Imaging Molecular Targets and Metabolic Pathways in Breast Cancer for Improved Clinical Management: Current Practice and Future Perspectives
Abstract
1. Introduction
2. Current Standard-of-Care PET Imaging Techniques in Breast Cancer Management
2.1. [18F]FDG PET/CT
2.1.1. [18F]FDG Uptake Patterns Based on the Histological and Immunohistochemical Subtype of Breast Cancer
2.1.2. The Role of [18F]FDG PET/CT in Breast Cancer
Staging
Response Assessment
Prognostication
Recurrence Assessment
2.1.3. Limitations of [18F]FDG PET/CT Relevant to Tumor Heterogeneity
2.2. Estrogen Receptor Imaging
2.2.1. Estrogen Receptor Signaling and Therapeutic Targets
2.2.2. Role of [18F]FES Breast Cancer
Diagnosis and Staging
Response Assessment and Prognostication
Recurrence Assessment
3. Promising PET Radiopharmaceuticals for Breast Cancer Imaging
3.1. Progesterone and HER2 Receptor Expression
3.2. Imaging the Tumor Micro-Environment (TME)
3.2.1. Cancer-Associated Fibroblasts (CAF) and Fibroblast Activated Protein (FAP)
3.2.2. Neovasculature/Angiogenesis
Prostate-Specific Membrane Antigen (PSMA)
Integrins Recognizing Arginine-Glycine-Aspartate (RGD)
3.3. Other Imaging Targets (Cellular Proliferation, Hypoxia, Fatty Acid Synthesis and Somatostatin Receptor Imaging)
3.4. Poly (ADP-Ribosyl) Polymerase (PARP) Imaging
4. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer Statistics, 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- Loibl, S.; Poortmans, P.; Morrow, M.; Denkert, C.; Curigliano, G. Breast Cancer. Lancet 2021, 397, 1750–1769. [Google Scholar] [CrossRef]
- Gennari, A.; André, F.; Barrios, C.; Cortés, J.; de Azambuja, E.; DeMichele, A.; Dent, R.; Fenlon, D.; Gligorov, J.; Hurvitz, S.; et al. ESMO Clinical Practice Guideline for the Diagnosis, Staging and Treatment of Patients with Metastatic Breast Cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar] [CrossRef]
- Groheux, D. FDG-PET/CT for Primary Staging and Detection of Recurrence of Breast Cancer. Semin. Nucl. Med. 2022, 52, 508–519. [Google Scholar] [CrossRef] [PubMed]
- Weigelt, B.; Geyer, F.C.; Reis-Filho, J.S. Histological Types of Breast Cancer: How Special Are They? Mol. Oncol. 2010, 4, 192–208. [Google Scholar] [CrossRef]
- Dalenc, F.; Lusque, A.; Rouge, T.D.L.M.; Pistilli, B.; Brain, E.; Pasquier, D.; Debled, M.; Thery, J.-C.; Gonçalves, A.; Desmoulins, I.; et al. Impact of Lobular versus Ductal Histology on Overall Survival in Metastatic Breast Cancer: A French Retrospective Multicentre Cohort Study. Eur. J. Cancer 2022, 164, 70–79. [Google Scholar] [CrossRef]
- Hammond, M.E.H.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer. J. Clin. Oncol. 2010, 28, 2784–2795. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.H.; Hicks, D.G.; Dowsett, M.; McShane, L.M.; Allison, K.H.; Allred, D.C.; Bartlett, J.M.S.; Bilous, M.; Fitzgibbons, P.; et al. Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast. J. Clin. Oncol. 2013, 31, 3997–4013. [Google Scholar] [CrossRef]
- Waks, A.G.; Winer, E.P. Breast Cancer Treatment: A Review. JAMA J. Am. Med. Assoc. 2019, 321, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Goldhirsch, A.; Wood, W.C.; Coates, A.S.; Gelber, R.D.; Thürlimann, B.; Senn, H.J. Strategies for Subtypes-Dealing with the Diversity of Breast Cancer: Highlights of the St Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann. Oncol. 2011, 22, 1736–1747. [Google Scholar] [CrossRef]
- Grabher, B.J. Breast Cancer: Evaluating Tumor Estrogen Receptor Status with Molecular Imaging to Increase Response to Therapy and Improve Patient Outcomes. J. Nucl. Med. Technol. 2020, 48, 191–201. [Google Scholar] [CrossRef]
- Ciriello, G.; Gatza, M.L.; Beck, A.H.; Wilkerson, M.D.; Rhie, S.K.; Pastore, A.; Zhang, H.; McLellan, M.; Yau, C.; Kandoth, C.; et al. Comprehensive Molecular Portraits of Invasive Lobular Breast Cancer. Cell 2015, 163, 506–519. [Google Scholar] [CrossRef]
- Burstein, H.J. Systemic Therapy for Estrogen Receptor–Positive, HER2-Negative Breast Cancer. N. Engl. J. Med. 2020, 383, 2557–2570. [Google Scholar] [CrossRef] [PubMed]
- Von Minckwitz, G.; Procter, M.; de Azambuja, E.; Zardavas, D.; Benyunes, M.; Viale, G.; Suter, T.; Arahmani, A.; Rouchet, N.; Clark, E.; et al. Adjuvant Pertuzumab and Trastuzumab in Early HER2-Positive Breast Cancer. N. Engl. J. Med. 2017, 377, 122–131. [Google Scholar] [CrossRef]
- Schlam, I.; Tarantino, P.; Morganti, S.; Lynce, F.; Trapani, D.; Mayer, E.L.; Garrido-Castro, A.C.; Waks, A.; Tolaney, S.M. Emerging Targeted Therapies for Early Breast Cancer. Drugs 2022, 82, 1437–1451. [Google Scholar] [CrossRef] [PubMed]
- Freelander, A.; Brown, L.J.; Parker, A.; Segara, D.; Portman, N.; Lau, B.; Lim, E. Molecular Biomarkers for Contemporary Therapies in Hormone Receptor-Positive Breast Cancer. Genes 2021, 12, 285. [Google Scholar] [CrossRef] [PubMed]
- Cortesi, L.; Rugo, H.S.; Jackisch, C. An Overview of PARP Inhibitors for the Treatment of Breast Cancer. Target. Oncol. 2021, 16, 255–282. [Google Scholar] [CrossRef]
- Tutt, A.N.; Garber, J.E.; Kaufman, B.; Viale, G.; Fumagalli, D.; Rastogi, P.; Gelber, R.D.; de Azambuja, E.; Fielding, A.; Balmaña, J.; et al. Adjuvant Olaparib for Patients with BRCA1- or BRCA2-Mutated Breast Cancer. N. Engl. J. Med. 2021, 384, 2394–2405. [Google Scholar] [CrossRef]
- Robson, M.; Im, S.A.; Senkus, E.; Xu, B.; Domchek, S.M.; Masuda, N.; Delaloge, S.; Li, W.; Tung, N.; Armstrong, A.; et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N. Engl. J. Med. 2017, 377, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Simon, R.; Mirlacher, M.; Sauter, G. Immunohistochemical Analysis of Tissue Microarrays; Springer: Berlin/Heidelberg, Germany, 2010; pp. 113–126. [Google Scholar] [CrossRef]
- Burrell, R.A.; Swanton, C. Tumour Heterogeneity and the Evolution of Polyclonal Drug Resistance. Mol. Oncol. 2014, 8, 1095–1111. [Google Scholar] [CrossRef]
- Kavan, S.; Kruse, T.A.; Vogsen, M.; Hildebrandt, M.G.; Thomassen, M. Heterogeneity and Tumor Evolution Reflected in Liquid Biopsy in Metastatic Breast Cancer Patients: A Review. Cancer Metastasis Rev. 2022, 41, 433–446. [Google Scholar] [CrossRef]
- Gerlinger, M.; Swanton, C. How Darwinian Models Inform Therapeutic Failure Initiated by Clonal Heterogeneity in Cancer Medicine. Br. J. Cancer 2010, 103, 1139–1143. [Google Scholar] [CrossRef]
- Krøigård, A.B.; Larsen, M.J.; Brasch-Andersen, C.; Lænkholm, A.V.; Knoop, A.S.; Jensen, J.D.; Bak, M.; Mollenhauer, J.; Thomassen, M.; Kruse, T.A. Genomic Analyses of Breast Cancer Progression Reveal Distinct Routes of Metastasis Emergence. Sci. Rep. 2017, 7, 43813. [Google Scholar] [CrossRef]
- Aurilio, G.; Disalvatore, D.; Pruneri, G.; Bagnardi, V.; Viale, G.; Curigliano, G.; Adamoli, L.; Munzone, E.; Sciandivasci, A.; De Vita, F.; et al. A Meta-Analysis of Oestrogen Receptor, Progesterone Receptor and Human Epidermal Growth Factor Receptor 2 Discordance between Primary Breast Cancer and Metastases. Eur. J. Cancer 2014, 50, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Mellouli, M.; Graja, S.; Ben Kridis, W.; Ayed, H.B.; Makni, S.; Triki, M.; Charfi, S.; Khanfir, A.; Boudawara, T.S.; Kallel, R. Discordance in Receptor Status between Primary and Metastatic Breast Cancer and Overall Survival: A Single-Center Analysis. Ann. Diagn. Pathol. 2022, 61, 152044. [Google Scholar] [CrossRef]
- Korhonen, T.; Kuukasjärvi, T.; Huhtala, H.; Alarmo, E.L.; Holli, K.; Kallioniemi, A.; Pylkkänen, L. The Impact of Lobular and Ductal Breast Cancer Histology on the Metastatic Behavior and Long Term Survival of Breast Cancer Patients. Breast 2013, 22, 1119–1124. [Google Scholar] [CrossRef]
- Nayar, U.; Cohen, O.; Kapstad, C.; Cuoco, M.S.; Waks, A.G.; Wander, S.A.; Painter, C.; Freeman, S.; Persky, N.S.; Marini, L.; et al. Acquired HER2 Mutations in ER + Metastatic Breast Cancer Confer Resistance to Estrogen Receptor–Directed Therapies. Nat. Genet. 2019, 51, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Iranmakani, S.; Mortezazadeh, T.; Sajadian, F.; Ghaziani, M.F.; Ghafari, A.; Khezerloo, D.; Musa, A.E. A Review of Various Modalities in Breast Imaging: Technical Aspects and Clinical Outcomes. Egypt. J. Radiol. Nucl. Med. 2020, 51, 1–22. [Google Scholar] [CrossRef]
- Pesapane, F.; Downey, K.; Rotili, A.; Cassano, E.; Koh, D.M. Imaging Diagnosis of Metastatic Breast Cancer. In Insights into Imaging; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar] [CrossRef]
- PubMed. A Definition of Molecular Imaging. Available online: https://pubmed.ncbi.nlm.nih.gov/17536102/ (accessed on 13 July 2023).
