Astrocytes of the Anterior Commissure Regulate the Axon Guidance Pathways of Newly Generated Neocortical Neurons in the Opossum Monodelphis domestica
Abstract
1. Introduction
2. Results
2.1. BrdU-Labeled Cells in the Cerebral Cortex and Anterior Commissure
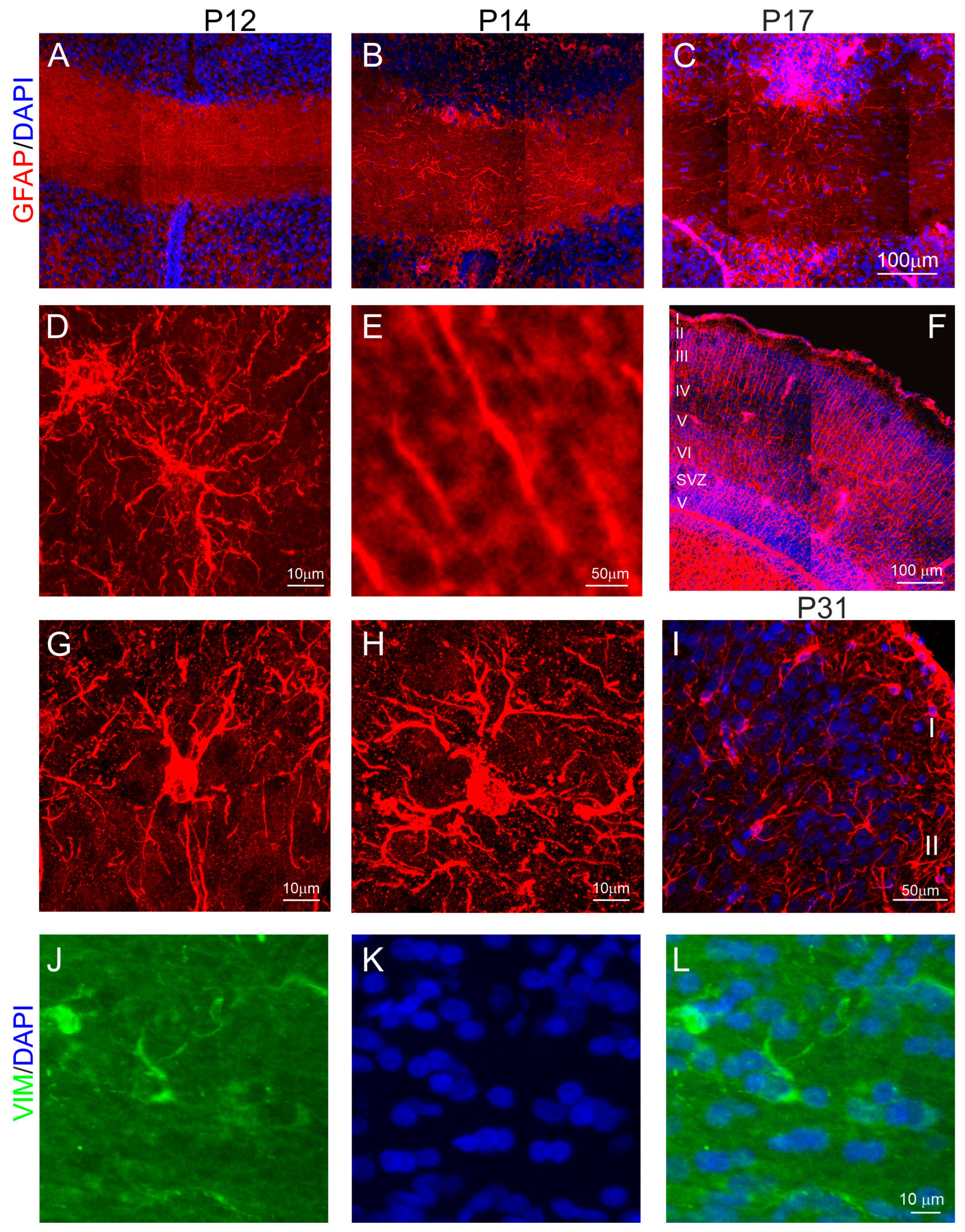
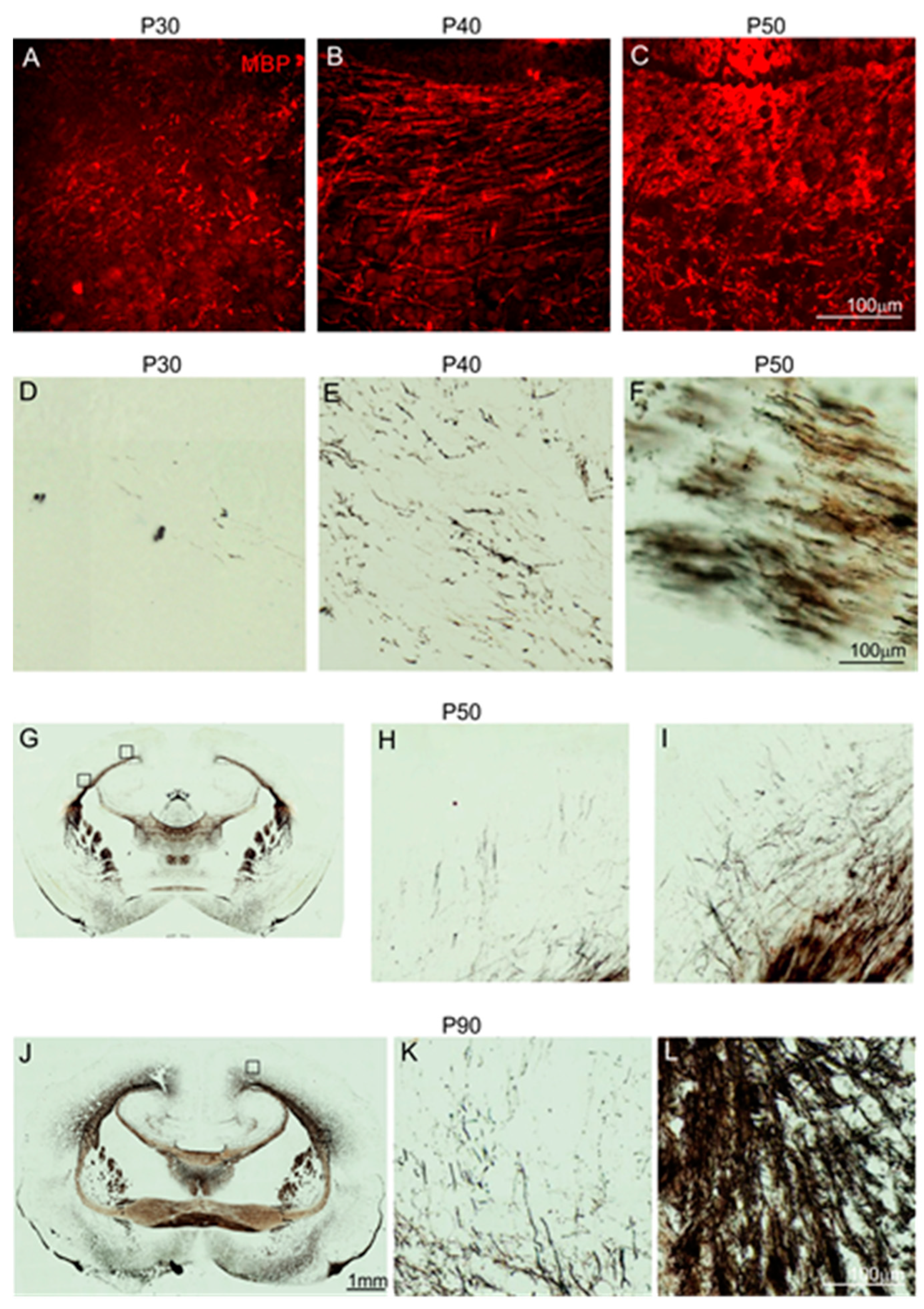
2.2. Cellular Organization of the Anterior Commissure
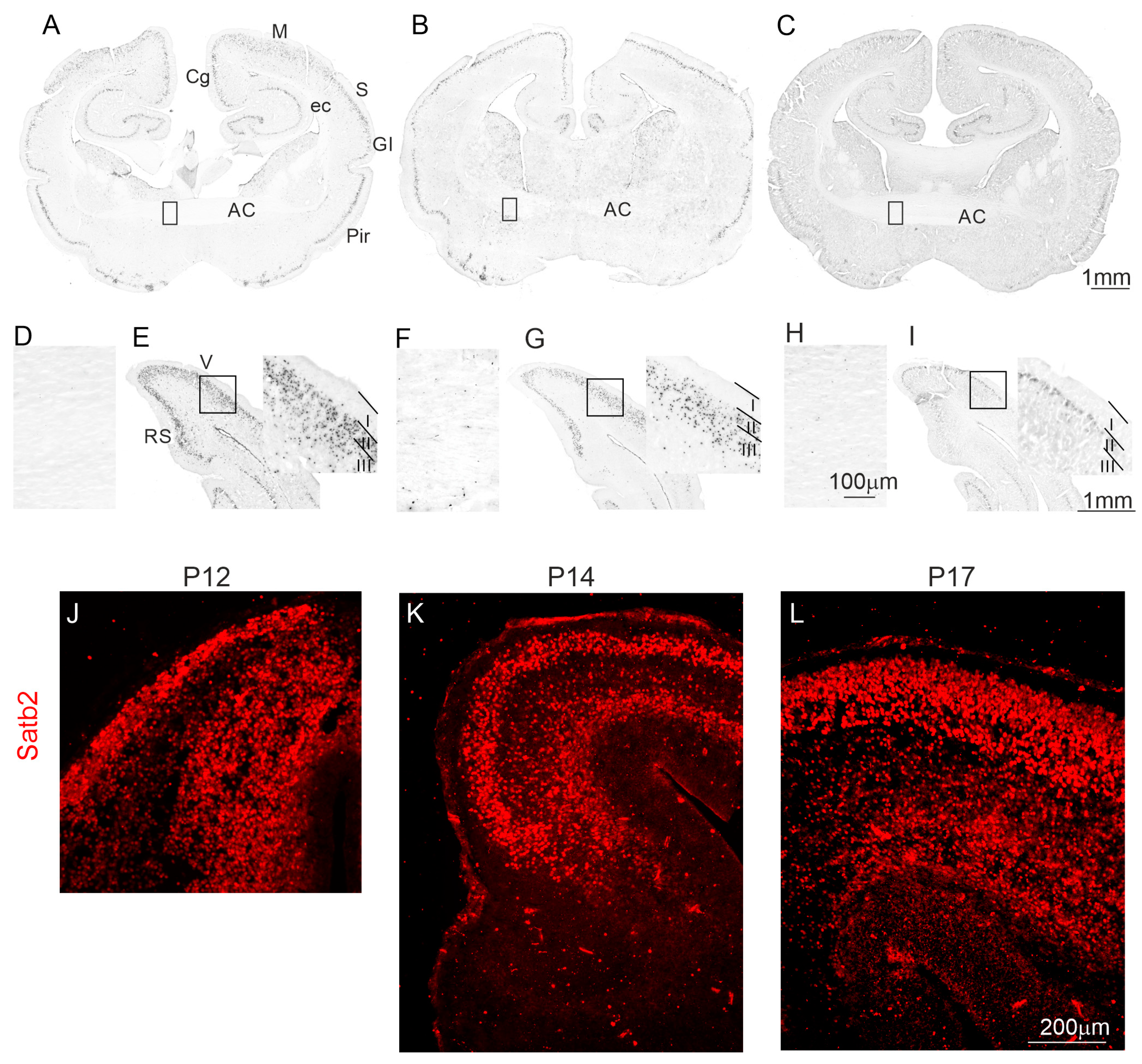
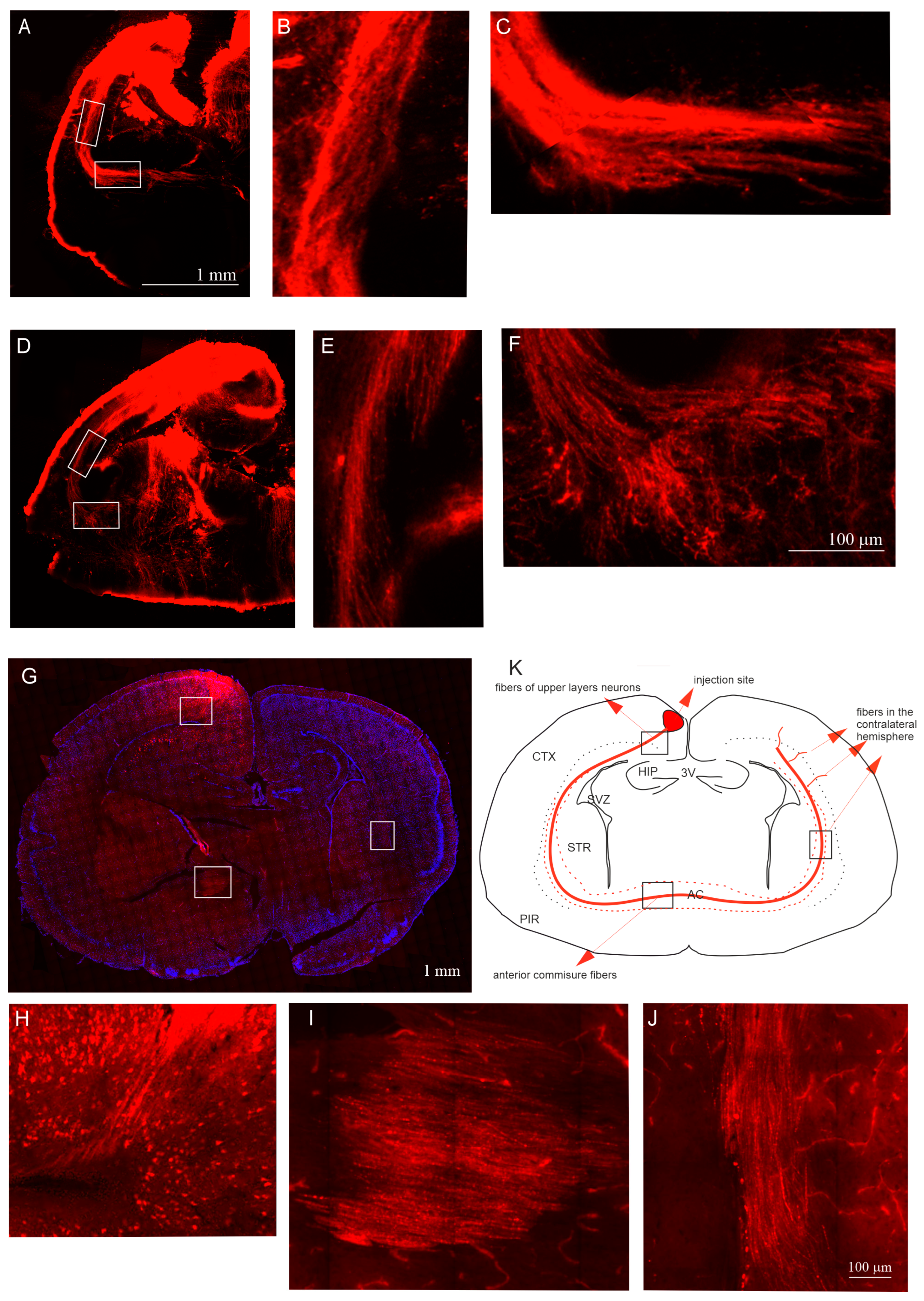
2.3. The Pattern of Neocortical Axons Forming the Anterior Commissure
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Injections of BrdU, DiI and Viruses
4.3. Brain Tissue Preparation
4.4. Myelin Staining by Gallyas Silver Impregnation
4.5. Immunofluorescent Labeling
4.6. Immunolabeling for BrdU
Double Immunolabeling
4.7. Western Blot
4.8. Data Analysis and Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, Z.X.; Yuan, C.X.; Meng, Q.J.; Ji, Q. A Jurassic eutherian mammal and divergence of marsupials and placentals. Nature 2011, 476, 442–445. [Google Scholar] [CrossRef]
- Rose, R.W. Embryonic growth rates of marsupials with a note on monotremes. J. Zool. Lond. 1989, 218, 11–16. [Google Scholar] [CrossRef]
- Bartkowska, K.; Tepper, B.; Turlejski, K.; Djavadian, R. Postnatal and Adult Neurogenesis in Mammals, Including Marsupials. Cells 2022, 11, 2735–2741. [Google Scholar] [CrossRef]
- Saunders, N.R.; Adam, E.; Reader, M.; Møllgrd, K. Monodelphis domestica (grey short-tailed opossum): An accessible model for studies of early neocortical development. Anat. Embryol. 1989, 180, 227–236. [Google Scholar] [CrossRef]
- Ashwell, K.W.S. The Neurobiology of Australian Marsupials. In Brain Evolution in the Other Mammalian Radiation; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2010; pp. 18–119. [Google Scholar]
- Bartkowska, K.; Gajerska, M.; Turlejski, K.; Djavadian, R.L. Expression of TrkC receptors in the developing brain of the Monodelphis opossum and its effect on the development of cortical cells. PLoS ONE 2013, 8, e74346. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, M.L.; Cavanagh, M.E.; Dziegielewska, K.M.; Hinds, L.A.; Saunders, N.R. Tyndale-Biscoe CH. Postnatal development of the telencephalon of the tammar wallaby (Macropus eugenii). An accessible model of neocortical differentiation. Anat. Embryol. 1985, 173, 81–94. [Google Scholar] [CrossRef]
