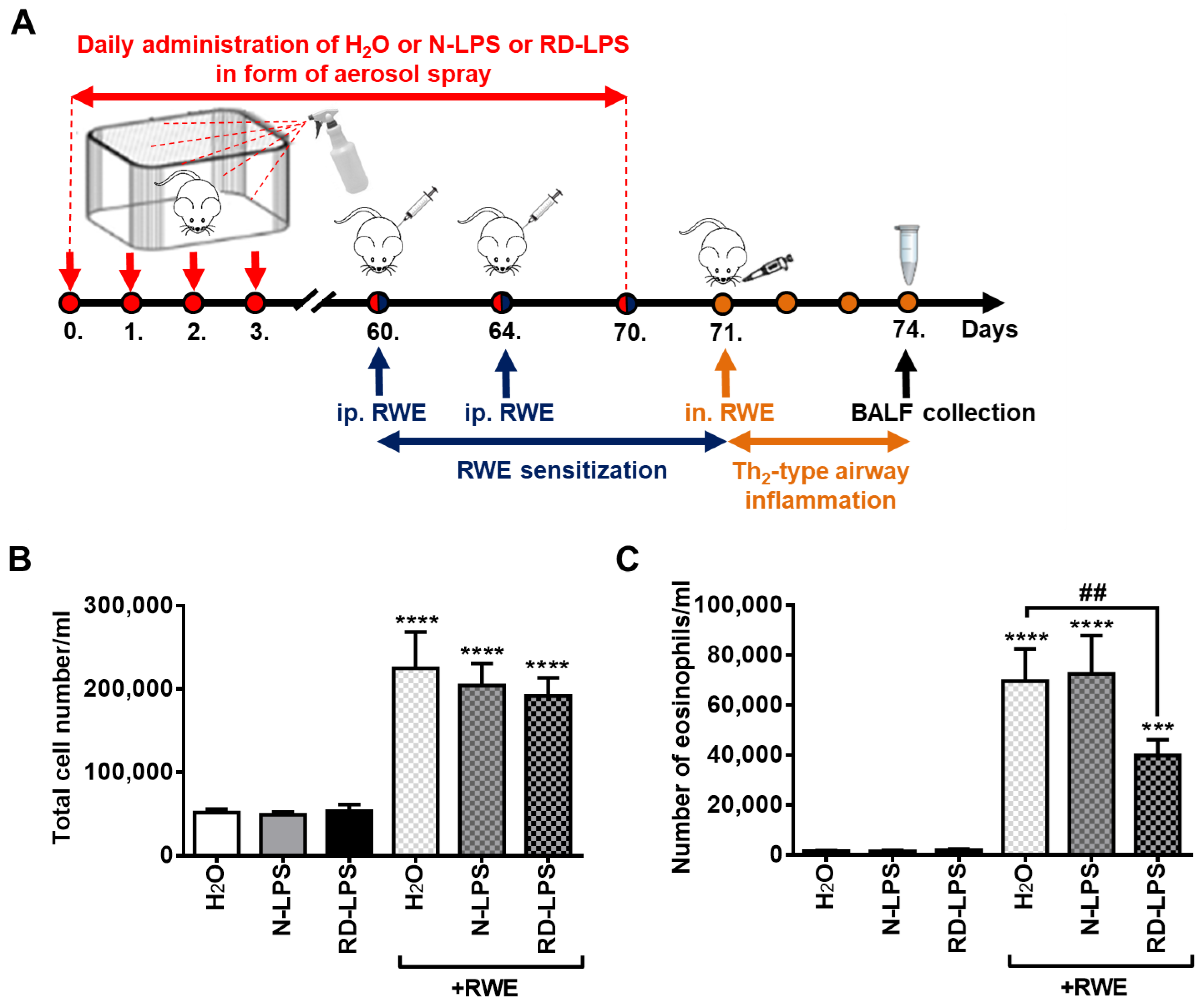
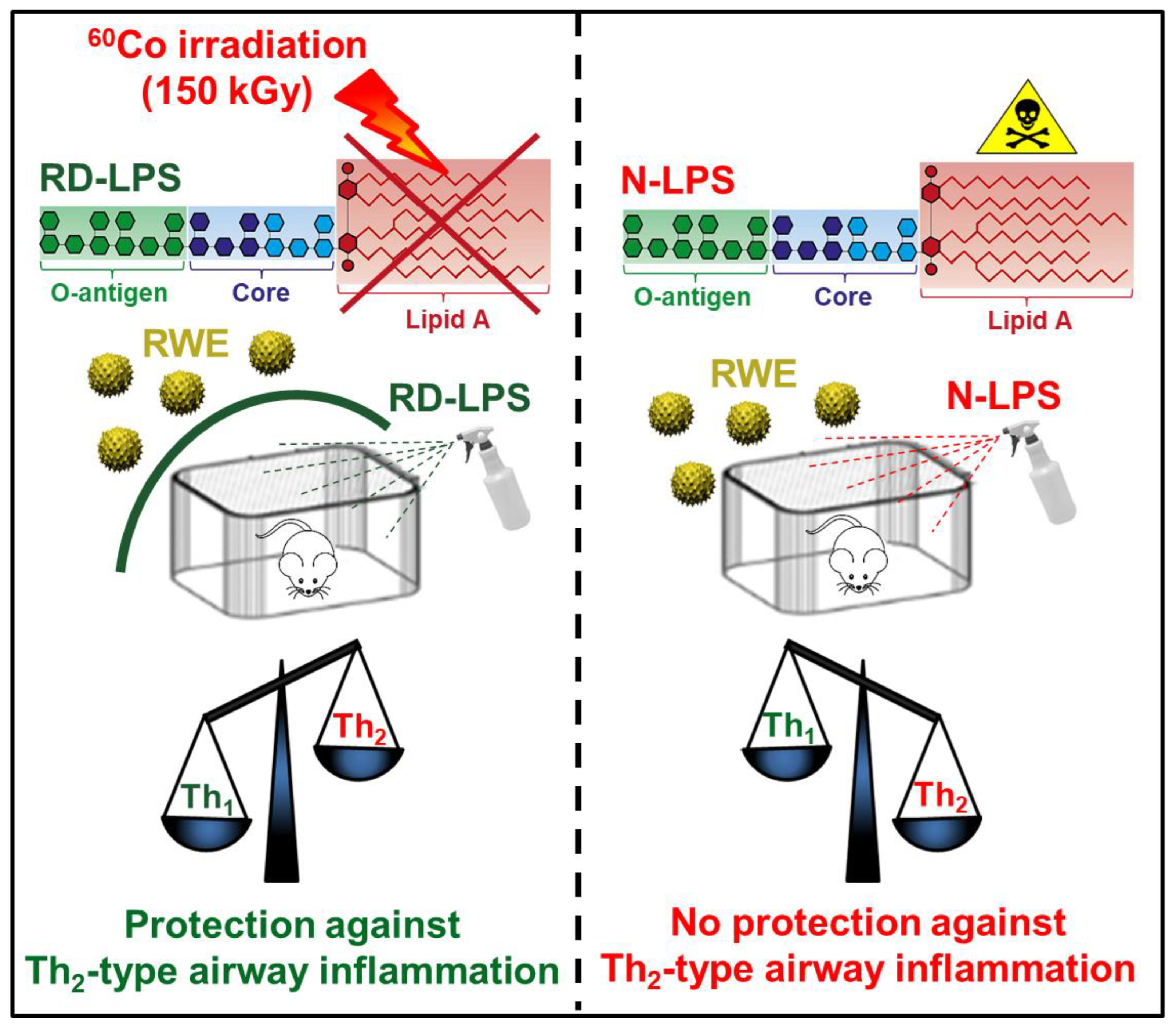
2.1. Long-Term Environmental Exposure to RD-LPS Mitigates Pollen-Induced Eosinophilic Airway Inflammation in Mice
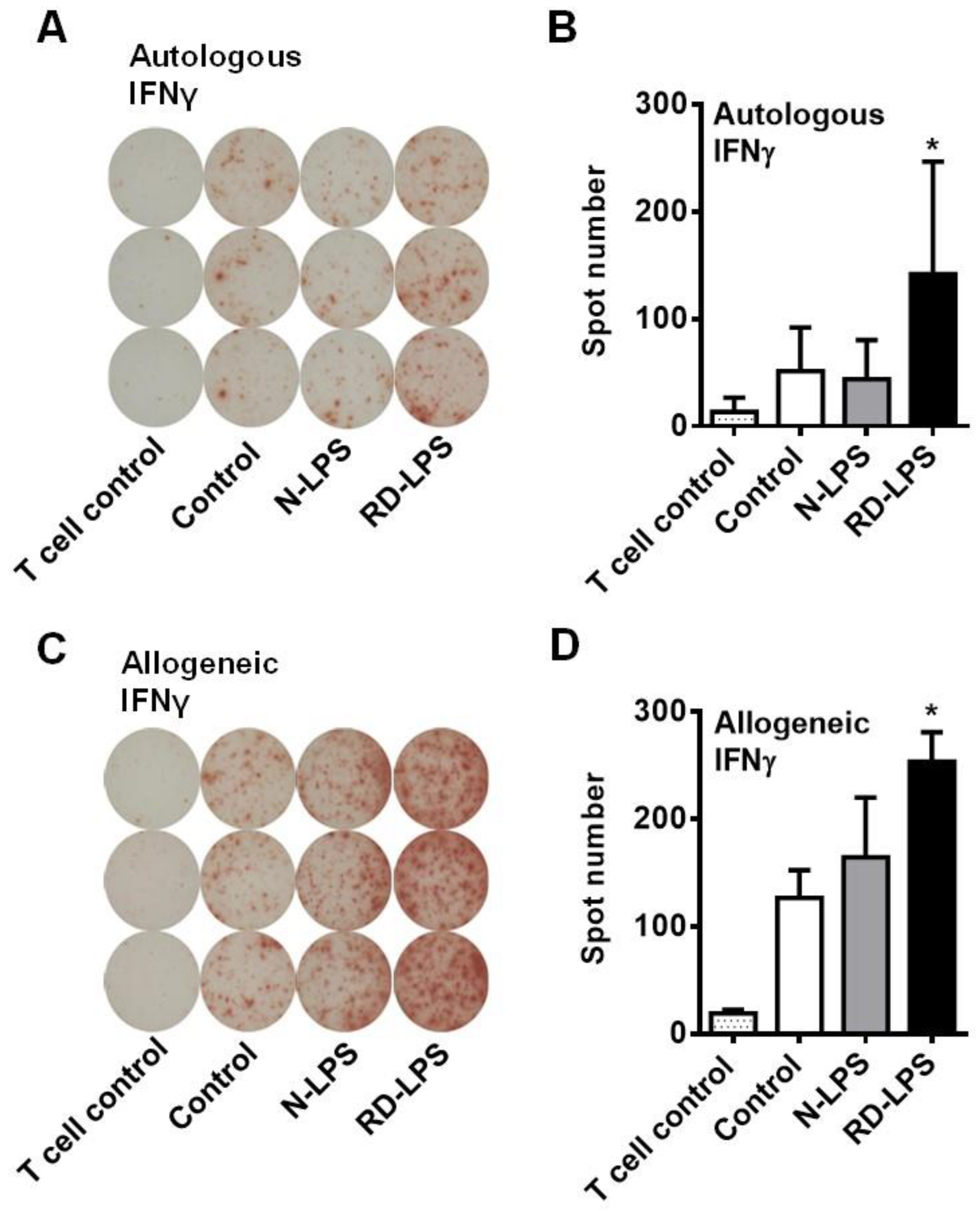
2.2. RD-LPS-Exposed Human moDCs Are More Potent Th1 Inducers Than the N-LPS-Treated Ones
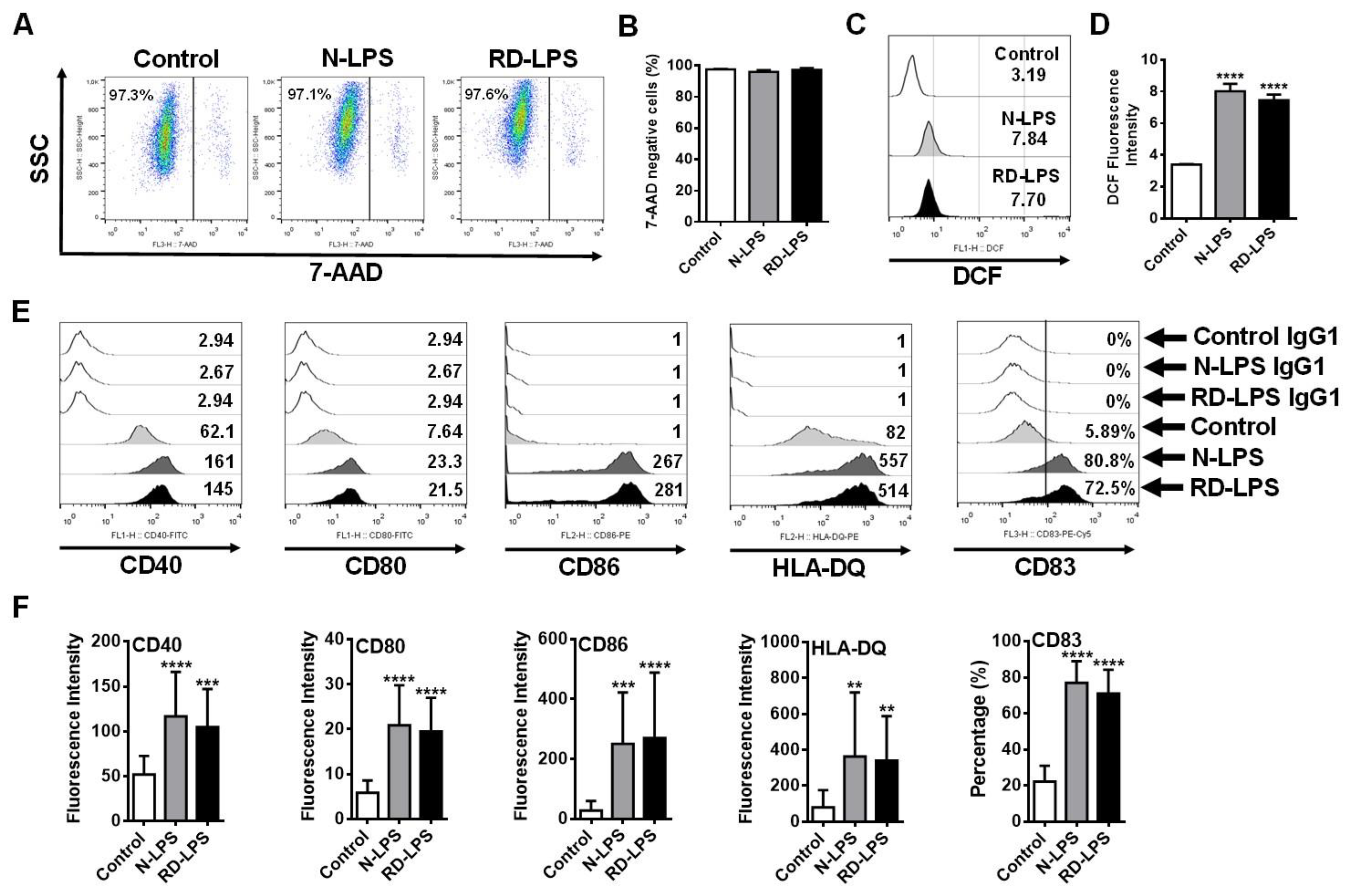
2.3. N-LPS and RD-LPS Induce the Maturation of Human moDCs in a Similar Manner
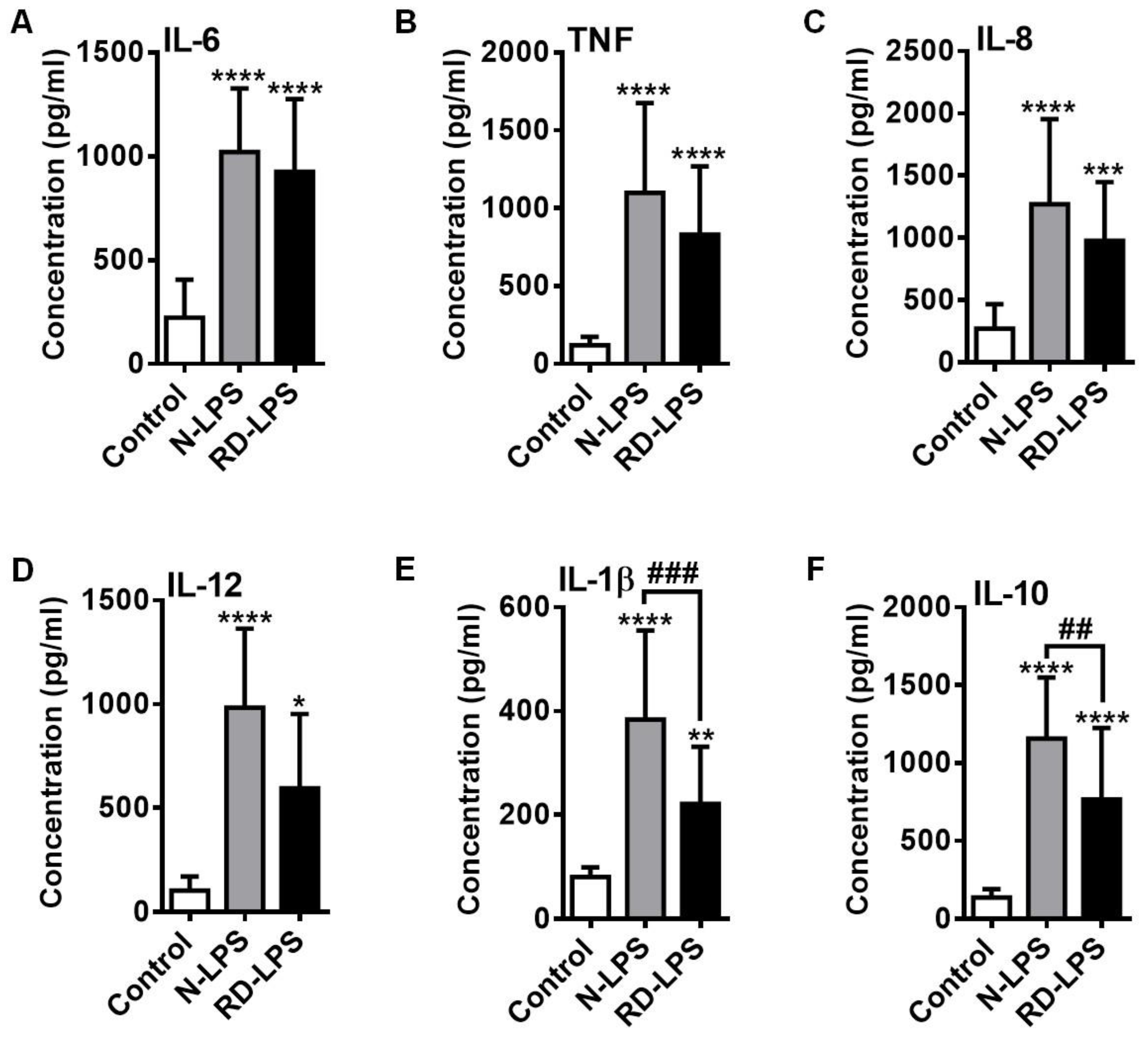
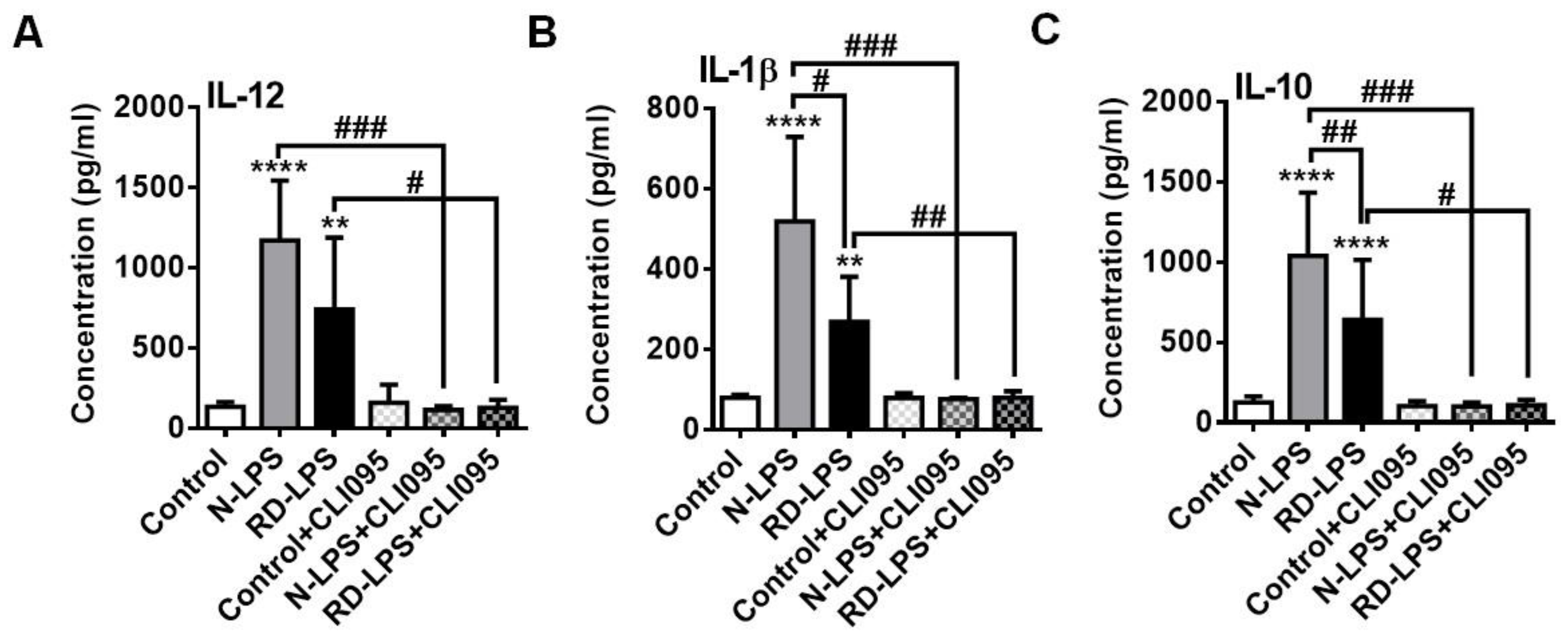
2.4. RD-LPS-Exposed Human moDCs Are Characterized by a Reduced Ability to Produce Various Cytokines Compared to Those Treated with the Native Form
2.5. Both N-LPS and RD-LPS Induce Endotoxin Tolerance in Human moDCs
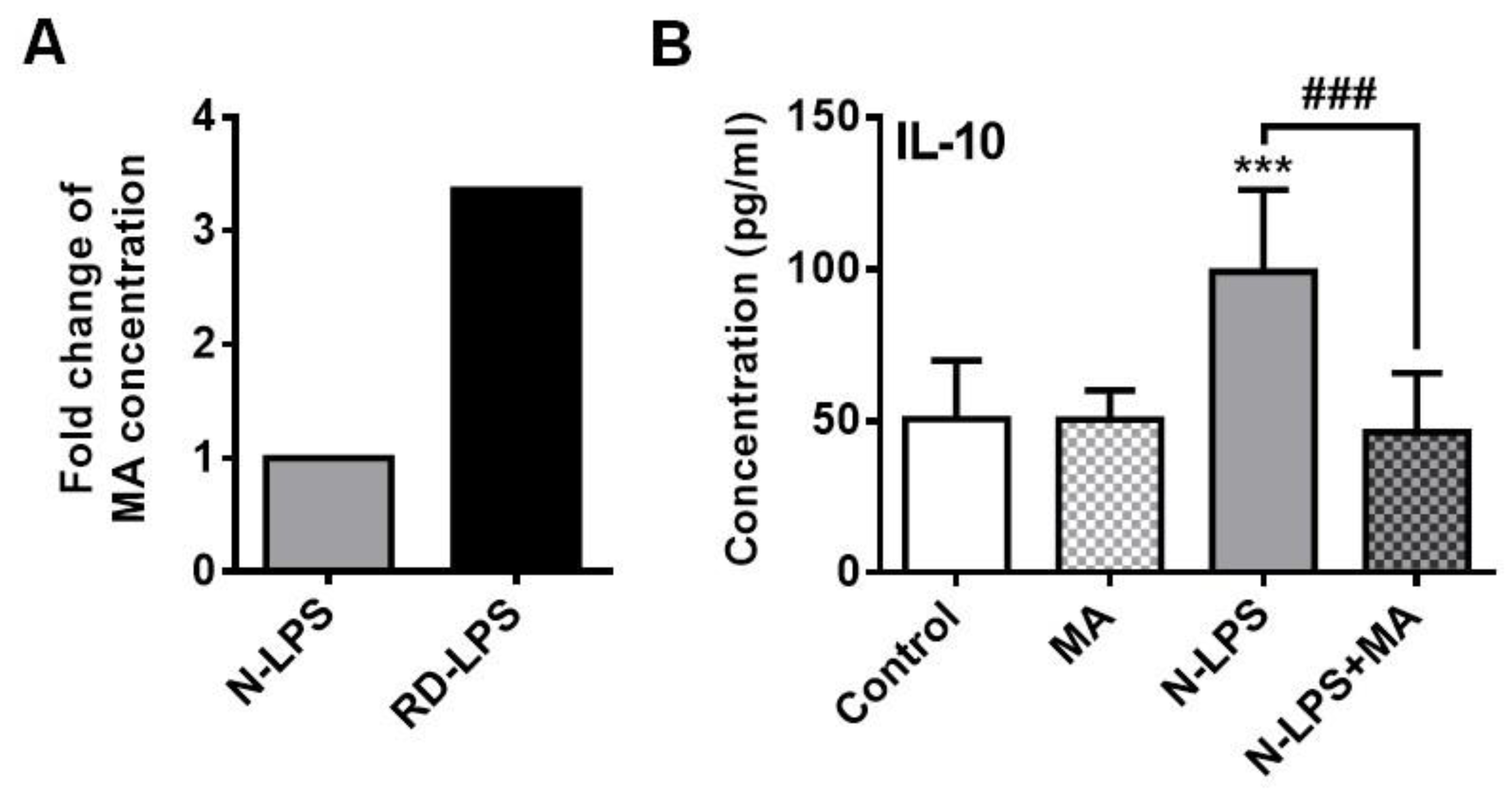
2.6. The Myristic Acid Content of Radiation-Detoxified Endotoxin May Influence Its Immunomodulatory Effects
3. Discussion
4. Materials and Methods
4.1. Bacterial Endotoxin Preparations
4.2. In Vivo Mice Experiments
4.3. Isolation of Human Cells
4.4. Generation of moDCs from Human CD14+ Monocytes
4.5. In Vitro Treatments Conditions
4.6. Assessment of Intracellular ROS Level in Human moDCs
4.7. Phenotypic Analysis of Human moDCs
4.8. Detection of Cytokine and Chemokine Release of Human moDCs
4.9. Enzyme-Linked Immunospot (ELISPOT) Assay
4.10. Ultra-High-Pressure Liquid Chromatography (UHPLC) Coupled to Electrospray Ionization Quadrupole Time-of-Flight Mass Spectrometry (ESI-QTOF-MS)
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Denton, E.; O’Hehir, R.E.; Hew, M. The changing global prevalence of asthma and atopic dermatitis. Allergy 2023, 78, 2079–2080. [Google Scholar] [CrossRef]
- Von Ehrenstein, O.S.; Von Mutius, E.; Illi, S.; Baumann, L.; Bohm, O.; von Kries, R. Reduced risk of hay fever and asthma among children of farmers. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2000, 30, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Riedler, J.; Braun-Fahrlander, C.; Eder, W.; Schreuer, M.; Waser, M.; Maisch, S.; Carr, D.; Schierl, R.; Nowak, D.; von Mutius, E.; et al. Exposure to farming in early life and development of asthma and allergy: A cross-sectional survey. Lancet 2001, 358, 1129–1133. [Google Scholar] [CrossRef] [PubMed]
- Ege, M.J.; Mayer, M.; Normand, A.C.; Genuneit, J.; Cookson, W.O.; Braun-Fahrlander, C.; Heederik, D.; Piarroux, R.; von Mutius, E.; Group, G.T.S. Exposure to environmental microorganisms and childhood asthma. N. Engl. J. Med. 2011, 364, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Illi, S.; Depner, M.; Genuneit, J.; Horak, E.; Loss, G.; Strunz-Lehner, C.; Buchele, G.; Boznanski, A.; Danielewicz, H.; Cullinan, P.; et al. Protection from childhood asthma and allergy in Alpine farm environments-the GABRIEL Advanced Studies. J. Allergy Clin. Immunol. 2012, 129, 1470–1477 e1476. [Google Scholar] [CrossRef] [PubMed]
- Strieker, S.; Weinmann, T.; Gerlich, J.; von Mutius, E.; Nowak, D.; Radon, K.; Wengenroth, L. Farm living and allergic rhinitis from childhood to young adulthood: Prospective results of the GABRIEL study. J. Allergy Clin. Immunol. 2022, 150, 1209–1215 e1202. [Google Scholar] [CrossRef] [PubMed]
- Pechlivanis, S.; Depner, M.; Kirjavainen, P.V.; Roduit, C.; Taubel, M.; Frei, R.; Skevaki, C.; Hose, A.; Barnig, C.; Schmausser-Hechfellner, E.; et al. Continuous Rather Than Solely Early Farm Exposure Protects From Hay Fever Development. J. Allergy Clin. Immunol. Pract. 2023, 11, 591–601. [Google Scholar] [CrossRef]