- Gradishar, W.J.; Moran, M.S.; Abraham, J.; Aft, R.; Agnese, D.; Allison, K.H.; Anderson, B.; Burstein, H.J.; Chew, H.; Dang, C.; et al. Breast Cancer, Version 3.2022. JNCCN J. Natl. Compr. Cancer Netw. 2022, 20, 691–722. [Google Scholar] [CrossRef]
- Vaupel, P.; Multhoff, G. Revisiting the Warburg Effect: Historical Dogma versus Current Understanding. J. Physiol. 2021, 599, 1745–1757. [Google Scholar] [CrossRef]
- Vallabhajosula, S. 18F-Labeled Positron Emission Tomographic Radiopharmaceuticals in Oncology: An Overview of Radiochemistry and Mechanisms of Tumor Localization. Semin. Nucl. Med. 2007, 37, 400–419. [Google Scholar] [CrossRef] [PubMed]
- Pascale, R.M.; Calvisi, D.F.; Simile, M.M.; Feo, C.F.; Feo, F. The Warburg Effect 97 Years after Its Discovery. Cancers 2020, 12, 2819. [Google Scholar] [CrossRef] [PubMed]
- Parghane, R.V.; Basu, S. PET-CTBased Quantitative Parameters for Assessment of Treatment Response and Disease Activity in Cancer and Noncancerous Disorders. PET Clin. 2022, 17, 465–478. [Google Scholar] [CrossRef] [PubMed]
- Buck, A.; Schirrmeister, H.; Kühn, T.; Shen, C.; Kalker, T.; Kotzerke, J.; Dankerl, A.; Glatting, G.; Reske, S.; Mattfeldt, T. FDG Uptake in Breast Cancer: Correlation with Biological and Clinical Prognostic Parameters. Eur. J. Nucl. Med. 2002, 29, 1317–1323. [Google Scholar] [CrossRef] [PubMed]
- Dashevsky, B.Z.; Goldman, D.A.; Parsons, M.; Gönen, M.; Corben, A.D.; Jochelson, M.S.; Hudis, C.A.; Morrow, M.; Ulaner, G.A. Appearance of Untreated Bone Metastases from Breast Cancer on FDG PET/CT: Importance of Histologic Subtype. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1666–1673. [Google Scholar] [CrossRef] [PubMed]
- Kitajima, K.; Fukushima, K.; Miyoshi, Y.; Nishimukai, A.; Hirota, S.; Igarashi, Y.; Katsuura, T.; Maruyama, K.; Hirota, S. Association between 18F-FDG Uptake and Molecular Subtype of Breast Cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1371–1377. [Google Scholar] [CrossRef]
- Sasada, S.; Masumoto, N.; Suzuki, E.; Sueoka, S.; Goda, N.; Kajitani, K.; Emi, A.; Kadoya, T.; Okada, M. Prediction of Biological Characteristics of Breast Cancer Using Dual-Phase FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2019, 46, 831–837. [Google Scholar] [CrossRef]
- Yoon, H.-J.; Kang, K.W.; Chun, I.K.; Cho, N.; Im, S.-A.; Jeong, S.; Lee, S.; Jung, K.C.; Lee, Y.-S.; Jeong, J.M.; et al. Correlation of Breast Cancer Subtypes, Based on Estrogen Receptor, Progesterone Receptor, and HER2, with Functional Imaging Parameters from 68Ga-RGD PET/CT and 18F-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 1534–1543. [Google Scholar] [CrossRef]
- Groheux, D.; Giacchetti, S.; Moretti, J.-L.; Porcher, R.; Espié, M.; Lehmann-Che, J.; de Roquancourt, A.; Hamy, A.-S.; Cuvier, C.; Vercellino, L.; et al. Correlation of High 18F-FDG Uptake to Clinical, Pathological and Biological Prognostic Factors in Breast Cancer. Eur. J. Nucl. Med. Mol. Imaging 2011, 38, 426–435. [Google Scholar] [CrossRef]
- Groheux, D.; Majdoub, M.; Tixier, F.; Le Rest, C.C.; Martineau, A.; Merlet, P.; Espié, M.; de Roquancourt, A.; Hindié, E.; Hatt, M.; et al. Do Clinical, Histological or Immunohistochemical Primary Tumour Characteristics Translate into Different 18F-FDG PET/CT Volumetric and Heterogeneity Features in Stage II/III Breast Cancer? Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1682–1691. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Lee, E.J.; Moon, S.H.; Kim, S.; Hyun, S.H.; Cho, Y.S.; Choi, J.Y.; Kim, B.T.; Lee, K.H. Strong Association of Epidermal Growth Factor Receptor Status with Breast Cancer FDG Uptake. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1438–1447. [Google Scholar] [CrossRef]
- Riedl, J.M.; Moik, F.; Esterl, T.; Kostmann, S.M.; Gerger, A.; Jost, P.J. Molecular Diagnostics Tailoring Personalized Cancer Therapy—An Oncologist’s View. Virchows Archiv. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
- Ulaner, G.A.; Castillo, R.; Wills, J.; Gönen, M.; Goldman, D.A. 18F–FDG-PET/CT for Systemic Staging of Patients with Newly Diagnosed ER-Positive and HER2-Positive Breast Cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1420–1427. [Google Scholar] [CrossRef] [PubMed]
- Groheux, D.; Hindié, E.; Delord, M.; Giacchetti, S.; Hamy, A.-S.; de Bazelaire, C.; de Roquancourt, A.; Vercellino, L.; Toubert, M.-E.; Merlet, P.; et al. Prognostic Impact of 18FDG-PET-CT Findings in Clinical Stage III and IIB Breast Cancer. J. Natl. Cancer Inst. 2012, 104, 1879–1887. [Google Scholar] [CrossRef] [PubMed]
- Groheux, D. FDG-PET/CT for Systemic Staging of Patients with Newly Diagnosed Breast Cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1417–1419. [Google Scholar] [CrossRef] [PubMed]
- Ulaner, G.A.; Juarez, J.; Riedl, C.C.; Goldman, D.A. 18F-FDG PET/CT for Systemic Staging of Newly Diagnosed Breast Cancer in Men. J. Nucl. Med. 2019, 60, 472–477. [Google Scholar] [CrossRef] [PubMed]
- Cochet, A.; Dygai-Cochet, I.; Riedinger, J.-M.; Humbert, O.; Berriolo-Riedinger, A.; Toubeau, M.; Guiu, S.; Coutant, C.; Coudert, B.; Fumoleau, P.; et al. 18F-FDG PET/CT Provides Powerful Prognostic Stratification in the Primary Staging of Large Breast Cancer When Compared with Conventional Explorations. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 428–437. [Google Scholar] [CrossRef] [PubMed]
- Boers, J.; Loudini, N.; Brunsch, C.L.; Koza, S.A.; de Vries, E.F.J.; Glaudemans, A.W.J.M.; Hospers, G.A.P.; Schröder, C.P. Value of 18F-FES PET in Solving Clinical Dilemmas in Breast Cancer Patients: A Retrospective Study. J. Nucl. Med. 2021, 62, 1214–1220. [Google Scholar] [CrossRef]
- Govindan, B.; Sabri, M.A.; Hai, A.; Banat, F.; Haija, M.A. A Review of Advanced Multifunctional Magnetic Nanostructures for Cancer Diagnosis and Therapy Integrated into an Artificial Intelligence Approach. Pharmaceutics 2023, 15, 868. [Google Scholar] [CrossRef]
- Xiao, Y.D.; Paudel, R.; Liu, J.; Ma, C.; Zhang, Z.S.; Zhou, S.K. MRI Contrast Agents: Classification and Application (Review). Int. J. Mol. Med. 2016, 38, 1319–1326. [Google Scholar] [CrossRef]