- Fenlon, L.R.; Suarez, R.; Lynton, Z.; Richards, L.J. The evolution, formation and connectivity of the anterior commissure. Semin. Cell Dev. Biol. 2021, 118, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Donahoo, A.L.; Richards, L.J. Understanding the mechanisms of callosal development through the use of transgenic mouse models. Semin. Pediatr. Neurol. 2009, 16, 127–142. [Google Scholar] [CrossRef] [PubMed]
- Henkemeyer, M.; Orioli, D.; Henderson, J.T.; Saxton, T.M.; Roder, J.; Pawson, T.; Klein, R. Nuk controls pathfinding of commissural axons in the mammalian central nervous system. Cell 1996, 86, 35–46. [Google Scholar] [CrossRef]
- Hu, Z.; Yue, X.; Shi, G.; Yue, Y.; Crockett, D.P.; Blair-Flynn, J.; Reuhl, K.; Tessarollo, L.; Zhou, R. Corpus callosum deficiency in transgenic mice expressing a truncated ephrin-A receptor. J. Neurosci. 2003, 23, 10963–10970. [Google Scholar] [CrossRef]
- Srivatsa, S.; Parthasarathy, S.; Britanova, O.; Bormuth, I.; Donahoo, A.L.; Ackerman, S.L.; Richards, L.J.; Tarabykin, V. Unc5C and DCC act downstream of Ctip2 and Satb2 and contribute to corpus callosum formation. Nat. Commun. 2014, 5, 3708. [Google Scholar] [CrossRef]
- Bagri, A.; Marín, O.; Plump, A.S.; Mak, J.; Pleasure, S.J.; Rubenstein, J.L.; Tessier-Lavigne, M. Slit proteins prevent midline crossing and determine the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron 2002, 33, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Piper, M.; Plachez, C.; Zalucki, O.; Fothergill, T.; Goudreau, G.; Erzurumlu, R.; Gu, C.; Richards, L.J. Neuropilin 1-Sema signaling regulates crossing of cingulate pioneering axons during development of the corpus callosum. Cereb. Cortex 2009, 19 (Suppl. S1), i11–i21. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.F.; Kondo, S.; Abdel-Mannan, O.; Chodroff, R.A.; Sirey, T.M.; Bluy, L.E.; Webber, N.; DeProto, J.; Karlen, S.J.; Krubitzer, L.; et al. The subventricular zone is the developmental milestone of a 6-layered neocortex: Comparisons in metatherian and eutherian mammals. Cereb. Cortex 2010, 20, 1071–1081. [Google Scholar] [CrossRef] [PubMed]
