Leptin Promotes the Proliferation and Neuronal Differentiation of Neural Stem Cells through the Cooperative Action of MAPK/ERK1/2, JAK2/STAT3 and PI3K/AKT Signaling Pathways
Abstract
1. Introduction
2. Results
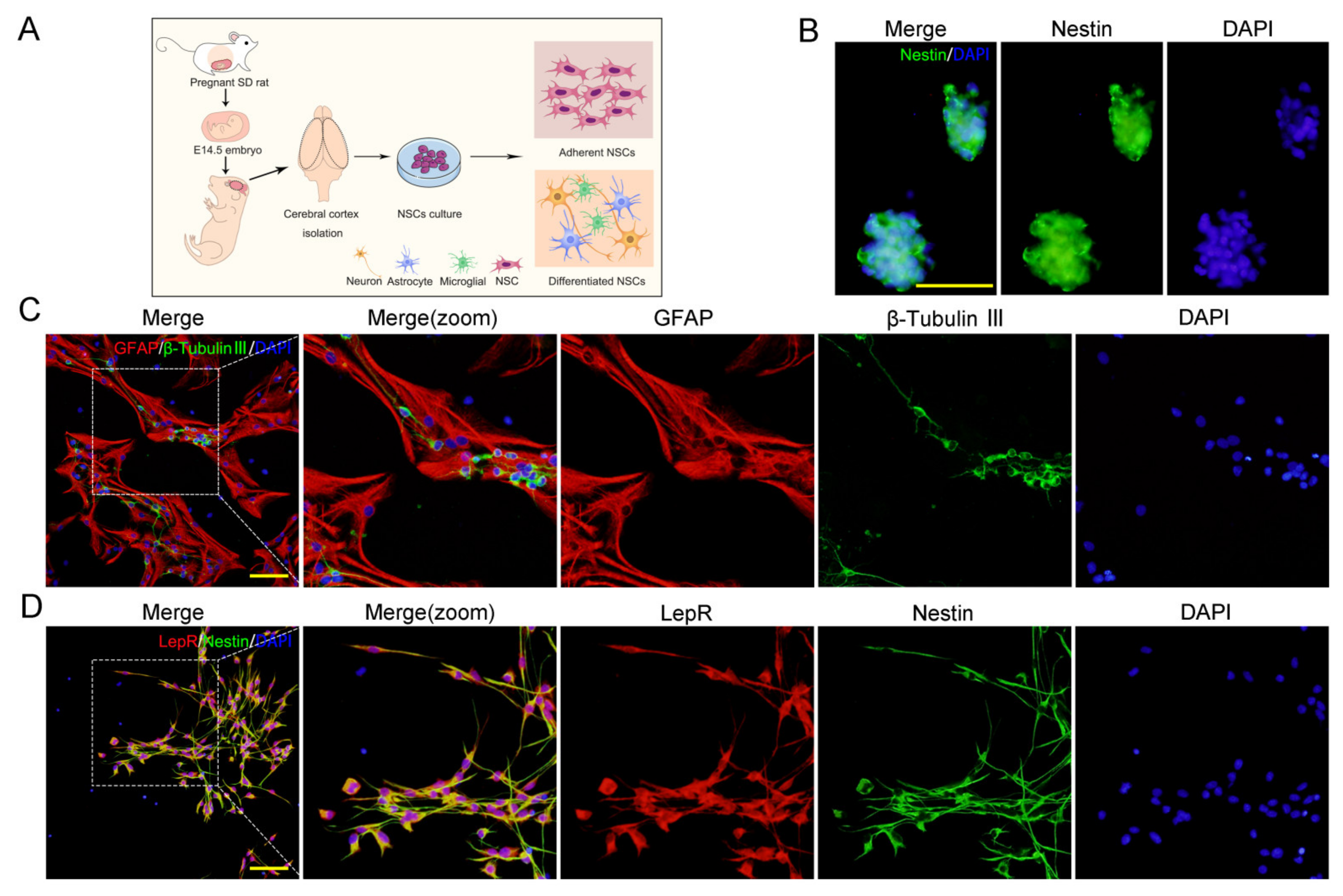
2.1. LepRs Were Wildly Expressed in NSCs
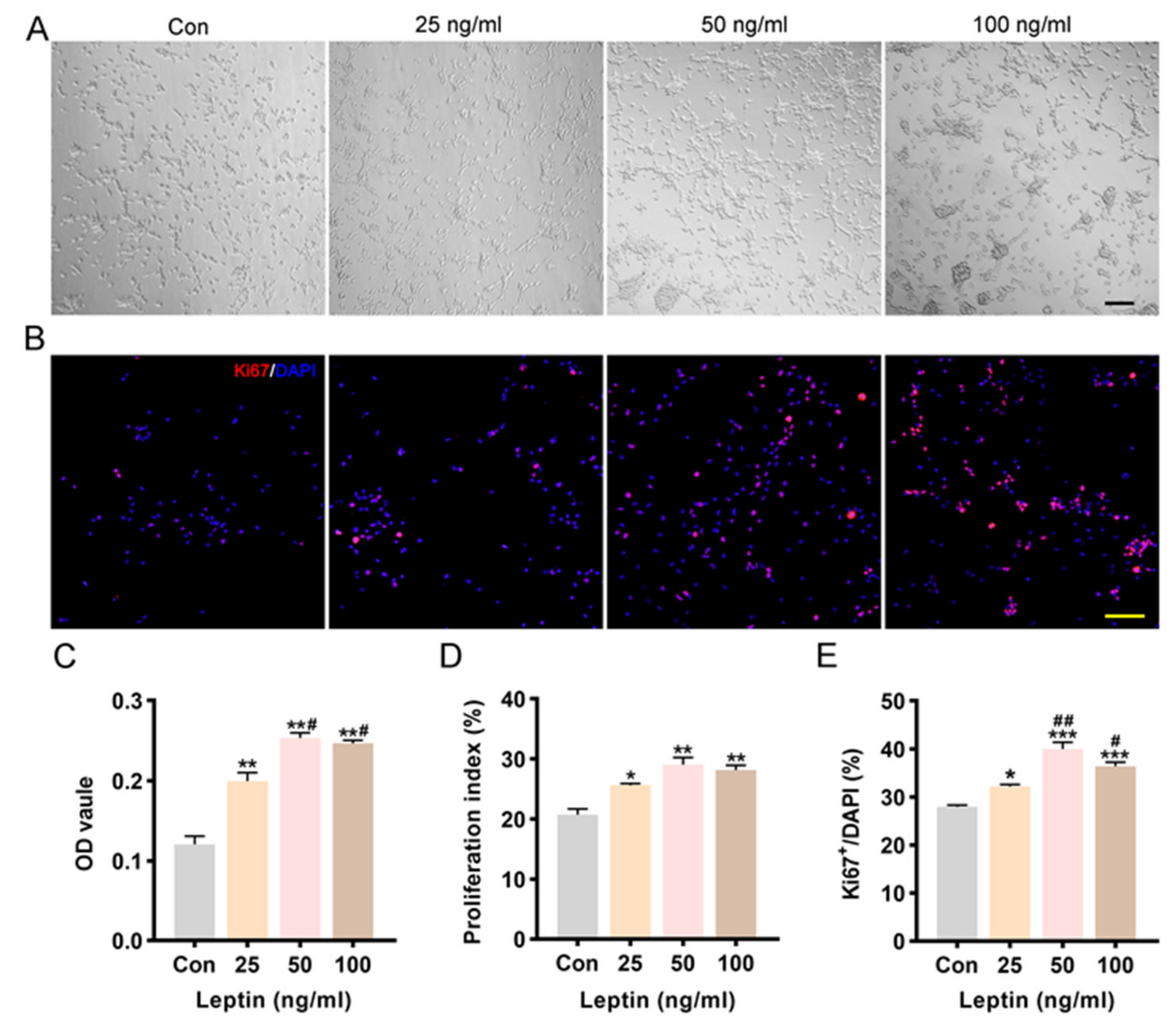
2.2. Leptin Promoted Cell Proliferation and Increased Cell Viability
2.3. MAPK/ERK 1/2 and PI3K/AKT Involves in Leptin-Induced Survival and Proliferation of NSCs
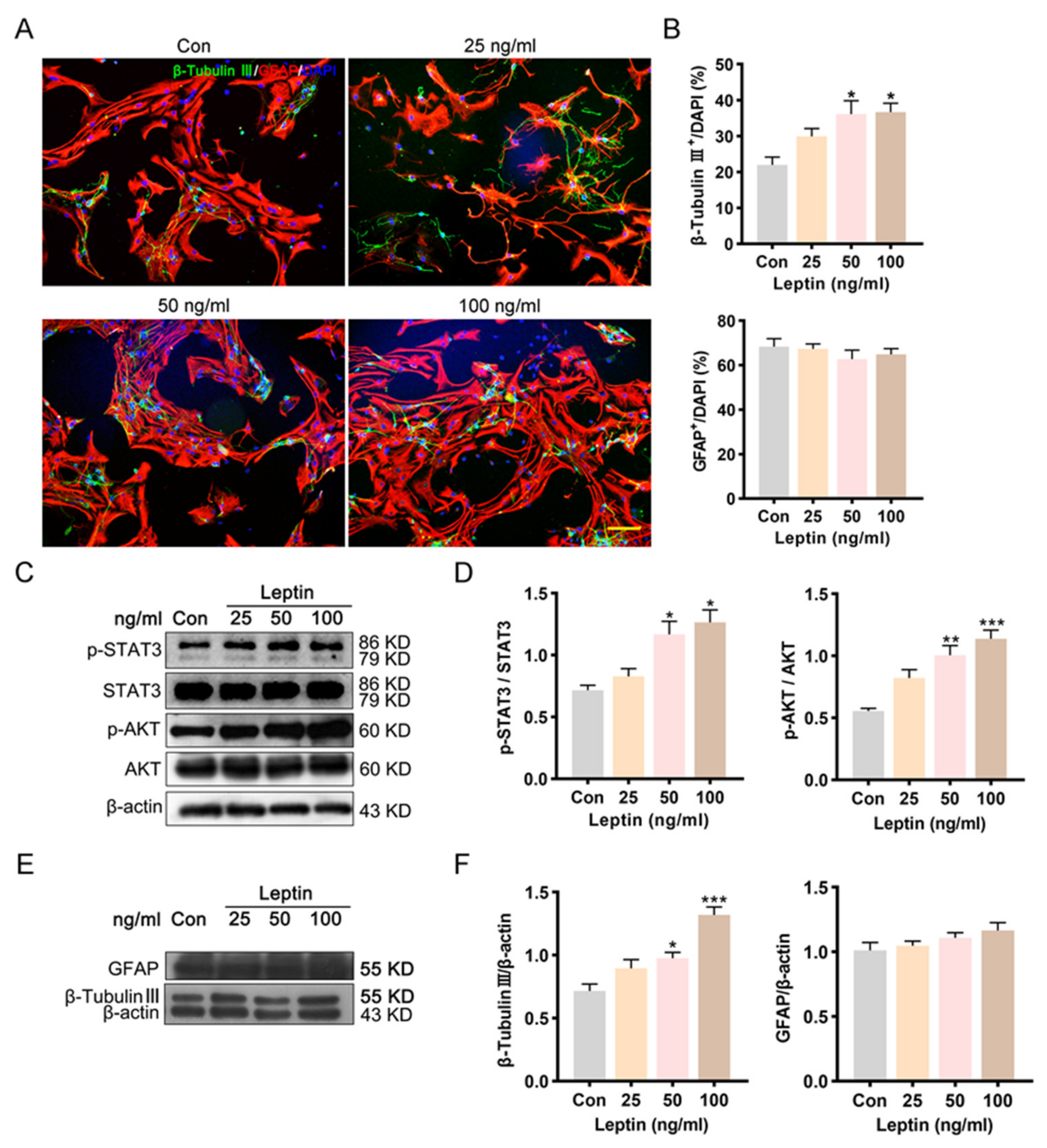
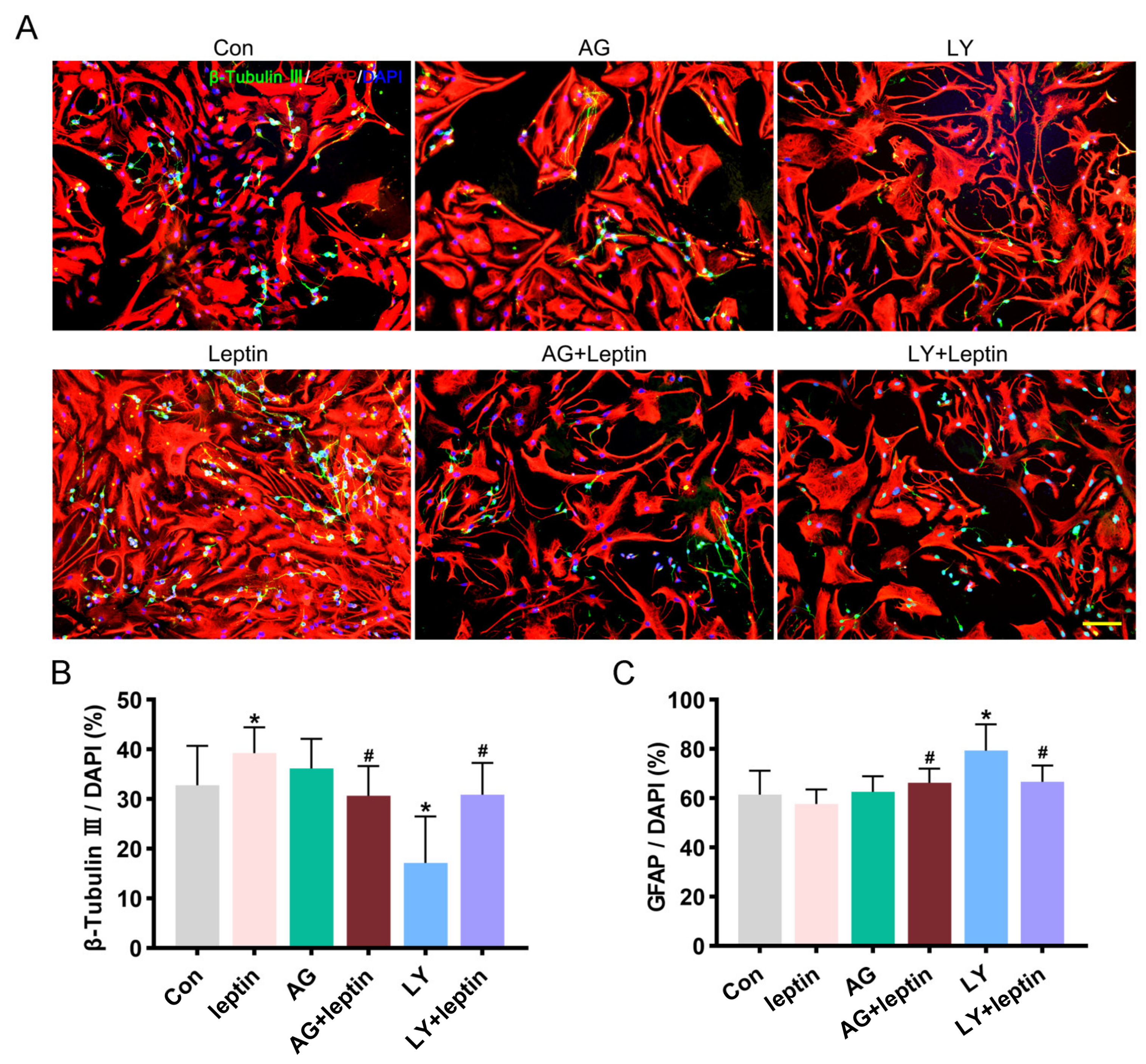
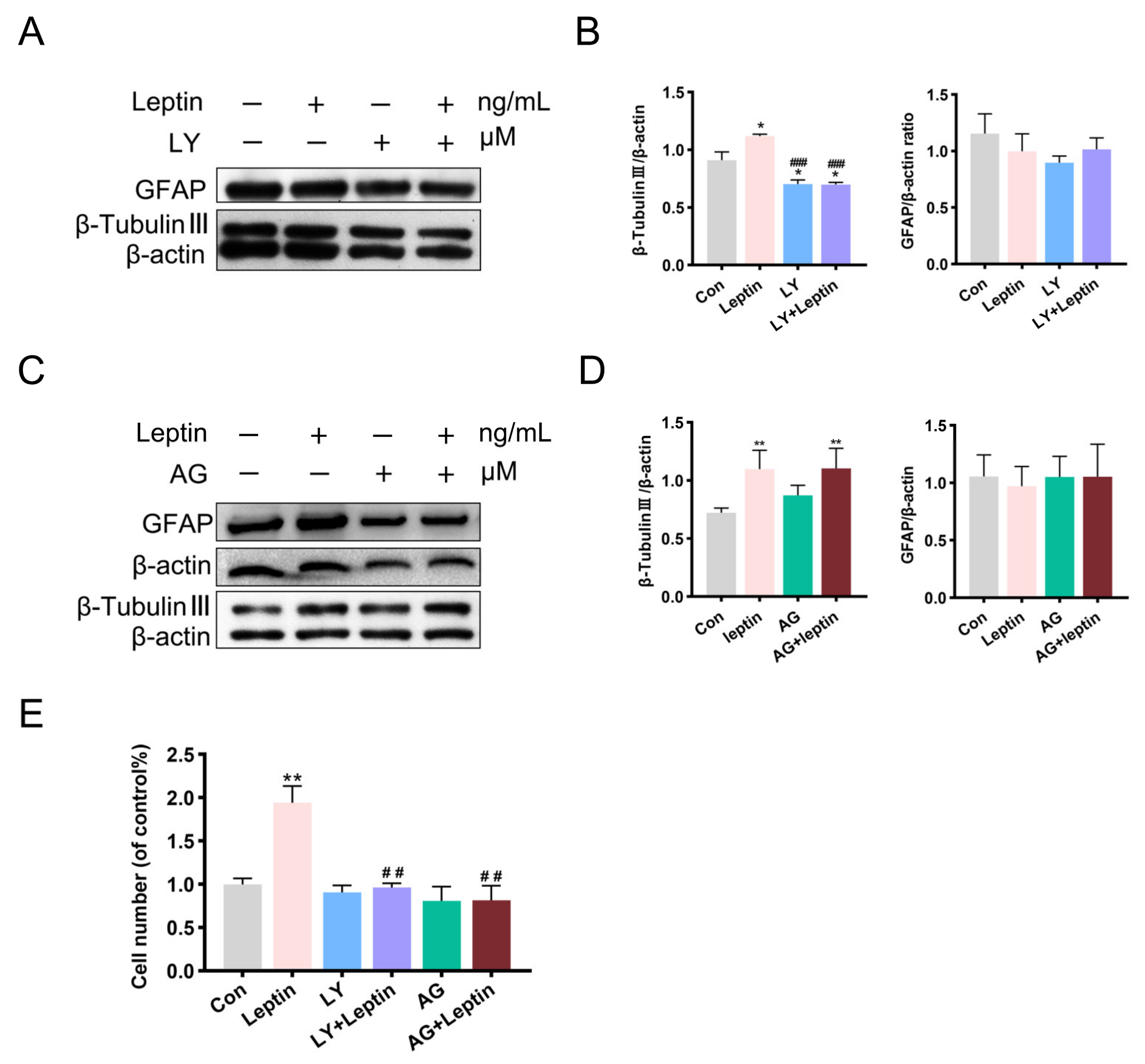
2.4. PI3K/AKT and JAK2/STAT3 Signaling Pathways Contribute to Leptin-Induced Neuronal Differentiation In Vitro
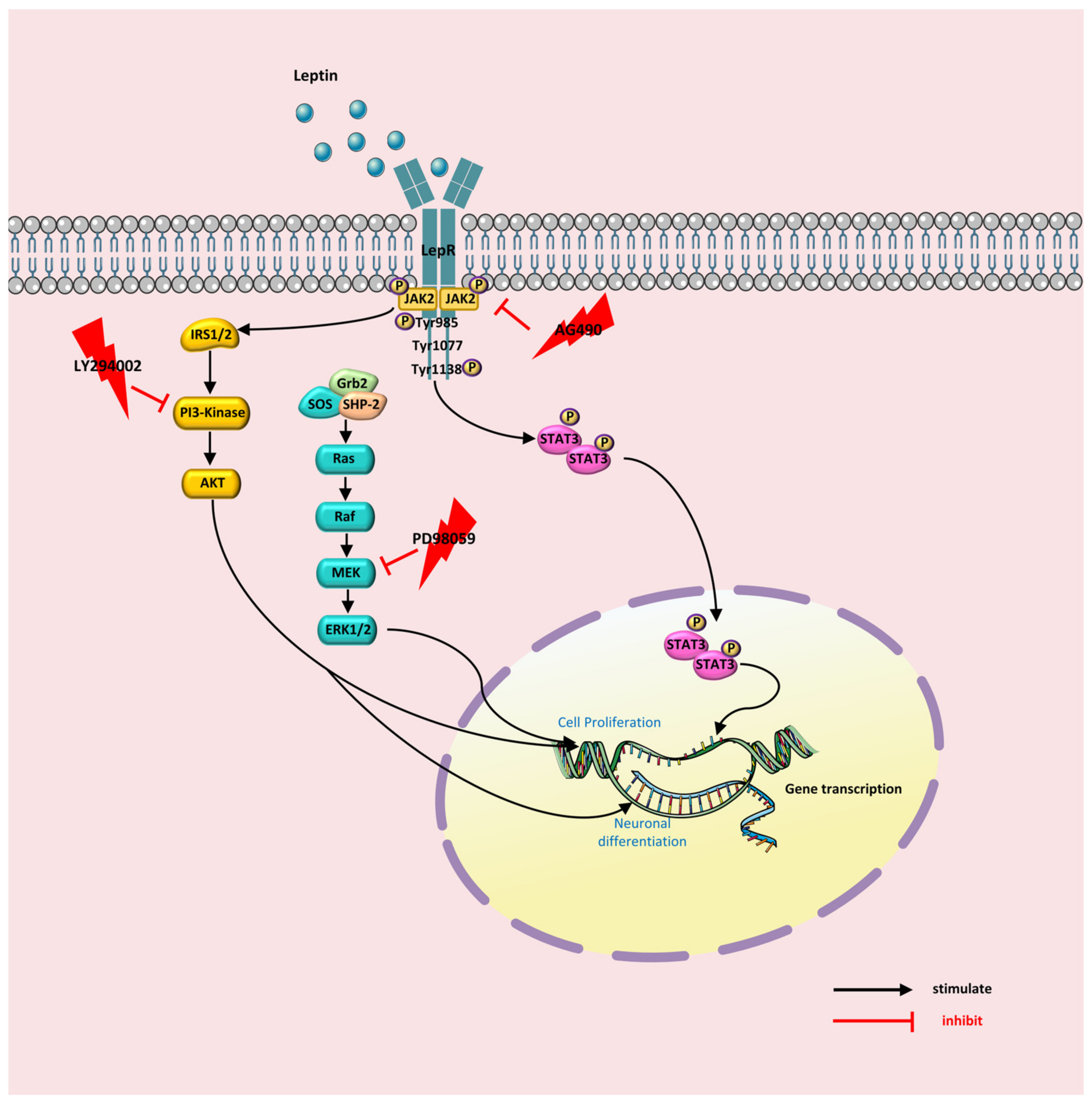
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Culture and Identification of Embryonic NSCs
4.3. Viability of NSCs
4.4. Proliferation of NSCs
4.5. Differentiation of NSCs
4.6. Western Blot Analysis
4.7. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Münzberg, H.; Morrison, C.D. Structure, production and signaling of leptin. Metabolism 2015, 64, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Morash, B.; Li, A.; Murphy, P.R.; Wilkinson, M.; Ur, E. Leptin gene expression in the brain and pituitary gland. Endocrinology 1999, 140, 5995–5998. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, J.; Hatta, T.; Naora, H.; Otani, H. Expression of the long form of leptin receptor (Ob-Rb) mRNA in the brain of mouse embryos and newborn mice. Brain Res. 2000, 868, 251–258. [Google Scholar] [CrossRef] [PubMed]
- van der Kroon, P.H.; Speijers, G.J. Brain deviations in adult obese-hyperglycemic mice (ob/ob). Metabolism 1979, 28, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Bereiter, D.A.; Jeanrenaud, B. Altered neuroanatomical organization in the central nervous system of the genetically obese (ob/ob) mouse. Brain Res. 1979, 165, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Rossier, J.; Rogers, J.; Shibasaki, T.; Guillemin, R.; Bloom, F.E. Opioid peptides and alpha-melanocyte-stimulating hormone in genetically obese (ob/ob) mice during development. Proc. Natl. Acad. Sci. USA 1979, 76, 2077–2080. [Google Scholar] [CrossRef]
- Bereiter, D.A.; Jeanrenaud, B. Altered dendritic orientation of hypothalamic neurons from genetically obese (ob/ob) mice. Brain Res. 1980, 202, 201–206. [Google Scholar] [CrossRef]
- Sena, A.; Sarliève, L.L.; Rebel, G. Brain myelin of genetically obese mice. J. Neurol. Sci. 1985, 68, 233–243. [Google Scholar] [CrossRef]
- Ahima, R.S.; Bjorbaek, C.; Osei, S.; Flier, J.S. Regulation of neuronal and glial proteins by leptin: Implications for brain development. Endocrinology 1999, 140, 2755–2762. [Google Scholar] [CrossRef]
- Valerio, A.; Dossena, M.; Bertolotti, P.; Boroni, F.; Sarnico, I.; Faraco, G.; Chiarugi, A.; Frontini, A.; Giordano, A.; Liou, H.C.; et al. Leptin is induced in the ischemic cerebral cortex and exerts neuroprotection through NF-kappaB/c-Rel-dependent transcription. Stroke 2009, 40, 610–617. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, S.; Signore, A.P.; Chen, J. Neuroprotective effects of leptin against ischemic injury induced by oxygen-glucose deprivation and transient cerebral ischemia. Stroke 2007, 38, 2329–2336. [Google Scholar] [CrossRef] [PubMed]
- Avraham, Y.; Dayan, M.; Lassri, V.; Vorobiev, L.; Davidi, N.; Chernoguz, D.; Berry, E.; Leker, R.R. Delayed leptin administration after stroke induces neurogenesis and angiogenesis. J. Neurosci. Res. 2013, 91, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Deng, Z.; Liao, J.; Song, C.; Liang, C.; Xue, H.; Wang, L.; Zhang, K.; Yan, G. Leptin attenuates cerebral ischemia injury through the promotion of energy metabolism via the PI3K/Akt pathway. J. Cereb. Blood Flow Metab. 2013, 33, 567–574. [Google Scholar] [CrossRef]