- von Mutius, E. 99th Dahlem conference on infection, inflammation and chronic inflammatory disorders: Farm lifestyles and the hygiene hypothesis. Clin. Exp. Immunol. 2010, 160, 130–135. [Google Scholar] [CrossRef]
- Frei, R.; Heye, K.; Roduit, C. Environmental influences on childhood allergies and asthma—The Farm effect. Pediatr. Allergy Immunol. 2022, 33, e13807. [Google Scholar] [CrossRef]
- Klein, G.; Raina, S. Regulated Assembly of LPS, Its Structural Alterations and Cellular Response to LPS Defects. Int. J. Mol. Sci. 2019, 20, 356. [Google Scholar] [CrossRef]
- Klein, G.; Wieczorek, A.; Szuster, M.; Raina, S. Checkpoints That Regulate Balanced Biosynthesis of Lipopolysaccharide and Its Essentiality in Escherichia coli. Int. J. Mol. Sci. 2021, 23, 189. [Google Scholar] [CrossRef]
- Mazgaeen, L.; Gurung, P. Recent Advances in Lipopolysaccharide Recognition Systems. Int. J. Mol. Sci. 2020, 21, 379. [Google Scholar] [CrossRef]
- Rylander, R. Endotoxin in the environment—exposure and effects. J. Endotoxin. Res. 2002, 8, 241–252. [Google Scholar] [CrossRef] [PubMed]
- Barnig, C.; Reboux, G.; Roussel, S.; Casset, A.; Sohy, C.; Dalphin, J.C.; de Blay, F. Indoor dust and air concentrations of endotoxin in urban and rural environments. Lett. Appl. Microbiol. 2013, 56, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Sipka, S.; Beres, A.; Bertok, L.; Varga, T.; Bruckner, G. Comparison of endotoxin levels in cow’s milk samples derived from farms and shops. Innate Immun. 2015, 21, 531–536. [Google Scholar] [CrossRef]
- Gehring, U.; Bischof, W.; Fahlbusch, B.; Wichmann, H.E.; Heinrich, J. House dust endotoxin and allergic sensitization in children. Am. J. Respir. Crit. Care Med. 2002, 166, 939–944. [Google Scholar] [CrossRef] [PubMed]
- Gereda, J.E.; Leung, D.Y.; Thatayatikom, A.; Streib, J.E.; Price, M.R.; Klinnert, M.D.; Liu, A.H. Relation between house-dust endotoxin exposure, type 1 T-cell development, and allergen sensitisation in infants at high risk of asthma. Lancet 2000, 355, 1680–1683. [Google Scholar] [CrossRef] [PubMed]
- Schuijs, M.J.; Willart, M.A.; Vergote, K.; Gras, D.; Deswarte, K.; Ege, M.J.; Madeira, F.B.; Beyaert, R.; van Loo, G.; Bracher, F.; et al. Farm dust and endotoxin protect against allergy through A20 induction in lung epithelial cells. Science 2015, 349, 1106–1110. [Google Scholar] [CrossRef] [PubMed]
- Douwes, J.; Pearce, N.; Heederik, D. Does environmental endotoxin exposure prevent asthma? Thorax 2002, 57, 86–90. [Google Scholar] [CrossRef]
- Liu, A.H. Endotoxin exposure in allergy and asthma: Reconciling a paradox. J. Allergy Clin. Immunology 2002, 109, 379–392. [Google Scholar] [CrossRef]
- Doreswamy, V.; Peden, D.B. Modulation of asthma by endotoxin. Clin. Exp. Allergy 2011, 41, 9–19. [Google Scholar] [CrossRef]
- Park, J.H.; Gold, D.R.; Spiegelman, D.L.; Burge, H.A.; Milton, D.K. House dust endotoxin and wheeze in the first year of life. Am. J. Respir. Crit. Care Med. 2001, 163, 322–328. [Google Scholar] [CrossRef]