- Wood, M.L.; Hardy, P.A. Proton Relaxation Enhancement. J. Magn. Reson. Imaging 1993, 3, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Marckmann, P.; Skov, L.; Rossen, K.; Dupont, A.; Damholt, M.B.; Heaf, J.G.; Thomsen, H.S. Nephrogenic Systemic Fibrosis: Suspected Causative Role of Gadodiamide Used for Contrast-Enhanced Magnetic Resonance Imaging. J. Am. Soc. Nephrol. 2006, 17, 2359–2362. [Google Scholar] [CrossRef]
- Avasthi, A.; Caro, C.; Pozo-Torres, E.; Leal, M.P.; García-Martín, M.L. Magnetic Nanoparticles as MRI Contrast Agents; Springer International Publishing: Berlin/Heidelberg, Germany, 2020; Volume 378. [Google Scholar] [CrossRef]
- Caspani, S.; Magalhães, R.; Araújo, J.P.; Sousa, C.T. Magnetic Nanomaterials as Contrast Agents for MRI. Materials 2020, 13, 2586. [Google Scholar] [CrossRef] [PubMed]
- Dodelzon, K.; McGinty, G.; Melsaether, A. PET/MRI in Breast Cancer Patients: Added Value, Barriers to Implementation, and Solutions. Clin. Imaging 2020, 68, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Morawitz, J.; Bruckmann, N.-M.; Dietzel, F.; Ullrich, T.; Bittner, A.-K.; Hoffmann, O.; Ruckhäberle, E.; Mohrmann, S.; Häberle, L.; Ingenwerth, M.; et al. Comparison of Nodal Staging between CT, MRI, and [18F]-FDG PET/MRI in Patients with Newly Diagnosed Breast Cancer. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 992–1001. [Google Scholar] [CrossRef]
- Eisenhauer, E.A.; Therasse, P.; Bogaerts, J.; Schwartz, L.H.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (Version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef]
- Joo Hyun, O.; Lodge, M.A.; Wahl, R.L. Practical Percist: A Simplified Guide to PET Response Criteria in Solid Tumors 1.0. Radiology 2016, 280, 576–584. [Google Scholar] [CrossRef]
- Riedl, C.C.; Pinker, K.; Ulaner, G.A.; Ong, L.T.; Baltzer, P.; Jochelson, M.S.; McArthur, H.L.; Gönen, M.; Dickler, M.; Weber, W.A. Comparison of FDG-PET/CT and Contrast-Enhanced CT for Monitoring Therapy Response in Patients with Metastatic Breast Cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1428–1437. [Google Scholar] [CrossRef]
- Vogsen, M.; Harbo, F.; Jakobsen, N.M.; Nissen, H.J.; Dahlsgaard-Wallenius, S.E.; Gerke, O.; Jensen, J.D.; Asmussen, J.T.; Jylling, A.M.B.; Braad, P.-E.; et al. Response Monitoring in Metastatic Breast Cancer: A Prospective Study Comparing 18F-FDG PET/CT with Conventional CT. J. Nucl. Med. 2023, 64, 355–361. [Google Scholar] [CrossRef]
- Ribi, K.; Kalbermatten, N.; Eicher, M.; Strasser, F. Towards a Novel Approach Guiding the Decision-Making Process for Anticancer Treatment in Patients with Advanced Cancer: Framework for Systemic Anticancer Treatment with Palliative Intent. ESMO Open 2022, 7, 100496. [Google Scholar] [CrossRef]
- Koolen, B.B.; Peeters, M.J.T.F.D.V.; Wesseling, J.; Lips, E.H.; Vogel, W.V.; Aukema, T.S.; van Werkhoven, E.; Gilhuijs, K.G.A.; Rodenhuis, S.; Rutgers, E.J.T.; et al. Association of Primary Tumour FDG Uptake with Clinical, Histopathological and Molecular Characteristics in Breast Cancer Patients Scheduled for Neoadjuvant Chemotherapy. Eur. J. Nucl. Med. Mol. Imaging 2012, 39, 1830–1838. [Google Scholar] [CrossRef]
- García Vicente, A.M.; Soriano Castrejón, Á.; López-Fidalgo, J.F.; Amo-Salas, M.; del Muñoz Sanchez, M.M.; Álvarez Cabellos, R.; Espinosa Aunión, R. Basal 18 F-Fluoro-2-Deoxy-d-Glucose Positron Emission Tomography/Computed Tomography as a Prognostic Biomarker in Patients with Locally Advanced Breast Cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1804–1813. [Google Scholar] [CrossRef]
- Nakajo, M.; Kajiya, Y.; Kaneko, T.; Kaneko, Y.; Takasaki, T.; Tani, A.; Ueno, M.; Koriyama, C.; Nakajo, M. FDG PET/CT and Diffusion-Weighted Imaging for Breast Cancer: Prognostic Value of Maximum Standardized Uptake Values and Apparent Diffusion Coefficient Values of the Primary Lesion. Eur. J. Nucl. Med. Mol. Imaging 2010, 37, 2011–2020. [Google Scholar] [CrossRef]
- Gil-Rendo, A.; Martínez-Regueira, F.; Zornoza, G.; García-Velloso, M.J.; Beorlegui, C.; Rodriguez-Spiteri, N. Association between [18F]Fluorodeoxyglucose Uptake and Prognostic Parameters in Breast Cancer. Br. J. Surg. 2009, 96, 166–170. [Google Scholar] [CrossRef] [PubMed]
- Evangelista, L.; Cervino, A.R.; Ghiotto, C.; Saibene, T.; Michieletto, S.; Fernando, B.; Orvieto, E.; Guarneri, V.; Conte, P. Could Semiquantitative FDG Analysis Add Information to the Prognosis in Patients with Stage II/III Breast Cancer Undergoing Neoadjuvant Treatment? Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1648–1655. [Google Scholar] [CrossRef] [PubMed]
- Groheux, D.; Biard, L.; Lehmann-Che, J.; Teixeira, L.; Bouhidel, F.A.; Poirot, B.; Bertheau, P.; Merlet, P.; Espié, M.; Resche-Rigon, M.; et al. Tumor Metabolism Assessed by FDG-PET/CT and Tumor Proliferation Assessed by Genomic Grade Index to Predict Response to Neoadjuvant Chemotherapy in Triple Negative Breast Cancer. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 1279–1288. [Google Scholar] [CrossRef]
- Kim, Y.I.; Kim, Y.J.; Paeng, J.C.; Cheon, G.J.; Lee, D.S.; Chung, J.K.; Kang, K.W. Prediction of Breast Cancer Recurrence Using Lymph Node Metabolic and Volumetric Parameters from 18F-FDG PET/CT in Operable Triple-Negative Breast Cancer. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 1787–1795. [Google Scholar] [CrossRef] [PubMed]
- Humbert, O.; Berriolo-Riedinger, A.; Cochet, A.; Gauthier, M.; Charon-Barra, C.; Guiu, S.; Desmoulins, I.; Toubeau, M.; Dygai-Cochet, I.; Coutant, C.; et al. Prognostic Relevance at 5 Years of the Early Monitoring of Neoadjuvant Chemotherapy Using 18F-FDG PET in Luminal HER2-Negative Breast Cancer. Eur. J. Nucl. Med. Mol. Imaging 2014, 41, 416–427. [Google Scholar] [CrossRef]
- Scholzen, T.; Gerdes, J.; Klinkhammer-schalke, E.C.I.M. Ki-67 Is a Prognostic Parameter in Breast Cancer Patients: Results of a Large Population-Based Cohort of a Cancer Registry. Breast Cancer Res. Treat. 2013, 139, 539–552. [Google Scholar] [CrossRef]
- Shimoda, W.; Hayashi, M.; Murakami, K.; Oyama, T.; Sunagawa, M. The Relationship between FDG Uptake in PET Scans and Biological Behavior in Breast Cancer. Breast Cancer 2007, 14, 260–268. [Google Scholar] [CrossRef]
- Scholzen, T.; Gerdes, J. The Ki-67 Protein: From the Known and the Unknown. J. Cell. Physiol. 2000, 182, 311–322. [Google Scholar] [CrossRef]
