1. Introduction
The quality and yield of grape berries are greatly influenced by the adaptability of grapevines (
Vitis vinifera L.) to their environment. A crucial aspect of grape berry development is their secondary metabolism, which plays a vital role in determining the quality characteristics of both the berries and the resulting wine. Phenolic compounds, the most abundant group of secondary metabolites in grapevines, contribute significantly to these traits [
1,
2]. Beyond their impacts on the color, aroma, texture, and stability of the berries and wine, phenolic compounds also possess notable antioxidant properties. To enhance the composition of the phenolic compounds of interest, it is crucial to gain a comprehensive understanding of the intricate and interconnected pathways, such as phenylpropanoids and flavonoids. By comprehending their functioning, we can effectively modulate these pathways. In addition to external environmental factors, intrinsic molecular mechanisms, including transcription factors (TFs), play a pivotal role in regulating these pathways. TFs can act independently or in conjunction with other TFs, coordinating their efforts to achieve different metabolic outcomes based on the plant’s requirements or in response to environmental stress [
2].
A critical component of this coordinated regulatory network is the ternary MBW transcription complex, which consists of a basic helix-loop-helix (bHLH) protein, a WD repeat protein (WDR), and a MYB transcription factor [
3]. While all three components are necessary to activate anthocyanin biosynthesis, it is the MYB transcription factor that confers specificity to this complex. It determines the patterns and spatial localization of anthocyanin biosynthesis [
4]. Therefore, the MYB transcription factor plays a crucial role in regulating the production and distribution of anthocyanins. In grapevines, various transcription factors have been identified as positive regulators (activators) or repressors of specific metabolic pathways. For instance, the anthocyanin-related TFs MYBA1/A2 and the proanthocyanidin-related TFs MYBPA1/A2 function as activators [
4,
5]. Conversely, the R3-MYB and R2R3-MYB repressors play a negative regulatory role in anthocyanin synthesis. R3-MYB factors compete with activators for binding to bHLH transcription factors, preventing the formation of the MBW activation complex [
6]. The R2R3-MYB repressors also interact with bHLH factors but possess a distinctive C2 repressor motif clade and an ethylene response factor (ERF)-associated amphiphilic repression (EAR) motif, which are involved in transcriptional repression [
7]. Therefore, the regulation of anthocyanin biosynthesis involves a coordinated interplay between transcriptional activators and repressors.
In grapevines, the R2R3-MYB transcriptional repressors, including MYBC2-L1, MYBC2-L2, MYBC2-L3, and MYB4-like, have been shown to finely modulate different branches of the phenylpropanoid pathway in petunia plants. However, only MYBC2-L1 negatively impacts both anthocyanin and proanthocyanidin synthesis in grapevines [
7]. MYBC2-L1 was previously identified as a repressor of proanthocyanidin-related genes (
LAR and
ANR) by interacting with the transcriptional activator MYBPA1 to maintain a balance in proanthocyanidin content [
8]. Recent studies by Xie et al. [
9] confirmed that MYBA1 (an anthocyanin transcriptional activator) and MYBC2-L1 (a repressor) function in a coordinated manner, providing regulatory feedback for the cell-specific production of anthocyanins in grape berries, although the underlying mechanism is not yet fully understood. Additionally, studies in forage legumes suggest that MYBC2-L1 can affect anthocyanin synthesis directly by repressing structural genes (
LDOX,
DFR, or
UFGT1) or indirectly by downregulating the expression of MYBA1 or bHLH activator genes [
3]. Interestingly, despite being a repressor,
MYBC2-L1 expression has been observed to correlate with the expression of
MYBA1 and anthocyanin accumulation, indicating a complex regulatory relationship.
The regulation of the flavonoid pathway not only involves the complex network of transcription factors but also post-transcriptional regulation mediated by microRNAs and miRNA-encoded micropeptides (miPEPs) [
10]. These miPEPs enhance the transcription and accumulation of the corresponding primary miRNA, leading to an intensified downregulation of the targeted genes through a positive feedback loop [
11]. Although miPEPs are a recent discovery in plants, a few have been described in various species. For example, miPEP171b promotes lateral root development in
Medicago truncatula [
11], while the exogenous application of miPEP172c enhances nodule formation and reduces the need for nitrogen fertilization in
Glycine max [
12]. In Arabidopsis, miPEP858 modulates the expression of the target genes involved in plant growth, development, and the phenylpropanoid pathway by inducing the expression of miR858 [
13]. In grapevines, a miPEP called miPEP171d1 has been identified and described as promoting root organ development and enhancing adventitious root formation. A promising feature in addressing one of the biggest challenges in clonal propagation of
Vitis vinifera is the difficulty of the critical step of rooting [
14].
In a previous study, we demonstrated that miPEP164c negatively regulates the synthesis of proanthocyanidins and simultaneously stimulates anthocyanin synthesis by targeting the corresponding miRNA (
miR164c) and repressing the
VvMYBPA1,
VviLAR1, and
VviANR genes [
15]. The inhibition of the proanthocyanidin pathway leads to the upregulation of the anthocyanin pathway, as both pathways compete for the same substrate. This finding highlighted the potential of exogenously applied miPEPs in modulating secondary metabolic pathways and increasing the synthesis of secondary metabolites, such as anthocyanins, crucial for grape quality and possessing bioactive properties. Based on these observations, we identified miPEP166c in silico, which potentially enhances the synthesis of anthocyanins and proanthocyanidins by targeting the transcriptional repressor VviMYBC2-L1 via its corresponding miRNA,
miR166c. Therefore, in this study, we aimed to investigate whether the exogenous application of miPEP166c could increase the synthesis and accumulation of anthocyanins and proanthocyanidins by inhibiting VviMYBC2-L1.
3. Discussion
Despite being a relatively recent discovery, miPEPs have emerged as a promising avenue for plant gene regulation. Their unique ability to exhibit specificity towards their associated miRNAs makes them particularly valuable, offering a swift and transient response to adverse abiotic conditions or the modulation of specific quality-related traits in plants [
16]. This distinctive feature of miPEPs circumvents the need for laborious and costly genetic transformations in crops due to their being a relatively easy-to-implement technology in which many physiological phenotypes of agronomical interest can be achieved by simply applying a specific miPEP [
16]. Extending their influence across various plant developmental processes, miPEPs present an opportunity to enhance agronomically important characteristics. In addition to their recognized potential as a valuable agronomic tool, miPEPs can be explored in vitro within a biotechnological context. Their ease of use and relatively cost-effective modulation of secondary metabolic pathways can result in the overproduction of various bioactive compounds possessing nutraceutical characteristics. These compounds hold significant interest for diverse industries, including pharmacology and cosmetics. In the present study, we aimed to validate the application of miPEP166c in suspension-cultured grape berry cells of Gamay Fréaux to effectively modulate secondary metabolic pathways to increase the synthesis of specific metabolites of interest, notably anthocyanins and proanthocyanidins. These compounds play a pivotal role in shaping the quality of grape berries and, consequently, contribute significantly to the quality of wines [
9].