- Mancini, M.; Soldovieri, M.V.; Gessner, G.; Wissuwa, B.; Barrese, V.; Boscia, F.; Secondo, A.; Miceli, F.; Franco, C.; Ambrosino, P.; et al. Critical role of large-conductance calcium- and voltage-activated potassium channels in leptin-induced neuroprotection of N-methyl-d-aspartate-exposed cortical neurons. Pharmacol. Res. 2014, 87, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Amantea, D.; Tassorelli, C.; Russo, R.; Petrelli, F.; Morrone, L.A.; Bagetta, G.; Corasaniti, M.T. Neuroprotection by leptin in a rat model of permanent cerebral ischemia: Effects on STAT3 phosphorylation in discrete cells of the brain. Cell Death Dis. 2011, 2, e238. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, J. Leptin protects hippocampal CA1 neurons against ischemic injury. J. Neurochem. 2008, 107, 578–587. [Google Scholar] [CrossRef]
- Genchi, A.; Brambilla, E.; Sangalli, F.; Radaelli, M.; Bacigaluppi, M.; Furlan, R.; Andolfo, A.; Drago, D.; Magagnotti, C.; Scotti, G.M.; et al. Neural stem cell transplantation in patients with progressive multiple sclerosis: An open-label, phase 1 study. Nat. Med. 2023, 29, 75–85. [Google Scholar] [CrossRef]
- Huang, L.; Zhang, L. Neural stem cell therapies and hypoxic-ischemic brain injury. Prog. Neurobiol. 2019, 173, 1–17. [Google Scholar] [CrossRef]
- Zhao, T.; Zhu, T.; Xie, L.; Li, Y.; Xie, R.; Xu, F.; Tang, H.; Zhu, J. Neural Stem Cells Therapy for Ischemic Stroke: Progress and Challenges. Transl. Stroke Res. 2022, 13, 665–675. [Google Scholar] [CrossRef]
- Stonesifer, C.; Corey, S.; Ghanekar, S.; Diamandis, Z.; Acosta, S.A.; Borlongan, C.V. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog. Neurobiol. 2017, 158, 94–131. [Google Scholar] [CrossRef]