- Tchou, J.; Sonnad, S.S.; Bergey, M.R.; Basu, S.; Tomaszewski, J.; Alavi, A.; Schnall, M. Degree of Tumor FDG Uptake Correlates with Proliferation Index in Triple Negative Breast Cancer. Mol. Imaging Biol. 2010, 12, 657–662. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xu, X.; Tian, B.; Wang, Y.; Du, L.; Sun, T.; Shi, Y.; Zhao, X.; Jing, J. The Diagnostic Value of Serum Tumor Markers CEA, CA19-9, CA125, CA15-3, and TPS in Metastatic Breast Cancer. Clin. Chim. Acta 2017, 470, 51–55. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.J. Serum Tumor Markers in Breast Cancer: Are They of Clinical Value? Clin. Chem. 2006, 52, 345–351. [Google Scholar] [CrossRef]
- Grassetto, G.; Fornasiero, A.; Otello, D.; Bonciarelli, G.; Rossi, E.; Nashimben, O.; Minicozzi, A.M.; Crepaldi, G.; Pasini, F.; Facci, E.; et al. 18F-FDG-PET/CT in Patients with Breast Cancer and Rising Ca 15-3 with Negative Conventional Imaging: A Multicentre Study. Eur. J. Radiol. 2011, 80, 828–833. [Google Scholar] [CrossRef]
- Aukema, T.S.; Rutgers, E.J.T.; Vogel, W.V.; Teertstra, H.J.; Oldenburg, H.S.; Vrancken Peeters, M.T.F.D.; Wesseling, J.; Russell, N.S.; Valdés Olmos, R.A. The Role of FDG PET/CT in Patients with Locoregional Breast Cancer Recurrence: A Comparison to Conventional Imaging Techniques. Eur. J. Surg. Oncol. 2010, 36, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, G.P.; Baur-Melnyk, A.; Haug, A.; Heinemann, V.; Bauerfeind, I.; Reiser, M.F.; Schoenberg, S.O. Comprehensive Imaging of Tumor Recurrence in Breast Cancer Patients Using Whole-Body MRI at 1.5 and 3 T Compared to FDG-PET-CT. Eur. J. Radiol. 2008, 65, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Plecha, D.M.; Faulhaber, P. PET/MRI of the Breast. Eur. J. Radiol. 2017, 94, A26–A34. [Google Scholar] [CrossRef]
- Gilardi, L.; Colleoni, M.; Paganelli, G. PET/CT and Breast Cancer Subtypes. Eur. J. Nucl. Med. Mol. Imaging 2013, 40, 1301–1303. [Google Scholar] [CrossRef][Green Version]
- Giuliano, A.E.; Edge, S.B.; Hortobagyi, G.N. Eighth Edition of the AJCC Cancer Staging Manual: Breast Cancer. Ann. Surg. Oncol. 2018, 25, 1783–1785. [Google Scholar] [CrossRef]
- Saha Roy, S.; Vadlamudi, R.K. Role of Estrogen Receptor Signaling in Breast Cancer Metastasis. Int. J. Breast Cancer 2012, 2012, 654698. [Google Scholar] [CrossRef]
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. 2020, 38, 1346–1366. [Google Scholar] [CrossRef]
- Early Breast Cancer Trialists’ Collaborative Group (EBCTCG). Relevance of Breast Cancer Hormone Receptors and Other Factors to the Efficacy of Adjuvant Tamoxifen: Patient-Level Meta-Analysis of Randomised Trials. Lancet 2011, 378, 771–784. [Google Scholar] [CrossRef]
- Yi, M.; Huo, L.; Koenig, K.B.; Mittendorf, E.A.; Meric-Bernstam, F.; Kuerer, H.M.; Bedrosian, I.; Buzdar, A.U.; Symmans, W.F.; Crow, J.R.; et al. Which Threshold for ER Positivity? A Retrospective Study Based on 9639 Patients. Ann. Oncol. 2014, 25, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Hennigs, A.; Riedel, F.; Gondos, A.; Sinn, P.; Schirmacher, P.; Marmé, F.; Jäger, D.; Kauczor, H.-U.; Stieber, A.; Lindel, K.; et al. Prognosis of Breast Cancer Molecular Subtypes in Routine Clinical Care: A Large Prospective Cohort Study. BMC Cancer 2016, 16, 734. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Qadir, H.; Amir, E.; Fischer, H.D.; Fu, L.; Austin, P.C.; Harvey, P.J.; Rochon, P.A.; Lee, D.S.; Anderson, G.M. The Risk of Myocardial Infarction with Aromatase Inhibitors Relative to Tamoxifen in Post-Menopausal Women with Early Stage Breast Cancer. Eur. J. Cancer 2016, 68, 11–21. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Mankoff, D.A.; Clark, A.S.; Fowler, A.M.; Linden, H.M.; Peterson, L.M.; Dehdashti, F.; Kurland, B.F.; Mortimer, J.; Mouabbi, J.; et al. Summary: Appropriate Use Criteria for Estrogen Receptor-Targeted PET Imaging with 16α-18F-Fluoro-17β-Fluoroestradiol. J. Nucl. Med. 2023, 64, 351–354. [Google Scholar] [CrossRef]
- Ulaner, G.A. 16α-18F-Fluoro-17β-Fluoroestradiol (FES): Clinical Applications for Patients with Breast Cancer. Semin. Nucl. Med. 2022, 52, 574–583. [Google Scholar] [CrossRef] [PubMed]
- Kurland, B.F.; Wiggins, J.R.; Coche, A.; Fontan, C.; Bouvet, Y.; Webner, P.; Divgi, C.; Linden, H.M. Whole-Body Characterization of Estrogen Receptor Status in Metastatic Breast Cancer with 16α-18F-Fluoro-17β-Estradiol Positron Emission Tomography: Meta-Analysis and Recommendations for Integration into Clinical Applications. Oncologist 2020, 25, 835–844. [Google Scholar] [CrossRef]
- Ulaner, G.A.; Jhaveri, K.; Chandarlapaty, S.; Hatzoglou, V.; Riedl, C.C.; Lewis, J.S.; Mauguen, A. Head-to-Head Evaluation of 18F-FES and 18F-FDG PET/CT in Metastatic Invasive Lobular Breast Cancer. J. Nucl. Med. 2021, 62, 326–331. [Google Scholar] [CrossRef]
- Denton, M.; Taubman, K.; Sutherland, T. 18F-Fluoroestradiol PET in the Evaluation of Probable Oligometastatic Breast Cancer. J. Med. Imaging Radiat. Oncol. 2021, 65, 333–334. [Google Scholar] [CrossRef]
- Koleva-Kolarova, R.G.; Greuter, M.J.W.; van Kruchten, M.; Vermeulen, K.M.; Feenstra, T.; Buskens, E.; Glaudemans, A.W.J.M.; de Vries, E.F.J.; de Vries, E.G.E.; Hospers, G.A.P.; et al. The Value of PET/CT with FES or FDG Tracers in Metastatic Breast Cancer: A Computer Simulation Study in ER-Positive Patients. Br. J. Cancer 2015, 112, 1617–1625. [Google Scholar] [CrossRef]
- Peterson, L.M.; Kurland, B.F.; Schubert, E.K.; Link, J.M.; Gadi, V.; Specht, J.M.; Eary, J.F.; Porter, P.; Shankar, L.K.; Mankoff, D.A.; et al. A Phase 2 Study of 16α-[18F]-Fluoro-17β-Estradiol Positron Emission Tomography (FES-PET) as a Marker of Hormone Sensitivity in Metastatic Breast Cancer (MBC). Mol. Imaging Biol. 2014, 16, 431–440. [Google Scholar] [CrossRef]
- Chae, S.Y.; Ahn, S.H.; Kim, S.-B.; Han, S.; Lee, S.H.; Oh, S.J.; Lee, S.J.; Kim, H.J.; Ko, B.S.; Lee, J.W.; et al. Diagnostic Accuracy and Safety of 16α-[18F]Fluoro-17β-Oestradiol PET-CT for the Assessment of Oestrogen Receptor Status in Recurrent or Metastatic Lesions in Patients with Breast Cancer: A Prospective Cohort Study. Lancet Oncol. 2019, 20, 546–555. [Google Scholar] [CrossRef]
- Katzenellenbogen, J.A. The Quest for Improving the Management of Breast Cancer by Functional Imaging: The Discovery and Development of 16α-[18F]Fluoroestradiol (FES), a PET Radiotracer for the Estrogen Receptor, a Historical Review. Nucl. Med. Biol. 2021, 92, 24–37. [Google Scholar] [CrossRef]