3.1. The Exogenous Application of miPEP166c Promotes the Accumulation of miR166c and Modifies the Dynamics of VviMYBC2-L1–VviMYBA1/2 Interactions in the Regulation of PAs and Anthocyanin Synthesis
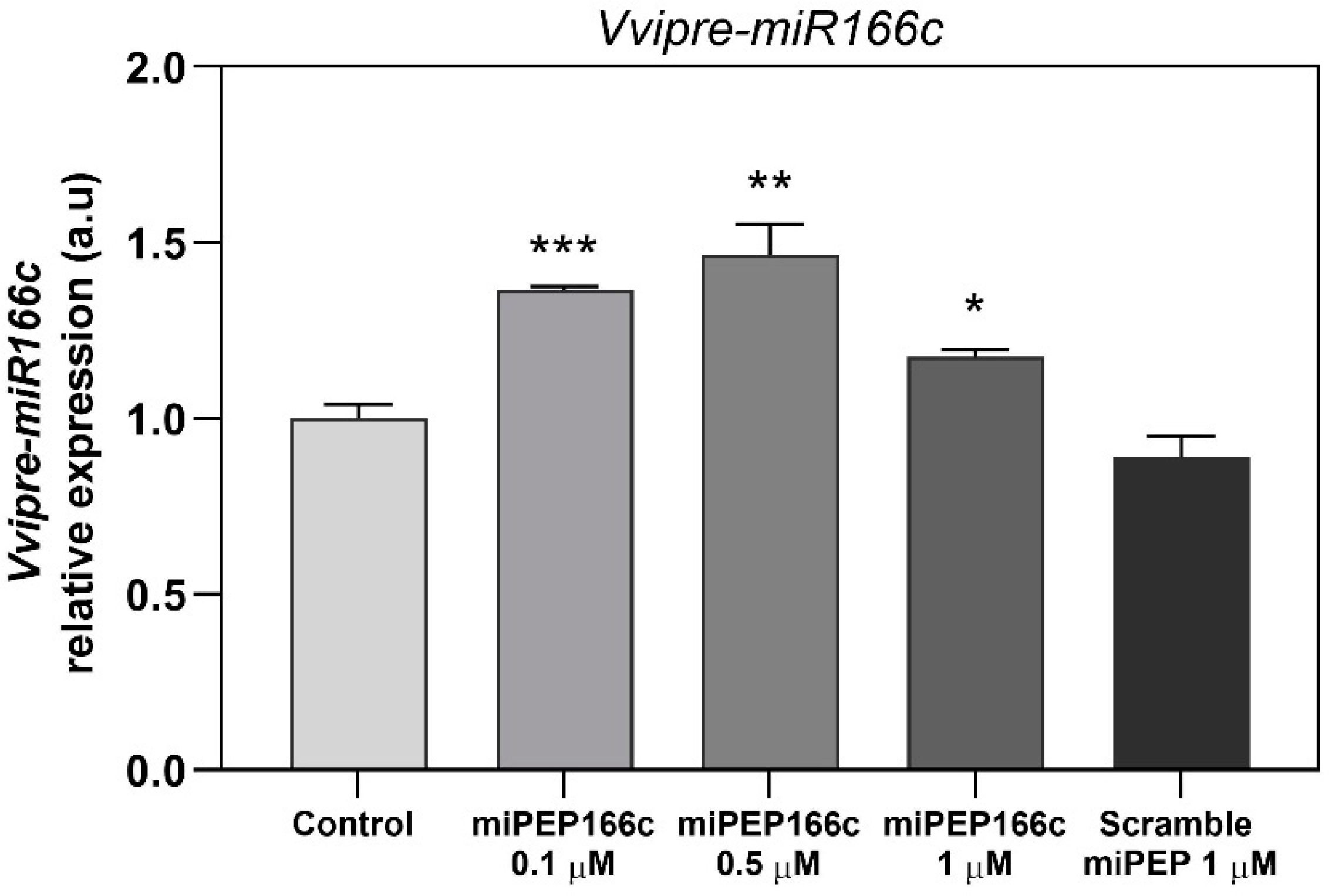
The outcomes of our study revealed that supplementing Gamay cv. grape berry cell suspensions with exogenous miPEP166c led to an augmented accumulation of its corresponding pre-miR166c. Notably, all tested concentrations of miPEP166c elicited a substantial upregulation in the abundance of pre-miR166c transcripts, representing the stem–loop form of miR166c. The most pronounced expression was observed at 0.5 µM of miPEP166c, resulting in a noteworthy 46% increase, as shown in
Figure 1.
VviMYBC2-L1 was initially characterized as a transcriptional repressor of proanthocyanidins (PAs) through its competitive interaction with the well-established transcriptional activator VviMYBPA1 [
8]. However, its involvement in the anthocyanin biosynthetic pathway was elucidated by Cavallini et al. [
7]. Unlike VviMYBC2-L2 and VviMYBC2-L3, which exhibited a strong correlation with PA-related genes primarily during the early stages of grape berry development, VviMYBC2-L1 demonstrated a unique ability to negatively regulate anthocyanin synthesis [
6,
7]. This effect was attributed to VviMYBC2-L1′s capacity to interact with other basic helix-loop-helix (bHLH) factors, competing with anthocyanin transcriptional activators for DNA-binding sites. Additionally, it directly repressed
VviUFGT1, further contributing to its role in anthocyanin downregulation [
7].