- Michel, O.; Kips, J.; Duchateau, J.; Vertongen, F.; Robert, L.; Collet, H.; Pauwels, R.; Sergysels, R. Severity of asthma is related to endotoxin in house dust. Am. J. Respir. Crit. Care Med. 1996, 154, 1641–1646. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, M.C.; Naspitz, C.K.; Fernandez-Caldas, E.; Lockey, R.F.; Mimica, I.; Sole, D. Endotoxin exposure and symptoms in asthmatic children. Pediatr. Allergy Immunol. 1997, 8, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Williams, L.K.; Ownby, D.R.; Maliarik, M.J.; Johnson, C.C. The role of endotoxin and its receptors in allergic disease. Ann. Allergy Asthma Immunol. 2005, 94, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Oh, S.Y.; Zheng, T.; Kim, Y.K. Immunomodulating effects of endotoxin in mouse models of allergic asthma. Clin. Exp. Allergy J. Br. Soc. Allergy Clin. Immunol. 2010, 40, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Schwarzer, M.; Srutkova, D.; Hermanova, P.; Leulier, F.; Kozakova, H.; Schabussova, I. Diet Matters: Endotoxin in the Diet Impacts the Level of Allergic Sensitization in Germ-Free Mice. PLoS ONE 2017, 12, e0167786. [Google Scholar] [CrossRef] [PubMed]
- Garcia, L.N.; Leimgruber, C.; Nicola, J.P.; Quintar, A.A.; Maldonado, C.A. Neonatal endotoxin stimulation is associated with a long-term bronchiolar epithelial expression of innate immune and anti-allergic markers that attenuates the allergic response. PLoS ONE 2020, 15, e0226233. [Google Scholar] [CrossRef] [PubMed]
- Fiuza, B.S.D.; Fonseca, H.F.; Meirelles, P.M.; Marques, C.R.; da Silva, T.M.; Figueiredo, C.A. Understanding Asthma and Allergies by the Lens of Biodiversity and Epigenetic Changes. Front. Immunol. 2021, 12, 623737. [Google Scholar] [CrossRef]
- Hanski, I.; von Hertzen, L.; Fyhrquist, N.; Koskinen, K.; Torppa, K.; Laatikainen, T.; Karisola, P.; Auvinen, P.; Paulin, L.; Makela, M.J.; et al. Environmental biodiversity, human microbiota, and allergy are interrelated. Proc. Natl. Acad. Sci. USA 2012, 109, 8334–8339. [Google Scholar] [CrossRef]
- Deckers, J.; Marsland, B.J.; von Mutius, E. Protection against allergies: Microbes, immunity, and the farming effect. Eur. J. Immunol. 2021, 51, 2387–2398. [Google Scholar] [CrossRef]
- Alashkar Alhamwe, B.; Gao, Z.; Alhamdan, F.; Harb, H.; Pichene, M.; Garnier, A.; El Andari, J.; Kaufmann, A.; Graumann, P.L.; Kesper, D.; et al. Intranasal administration of Acinetobacter lwoffii in a murine model of asthma induces IL-6-mediated protection associated with cecal microbiota changes. Allergy 2023, 78, 1245–1257. [Google Scholar] [CrossRef] [PubMed]
- Theodorou, J.; Nowak, E.; Bock, A.; Salvermoser, M.; Beerweiler, C.; Zeber, K.; Kulig, P.; Tsang, M.S.; Wong, C.K.; Wong, G.W.K.; et al. Mitogen-activated protein kinase signaling in childhood asthma development and environment-mediated protection. Pediatr. Allergy Immunol. 2022, 33, e13657. [Google Scholar] [CrossRef] [PubMed]
- Pascal, M.; Perez-Gordo, M.; Caballero, T.; Escribese, M.M.; Lopez Longo, M.N.; Luengo, O.; Manso, L.; Matheu, V.; Seoane, E.; Zamorano, M.; et al. Microbiome and Allergic Diseases. Front. Immunol. 2018, 9, 1584. [Google Scholar] [CrossRef]
- Lehtimaki, J.; Thorsen, J.; Rasmussen, M.A.; Hjelmso, M.; Shah, S.; Mortensen, M.S.; Trivedi, U.; Vestergaard, G.; Bonnelykke, K.; Chawes, B.L.; et al. Urbanized microbiota in infants, immune constitution, and later risk of atopic diseases. J. Allergy Clin. Immunol. 2021, 148, 234–243. [Google Scholar] [CrossRef] [PubMed]
- Michel, O.; Nagy, A.M.; Schroeven, M.; Duchateau, J.; Neve, J.; Fondu, P.; Sergysels, R. Dose-response relationship to inhaled endotoxin in normal subjects. Am. J. Respir. Crit. Care Med. 1997, 156, 1157–1164. [Google Scholar] [CrossRef] [PubMed]