- Bauer, S. Cytokine control of adult neural stem cells. Ann. N. Y. Acad. Sci. 2009, 1153, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Bland, T.; Zhu, M.; Dillon, C.; Sahin, G.S.; Rodriguez-Llamas, J.L.; Appleyard, S.M.; Wayman, G.A. Leptin Controls Glutamatergic Synaptogenesis and NMDA-Receptor Trafficking via Fyn Kinase Regulation of NR2B. Endocrinology 2020, 161, bqz030. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J. Leptin regulation of neuronal morphology and hippocampal synaptic function. Front. Synaptic Neurosci. 2013, 5, 3. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.; Solovyova, N.; Irving, A. Leptin and its role in hippocampal synaptic plasticity. Prog. Lipid Res. 2006, 45, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Moult, P.R.; Milojkovic, B.; Harvey, J. Leptin reverses long-term potentiation at hippocampal CA1 synapses. J. Neurochem. 2009, 108, 685–696. [Google Scholar] [CrossRef] [PubMed]
- Dumon, C.; Diabira, D.; Chudotvorova, I.; Bader, F.; Sahin, S.; Zhang, J.; Porcher, C.; Wayman, G.; Medina, I.; Gaiarsa, J.L. The adipocyte hormone leptin sets the emergence of hippocampal inhibition in mice. Elife 2018, 7, e36726. [Google Scholar] [CrossRef]
- Dumon, C.; Pisella, L.; Diabira, D.; Belaidouni, Y.; Wayman, G.A.; Gaiarsa, J.L. Developmental Switch of Leptin Action on Network Driven Activity in the Neonatal Rat Hippocampus. Front. Cell. Neurosci. 2019, 13, 254. [Google Scholar] [CrossRef]
- Taga, T.; Kishimoto, T. Gp130 and the interleukin-6 family of cytokines. Annu. Rev. Immunol. 1997, 15, 797–819. [Google Scholar] [CrossRef]
- Udagawa, J.; Ono, A.; Kawamoto, M.; Otani, H. Leptin and its intracellular signaling pathway maintains the neurosphere. Neuroreport 2010, 21, 1140–1145. [Google Scholar] [CrossRef]
- Engelman, J.A.; Luo, J.; Cantley, L.C. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat. Rev. Genet. 2006, 7, 606–619. [Google Scholar] [CrossRef]