In their work with Yan73 berries, a teinturier variety, Xie et al. [
5] observed that although VviMYBC2-L1 acts as a transcriptional repressor of anthocyanins, its expression pattern correlates with that of the transcriptional activator VviMYBA1 and the accumulation of anthocyanins. Similar expression profiles were noted in other species, such as the strawberry
FaMYB112 and the peach
PpMYB18, where the expression of anthocyanin repressors peaked during the ripening stage or fruit development stages associated with anthocyanin and proanthocyanidin synthesis [
17,
18]. This apparent discrepancy in expression patterns and anthocyanin accumulation prompted the proposal of a negative feedback loop controlling anthocyanin biosynthesis: activators activate repressors, repressors repress activators, and repressors repress repressors. The conclusion drawn was that VviMYBA1 (transcriptional activator) and VviMYBC2-L1 (transcriptional repressor) coordinate to regulate Yan73 berry’s flesh color [
9]. Our findings align with this perspective, as the expression of
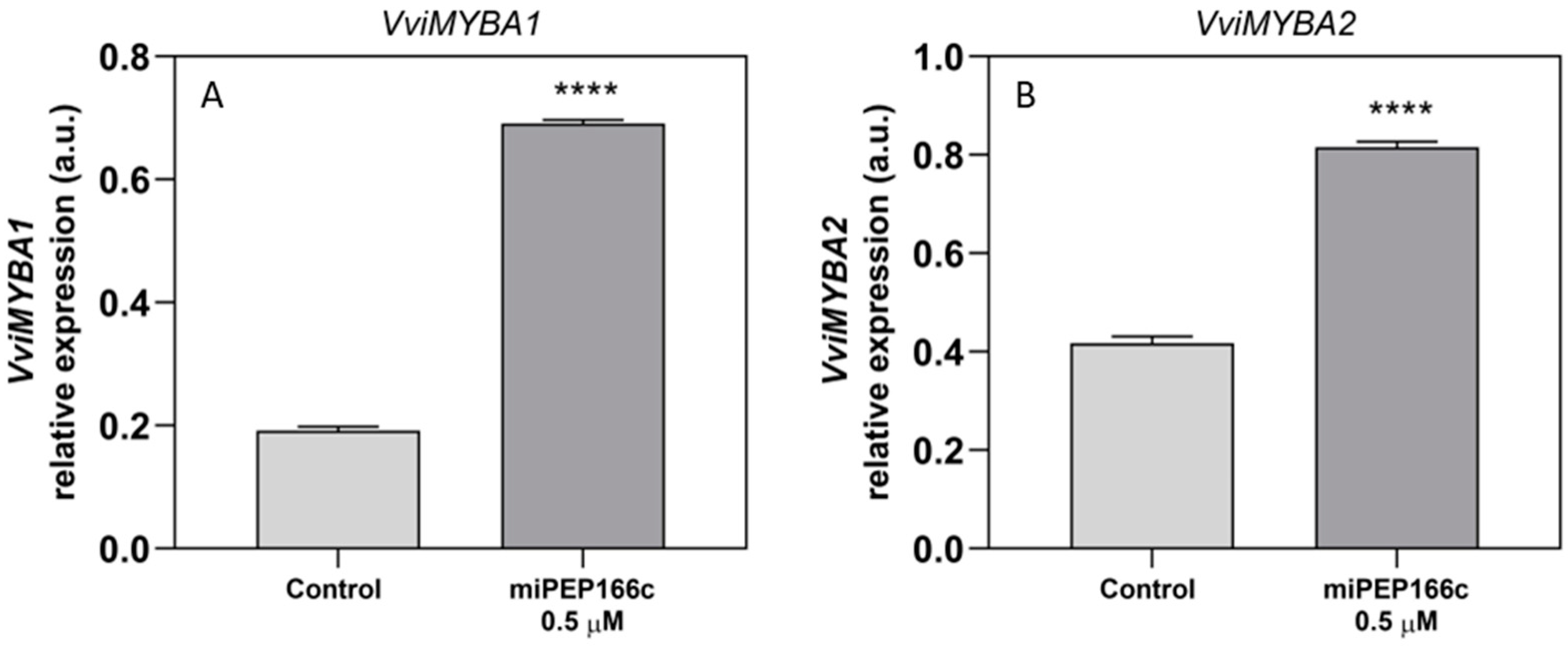
VviMYBC2-L1 did not appear to be influenced by the miPEP166c treatment. This observation is likely due to the significant upregulation of transcript levels in both anthocyanin transcriptional activators (VviMYBA1 and VviMYBA2) and the proanthocyanidin transcriptional activator (VviMYBPA1) induced by miPEP166c. This supports the proposed theory that transcriptional activators may induce the upregulation of transcriptional repressors to maintain a balance in the synthesis of anthocyanins and proanthocyanidins [
9]. Thus, a miR166c-mediated repression of
VviMYBC2-L1 was probably counterbalanced by a VviMYBA1/2-triggered increase in
VviMYBC2-L1 transcripts, and for that reason, no effect of miPEP166c on its transcript’s abundance was detected. However, the accentuated expression and availability of VvMYBA1/2 were per se sufficient to diminish the repressive action of VviMYBC2-L1 on the synthesis of proanthocyanidins and anthocyanins.
3.2. Exogenous miPEP166c Leads to Enhanced Synthesis of Anthocyanins and Proanthocyanidins in Gamay cv. Grape Berry Cells