- Dehdashti, F.; Mortimer, J.E.; Trinkaus, K.; Naughton, M.J.; Ellis, M.; Katzenellenbogen, J.A.; Welch, M.J.; Siegel, B.A. PET-Based Estradiol Challenge as a Predictive Biomarker of Response to Endocrine Therapy in Women with Estrogen-Receptor-Positive Breast Cancer. Breast Cancer Res. Treat. 2009, 113, 509–517. [Google Scholar] [CrossRef]
- Yang, Z.; Xie, Y.; Liu, C.; Liu, X.; Song, S.; Zhang, Y.; Ge, R.; Wang, B.; Yang, Z. The Clinical Value of 18F-Fluoroestradiol in Assisting Individualized Treatment Decision in Dual Primary Malignancies. Quant. Imaging Med. Surg. 2021, 11, 3956–3965. [Google Scholar] [CrossRef]
- Chae, S.Y.; Son, H.J.; Lee, D.Y.; Shin, E.; Oh, J.S.; Seo, S.Y.; Baek, S.; Kim, J.Y.; Na, S.J.; Moon, D.H. Comparison of Diagnostic Sensitivity of [18F]Fluoroestradiol and [18F]Fluorodeoxyglucose Positron Emission Tomography/Computed Tomography for Breast Cancer Recurrence in Patients with a History of Estrogen Receptor-Positive Primary Breast Cancer. EJNMMI Res. 2020, 10, 54. [Google Scholar] [CrossRef] [PubMed]
- Cortés, J.; Kim, S.-B.; Chung, W.-P.; Im, S.-A.; Park, Y.H.; Hegg, R.; Kim, M.H.; Tseng, L.-M.; Petry, V.; Chung, C.-F.; et al. Trastuzumab Deruxtecan versus Trastuzumab Emtansine for Breast Cancer. N. Engl. J. Med. 2022, 386, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Larsimont, D.; Gancberg, D.; Di Leo, A.; Cardoso, F.; Rouas, G.; Pedrocchi, M.; Paesmans, M.; Verhest, A.; Bernard-Marty, C.; Piccart, M.J. Comparison of HER-2 Status between Primary Breast Cancer and Corresponding Distant Metastatic Sites. Ann. Oncol. 2002, 13, 1036–1043. [Google Scholar] [CrossRef]
- Regitnig, P.; Schippinger, W.; Lindbauer, M.; Samonigg, H.; Lax, S.F. Change of HER-2/Neu Status in a Subset of Distant Metastases from Breast Carcinomas. J. Pathol. 2004, 203, 918–926. [Google Scholar] [CrossRef]
- Bensch, F.; Brouwers, A.H.; Lub-de Hooge, M.N.; de Jong, J.R.; van der Vegt, B.; Sleijfer, S.; de Vries, E.G.E.; Schröder, C.P. 89Zr-Trastuzumab PET Supports Clinical Decision Making in Breast Cancer Patients, When HER2 Status Cannot Be Determined by Standard Work Up. Eur. J. Nucl. Med. Mol. Imaging 2018, 45, 2300–2306. [Google Scholar] [CrossRef]
- Brouwers, A.H.; van Sluis, J.; van Snick, J.H.; Schröder, C.P.; Baas, I.O.; Boellaard, R.; Glaudemans, A.W.J.M.; Borra, R.J.H.; Lammertsma, A.A.; Dierckx, R.A.J.O.; et al. First-Time Imaging of [89Zr]Trastuzumab in Breast Cancer Using a Long Axial Field-of-View PET/CT Scanner. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 3593–3595. [Google Scholar] [CrossRef]
- Wu, Y.; Li, L.; Wang, Z.; Shi, J.; Hu, Z.; Gao, S.; Miao, W.; Ma, Q.; Dong, C.; Wang, F. Imaging and Monitoring HER2 Expression in Breast Cancer during Trastuzumab Therapy with a Peptide Probe 99mTc-HYNIC-H10F. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 2613–2623. [Google Scholar] [CrossRef]
- Bragina, O.; von Witting, E.; Garousi, J.; Zelchan, R.; Sandström, M.; Orlova, A.; Medvedeva, A.; Doroshenko, A.; Vorobyeva, A.; Lindbo, S.; et al. Phase I Study of 99mTc-ADAPT6, a Scaffold Protein-Based Probe for Visualization of HER2 Expression in Breast Cancer. J. Nucl. Med. 2021, 62, 493–499. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Salem, K.; Tevaarwerk, A.J.; Strigel, R.M.; Fowler, A.M. Recent Advances in Imaging Steroid Hormone Receptors in Breast Cancer. J. Nucl. Med. 2020, 61, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Simon, M.C. The Tumor Microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef]
- Hanahan, D.; Coussens, L.M. Accessories to the Crime: Functions of Cells Recruited to the Tumor Microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; McAndrews, K.M.; Kalluri, R. Clinical and Therapeutic Relevance of Cancer-Associated Fibroblasts. Nat. Rev. Clin. Oncol. 2021, 18, 792–804. [Google Scholar] [CrossRef]
- Sugimoto, H.; Mundel, T.M.; Kieran, M.W.; Kalluri, R. Identification of Fibroblast Heterogeneity in the Tumor Microenvironment. Cancer Biol. Ther. 2006, 5, 1640–1646. [Google Scholar] [CrossRef]
- Chhabra, Y.; Weeraratna, A.T. Fibroblasts in Cancer: Unity in Heterogeneity. Cell 2023, 186, 1580–1609. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.Y.; Wei, W.F.; Wu, H.Z.; Fan, L.S.; Wang, W. Cancer-Associated Fibroblast Heterogeneity: A Factor That Cannot Be Ignored in Immune Microenvironment Remodeling. Front. Immunol. 2021, 12, 671595. [Google Scholar] [CrossRef] [PubMed]
- Musielak, M.; Piwocka, O.; Kulcenty, K.; Ampuła, K.; Adamczyk, B.; Piotrowski, I.; Fundowicz, M.; Kruszyna-Mochalska, M.; Suchorska, W.M.; Malicki, J. Biological Heterogeneity of Primary Cancer-Associated Fibroblasts Determines the Breast Cancer Microenvironment. Am. J. Cancer Res. 2022, 12, 4411–4427. [Google Scholar] [PubMed]
- Piwocka, O.; Musielak, M.; Piotrowski, I.; Kulcenty, K.; Adamczyk, B.; Fundowicz, M.; Suchorska, W.M.; Malicki, J. Primary Cancer-Associated Fibroblasts Exhibit High Heterogeneity among Breast Cancer Subtypes. Rep. Pract. Oncol. Radiother. 2023, 28, 159–171. [Google Scholar] [CrossRef]
- Hu, D.; Li, Z.; Zheng, B.; Lin, X.; Pan, Y.; Gong, P.; Zhuo, W.; Hu, Y.; Chen, C.; Chen, L.; et al. Cancer-Associated Fibroblasts in Breast Cancer: Challenges and Opportunities. Cancer Commun. 2022, 42, 401–434. [Google Scholar] [CrossRef]
- Kratochwil, C.; Flechsig, P.; Lindner, T.; Abderrahim, L.; Altmann, A.; Mier, W.; Adeberg, S.; Rathke, H.; Röhrich, M.; Winter, H.; et al. 68Ga-FAPI PET/CT: Tracer Uptake in 28 Different Kinds of Cancer. J. Nucl. Med. 2019, 60, 801–805. [Google Scholar] [CrossRef]
- Ding, F.; Huang, C.; Liang, C.; Wang, C.; Liu, J.; Tang, D. 68Ga-FAPI-04 vs. 18F-FDG in a Longitudinal Preclinical PET Imaging of Metastatic Breast Cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 49, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhao, L.; Ruan, D.; Pang, Y.; Hao, B.; Dai, Y.; Wu, X.; Guo, W.; Fan, C.; Wu, J.; et al. Usefulness of [68Ga]Ga-DOTA-FAPI-04 PET/CT in Patients Presenting with Inconclusive [18F]FDG PET/CT Findings. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 73–86. [Google Scholar] [CrossRef]