- Xi, L. Nitric oxide-dependent mechanism of anti-ischemic myocardial protection induced by monophosphoryl lipid A. Zhongguo Yao Li Xue Bao = Acta Pharmacol. Sin. 1999, 20, 865–871. [Google Scholar]
- Toyoda, T.; Kassell, N.F.; Lee, K.S. Induction of tolerance against ischemia/reperfusion injury in the rat brain by preconditioning with the endotoxin analog diphosphoryl lipid A. J. Neurosurg. 2000, 92, 435–441. [Google Scholar] [CrossRef]
- Zariri, A.; van der Ley, P. Biosynthetically engineered lipopolysaccharide as vaccine adjuvant. Expert Rev. Vaccines 2015, 14, 861–876. [Google Scholar] [CrossRef]
- Li, X.; Qu, S.; Song, X.; Wu, C.; Shen, J.; Zhu, K. In Situ Neutralization and Detoxification of LPS to Attenuate Hyperinflammation. Adv. Sci. (Weinh) 2023, 10, e2302950. [Google Scholar] [CrossRef]
- Najafzadeh, F.; Tanomand, A.; Haddadi, A.; Majidi, J. Immunological Properties of Exotoxin A Toxoid—Detoxified Lipopolysaccharide—Gold Nanoparticles Conjugate Against Pseudomonas aeruginosa Infection. Iran J. Immunol. 2021, 18, 292–303. [Google Scholar] [CrossRef]
- Bertok, L. Radio-detoxified endotoxin as a potent stimulator of nonspecific resistance. Perspect. Biol. Med. 1980, 24, 61–66. [Google Scholar] [CrossRef]
- Bertok, L. Radio-detoxified endotoxin activates natural immunity: A review. Pathophysiol. Off. J. Int. Soc. Pathophysiol./ISP 2005, 12, 85–95. [Google Scholar] [CrossRef]
- Ciprandi, G.; Vizzaccaro, A.; Cirillo, I.; Tosca, M.; Massolo, A.; Passalacqua, G. Nasal eosinophils display the best correlation with symptoms, pulmonary function and inflammation in allergic rhinitis. Int. Arch. Allergy Immunol. 2005, 136, 266–272. [Google Scholar] [CrossRef]
- Lambrecht, B.N.; De Veerman, M.; Coyle, A.J.; Gutierrez-Ramos, J.C.; Thielemans, K.; Pauwels, R.A. Myeloid dendritic cells induce Th2 responses to inhaled antigen, leading to eosinophilic airway inflammation. J. Clin. Investig. 2000, 106, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Matsue, H.; Edelbaum, D.; Shalhevet, D.; Mizumoto, N.; Yang, C.; Mummert, M.E.; Oeda, J.; Masayasu, H.; Takashima, A. Generation and function of reactive oxygen species in dendritic cells during antigen presentation. J. Immunol. 2003, 171, 3010–3018. [Google Scholar] [CrossRef]
- Dalod, M.; Chelbi, R.; Malissen, B.; Lawrence, T. Dendritic cell maturation: Functional specialization through signaling specificity and transcriptional programming. EMBO J. 2014, 33, 1104–1116. [Google Scholar] [CrossRef]
- Cella, M.; Sallusto, F.; Lanzavecchia, A. Origin, maturation and antigen presenting function of dendritic cells. Curr. Opin. Immunol. 1997, 9, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann, T.; Bockmann, R.A.; Grubmuller, H.; Uchanska-Ziegler, B.; Ziegler, A.; Alexiev, U. Differential peptide dynamics is linked to major histocompatibility complex polymorphism. J. Biol. Chem. 2004, 279, 28197–28201. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, N.; Tsuchimori, N.; Matsumoto, T.; Ii, M. TAK-242 (resatorvid), a small-molecule inhibitor of Toll-like receptor (TLR) 4 signaling, binds selectively to TLR4 and interferes with interactions between TLR4 and its adaptor molecules. Mol. Pharmacol. 2011, 79, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Medvedev, A.E.; Lentschat, A.; Wahl, L.M.; Golenbock, D.T.; Vogel, S.N. Dysregulation of LPS-induced Toll-like receptor 4-MyD88 complex formation and IL-1 receptor-associated kinase 1 activation in endotoxin-tolerant cells. J. Immunol. 2002, 169, 5209–5216. [Google Scholar] [CrossRef]