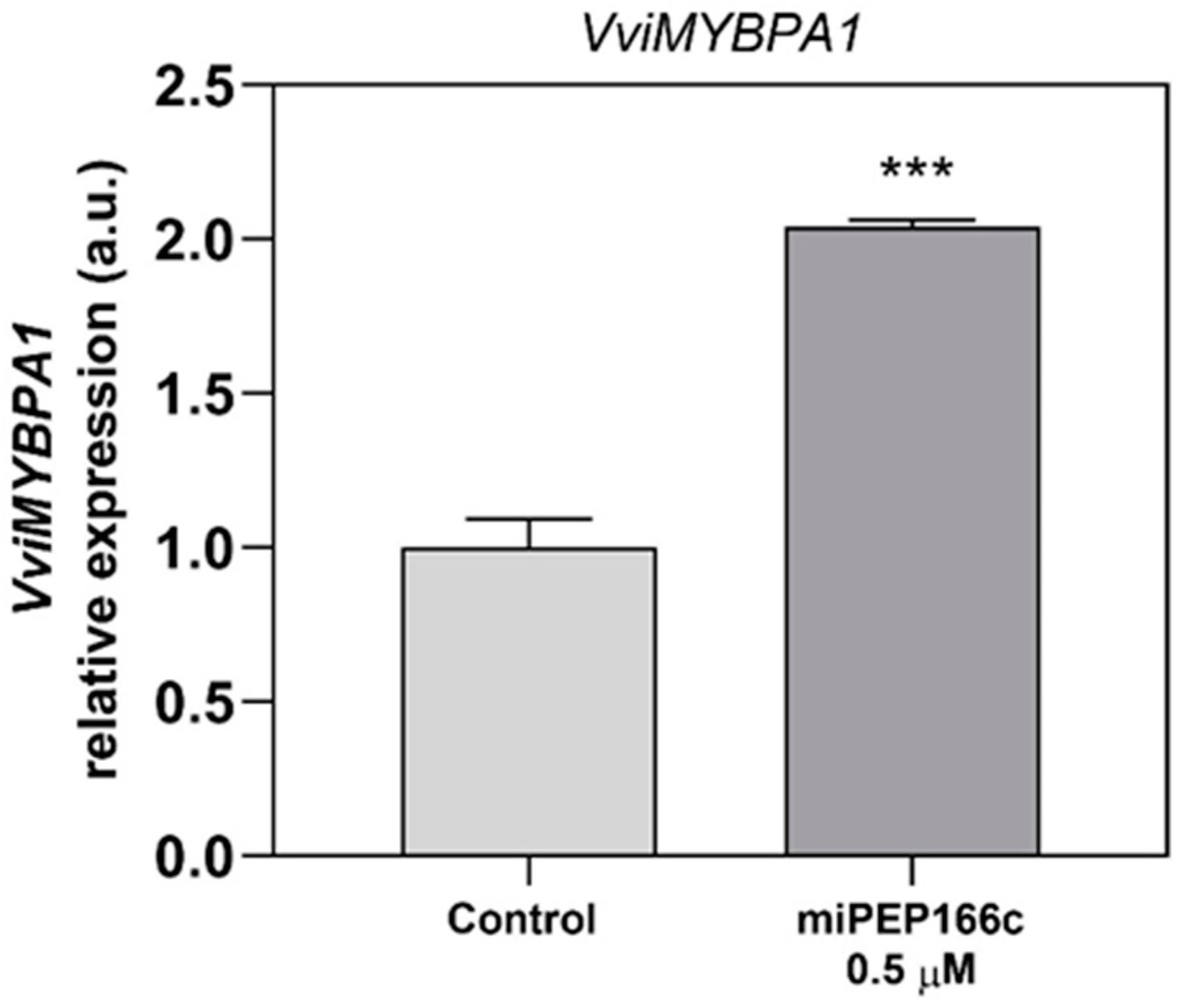
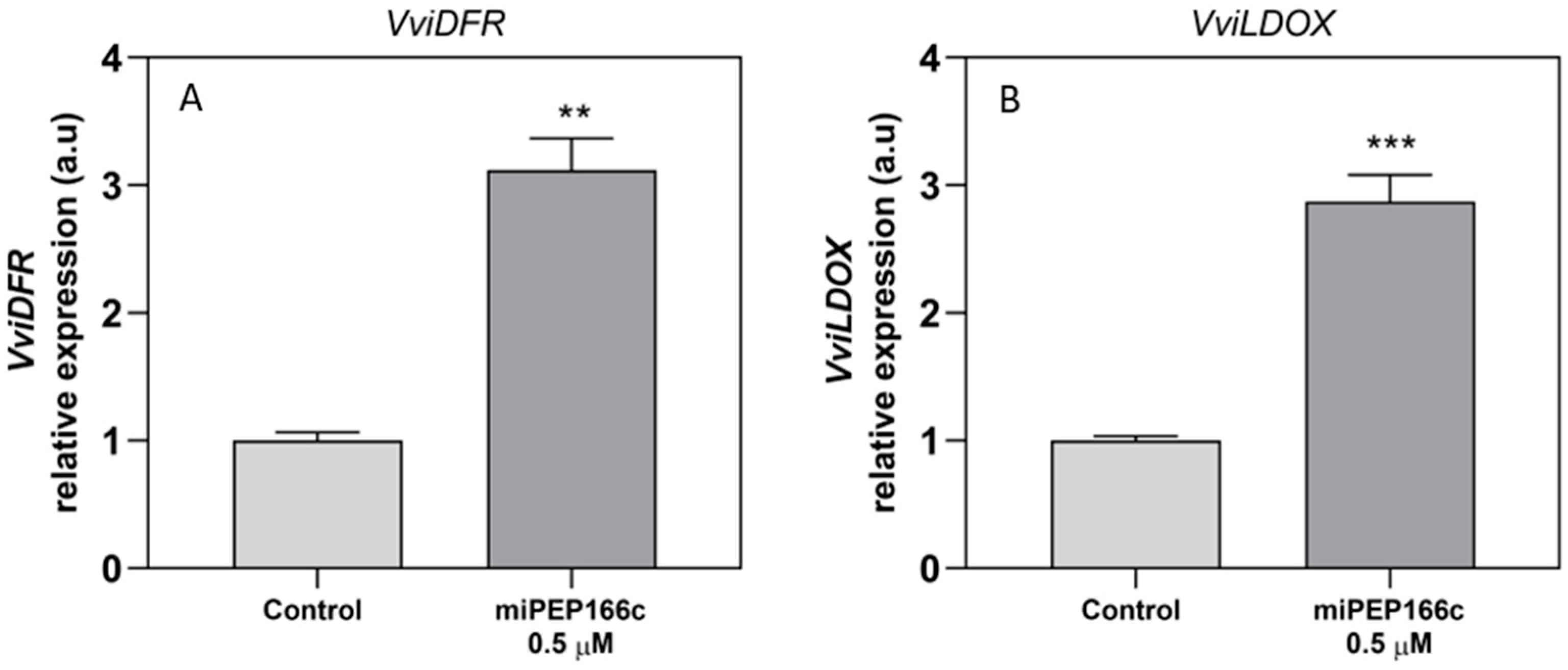
By upregulating the expression of
VviMYBPA1 (
Figure 4), the exogenous treatment with miPEP166c significantly induced the proanthocyanidin biosynthetic pathway, as observed by the transcriptional and biochemical changes in the key genes and enzymes that lead to the synthesis of flavan-3-ols (proanthocyanidin monomers). In this study, we also observed an upregulation in the transcripts of
VviDFR and
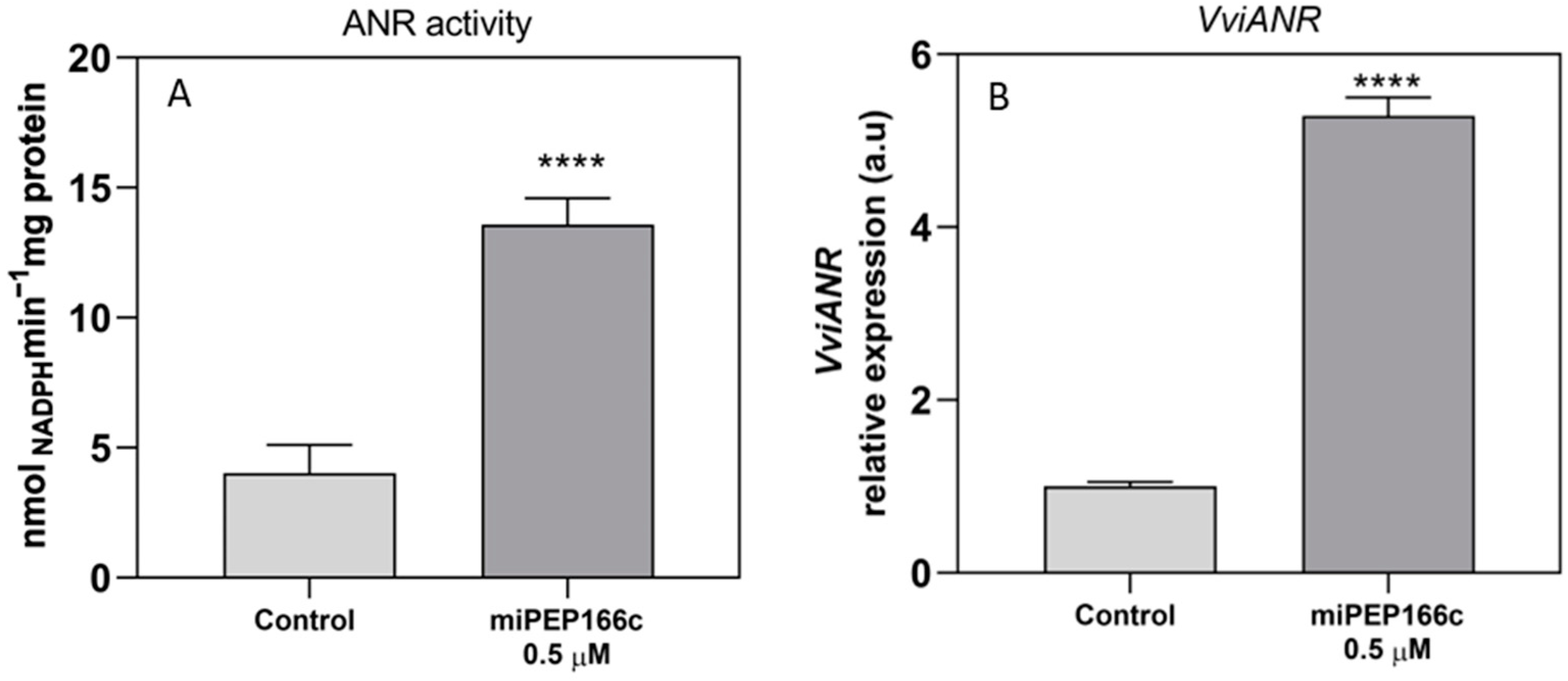
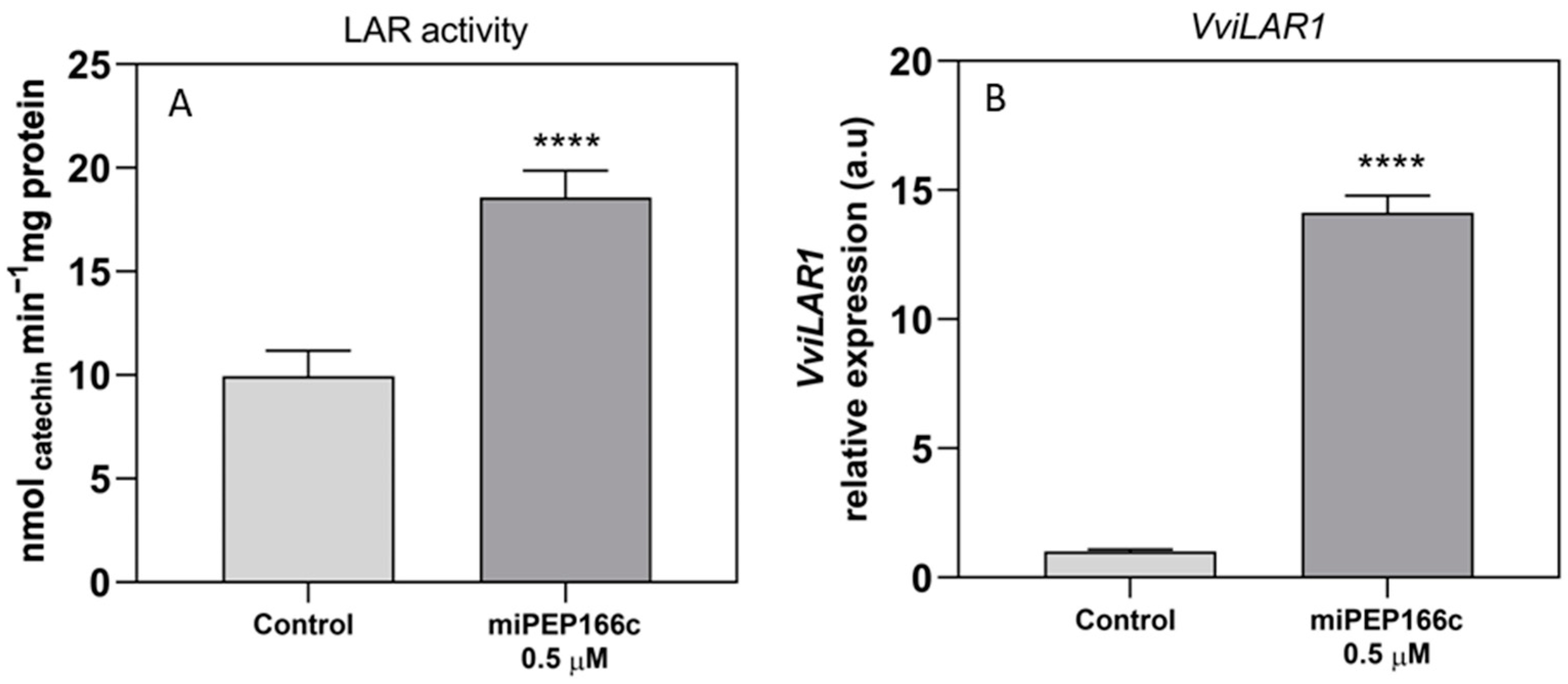
VviLDOX, which encode enzymes involved in earlier steps of the flavonoid pathway that synthesize important substrate molecules for both the anthocyanin and proanthocyanidin pathways. In accordance with the higher content of proanthocyanidins, the maximum velocities of VviLAR and VviANR activities were significantly increased by the application of miPEP166c to the cell cultures, and their respective genes were upregulated, as observed in
Figure 5 and
Figure 6.