- Ren, H.; Jia, L.; Zhao, T.; Zhang, H.; Chen, J.; Yang, S.; Liu, J.; Yu, M.; Hao, J. Hypoxia inducible factor (HIF)-1α directly activates leptin receptor (Ob-R) in pancreatic cancer cells. Cancer Lett. 2014, 354, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.L.; Rui, L. Recent advances in understanding leptin signaling and leptin resistance. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E1247–E1259. [Google Scholar] [CrossRef] [PubMed]
- Xu, A.W.; Kaelin, C.B.; Takeda, K.; Akira, S.; Schwartz, M.W.; Barsh, G.S. PI3K integrates the action of insulin and leptin on hypothalamic neurons. J. Clin. Investig. 2005, 115, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J. Leptin: A multifaceted hormone in the central nervous system. Mol. Neurobiol. 2003, 28, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.; Roseberry, A.G.; Liu, H.; Diano, S.; Shanabrough, M.; Cai, X.; Friedman, J.M.; Horvath, T.L. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science 2004, 304, 110–115. [Google Scholar] [CrossRef]
- Sahin, G.S.; Luis Rodriguez-Llamas, J.; Dillon, C.; Medina, I.; Appleyard, S.M.; Gaiarsa, J.L.; Wayman, G.A. Leptin increases GABAergic synaptogenesis through the Rho guanine exchange factor β-PIX in developing hippocampal neurons. Sci. Signal 2021, 14, eabe4111. [Google Scholar] [CrossRef]
- Moon, H.S.; Dincer, F.; Mantzoros, C.S. Amylin-induced downregulation of hippocampal neurogenesis is attenuated by leptin in a STAT3/AMPK/ERK-dependent manner in mice. Diabetologia 2013, 56, 627–634. [Google Scholar] [CrossRef]
- Alonzi, T.; Middleton, G.; Wyatt, S.; Buchman, V.; Betz, U.A.; Müller, W.; Musiani, P.; Poli, V.; Davies, A.M. Role of STAT3 and PI 3-kinase/Akt in mediating the survival actions of cytokines on sensory neurons. Mol. Cell Neurosci. 2001, 18, 270–282. [Google Scholar] [CrossRef]
- Pérez-González, R.; Antequera, D.; Vargas, T.; Spuch, C.; Bolós, M.; Carro, E. Leptin induces proliferation of neuronal progenitors and neuroprotection in a mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2011, 24 (Suppl. S2), 17–25. [Google Scholar] [CrossRef]