- Erridge, C.; Bennett-Guerrero, E.; Poxton, I.R. Structure and function of lipopolysaccharides. Microbes Infect. 2002, 4, 837–851. [Google Scholar] [CrossRef]
- Caroff, M.; Novikov, A. Lipopolysaccharides: Structure, function and bacterial identification. OCL 2020, 27, 31. [Google Scholar] [CrossRef]
- Csako, G.; Elin, R.J.; Hochstein, H.D.; Tsai, C.M. Physical and biological properties of U.S. standard endotoxin EC after exposure to ionizing radiation. Infect. Immun. 1983, 41, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Elekes, E.; Luderitz, O.; Galanos, C.; Bertok, L. Alterations in the chemical composition and antigenicity of Escherichia coli O89 lipopolysaccharide and lipid A after 60Co gamma irradiation. Acta Microbiol. Hung. 1988, 35, 301–305. [Google Scholar] [PubMed]
- Park, B.S.; Song, D.H.; Kim, H.M.; Choi, B.S.; Lee, H.; Lee, J.O. The structural basis of lipopolysaccharide recognition by the TLR4-MD-2 complex. Nature 2009, 458, 1191–1195. [Google Scholar] [CrossRef] [PubMed]
- Erridge, C.; Stewart, J.; Bennett-Guerrero, E.; McIntosh, T.J.; Poxton, I.R. The biological activity of a liposomal complete core lipopolysaccharide vaccine. J. Endotoxin Res. 2002, 8, 39–46. [Google Scholar] [CrossRef]
- Horner, A.A. Toll-like receptor ligands and atopy: A coin with at least two sides. J. Allergy Clin. Immunol. 2006, 117, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- von Mutius, E. Allergies, infections and the hygiene hypothesis—The epidemiological evidence. Immunobiology 2007, 212, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Inagawa, H.; Kohchi, C.; Soma, G.-I. Oral Administration of Lipopolysaccharides for the Prevention of Various Diseases: Benefit and Usefulness. Anticancer. Res. 2011, 31, 2431–2436. [Google Scholar]
- Gerretsen, V.I.V.; Schuijs, M.J. The role of LPS and CpG in the farm effect against allergies, and beyond. Allergol. Sel. 2022, 6, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Lloyd, C.M.; Saglani, S. Opening the Window of Immune Opportunity: Treating Childhood Asthma. Trends Immunol. 2019, 40, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Liu, B.; Niu, C.; Wang, T.; Wang, Y.; Geng, G.; Tian, D.; Dai, J.; Fu, Z. Low-Dose LPS Induces Tolerogenic Treg Skewing in Asthma. Front. Immunol. 2020, 11, 2150. [Google Scholar] [CrossRef]
- Sallusto, F.; Lanzavecchia, A. The instructive role of dendritic cells on T-cell responses. Arthritis Res. 2002, 4 (Suppl S3), S127–S132. [Google Scholar] [CrossRef] [PubMed]
- Haitchi, H.M.; Holgate, S.T. New strategies in the treatment and prevention of allergic diseases. Expert. Opin. Investig. Drugs 2004, 13, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Csako, G.; Suba, E.A.; Tsai, C.M.; Mocca, L.F.; Elin, R.J. Dose-dependent changes in the antigenicity of bacterial endotoxin exposed to ionizing radiation. J. Clin. Lab. Immunol. 1987, 24, 193–198. [Google Scholar]
- Gorman, A.; Golovanov, A.P. Lipopolysaccharide Structure and the Phenomenon of Low Endotoxin Recovery. Eur. J. Pharm. Biopharm. 2022, 180, 289–307. [Google Scholar] [CrossRef]
- Fux, A.C.; Casonato Melo, C.; Michelini, S.; Swartzwelter, B.J.; Neusch, A.; Italiani, P.; Himly, M. Heterogeneity of Lipopolysaccharide as Source of Variability in Bioassays and LPS-Binding Proteins as Remedy. Int. J. Mol. Sci. 2023, 24, 8395. [Google Scholar] [CrossRef]