- Shang, Q.; Hao, B.; Xu, W.; Meng, T.; Pang, Y.; Sun, L.; Chen, H. 68Ga-FAPI PET/CT Detected Non-FDG-Avid Bone Metastases in Breast Cancer. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2096–2097. [Google Scholar] [CrossRef]
- Ballal, S.; Yadav, M.P.; Moon, E.S.; Kramer, V.S.; Roesch, F.; Kumari, S.; Tripathi, M.; ArunRaj, S.T.; Sarswat, S.; Bal, C. Biodistribution, Pharmacokinetics, Dosimetry of [68Ga]Ga-DOTA.SA.FAPi, and the Head-to-Head Comparison with [18F]F-FDG PET/CT in Patients with Various Cancers. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 1915–1931. [Google Scholar] [CrossRef]
- Chen, H.; Pang, Y.; Wu, J.; Zhao, L.; Hao, B.; Wu, J.; Wei, J.; Wu, S.; Zhao, L.; Luo, Z.; et al. Comparison of [68Ga]Ga-DOTA-FAPI-04 and [18F] FDG PET/CT for the Diagnosis of Primary and Metastatic Lesions in Patients with Various Types of Cancer. Eur. J. Nucl. Med. Mol. Imaging 2020, 47, 1820–1832. [Google Scholar] [CrossRef] [PubMed]
- Ballal, S.; Yadav, M.P.; Kramer, V.; Moon, E.S.; Roesch, F.; Tripathi, M.; Mallick, S.; ArunRaj, S.T.; Bal, C. A Theranostic Approach of [68Ga]Ga-DOTA.SA.FAPi PET/CT-Guided [177Lu]Lu-DOTA.SA.FAPi Radionuclide Therapy in an End-Stage Breast Cancer Patient: New Frontier in Targeted Radionuclide Therapy. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 942–944. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, Y.; Kuratsukuri, K.; Landas, S.; Imaida, K.; Rovito, P.M.; Wang, C.Y.; Haas, G.P. Expression of Prostate-Specific Membrane Antigen in Normal and Malignant Human Tissues. World J. Surg. 2006, 30, 628–636. [Google Scholar] [CrossRef] [PubMed]
- Uijen, M.J.M.; Derks, Y.H.W.; Merkx, R.I.J.; Schilham, M.G.M.; Roosen, J.; Privé, B.M.; van Lith, S.A.M.; van Herpen, C.M.L.; Gotthardt, M.; Heskamp, S.; et al. PSMA Radioligand Therapy for Solid Tumors Other than Prostate Cancer: Background, Opportunities, Challenges, and First Clinical Reports. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4350–4368. [Google Scholar] [CrossRef] [PubMed]
- Sathekge, M.; Lengana, T.; Modiselle, M.; Vorster, M.; Zeevaart, J.R.; Maes, A.; Ebenhan, T.; Van de Wiele, C. 68Ga-PSMA-HBED-CC PET Imaging in Breast Carcinoma Patients. Eur. J. Nucl. Med. Mol. Imaging 2017, 44, 689–694. [Google Scholar] [CrossRef]
- Erhamamci, S.; Aslan, N. Male Breast Cancer with Axillary Lymph Metastasis Incidentally Detected by 68Ga-PSMA PET/CT in a Patient with Prostate Cancer. Rev. Española Med. Nucl. Imagen Mol. Engl. Ed. 2021, 40, 186–187. [Google Scholar] [CrossRef]
- Sathekge, M.; Modiselle, M.; Vorster, M.; Mokgoro, N.; Nyakale, N.; Mokaleng, B.; Ebenhan, T. 68Ga-PSMA Imaging of Metastatic Breast Cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1482–1483. [Google Scholar] [CrossRef] [PubMed]
- Marafi, F.; Sasikumar, A.; Alfeeli, M.; Fathallah, W. 18F-PSMA 1007 Uptake in Brain Metastases from Breast Cancer. Clin. Nucl. Med. 2020, 45, e77–e79. [Google Scholar] [CrossRef]
- Nomura, N.; Pastorino, S.; Jiang, P.; Lambert, G.; Crawford, J.R.; Gymnopoulos, M.; Piccioni, D.; Juarez, T.; Pingle, S.C.; Makale, M.; et al. Prostate Specific Membrane Antigen (PSMA) Expression in Primary Gliomas and Breast Cancer Brain Metastases. Cancer Cell Int. 2014, 14, 26. [Google Scholar] [CrossRef]
- Ludwig, B.S.; Kessler, H.; Kossatz, S.; Reuning, U. Rgd-Binding Integrins Revisited: How Recently Discovered Functions and Novel Synthetic Ligands (Re-)Shape an Ever-Evolving Field. Cancers 2021, 13, 1711. [Google Scholar] [CrossRef]
- Ji, B.; Chen, B.; Wang, T.; Song, Y.; Chen, M.; Ji, T.; Wang, X.; Gao, S.; Ma, Q. 99mTc-3PRGD2 SPECT to Monitor Early Response to Neoadjuvant Chemotherapy in Stage II and III Breast Cancer. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 1362–1370. [Google Scholar] [CrossRef]
- Wu, J.; Tian, J.; Zhang, Y.; Ji, H.; Sun, J.; Wang, X.; Sun, C.; Wang, L.; Teng, Z.; Lu, G.; et al. 18F-Alfatide II for the Evaluation of Axillary Lymph Nodes in Breast Cancer Patients: Comparison with 18F-FDG. Eur. J. Nucl. Med. Mol. Imaging 2014, 49, 1534–1543. [Google Scholar] [CrossRef]
- Wu, J.; Wang, S.; Zhang, X.; Teng, Z.; Wang, J.; Yung, B.C.; Niu, G.; Zhu, H.; Lu, G.; Chen, X. 18F-Alfatide II PET/CT for Identification of Breast Cancer: A Preliminary Clinical Study. J. Nucl. Med. 2018, 59, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- Smyczek-Gargya, B.; Fersis, N.; Dittmann, H.; Vogel, U.; Reischl, G.; Machulla, H.J.; Wallwiener, D.; Bares, R.; Dohmen, B.M. PET with [18F]Fluorothymidine for Imaging of Primary Breast Cancer: A Pilot Study. Eur. J. Nucl. Med. Mol. Imaging 2004, 31, 720–724. [Google Scholar] [CrossRef] [PubMed]
- Crippa, F.; Agresti, R.; Sandri, M.; Mariani, G.; Padovano, B.; Alessi, A.; Bianchi, G.; Bombardieri, E.; Maugeri, I.; Rampa, M.; et al. 18F-FLT PET/CT as an Imaging Tool for Early Prediction of Pathological Response in Patients with Locally Advanced Breast Cancer Treated with Neoadjuvant Chemotherapy: A Pilot Study. Eur. J. Nucl. Med. Mol. Imaging 2015, 42, 818–830. [Google Scholar] [CrossRef] [PubMed]
- Woolf, D.K.; Beresford, M.; Li, S.P.; Dowsett, M.; Sanghera, B.; Wong, W.L.; Sonoda, L.; Detre, S.; Amin, V.; Ah-See, M.-L.; et al. Evaluation of FLT-PET-CT as an Imaging Biomarker of Proliferation in Primary Breast Cancer. Br. J. Cancer 2014, 110, 2847–2854. [Google Scholar] [CrossRef] [PubMed]
- Pio, B.S.; Park, C.K.; Pietras, R.; Hsueh, W.A.; Satyamurthy, N.; Pegram, M.D.; Czernin, J.; Phelps, M.E.; Silverman, D.H.S. Usefulness of 3′-[F-18]Fluoro-3′-Deoxythymidine with Positron Emission Tomography in Predicting Breast Cancer Response to Therapy. Mol. Imaging Biol. 2006, 8, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Daimiel, I. Insights into Hypoxia: Non-Invasive Assessment through Imaging Modalities and Its Application in Breast Cancer. J. Breast Cancer 2019, 22, 155–171. [Google Scholar] [CrossRef]
- Rajendran, J.G.; Krohn, K.A. F-18 Fluoromisonidazole for Imaging Tumor Hypoxia: Imaging the Microenvironment for Personalized Cancer Therapy. Semin. Nucl. Med. 2015, 45, 151–162. [Google Scholar] [CrossRef]
- Mokoala, K.M.G.; Lawal, I.O.; Maserumule, L.C.; Hlongwa, K.N.; Ndlovu, H.; Reed, J.; Bida, M.; Maes, A.; van de Wiele, C.; Mahapane, J.; et al. A Prospective Investigation of Tumor Hypoxia Imaging With68Ga-Nitroimidazole PET/CT in Patients with Carcinoma of the Cervix Uteri and Comparison with 18F-FDG PET/CT: Correlation with Immunohistochemistry. J. Clin. Med. 2022, 11, 962. [Google Scholar] [CrossRef]