- Puzzolo, E.; Mallamaci, A. Cortico-cerebral histogenesis in the opossum Monodelphis domestica: Generation of a hexalaminar neocortex in the absence of a basal proliferative compartment. Neural. Dev. 2010, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Bartkowska, K.; Tepper, B.; Gawda, A.; Jarosik, M.; Sobolewska, P.; Turlejski, K.; Djavadian, R.L. Inhibition of TrkB- and TrkC-Signaling Pathways Affects Neurogenesis in the Opossum Developing Neocortex. Cereb. Cortex 2019, 29, 3666–3675. [Google Scholar] [CrossRef]
- Kushwaha, R.S.; VandeBerg, J.F.; VandeBerg, J.L. Effect of dietary cholesterol with or without saturated fat on plasma lipoprotein cholesterol levels in the laboratory opossum (Monodelphis domestica) model for diet-induced hyperlipidaemia. Br. J. Nutr. 2004, 92, 63–70. [Google Scholar] [CrossRef][Green Version]
- Kolb, B.; Pedersen, B.; Ballermann, M.; Gibb, R.; Whishaw, I.Q. Embryonic and postnatal injections of bromodeoxyuridine produce age-dependent morphological and behavioral abnormalities. J. Neurosci. 1999, 19, 2337–2346. [Google Scholar] [CrossRef]
- Kuwagata, M.; Ogawa, T.; Nagata, T.; Shioda, S. The evaluation of early embryonic neurogenesis after exposure to the genotoxic agent 5-bromo-2′-deoxyuridine in mice. Neurotoxicology 2007, 28, 780–789. [Google Scholar] [CrossRef]
- Turovsky, E.A.; Turovskaya, M.V.; Fedotova, E.I.; Babaev, A.A.; Tarabykin, V.S.; Varlamova, E.G. Role of Satb1 and Satb2 Transcription Factors in the Glutamate Receptors Expression and Ca2+ Signaling in the Cortical Neurons In Vitro. Int. J. Mol. Sci. 2021, 22, 5968. [Google Scholar] [CrossRef]
- Nomura, T.; Yamashita, W.; Gotoh, H.; Ono, K. Species-Specific Mechanisms of Neuron Subtype Specification Reveal Evolutionary Plasticity of Amniote Brain Development. Cell Rep. 2018, 22, 3142–3151. [Google Scholar] [CrossRef] [PubMed]