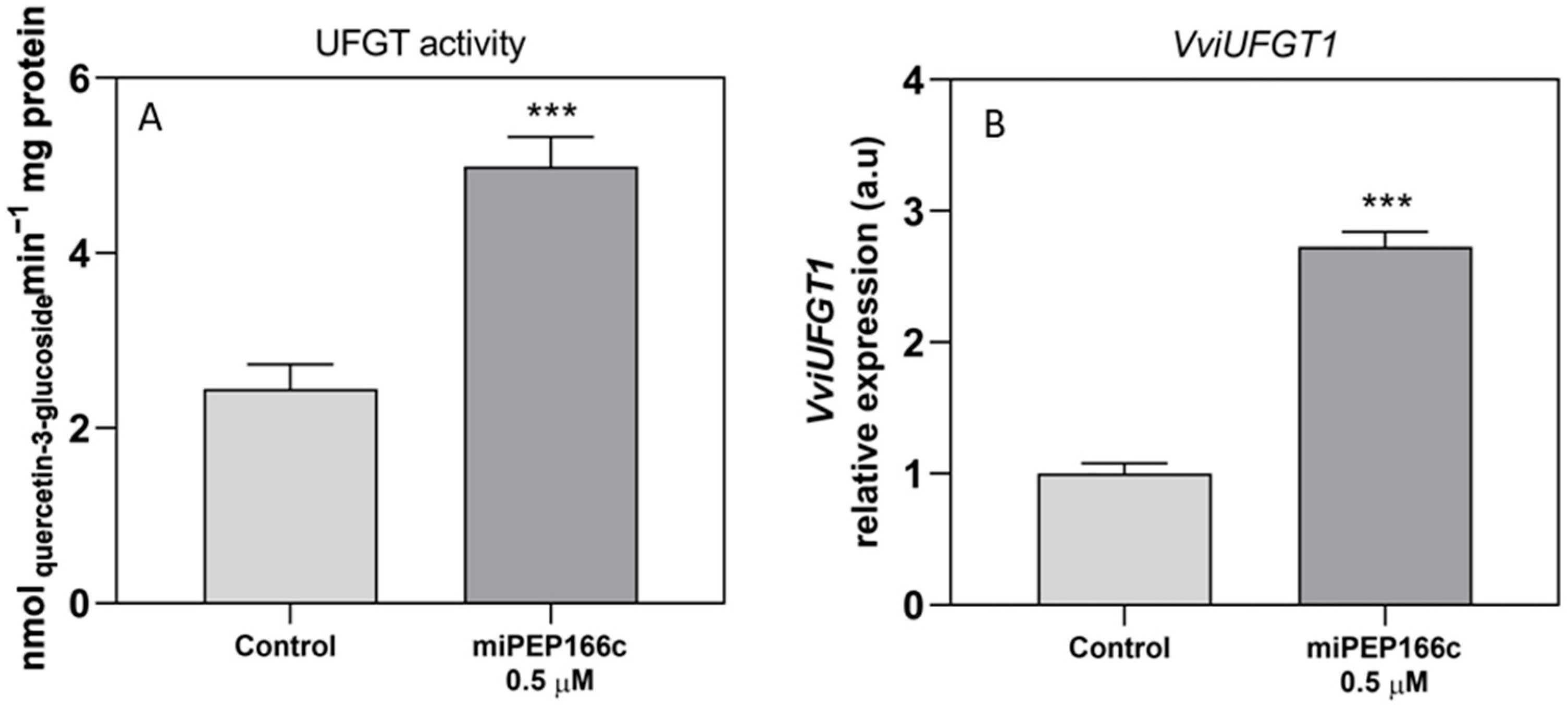
The observed increase in the content of anthocyanins in miPEP166c-treated Gamay cells, together with the abundance of transcripts of both anthocyanin transcriptional activators (VviMYBA1 and VviMYBA2), was correlated with the increase in the expression levels of key anthocyanin gene
VviUFGT1, accompanied by an increased enzyme activity of UFGT as well, as shown in
Figure 9. This evidence demonstrates that the anthocyanin-synthetic pathway was stimulated by the exogenous addition of miPEP166c. Moreover, as demonstrated in
Figure 10, anthocyanin stabilization and transport into the vacuole were also stimulated, as denoted by increased
VviGST4 and
VviMATE1 transcripts.
In sum, we have demonstrated that miPEP166c exogenous applications in Gamay Fréaux cv. grape berry cells significantly stimulated the anthocyanin and proanthocyanidin biosynthetic pathways by targeting VviMYBC2-L1 directly, diminishing its repressive action, and, for that reason, indirectly inducing the transcriptional activators VviMYBA1/2 and VviMYBPA1 in this complex regulatory network.
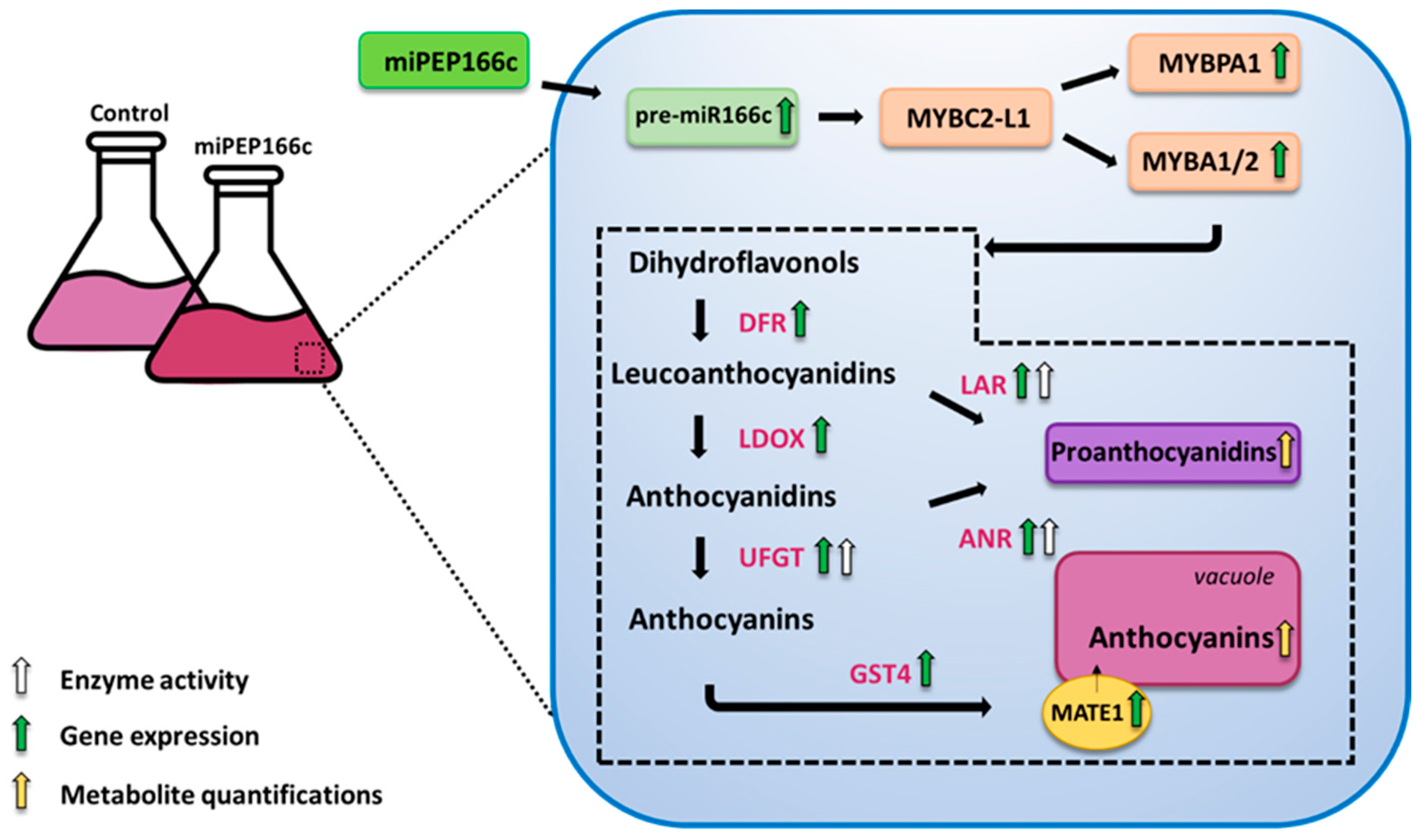
As illustrated in
Figure 11, by increasing the transcript abundance of
pre-miR166c and thus regulating the VviMYBC2-L1–VviMYBA1/2–VviMYBPA1 regulatory complex, miPEP166c enhanced the synthesis of anthocyanins and proanthocyanidins and their underlying molecular mechanisms.