- Cheng, J.; Lei, L.; Xu, J.; Sun, Y.; Zhang, Y.; Wang, X.; Pan, L.; Shao, Z.; Zhang, Y.; Liu, G. 18F-Fluoromisonidazole PET/CT: A Potential Tool for Predicting Primary Endocrine Therapy Resistance in Breast Cancer. J. Nucl. Med. 2013, 54, 333–340. [Google Scholar] [CrossRef]
- Asano, A.; Ueda, S.; Kuji, I.; Yamane, T.; Takeuchi, H.; Hirokawa, E.; Sugitani, I.; Shimada, H.; Hasebe, T.; Osaki, A.; et al. Intracellular Hypoxia Measured by 18F-Fluoromisonidazole Positron Emission Tomography Has Prognostic Impact in Patients with Estrogen Receptor-Positive Breast Cancer. Breast Cancer Res. 2018, 20, 78. [Google Scholar] [CrossRef] [PubMed]
- Napier, T.S.; Lynch, S.E.; Lu, Y.; Song, P.N.; Burns, A.C.; Sorace, A.G. Molecular Imaging of Oxygenation Changes during Immunotherapy in Combination with Paclitaxel in Triple Negative Breast Cancer. Biomedicines 2023, 11, 125. [Google Scholar] [CrossRef]
- Scott, N.P.; Teoh, E.J.; Flight, H.; Jones, B.E.; Niederer, J.; Mustata, L.; MacLean, G.M.; Roy, P.G.; Remoundos, D.D.; Snell, C.; et al. Characterising 18F-Fluciclovine Uptake in Breast Cancer through the Use of Dynamic PET/CT Imaging. Br. J. Cancer 2022, 126, 598–605. [Google Scholar] [CrossRef] [PubMed]
- Dalm, S.U.; Haeck, J.; Doeswijk, G.N.; De Blois, E.; De Jong, M.; Van Deurzen, C.H.M. SSTR-Mediated Imaging in Breast Cancer: Is There a Role for Radiolabeled Somatostatin Receptor Antagonists? J. Nucl. Med. 2017, 58, 1609–1614. [Google Scholar] [CrossRef] [PubMed]
- Sallmann, F.R.; Vodenicharov, M.D.; Wang, Z.Q.; Poirier, G.G. Characterization of SPARP-1. An Alternative Product of PARP-1 Gene with Poly(ADP-Ribose) Polymerase Activity Independent of DNA Strand Breaks. J. Biol. Chem. 2000, 275, 15504–15511. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Vyas, A.; Kassab, M.A.; Singh, A.K.; Yu, X. The Role of Poly ADP-Ribosylation in the First Wave of DNA Damage Response. Nucleic Acids Res. 2017, 45, 8129–8141. [Google Scholar] [CrossRef]
- Rose, M.; Burgess, J.T.; O’Byrne, K.; Richard, D.J.; Bolderson, E. PARP Inhibitors: Clinical Relevance, Mechanisms of Action and Tumor Resistance. Front. Cell Dev. Biol. 2020, 8, 564601. [Google Scholar] [CrossRef]
- Rottenberg, S.; Jaspers, J.E.; Kersbergen, A.; Van Der Burg, E.; Nygren, A.O.H.; Zander, S.A.L.; Derksen, P.W.B.; De Bruin, M.; Zevenhoven, J.; Lau, A.; et al. High Sensitivity of BRCA1-Deficient Mammary Tumors to the PARP Inhibitor AZD2281 Alone and in Combination with Platinum Drugs. Proc. Natl. Acad. Sci. USA 2008, 105, 17079–17084. [Google Scholar] [CrossRef]
- Ossovskaya, V.; Koo, I.C.; Kaldjian, E.P.; Alvares, C.; Sherman, B.M. Upregulation of Poly (ADP-Ribose) Polymerase-1 (PARP1) in Triple-Negative Breast Cancer and Other Primary Human Tumor Types. Genes Cancer 2010, 1, 812–821. [Google Scholar] [CrossRef]
- Topatana, W.; Juengpanich, S.; Li, S.; Cao, J.; Hu, J.; Lee, J.; Suliyanto, K.; Ma, D.; Zhang, B.; Chen, M.; et al. Advances in Synthetic Lethality for Cancer Therapy: Cellular Mechanism and Clinical Translation. J. Hematol. Oncol. 2020, 13, 118. [Google Scholar] [CrossRef] [PubMed]
- Helleday, T. The Underlying Mechanism for the PARP and BRCA Synthetic Lethality: Clearing up the Misunderstandings. Mol. Oncol. 2011, 5, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Won, K.A.; Spruck, C. Triple-negative Breast Cancer Therapy: Current and Future Perspectives. Int. J. Oncol. 2020, 57, 1245–1261. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, D.R.; Dougherty, B.A.; Lai, Z.; Fielding, A.; Grinsted, L.; Spencer, S.; O’connor, M.J.; Ho, T.W.; Robertson, J.D.; Lanchbury, J.S.; et al. Candidate Biomarkers of PARP Inhibitor Sensitivity in Ovarian Cancer beyond the BRCA Genes. Br. J. Cancer 2018, 119, 1401–1409. [Google Scholar] [CrossRef] [PubMed]
- Francica, P.; Rottenberg, S. Mechanisms of PARP Inhibitor Resistance in Cancer and Insights into the DNA Damage Response. Genome Med. 2018, 10, 101. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, J. Current Status and Progress in Using Radiolabelled PARP-1 Inhibitors for Imaging PARP-1 Expression in Tumours. Eur. J. Med. Chem. 2022, 242, 114690. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, W.; Li, K.; Chen, K.; He, S.; Zhang, J.; Gu, B.; Xu, X.; Song, S. PET Imaging of PARP Expression Using 68Ga-Labelled Inhibitors. Eur. J. Nucl. Med. Mol. Imaging 2023, 50, 2606–2620. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Schwarz, S.W.; Schubert, E.K.; Chen, D.L.; Doot, R.K.; Makvandi, M.; Lin, L.L.; McDonald, E.S.; Mankoff, D.A.; Mach, R.H. The Development of 18 F Fluorthanatrace: A PET Radiotracer for Imaging Poly (ADP-Ribose) Polymerase-1. Radiol. Imaging Cancer 2022, 4, e210070. [Google Scholar] [CrossRef]
- McDonald, E.S.; Doot, R.K.; Pantel, A.R.; Farwell, M.D.; Mach, R.H.; Maxwell, K.N.; Mankoff, D.A. Positron Emission Tomography Imaging of Poly-(Adenosine Diphosphate-Ribose) Polymerase 1 Expression in Breast Cancer: A Nonrandomized Clinical Trial. JAMA Oncol. 2020, 6, 921–923. [Google Scholar] [CrossRef]
- Effron, S.S.; Makvandi, M.; Lin, L.; Xu, K.; Li, S.; Lee, H.; Hou, C.; Pryma, D.A.; Koch, C.; Mach, R.H. PARP-1 Expression Quantified by [18F]FluorThanatrace: A Biomarker of Response to PARP Inhibition Adjuvant to Radiation Therapy. Cancer Biother. Radiopharm. 2017, 32, 9–15. [Google Scholar] [CrossRef]
- Laird, J.; Lok, B.H.; Carney, B.; Kossatz, S.; de Stanchina, E.; Reiner, T.; Poirier, J.T.; Rudin, C.M. Positron-Emission Tomographic Imaging of a Fluorine 18–Radiolabeled Poly(ADP-Ribose) Polymerase 1 Inhibitor Monitors the Therapeutic Efficacy of Talazoparib in SCLC Patient–Derived Xenografts. J. Thorac. Oncol. 2019, 14, 1743–1752. [Google Scholar] [CrossRef]
- Young, R.J.; França, P.D.D.S.; Pirovano, G.; Piotrowski, A.F.; Nicklin, P.J.; Riedl, C.C.; Schwartz, J.; Bale, T.A.; Donabedian, P.L.; Kossatz, S.; et al. Preclinical and First-in-Human-Brain-Cancer Applications of [18F]Poly (ADP-Ribose) Polymerase Inhibitor PET/MR. Neurooncol. Adv. 2020, 2, vdaa119. [Google Scholar] [CrossRef]