- Britanova, O.; Akopov, S.; Lukyanov, S.; Gruss, P.; Tarabykin, V. Novel transcription factor Satb2 interacts with matrix attachment region DNA elements in a tissue-specific manner and demonstrates cell-type-dependent expression in the developing mouse CNS. Eur. J. Neurosci. 2005, 21, 658–668. [Google Scholar] [CrossRef] [PubMed]
- Szemes, M.; Gyorgy, A.; Paweletz, C.; Dobi, A.; Agoston, D.V. Isolation and characterization of SATB2, a novel AT-rich DNA binding protein expressed in development- and cell-specific manner in the rat brain. Neurochem. Res. 2006, 31, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Kohno, T.; Ishii, K.; Hirota, Y. Reelin-Nrp1 Interaction Regulates Neocortical Dendrite Development in a Context-Specific Manner. J. Neurosci. 2020, 40, 8248–8261. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wen, Y.; She, L.; Sui, Y.N.; Liu, L.; Richards, L.J.; Poo, M.M. Axon position within the corpus callosum determines contralateral cortical projection. Proc. Natl. Acad. Sci. USA 2013, 110, E2714–E2723. [Google Scholar] [CrossRef]
- Noctor, S.C.; Flint, A.C.; Weissman, T.A.; Dammerman, R.S.; Kriegstein, A.R. Neurons derived from radial glial cells establish radial units in neocortex. Nature 2001, 409, 714–720. [Google Scholar] [CrossRef] [PubMed]
- Rakic, P. Evolution of the neocortex: A perspective from developmental biology. Nat. Rev. Neurosci. 2009, 10, 724–735. [Google Scholar] [CrossRef]
- Clavreul, S.; Abdeladim, L.; Hernández-Garzón, E. Cortical astrocytes develop in a plastic manner at both clonal and cellular levels. Nat. Commun. 2019, 10, 4884. [Google Scholar] [CrossRef]
- Tabata, H. Diverse subtypes of astrocytes and their development during corticogenesis. Front. Neurosci. 2015, 9, 114. [Google Scholar] [CrossRef]
- Polleux, F.; Dehay, C.; Kennedy, H. The timetable of laminar neurogenesis contributes to the specification of cortical areas in mouse isocortex. J. Comp. Neurol. 1997, 385, 95–116. [Google Scholar] [CrossRef]