- Yook, J.S.; Rakwal, R.; Shibato, J.; Takahashi, K.; Koizumi, H.; Shima, T.; Ikemoto, M.J.; Oharomari, L.K.; McEwen, B.S.; Soya, H. Leptin in hippocampus mediates benefits of mild exercise by an antioxidant on neurogenesis and memory. Proc. Natl. Acad. Sci. USA 2019, 116, 10988–10993. [Google Scholar] [CrossRef]
- Finkel, Z.; Esteban, F.; Rodriguez, B.; Fu, T.; Ai, X.; Cai, L. Diversity of Adult Neural Stem and Progenitor Cells in Physiology and Disease. Cells 2021, 10, 2045. [Google Scholar] [CrossRef] [PubMed]
- Calió, M.L.; Mosini, A.C.; Marinho, D.S.; Salles, G.N.; Massinhani, F.H.; Ko, G.M.; Porcionatto, M.A. Leptin enhances adult neurogenesis and reduces pathological features in a transgenic mouse model of Alzheimer’s disease. Neurobiol. Dis. 2021, 148, 105219. [Google Scholar] [CrossRef] [PubMed]
- Udagawa, J.; Hashimoto, R.; Suzuki, H.; Hatta, T.; Sotomaru, Y.; Hioki, K.; Kagohashi, Y.; Nomura, T.; Minami, Y.; Otani, H. The role of leptin in the development of the cerebral cortex in mouse embryos. Endocrinology 2006, 147, 647–658. [Google Scholar] [CrossRef]
- Shan, J.; Nguyen, T.B.; Totary-Jain, H.; Dansky, H.; Marx, S.O.; Marks, A.R. Leptin-enhanced neointimal hyperplasia is reduced by mTOR and PI3K inhibitors. Proc. Natl. Acad. Sci. USA 2008, 105, 19006–19011. [Google Scholar] [CrossRef] [PubMed]
- Frankenberry, K.A.; Skinner, H.; Somasundar, P.; McFadden, D.W.; Vona-Davis, L.C. Leptin receptor expression and cell signaling in breast cancer. Int. J. Oncol. 2006, 28, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, S.K.; Kim, H.; Kim, T.M. Exosomes Secreted from Induced Pluripotent Stem Cell-Derived Mesenchymal Stem Cells Accelerate Skin Cell Proliferation. Int. J. Mol. Sci. 2018, 19, 3119. [Google Scholar] [CrossRef]