- Donabedian, P.L.; Kossatz, S.; Engelbach, J.A.; Jannetti, S.A.; Carney, B.; Young, R.J.; Weber, W.A.; Garbow, J.R.; Reiner, T. Discriminating Radiation Injury from Recurrent Tumor with [18F]PARPi and Amino Acid PET in Mouse Models. EJNMMI Res. 2018, 8, 59. [Google Scholar] [CrossRef]
- Tang, J.; Salloum, D.; Carney, B.; Brand, C.; Kossatz, S.; Sadique, A.; Lewis, J.S.; Weber, W.A.; Wendel, H.G.; Reiner, T. Targeted PET Imaging Strategy to Differentiate Malignant from Inflamed Lymph Nodes in Diffuse Large B-Cell Lymphoma. Proc. Natl. Acad. Sci. USA 2017, 114, E7441–E7449. [Google Scholar] [CrossRef] [PubMed]
- Demétrio de Souza França, P.; Roberts, S.; Kossatz, S.; Guru, N.; Mason, C.; Zanoni, D.K.; Abrahão, M.; Schöder, H.; Ganly, I.; Patel, S.G.; et al. Fluorine-18 Labeled Poly (ADP-Ribose) Polymerase1 Inhibitor as a Potential Alternative to 2-Deoxy-2-[18F]Fluoro-D-Glucose Positron Emission Tomography in Oral Cancer Imaging. Nucl. Med. Biol. 2020, 84–85, 80–87. [Google Scholar] [CrossRef] [PubMed]
- Schöder, H.M.; França, P.D.D.S.; Nakajima, R.; Burnazi, E.M.; Roberts, S.; Brand, C.; Grkovski, M.; Mauguen, A.; Dunphy, M.P.; Ghossein, R.A.; et al. Safety and Feasibility of PARP1/2 Imaging with 18F-PARPi in Patients with Head and Neck Cancer. Clin. Cancer Res. 2020, 26, 3110–3116. [Google Scholar] [CrossRef]
- Riad, A.; Gitto, S.B.; Lee, H.; Winters, H.D.; Martorano, P.M.; Hsieh, C.-J.; Xu, K.; Omran, D.K.; Powell, D.J., Jr.; Mach, R.H.; et al. PARP Theranostic Auger Emitters Are Cytotoxic in BRCA Mutant Ovarian Cancer and Viable Tumors from Ovarian Cancer Patients Enable Ex-Vivo Screening of Tumor Response. Molecules 2020, 25, 6029. [Google Scholar] [CrossRef]
- Makvandi, M.; Lee, H.; Puentes, L.N.; Reilly, S.W.; Rathi, K.S.; Weng, C.-C.; Chan, H.S.; Hou, C.; Raman, P.; Martinez, D.; et al. Targeting PARP-1 with Alpha-Particles Is Potently Cytotoxic to Human Neuroblastoma in Preclinical Models. Mol. Cancer Ther. 2019, 18, 1195–1204. [Google Scholar] [CrossRef] [PubMed]
- Sreekumar, S.; Zhou, D.; Mpoy, C.; Schenk, E.; Scott, J.; Arbeit, J.M.; Xu, J.; Rogers, B.E. Preclinical Efficacy of a PARP-1 Targeted Auger-Emitting Radionuclide in Prostate Cancer. Int. J. Mol. Sci. 2023, 24, 3083. [Google Scholar] [CrossRef]
- Jannetti, S.A.; Carlucci, G.; Carney, B.; Kossatz, S.; Shenker, L.; Carter, L.M.; Salinas, B.; Brand, C.; Sadique, A.; Donabedian, P.L.; et al. PARP-1-Targeted Radiotherapy in Mouse Models of Glioblastoma. J. Nucl. Med. 2018, 59, 1225–1233. [Google Scholar] [CrossRef]
- Sankaranarayanan, R.A.; Kossatz, S.; Weber, W.; Beheshti, M.; Morgenroth, A.; Mottaghy, F.M. Advancements in PARP1 Targeted Nuclear Imaging and Theranostic Probes. J. Clin. Med. 2020, 9, 2130. [Google Scholar] [CrossRef] [PubMed]
- Ndlovu, H.; Lawal, I.; Mokoala, K.; Disenyane, D.; Nkambule, N.; Bassa, S.; Mzizi, Y.; Bida, M.; Sathekge, M. Imaging PARP Upregulation with [ 123 I]I-PARPi SPECT/CT in Small Cell Neuroendocrine Carcinoma. J. Nucl. Med. 2023, in press. [Google Scholar] [CrossRef] [PubMed]
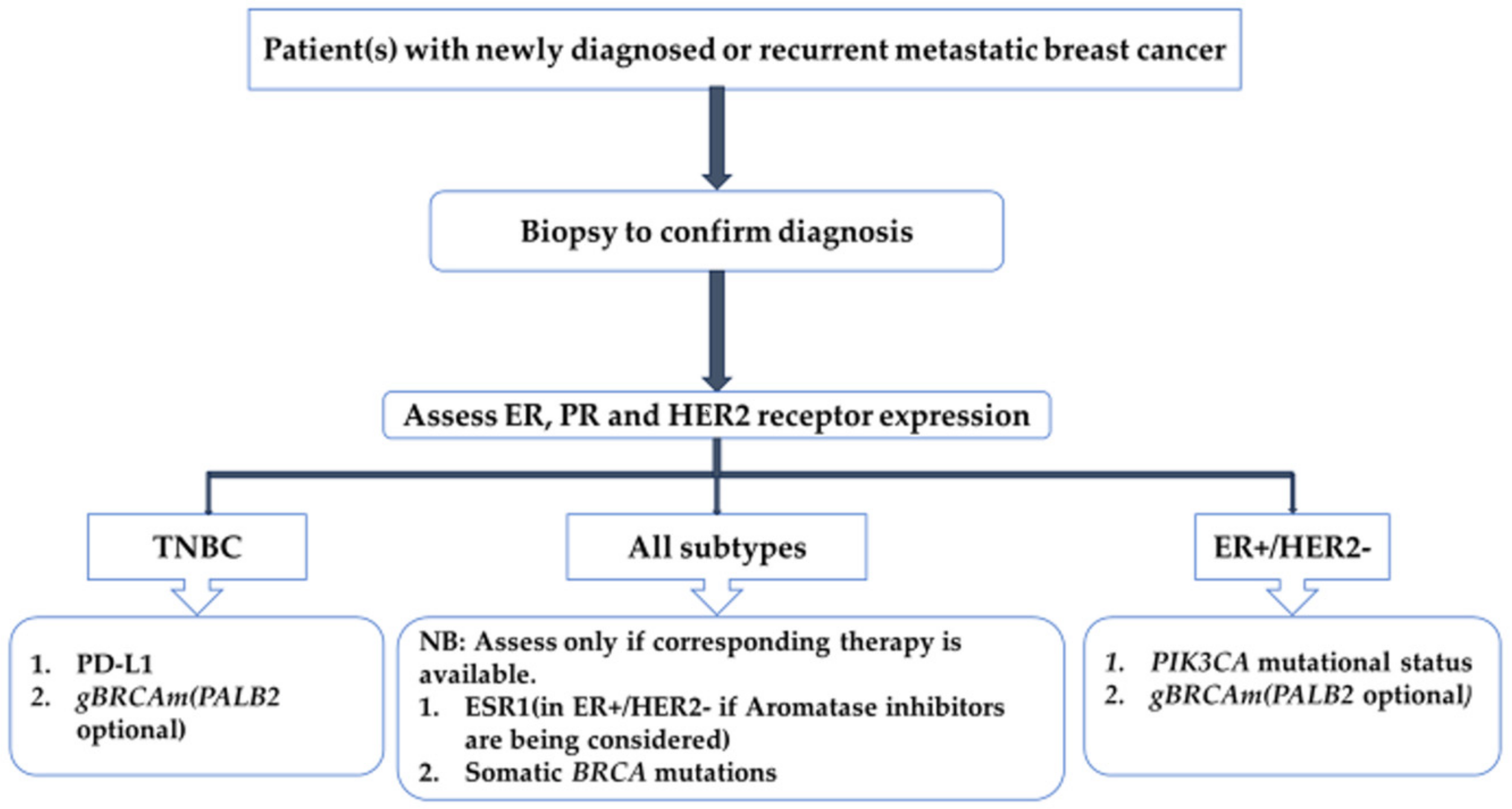


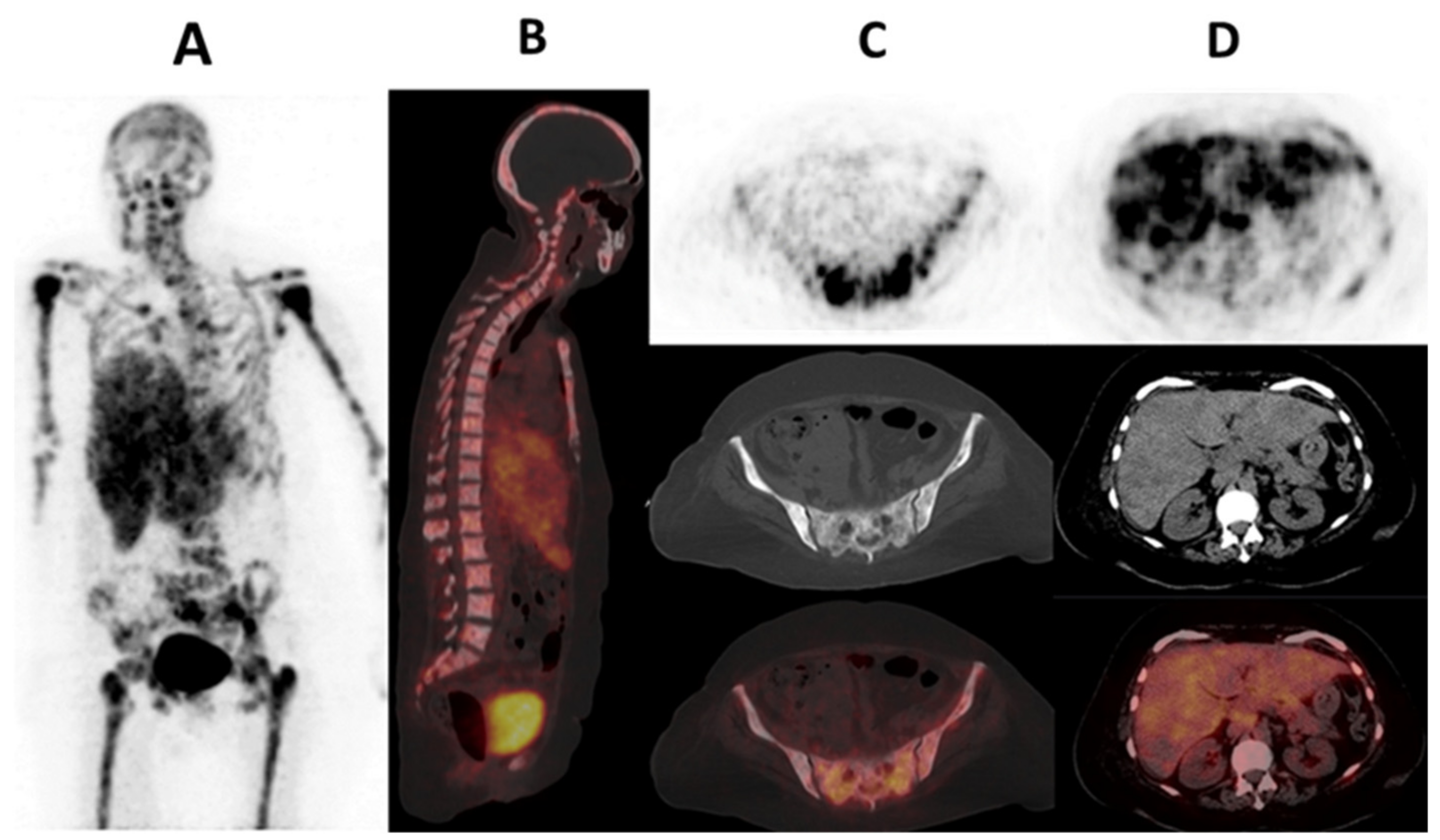
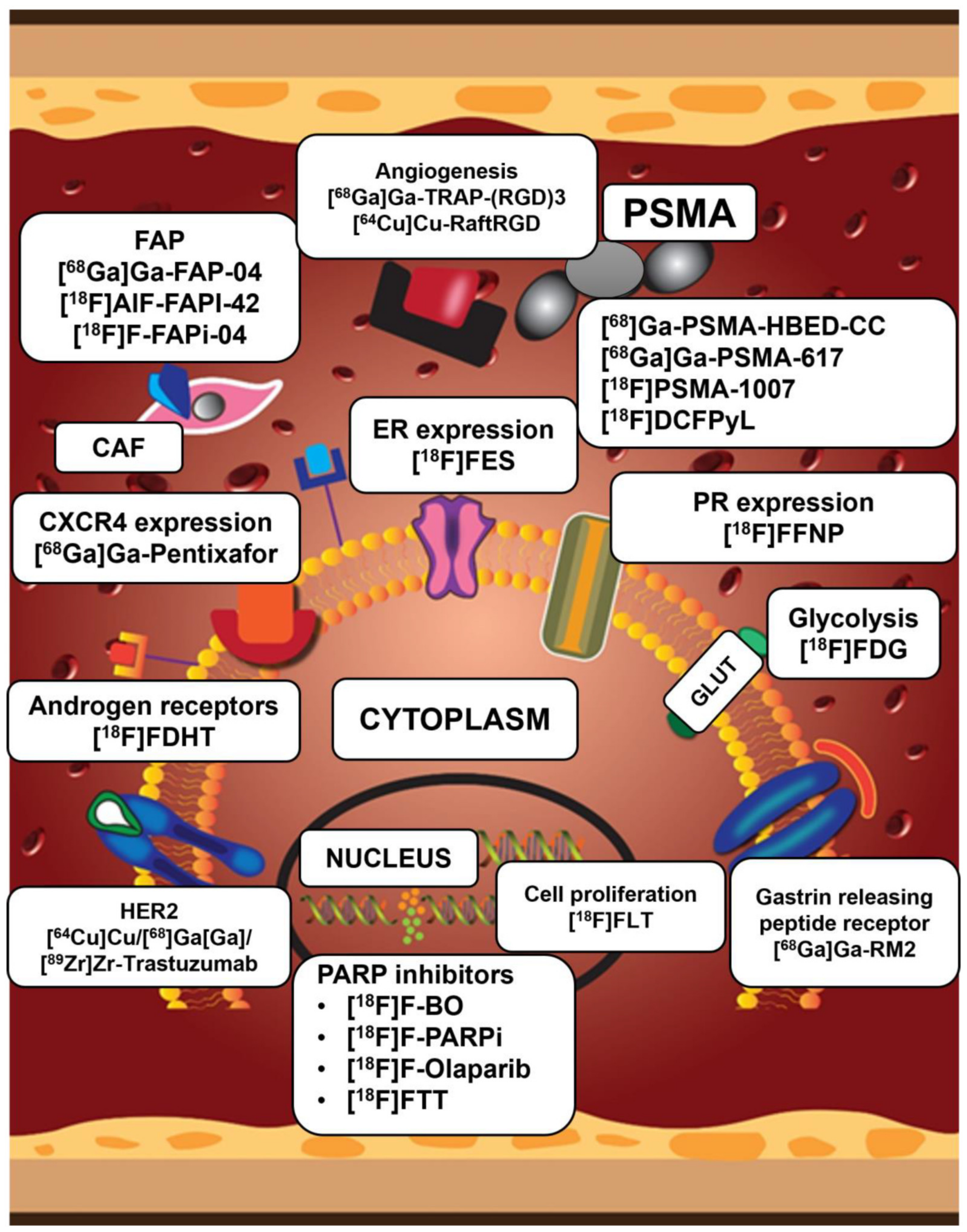
| Parameter | Definition |
|---|---|
| Standardized uptake value (SUV) | The ratio of the image-derived radioactivity concentration and the whole-body concentration of the injected activity. |
| Mean standardized uptake value (SUVmean) | Calculated by dividing mean tissue activity in the region or volume of interest by the injected activity normalized to patient body weight. |
| Maximum standard uptake value (SUVmax) | The maximum tissue activity within the region or volume of interest is divided by the injected activity normalized to the patient’s body weight. |
| Peak standardized uptake value (SUVpeak) | This is calculated as the average of the SUV within a small, fixed region of interest (ROI) centered on a high-activity part of the lesion. |
| Lean standardized uptake value (SUL) | The tissue activity divided by the lean body mass of the patient. |
| Mean tumor volume (MTV) | The volume of the lesion with non-physiological uptake of [18F]FDG. This will be summed up to obtain the total metabolic tumor volume. |
| Total lesion glycolysis (TLG) | SUVmean of a single lesion multiplied by its respective MTV. These will be summed up for all the lesions to obtain the TLG. |
| PET/SPECT Tracer | Tracer | |
|---|---|---|
| Olaparib-based molecular probes | PET | [18F]F-BO |
| [18F]F-PARPi-FL | ||
| [18F]F-PARPi | ||
| [18F]F-Olaparib | ||
| [18F]F-20 | ||
| [18F]F-9e and [18F]F-AZD2461 | ||
| [18F]FPyPARP | ||
| [68Ga]Ga-DOTA-Olaparib | ||
| [11C]C-Olaparib | ||
| [64Cu]Cu-DOTA-PARPi | ||
| SPECT | [123I]I-PARPi | |
| [131I]I-PARPi | ||
| [131I]I2-PARPi | ||
| [123I]I-MAPi | ||
| [125I]I-PARPi-01 | ||
| Rucaparib-based molecular probes | PET | [18F]FTT |
| [18F]F-WC-DZ-F | ||
| [18F]F-rucaparib | ||
| SPECT | [123/125I]I-KX1 | |
| Talazoparib-based molecular probes | PET | [18F]F-talazoparib |
| Molecular probes based on other PARP inhibitors | PET | [18F]F-SuPAR |
| SPECT | [125I]I-KX-02-019 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ndlovu, H.; Lawal, I.O.; Mokoala, K.M.G.; Sathekge, M.M. Imaging Molecular Targets and Metabolic Pathways in Breast Cancer for Improved Clinical Management: Current Practice and Future Perspectives. Int. J. Mol. Sci. 2024, 25, 1575. https://doi.org/10.3390/ijms25031575
Ndlovu H, Lawal IO, Mokoala KMG, Sathekge MM. Imaging Molecular Targets and Metabolic Pathways in Breast Cancer for Improved Clinical Management: Current Practice and Future Perspectives. International Journal of Molecular Sciences. 2024; 25(3):1575. https://doi.org/10.3390/ijms25031575
Chicago/Turabian StyleNdlovu, Honest, Ismaheel O. Lawal, Kgomotso M. G. Mokoala, and Mike M. Sathekge. 2024. "Imaging Molecular Targets and Metabolic Pathways in Breast Cancer for Improved Clinical Management: Current Practice and Future Perspectives" International Journal of Molecular Sciences 25, no. 3: 1575. https://doi.org/10.3390/ijms25031575
APA StyleNdlovu, H., Lawal, I. O., Mokoala, K. M. G., & Sathekge, M. M. (2024). Imaging Molecular Targets and Metabolic Pathways in Breast Cancer for Improved Clinical Management: Current Practice and Future Perspectives. International Journal of Molecular Sciences, 25(3), 1575. https://doi.org/10.3390/ijms25031575