- Heath, C.J.; Jones, E.G. Interhemispheric pathways in the absence of a corpus callosum. An experimental study of commissural connections in the marsupial phalanger. J. Anat. 1971, 109, 253–270. [Google Scholar]
- Cummings, D.M.; Malun, D.; Brunjes, P.C. Development of the anterior commissure in the opossum: Midline extracellular space and glia coincide with early axon decussation. J. Neurobiol. 1997, 32, 403–414. [Google Scholar] [CrossRef]
- Sansom, S.N.; Livesey, F.J. Gradients in the brain: The control of the development of form and function in the cerebral cortex. Cold Spring Harb. Perspect. Biol. 2009, 1, a002519. [Google Scholar] [CrossRef] [PubMed]
- Cabana, T.; Martin, G.F. The development of commissural connections of somatic motor-sensory areas of neocortex in the North American opossum. Anat. Embryol. 1985, 171, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Granger, E.M.; Masterton, R.B.; Glendenning, K.K. Origin of interhemispheric fibers in acallosal opossum (with a comparison to callosal origins in rat). J. Comp. Neurol. 1985, 241, 82–98. [Google Scholar] [CrossRef] [PubMed]
- Aboitiz, F.; Montiel, J. One hundred million years of interhemispheric communication: The history of the corpus callosum. Braz. J. Med. Biol. Res. 2003, 36, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Land, P.W.; Monaghan, A.P. Expression of the transcription factor, tailless, is required for formation of superficial cortical layers. Cereb. Cortex 2003, 13, 921–931. [Google Scholar] [CrossRef] [PubMed]
- Gyorgy, A.B.; Szemes, M.; de Juan Romero, C.; Tarabykin, V.; Agoston, D.V. SATB2 interacts with chromatin-remodeling molecules in differentiating cortical neurons. Eur. J. Neurosci. 2008, 27, 865–873. [Google Scholar] [CrossRef] [PubMed]
- Baranek, C.; Dittrich, M.; Parthasarathy, S.; Bonnon, C.G.; Britanova, O.; Lanshakov, D.; Boukhtouche, F.; Sommer, J.E.; Colmenares, C.; Tarabykin, V.; et al. Protooncogene Ski cooperates with the chromatin-remodeling factor Satb2 in specifying callosal neurons. Proc. Natl. Acad. Sci. USA 2012, 109, 3546–3551. [Google Scholar] [CrossRef]