- Chilton, P.M.; Embry, C.A.; Mitchell, T.C. Effects of Differences in Lipid A Structure on TLR4 Pro-Inflammatory Signaling and Inflammasome Activation. Front. Immunol. 2012, 3, 154. [Google Scholar] [CrossRef]
- Sali, W.; Patoli, D.; Pais de Barros, J.P.; Labbe, J.; Deckert, V.; Duheron, V.; Le Guern, N.; Blache, D.; Chaumont, D.; Lesniewska, E.; et al. Polysaccharide Chain Length of Lipopolysaccharides From Salmonella Minnesota Is a Determinant of Aggregate Stability, Plasma Residence Time and Proinflammatory Propensity in vivo. Front. Microbiol. 2019, 10, 1774. [Google Scholar] [CrossRef]
- Csako, G.; Suba, E.A.; Ahlgren, A.; Tsai, C.M.; Elin, R.J. Relation of structure to function for the U. S. reference standard endotoxin after exposure to 60Co radiation. J. Infect. Dis. 1986, 153, 98–108. [Google Scholar] [CrossRef] [PubMed]
- Csako, G.; Tsai, C.M.; Slomiany, B.L.; Herp, A.; Elin, R.J. Modification of the chemical composition and structure of the U.S. Reference Standard Endotoxin (RSE) by 60Co radiation. Radiat. Res. 1986, 105, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Ben-Sasson, S.Z.; Hu-Li, J.; Quiel, J.; Cauchetaux, S.; Ratner, M.; Shapira, I.; Dinarello, C.A.; Paul, W.E. IL-1 acts directly on CD4 T-cells to enhance their antigen-driven expansion and differentiation. Proc. Natl. Acad. Sci. USA 2009, 106, 7119–7124. [Google Scholar] [CrossRef] [PubMed]
- Revu, S.; Wu, J.; Henkel, M.; Rittenhouse, N.; Menk, A.; Delgoffe, G.M.; Poholek, A.C.; McGeachy, M.J. IL-23 and IL-1beta Drive Human Th17 Cell Differentiation and Metabolic Reprogramming in Absence of CD28 Costimulation. Cell Rep. 2018, 22, 2642–2653. [Google Scholar] [CrossRef] [PubMed]
- Boraschi, D. What Is IL-1 for? The Functions of Interleukin-1 Across Evolution. Front. Immunol. 2022, 13, 872155. [Google Scholar] [CrossRef] [PubMed]
- Caucheteux, S.M.; Hu-Li, J.; Guo, L.; Bhattacharyya, N.; Crank, M.; Collins, M.T.; Paul, W.E. IL-1beta enhances inflammatory Th2 differentiation. J. Allergy Clin. Immunol. 2016, 138, 898–901 e894. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the interleukin-10 receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T.R.; Schumacher, J.H.; Fiorentino, D.F.; Leverah, J.; Moore, K.W.; Bond, M.W. Isolation of monoclonal antibodies specific for IL-4, IL-5, IL-6, and a new Th2-specific cytokine (IL-10), cytokine synthesis inhibitory factor, by using a solid phase radioimmunoadsorbent assay. J. Immunol. 1990, 145, 2938–2945. [Google Scholar] [CrossRef]
- Schulke, S. Induction of Interleukin-10 Producing Dendritic Cells As a Tool to Suppress Allergen-Specific T Helper 2 Responses. Front. Immunol. 2018, 9, 455. [Google Scholar] [CrossRef]
- Iwasaki, A.; Kelsall, B.L. Freshly isolated Peyer’s patch, but not spleen, dendritic cells produce interleukin 10 and induce the differentiation of T helper type 2 cells. J. Exp. Med. 1999, 190, 229–239. [Google Scholar] [CrossRef]
- Palma, J.P.; Yauch, R.L.; Kang, H.K.; Lee, H.G.; Kim, B.S. Preferential induction of IL-10 in APC correlates with a switch from Th1 to Th2 response following infection with a low pathogenic variant of Theiler’s virus. J. Immunol. 2002, 168, 4221–4230. [Google Scholar] [CrossRef]
- Webster, H.C.; Gamino, V.; Andrusaite, A.T.; Ridgewell, O.J.; McCowan, J.; Shergold, A.L.; Heieis, G.A.; Milling, S.W.F.; Maizels, R.M.; Perona-Wright, G. Tissue-based IL-10 signalling in helminth infection limits IFNgamma expression and promotes the intestinal Th2 response. Mucosal. Immunol. 2022, 15, 1257–1269. [Google Scholar] [CrossRef]