4. Materials and Methods
4.1. Biological Materials
In this study, grape berry cell suspensions of Vitis vinifera L. cv. Gamay Fréaux cv. Tenturier were utilized. These cell suspensions were obtained and established through the dedifferentiation of grape berry mesocarp and were generously provided by Prof. Serge Delrot from the Institute of Vine and Wine Science (ISVV), University of Bordeaux. The grape berry cell suspensions were cultured in 250-mL flasks containing Gamborg B5 medium. The composition of the culture medium was as follows: 3 g/L Gamborg B5 salt mixture and vitamins, 30 g/L sucrose, 250 mg/L casein enzymatic hydrolysate, 0.1 mg/L α-napthaleneacetic acid (NAA), and 0.2 mg/L kinetin. The pH of the medium was maintained between 5.7 and 5.8. To provide optimal growth conditions, the flasks were placed in a controlled environment with a temperature of 23 °C. Constant agitation was applied using a rotator shaker set at 100 rpm. The cell suspensions were exposed to a 16 h light/8 h dark photoperiod, with a light intensity of 200 µmol photons m2/s. The light source wavelength was dominant at 450 nm (blue) and 620 nm (red), and the light source was a FLUORA OSRAM 36W/77 (14,000 lm) lamp. To sustain the cultures, sub-culturing was performed every 7 days, ensuring the continuous maintenance of the cell suspensions. This involved transferring a portion of the cells into fresh culture medium in new flasks to provide nutrients and maintain the growth of the cells. By following this cultivation protocol, the grape berry cell suspensions could be maintained and utilized for subsequent experiments in this study.
4.2. In Silico Analyses
To identify potential miPEPs targeting transcription factor VviMYBC2-L1, we conducted a series of in silico analyses by combining several bioinformatic tools and databases, such as the bioinformatic tool psRNATarget Finder [
19], which was used to blast the sequence of
VviMYBC2-L1 to retrieve information on which previously described grapevine miRNAs could putatively regulate it. The identified mature miRNAs were then screened in miRBase (a microRNA database) [
20] for their non-mature sequences of the regulatory miRNAs, possibly harboring small open reading frames (ORFs) corresponding to regulatory miPEPs. Finally, the obtained sequences were then run in a bioinformatic ORF finder tool that screens for putative ORFs that could translate into a small peptide by defining several parameters based on the few miPEPs identified in the literature so far [
10,
11,
12,
13,
14]. This analysis matched our gene of interest with a known grapevine miRNA, miR166c, and resulted in a putative ORF of 44 bp coding a potential miPEP for its regulation. To study whether this miPEP was active in grape berry cell suspensions, we tested its exogenous application in vitro.
4.3. Solubilization of miRNA-Encoded Peptides
Upon the in silico identification of miPEP166c, the micropeptide, along with a scrambled version, was obtained as 1 mg aliquots from Smart Bioscience (
www.sb-peptide.com, accessed on 8 July 2020). The solubilization process of these micropeptides followed the recommended guidelines provided by the Smart Bioscience Peptide Solubility Guidelines. To solubilize the micropeptides, each aliquot was dissolved in 1 mL of pure water, resulting in a final concentration of 1 mg/mL. Subsequently, the solubilized micropeptides were sterilized through filtration to ensure their purity before further use in the experiments. The specific sequences of miPEP166c and scrambled miPEP166c were as follows: miPEP166c: MLSTNKNTIIHIYR, and scrambled miPEP166c: NIILTRNMTHIYSK. These solubilized micropeptide solutions were then ready for application and further investigation in the subsequent experimental procedures.
4.4. Exogenous Application of Micropeptides to Gamay Grape Cells
Following each sub-cultivation, cell cultures received the addition of miPEP166c solubilized in pure water at concentrations of 0.1 µM, 0.5 µM, and 1 µM. The cell suspensions, which included control cells treated with an equivalent volume of the control solution as well as cells treated with the scrambled version of miPEP, underwent a 10-day cultivation period with continuous agitation and a 16 h light/8 h dark photoperiod. Subsequently, the cells were harvested, filtered using a vacuum filtration apparatus, promptly frozen using liquid nitrogen, and stored at −80 °C. Cells were then freeze-dried for 3 days to remove water content and used as a fine powder in all experiments.
4.5. Quantification of Anthocyanins
Anthocyanins were assessed using the method outlined by Conde et al. [
21]. Extraction involved utilizing 100 mg of grape berry cells from each experimental condition. Upon the addition of 1 mL of 90% methanol, the suspensions underwent vigorous shaking in an orbital shaker for 30 min at 200 rev/min, followed by centrifugation at 18,000×
g for 20 min. The resulting supernatants were collected, and 100 μL of each was combined with 900 µL of a 25 mM KCl solution (pH = 1.0). Absorbance readings at 520 nm and 700 nm were obtained using a Shimadzu UV-160A spectrophotometer (Kyoto, Japan). The quantification of total anthocyanins was determined in relation to cyanidin-3-glucoside equivalents, as follows:
where MW represents the molecular weight of cyanidin-3-glucoside (449.2 g mol
−1), DF stands for the dilution factor, and ε denotes the molar extinction coefficient of cyanidin-3-glucoside (26,900 M
−1 cm
−1). The concentration was then expressed per milligram of dry weight (DW) of the utilized cells.