- Silver, J.; Lorenz, S.E.; Wahlsten, D.; Coughlin, J. Axonal guidance during development of the great cerebral commissures: Descriptive and experimental studies, in vivo, on the role of preformed glial pathways. J. Comp. Neurol. 1982, 210, 10–29. [Google Scholar] [CrossRef]
- Rash, B.G.; Richards, L.J. A role for cingulate pioneering axons in the development of the corpus callosum. J. Comp. Neurol. 2001, 434, 147–157. [Google Scholar] [CrossRef]
- Alcamo, E.A.; Chirivella, L.; Dautzenberg, M.; Dobreva, G.; Fariñas, I.; Grosschedl, R.; McConnell, S.K. Satb2 regulates callosal projection neuron identity in the developing cerebral cortex. Neuron 2008, 57, 364–377. [Google Scholar] [CrossRef]
- Valentino, K.L.; Jones, E.G. The early formation of the corpus callosum: A light and electron microscopic study in foetal and neonatal rats. J. Neurocytol. 1982, 11, 583–609. [Google Scholar] [CrossRef] [PubMed]
- Santacana, M.; Heredia, M.; Valverde, F. Development of the main efferent cells of the olfactory bulb and of the bulbar component of the anterior commissure. Brain Res. Dev. Brain Res. 1992, 65, 75–83. [Google Scholar] [CrossRef][Green Version]
- Berbel, P.; Innocenti, G.M. The development of the corpus callosum in cats: A light- and electron-microscopic study. J. Comp. Neurol. 1988, 276, 132–156. [Google Scholar] [CrossRef]
- Pires-Neto, M.A.; Lent, R. The prenatal development of the anterior commissure in hamsters: Pioneer fibers lead the way. Brain Res. Dev. Brain Res. 1993, 72, 59–66. [Google Scholar] [CrossRef]
- Shang, F.; Ashwell, K.W.; Marotte, L.R.; Waite, P.M. Development of commissural neurons in the wallaby (Macropus eugenii). J. Comp. Neurol. 1997, 387, 507–523. [Google Scholar] [CrossRef]
- Ashwell, K.W.; Waite, P.M.; Marotte, L. Ontogeny of the projection tracts and commissural fibres in the forebrain of the tammar wallaby (Macropus eugenii): Timing in comparison with other mammals. Brain Behav. Evol. 1996, 47, 8–22. [Google Scholar] [CrossRef]
- Miller, F.D.; Gauthier, A.S. Timing is everything: Making neurons versus glia in the developing cortex. Neuron 2007, 54, 357–369. [Google Scholar] [CrossRef]