- Laouini, D.; Alenius, H.; Bryce, P.; Oettgen, H.; Tsitsikov, E.; Geha, R.S. IL-10 is critical for Th2 responses in a murine model of allergic dermatitis. J. Clin. Investig. 2003, 112, 1058–1066. [Google Scholar] [CrossRef]
- Natarajan, S.; Kim, J.; Bouchard, J.; Cruikshank, W.; Remick, D.G. Pulmonary endotoxin tolerance protects against cockroach allergen-induced asthma-like inflammation in a mouse model. Int. Arch. Allergy Immunol. 2012, 158, 120–130. [Google Scholar] [CrossRef]
- Matsushita, H.; Ohta, S.; Shiraishi, H.; Suzuki, S.; Arima, K.; Toda, S.; Tanaka, H.; Nagai, H.; Kimoto, M.; Inokuchi, A.; et al. Endotoxin tolerance attenuates airway allergic inflammation in model mice by suppression of the T-cell stimulatory effect of dendritic cells. Int. Immunol. 2010, 22, 739–747. [Google Scholar] [CrossRef]
- del Fresno, C.; Garcia-Rio, F.; Gomez-Pina, V.; Soares-Schanoski, A.; Fernandez-Ruiz, I.; Jurado, T.; Kajiji, T.; Shu, C.; Marin, E.; Gutierrez del Arroyo, A.; et al. Potent phagocytic activity with impaired antigen presentation identifying lipopolysaccharide-tolerant human monocytes: Demonstration in isolated monocytes from cystic fibrosis patients. J. Immunol. 2009, 182, 6494–6507. [Google Scholar] [CrossRef]
- Nahid, M.A.; Satoh, M.; Chan, E.K. MicroRNA in TLR signaling and endotoxin tolerance. Cell Mol. Immunol. 2011, 8, 388–403. [Google Scholar] [CrossRef]
- Radzikowska, U.; Rinaldi, A.O.; Celebi Sozener, Z.; Karaguzel, D.; Wojcik, M.; Cypryk, K.; Akdis, M.; Akdis, C.A.; Sokolowska, M. The Influence of Dietary Fatty Acids on Immune Responses. Nutrients 2019, 11, 2990. [Google Scholar] [CrossRef] [PubMed]
- Altieri, C.; Cardillo, D.; Bevilacqua, A.; Sinigaglia, M. Inhibition of Aspergillus spp. and Penicillium spp. by fatty acids and their monoglycerides. J. Food Prot. 2007, 70, 1206–1212. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ruan, W.; Li, J.; Xu, H.; Wang, J.; Gao, Y.; Wang, J. Biological control of phytopathogenic fungi by fatty acids. Mycopathologia 2008, 166, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Prasath, K.G.; Alexpandi, R.; Parasuraman, R.; Pavithra, M.; Ravi, A.V.; Pandian, S.K. Anti-inflammatory potential of myristic acid and palmitic acid synergism against systemic candidiasis in Danio rerio (Zebrafish). Biomed Pharmacother. 2021, 133, 111043. [Google Scholar] [CrossRef]
- Jia, M.; Wang, Y.; Wang, J.; Qin, D.; Wang, M.; Chai, L.; Fu, Y.; Zhao, C.; Gao, C.; Jia, J.; et al. Myristic acid as a checkpoint to regulate STING-dependent autophagy and interferon responses by promoting N-myristoylation. Nat. Commun. 2023, 14, 660. [Google Scholar] [CrossRef]
- Westphal, O.; Lüderitz, O.; Bister, F. Über die Extraktion von Bakterien mit Phenol/Wasser. Z. Für Naturforschung B 1952, 7, 148–155. [Google Scholar] [CrossRef]
- Apicella, M.A. Isolation and characterization of lipopolysaccharides. Methods Mol. Biol. 2008, 431, 3–13. [Google Scholar] [CrossRef]
- Rezania, S.; Amirmozaffari, N.; Tabarraei, B.; Jeddi-Tehrani, M.; Zarei, O.; Alizadeh, R.; Masjedian, F.; Zarnani, A.H. Extraction, Purification and Characterization of Lipopolysaccharide from Escherichia coli and Salmonella typhi. Avicenna J. Med. Biotechnol. 2011, 3, 3–9. [Google Scholar]