- Chen, X.; Wu, H.; Chen, H.; Wang, Q.; Xie, X.J.; Shen, J. Astragaloside VI Promotes Neural Stem Cell Proliferation and Enhances Neurological Function Recovery in Transient Cerebral Ischemic Injury via Activating EGFR/MAPK Signaling Cascades. Mol. Neurobiol. 2019, 56, 3053–3067. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, Y.; Ju, J.; Zhang, G.; Zhang, P.; Ji, P.; Jin, Q.; Cao, G.; Zuo, R.; Wang, H.; et al. miR-100-5p Promotes Epidermal Stem Cell Proliferation through Targeting MTMR3 to Activate PIP3/AKT and ERK Signaling Pathways. Stem Cells Int. 2022, 2022, 1474273. [Google Scholar] [CrossRef]
- Tai, C.I.; Schulze, E.N.; Ying, Q.L. Stat3 signaling regulates embryonic stem cell fate in a dose-dependent manner. Biol. Open 2014, 3, 958–965. [Google Scholar] [CrossRef][Green Version]
- Jung, K.H.; Chu, K.; Lee, S.T.; Kim, S.J.; Sinn, D.I.; Kim, S.U.; Kim, M.; Roh, J.K. Granulocyte colony-stimulating factor stimulates neurogenesis via vascular endothelial growth factor with STAT activation. Brain Res. 2006, 1073–1074, 190–201. [Google Scholar] [CrossRef]
- Yu, L.; Wang, J.; Xia, Y.; Hou, W.; Yao, X.; Guo, Y.; Wang, J.; Wei, H.; Wang, S. Adiponectin Promotes Neurogenesis after Transient Cerebral Ischemia through STAT3 Mediated BDNF Upregulation in Astrocytes. Neurochem. Res. 2023, 48, 641–657. [Google Scholar] [CrossRef]
- Garza, J.C.; Guo, M.; Zhang, W.; Lu, X.Y. Leptin increases adult hippocampal neurogenesis in vivo and in vitro. J. Biol. Chem. 2008, 283, 18238–18247. [Google Scholar] [CrossRef] [PubMed]
- Gage, F.H.; Coates, P.W.; Palmer, T.D.; Kuhn, H.G.; Fisher, L.J.; Suhonen, J.O.; Peterson, D.A.; Suhr, S.T.; Ray, J. Survival and differentiation of adult neuronal progenitor cells transplanted to the adult brain. Proc. Natl. Acad. Sci. USA 1995, 92, 11879–11883. [Google Scholar] [CrossRef]