- Ge, W.P.; Miyawaki, A.; Gage, F.H.; Jan, Y.N. Local generation of glia is a major astrocyte source in postnatal cortex. Nature 2012, 484, 376–380. [Google Scholar] [CrossRef]
- Falcone, C. Evolution of astrocytes: From invertebrates to vertebrates. Front. Cell Dev. Biol. 2022, 10, 931311. [Google Scholar] [CrossRef]
- Lu, Q.R.; Sun, T.; Zhu, Z.; Ma, N.; Garcia, M.; Stiles, C.D.; Rowitch, D.H. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell 2002, 109, 75–86. [Google Scholar] [CrossRef]
- Cai, J.; Chen, Y.; Cai, W.H.; Hurlock, E.C.; Wu, H.; Kernie, S.G.; Parada, L.F.; Lu, Q.R. A crucial role for Olig2 in white matter astrocyte development. Development 2007, 134, 1887–1899. [Google Scholar] [CrossRef]
- Cao, G.; Sun, C.; Shen, H.; Qu, D.; Shen, C.; Lu, H. Conditional Deletion of Foxg1 Delayed Myelination during Early Postnatal Brain Development. Int. J. Mol. Sci. 2023, 24, 13921. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Takebayashi, H.; Ikeda, K.; Furusho, M.; Nishizawa, T.; Watanabe, K.; Ikenaka, K. Regional- and temporal-dependent changes in the differentiation of Olig2 progenitors in the forebrain, and the impact on astrocyte development in the dorsal pallium. Dev. Biol. 2008, 320, 456–468. [Google Scholar] [CrossRef] [PubMed]
- Silver, J.; Edwards, M.A.; Levitt, P. Immunocytochemical demonstration of early appearing astroglial structures that form boundaries and pathways along axon tracts in the fetal brain. J. Comp. Neurol. 1993, 328, 415–436. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, J.L.; Makowiecki, K.; Cullen, C.L.; Young, K.M. Oligodendrogenesis and myelination regulate cortical development, plasticity and circuit function. Semin. Cell Dev. Biol. 2021, 118, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.M.; Ohkubo, Y.; Maragnoli, M.E.; Rašin, M.R.; Schwartz, M.L.; Šestan, N.; Vaccarino, F.M. Midline radial glia translocation and corpus callosum formation require FGF signaling. Nat. Neurosci. 2006, 9, 787–797. [Google Scholar] [CrossRef] [PubMed]