- Puljic, R.; Benediktus, E.; Plater-Zyberk, C.; Baeuerle, P.A.; Szelenyi, S.; Brune, K.; Pahl, A. Lipopolysaccharide-induced lung inflammation is inhibited by neutralization of GM-CSF. Eur. J. Pharmacol. 2007, 557, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Boldogh, I.; Bacsi, A.; Choudhury, B.K.; Dharajiya, N.; Alam, R.; Hazra, T.K.; Mitra, S.; Goldblum, R.M.; Sur, S. ROS generated by pollen NADPH oxidase provide a signal that augments antigen-induced allergic airway inflammation. J. Clin. Investig. 2005, 115, 2169–2179. [Google Scholar] [CrossRef]
- Sur, S.; Kita, H.; Gleich, G.J.; Chenier, T.C.; Hunt, L.W. Eosinophil recruitment is associated with IL-5, but not with RANTES, twenty-four hours after allergen challenge. J. Allergy Clin. Immunol. 1996, 97, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Sur, S.; Gleich, G.J.; Offord, K.P.; Swanson, M.C.; Ohnishi, T.; Martin, L.B.; Wagner, J.M.; Weiler, D.A.; Hunt, L.W. Allergen challenge in asthma: Association of eosinophils and lymphocytes with interleukin-5. Allergy 1995, 50, 891–898. [Google Scholar] [CrossRef] [PubMed]
- Sur, S.; Wild, J.S.; Choudhury, B.K.; Sur, N.; Alam, R.; Klinman, D.M. Long term prevention of allergic lung inflammation in a mouse model of asthma by CpG oligodeoxynucleotides. J. Immunol. 1999, 162, 6284–6293. [Google Scholar] [CrossRef]
- Van Hulst, G.; Bureau, F.; Desmet, C.J. Eosinophils as Drivers of Severe Eosinophilic Asthma: Endotypes or Plasticity? Int. J. Mol. Sci. 2021, 22, 10150. [Google Scholar] [CrossRef] [PubMed]
- Hunt, L.W.; Gleich, G.J.; Ohnishi, T.; Weiler, D.A.; Mansfield, E.S.; Kita, H.; Sur, S. Endotoxin contamination causes neutrophilia following pulmonary allergen challenge. Am. J. Respir. Crit. Care Med. 1994, 149, 1471–1475. [Google Scholar] [CrossRef] [PubMed]
- Varga, A.; Budai, M.M.; Milesz, S.; Bacsi, A.; Tozser, J.; Benko, S. Ragweed pollen extract intensifies lipopolysaccharide-induced priming of NLRP3 inflammasome in human macrophages. Immunology 2013, 138, 392–401. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bácsi, A.; Ágics, B.; Pázmándi, K.; Kocsis, B.; Sándor, V.; Bertók, L.; Bruckner, G.; Sipka, S. Radiation-Detoxified Form of Endotoxin Effectively Activates Th1 Responses and Attenuates Ragweed-Induced Th2-Type Airway Inflammation in Mice. Int. J. Mol. Sci. 2024, 25, 1581. https://doi.org/10.3390/ijms25031581
Bácsi A, Ágics B, Pázmándi K, Kocsis B, Sándor V, Bertók L, Bruckner G, Sipka S. Radiation-Detoxified Form of Endotoxin Effectively Activates Th1 Responses and Attenuates Ragweed-Induced Th2-Type Airway Inflammation in Mice. International Journal of Molecular Sciences. 2024; 25(3):1581. https://doi.org/10.3390/ijms25031581
Chicago/Turabian StyleBácsi, Attila, Beatrix Ágics, Kitti Pázmándi, Béla Kocsis, Viktor Sándor, Lóránd Bertók, Geza Bruckner, and Sándor Sipka. 2024. "Radiation-Detoxified Form of Endotoxin Effectively Activates Th1 Responses and Attenuates Ragweed-Induced Th2-Type Airway Inflammation in Mice" International Journal of Molecular Sciences 25, no. 3: 1581. https://doi.org/10.3390/ijms25031581
APA StyleBácsi, A., Ágics, B., Pázmándi, K., Kocsis, B., Sándor, V., Bertók, L., Bruckner, G., & Sipka, S. (2024). Radiation-Detoxified Form of Endotoxin Effectively Activates Th1 Responses and Attenuates Ragweed-Induced Th2-Type Airway Inflammation in Mice. International Journal of Molecular Sciences, 25(3), 1581. https://doi.org/10.3390/ijms25031581