- Lu, H.; Jiao, Q.; Wang, Y.; Yang, Z.; Feng, M.; Wang, L.; Chen, X.; Jin, W.; Liu, Y. The mental retardation-associated protein srGAP3 regulates survival, proliferation, and differentiation of rat embryonic neural stem/progenitor cells. Stem Cells Dev. 2013, 22, 1709–1716. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Tan, R.L.; Hu, X.X.; Cai, Z.L.; Sun, M.Q.; Ge, Q.; Ma, W.; Li, H.L.; Lu, H.X. Kinase inhibit region of SOCS3 attenuates IL6-induced proliferation and astrocytic differentiation of neural stem cells via cross talk between signaling pathways. CNS Neurosci. Ther. 2023, 29, 168–180. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tan, R.; Hu, X.; Wang, X.; Sun, M.; Cai, Z.; Zhang, Z.; Fu, Y.; Chen, X.; An, J.; Lu, H. Leptin Promotes the Proliferation and Neuronal Differentiation of Neural Stem Cells through the Cooperative Action of MAPK/ERK1/2, JAK2/STAT3 and PI3K/AKT Signaling Pathways. Int. J. Mol. Sci. 2023, 24, 15151. https://doi.org/10.3390/ijms242015151
Tan R, Hu X, Wang X, Sun M, Cai Z, Zhang Z, Fu Y, Chen X, An J, Lu H. Leptin Promotes the Proliferation and Neuronal Differentiation of Neural Stem Cells through the Cooperative Action of MAPK/ERK1/2, JAK2/STAT3 and PI3K/AKT Signaling Pathways. International Journal of Molecular Sciences. 2023; 24(20):15151. https://doi.org/10.3390/ijms242015151
Chicago/Turabian StyleTan, Ruolan, Xiaoxuan Hu, Xinyi Wang, Meiqi Sun, Zhenlu Cai, Zixuan Zhang, Yali Fu, Xinlin Chen, Jing An, and Haixia Lu. 2023. "Leptin Promotes the Proliferation and Neuronal Differentiation of Neural Stem Cells through the Cooperative Action of MAPK/ERK1/2, JAK2/STAT3 and PI3K/AKT Signaling Pathways" International Journal of Molecular Sciences 24, no. 20: 15151. https://doi.org/10.3390/ijms242015151
APA StyleTan, R., Hu, X., Wang, X., Sun, M., Cai, Z., Zhang, Z., Fu, Y., Chen, X., An, J., & Lu, H. (2023). Leptin Promotes the Proliferation and Neuronal Differentiation of Neural Stem Cells through the Cooperative Action of MAPK/ERK1/2, JAK2/STAT3 and PI3K/AKT Signaling Pathways. International Journal of Molecular Sciences, 24(20), 15151. https://doi.org/10.3390/ijms242015151