- Bignami, A.; Eng, L.F.; Dahl, D.; Uyeda, C.T. Localization of the glial fibrillary acidic protein in astrocytes by immunofluorescence. Brain Res. 1972, 43, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Shu, T.; Richards, L.J. Cortical axon guidance by the glial wedge during the development of the corpus callosum. J. Neurosci. 2001, 21, 2749–2758. [Google Scholar] [CrossRef]
- Chinn, G.A.; Hirokawa, K.E.; Chuang, T.M.; Urbina, C.; Patel, F.; Fong, J.; Funatsu, N.; Monuki, E.S. Agenesis of the Corpus Callosum Due to Defective Glial Wedge Formation in Lhx2 Mutant Mice. Cereb. Cortex 2015, 25, 2707–2718. [Google Scholar] [CrossRef] [PubMed]
- Nishikimi, M.; Oishi, K.; Nakajima, K. Axon guidance mechanisms for establishment of callosal connections. Neural. Plast. 2013, 2013, 149060. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartkowska, K.; Koguc-Sobolewska, P.; Djavadian, R.; Turlejski, K. Astrocytes of the Anterior Commissure Regulate the Axon Guidance Pathways of Newly Generated Neocortical Neurons in the Opossum Monodelphis domestica. Int. J. Mol. Sci. 2024, 25, 1476. https://doi.org/10.3390/ijms25031476
Bartkowska K, Koguc-Sobolewska P, Djavadian R, Turlejski K. Astrocytes of the Anterior Commissure Regulate the Axon Guidance Pathways of Newly Generated Neocortical Neurons in the Opossum Monodelphis domestica. International Journal of Molecular Sciences. 2024; 25(3):1476. https://doi.org/10.3390/ijms25031476
Chicago/Turabian StyleBartkowska, Katarzyna, Paulina Koguc-Sobolewska, Ruzanna Djavadian, and Krzysztof Turlejski. 2024. "Astrocytes of the Anterior Commissure Regulate the Axon Guidance Pathways of Newly Generated Neocortical Neurons in the Opossum Monodelphis domestica" International Journal of Molecular Sciences 25, no. 3: 1476. https://doi.org/10.3390/ijms25031476
APA StyleBartkowska, K., Koguc-Sobolewska, P., Djavadian, R., & Turlejski, K. (2024). Astrocytes of the Anterior Commissure Regulate the Axon Guidance Pathways of Newly Generated Neocortical Neurons in the Opossum Monodelphis domestica. International Journal of Molecular Sciences, 25(3), 1476. https://doi.org/10.3390/ijms25031476