4.6. Quantification of Proanthocyanidins
Proanthocyanidin levels were assessed through the DMACA assay, as refined by Wallace and Giusti [
22]. For proanthocyanidin extraction, 100 mg of frozen grape berry cells were treated with 1 mL of 90% methanol, vigorously shaken for 30 min in an orbital shaker at 200 rev/min, and then subjected to centrifugation at 18,000×
g for 15 min. The DMAC reagent (4-dimethylaminocinnamaldehyde) was freshly prepared by dissolving 2% of DMAC reagent (
w/v) in a 1:1 mixture of methanol and 6N H
2SO
4 (
v/v). This solution was kept on ice and shielded in aluminum foil to protect it from the light sensitivity inherent to DMAC. The assay mixture comprised 1.175 mL of methanol and 50 µL of DMAC (2%), and the reaction was initiated by adding 10 µL of each experimental condition. Following a 15-min reaction at room temperature under subdued light conditions, absorbance was measured at 640 nm using a Shimadzu UV-160A spectrophotometer (Kyoto, Japan). A standard curve using (+)-catechin and DMAC, with concentrations ranging from 50 to 500 µg/mL, was freshly prepared for each proanthocyanidin quantification. The results were expressed as catechin equivalents per gram of dry weight (DW).
4.7. Protein Extraction
The extraction of proteins was carried out following the procedure outlined by Badim et al. [
23]. Grape berry cell powder was combined with extraction buffer at an approximate ratio of 1:2 (
v/v) powder to buffer. The protein extraction buffer comprised 50 mM Tris-HCl (pH 8.9), 5 mM MgCl2, 1 mM EDTA, 1 mM phenylmethylsulphonyl fluoride (PMSF), 5 mM dithiothreitol (DTT), 0.1% (
v/v) Triton X-100, and 1% (
w/v) polyvinylpolypyrrolidone (PVPP). The homogenates were vortexed for 20 min and then centrifuged at 18,000×
g for 20 min at 4 °C. The resulting supernatants were kept on ice and utilized for all enzymatic assays. The total protein concentrations in the extracts were determined using the Bradford method [
24], with bovine serum albumin as the standard.
4.8. Enzymatic Activity Assays
The enzymatic activity of UDP-glucose: flavonoid 3-O glucosyltransferase (UFGT) was determined following the protocol described by Lister et al. [
25] with adaptations by Conde et al. [
21]. The assay mixture included a 300 mM Tris-HCl reaction buffer (pH 8), 200 µL of enzyme extract, and 1 mM UDP-glucose. The reaction was initiated with 1 mM quercetin as a substrate for enzyme activity (saturating concentration), reaching a final reaction volume of 500 µL. Each mixture underwent a 30-min incubation in the dark at room temperature with gentle shaking using an orbital shaker. Dilutions were prepared before and after the incubation period by combining 100 µL of each assay mixture with 900 µL of Tris-HCl reaction buffer, and absorbance was read at 350 nm immediately after (t = 0) and 30 min later (t = 30) to monitor the production of quercetin 3-glucoside (ε = 21,877 M
−1 cm
−1).
Leucoanthocyanidin reductase (LAR) enzymatic activity was assessed by spectrophotometrically tracking the conversion of dihydroquercetin into (+)-catechin following the method outlined by Gagné et al. [
26] with some modifications. The assay mixture comprised 1.7 mL of Tris-HCl buffer (0.1 M, pH 7.5), 300 µL of crude protein extract, and 2 µL of NADPH (100 mM), and the reaction was initiated by adding 1 µL of dihydroquercetin (10 mg mL
−1 in DMSO). The production of (+)-catechin (ε = 10,233 M
−1 cm
−1) was monitored at 280 nm for 30 min following the increase in absorbance of the reaction mixture.
The enzymatic activity of anthocyanidin reductase (ANR) was determined according to the protocol by Zhang et al. [
27]. The 1.5 mL assay mixture included 0.1 M PBS buffer (pH 6.5), 100 µL of enzyme extract, 1 mM ascorbic acid, and 0.07 mM cyanidin chloride. The reaction was initiated by adding 1 mM NADPH, followed by a 1/10 dilution with PBS reaction buffer for proper absorbance measurement. The enzyme activity was monitored by measuring the rate of NADPH oxidation (ε = 6.22 mM
−1 cm
−1) at 340 nm for 20 min at 45 °C. A control experiment was conducted under the same conditions without adding cyanidin chloride. All enzyme activities were measured using a Shimadzu UV-160A spectrophotometer (Kyoto, Japan).
4.9. RNA Extraction and cDNA Synthesis
RNA extraction was conducted following the methodology outlined by Reid et al. [
28], with additional purification steps incorporated from the GRS Total Plant RNA extraction kit (Grisp, Porto, Portugal). Approximately 200 mg of fresh cells treated with miPEP166c and control cells were employed for RNA extraction using a modified extraction buffer containing 2% (
w/v) CTAB, 2% (
w/v) PVP K-30, 300 mM Tris-HCl (pH 8.0), 25 mM EDTA, 2 M NaCl, and 40 mM DTT. Following in-column treatment with DNase I (Thermo Scientific
TM,, Waltham, MA, USA), cDNA was synthesized from 1 µg of total RNA using the Xpert cDNA Synthesis Master-mix Kit (Grisp, Porto, Portugal), following the manufacturer’s instructions. The concentration and purity of RNA were determined using Nanodrop, and its integrity was assessed on a 1% agarose gel stained with SYBR Safe (Invitrogen
TM, Life Technologies, Waltham, MA, USA).
4.10. Transcriptional Analyses through Real-Time qPCR
Quantitative real-time PCR was conducted utilizing Xpert Fast SYBR Blue (Grisp, Porto, Portugal) on a CFX96 Real-Time Detection System (Bio-Rad, Algés, Portugal). Each well contained 1 µL of cDNA in a final reaction volume of 10 µL. The specific primer pairs employed for each target gene are detailed in
Table S1. Melting curve analysis was executed to confirm the specificity of gene amplification.
VvACT1 (actin) and
VvGAPDH (glyceraldehyde-3-phosphate dehydrogenase) were chosen as reference genes, given their established stability and suitability for qPCR normalization in grapevine [
25]. Two independent runs with triplicates were performed for all experimental conditions tested. Expression values were normalized by the average expression of the reference genes, following the methodology described by Pfaffl [
29], and data analysis was carried out using the Bio-Rad CFX Manager software 3.1 (Bio-Rad, Algés, Portugal).
4.11. Statistical Analyses
Statistical analysis of the results was conducted using the Student’s t-test in Prism 9 (GraphPad Software, Inc., Boston, MA, USA). The data are expressed as the mean ± standard deviation of the mean (SD), and statistical comparisons were made with a 95% confidence interval. Significance levels between the mean values for each condition are indicated by asterisks based on the p-values, with * denoting p < 0.05, ** for p < 0.01, *** for p < 0.001, and **** for p < 0.0001. Each experimental approach utilized a minimum of three biological replicates and was repeated three times.