How the ‘Aerobic/Anaerobic Glycolysis’ Meme Formed a ‘Habit of Mind’ Which Impedes Progress in the Field of Brain Energy Metabolism
Abstract
1. Who’s Who of Muscle Carbohydrate Metabolism
2. Habit of Mind Fuels Resistance to and Disregard of Findings That May Debunk the Dogma
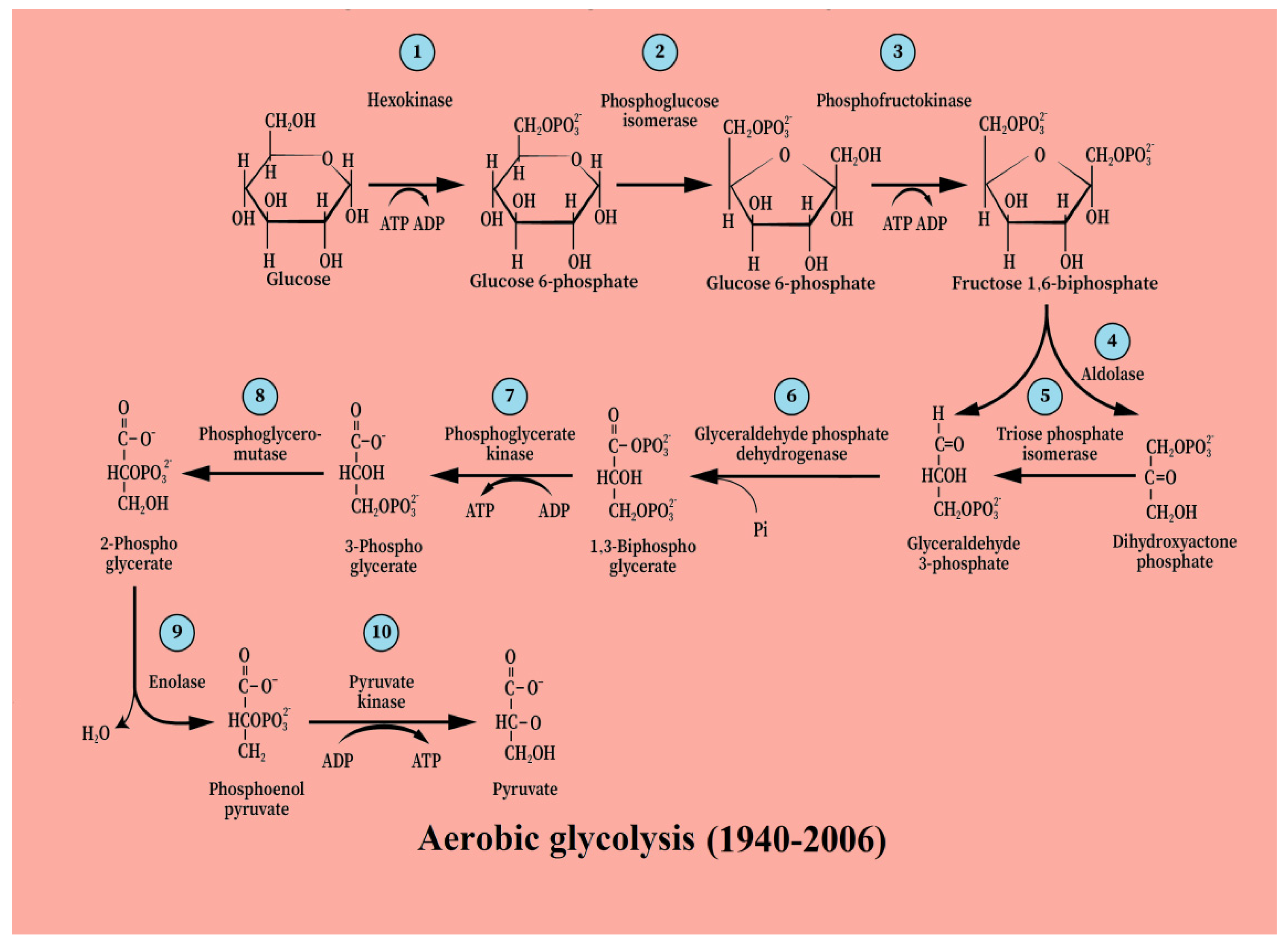
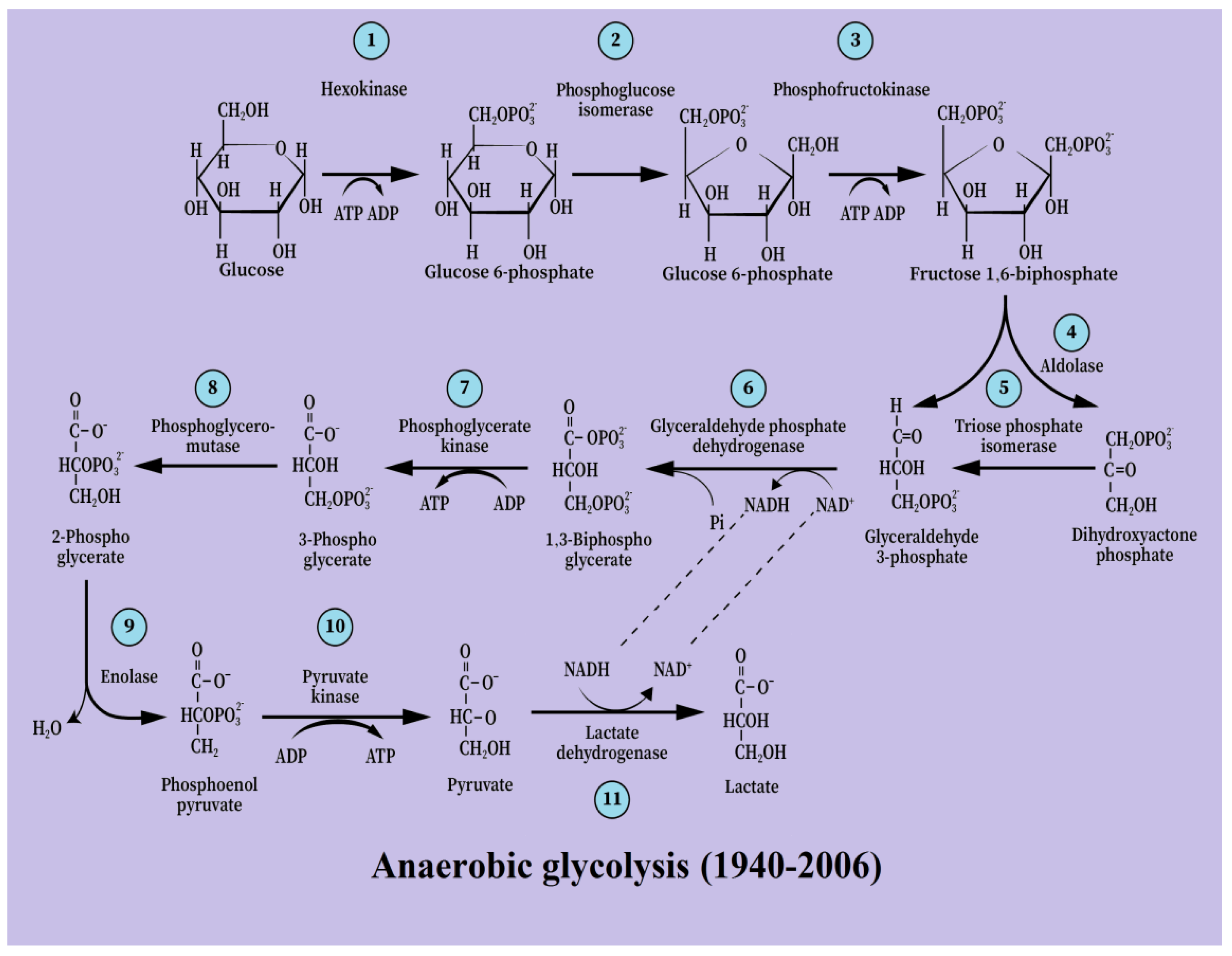
3. From [Anaerobic Glycolysis → Lactate] to [Aerobic Glycolysis → Lactate]
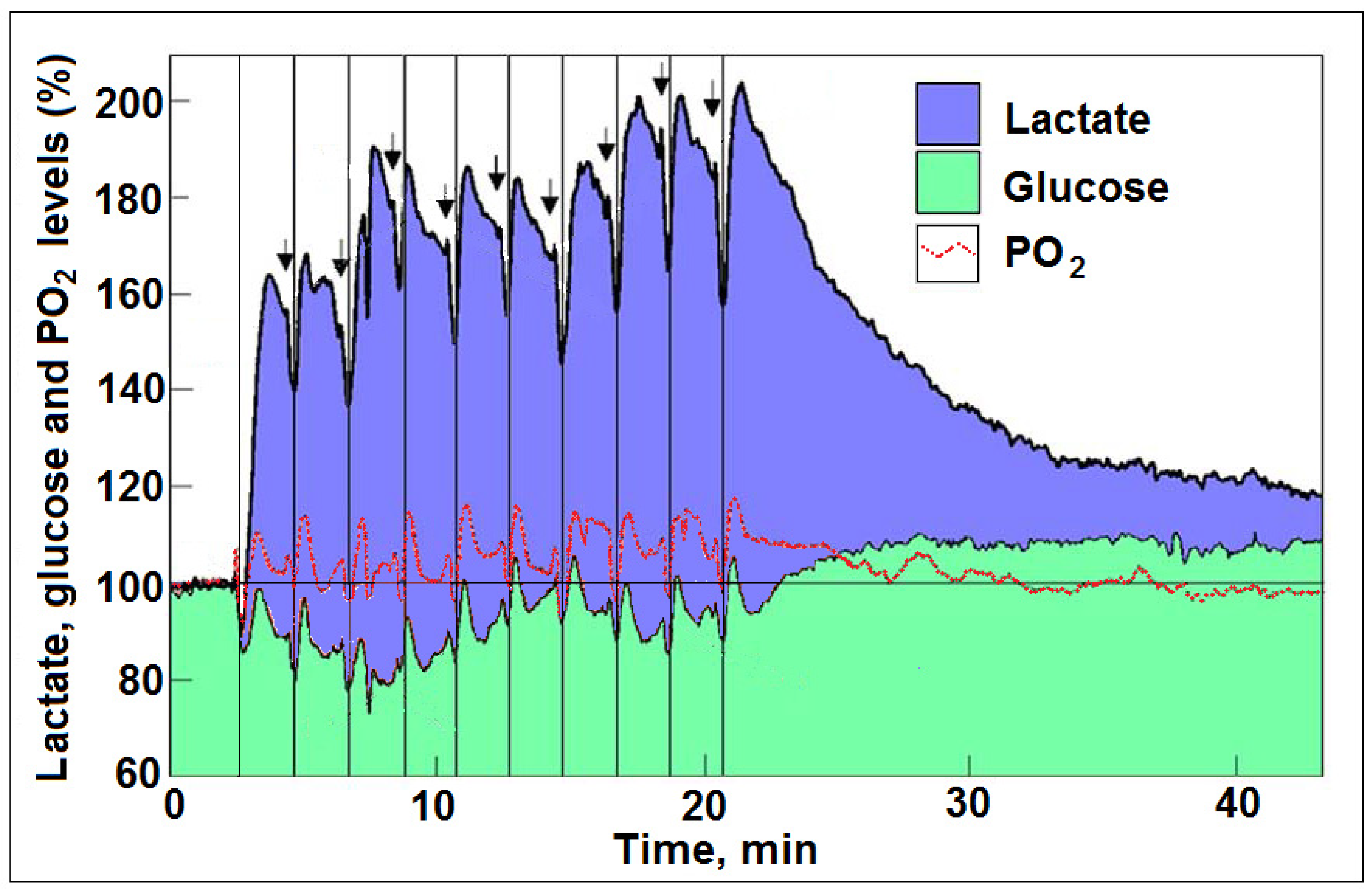
4. New Hypothesis to Circumvent a Flawed Dogma?
5. Glycolysis Always Ends with Lactate, the Aerobic Substrate of the Mitochondrial OXPHOS
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Krebs, H.A.; Johnson, W.A. The role of citric acid in intermediary metabolism in animal tissue. Enzymologia 1937, 4, 148–156. [Google Scholar]
- Holmes, B.E.; Holmes, E.G. Contributions to the study of brain metabolism. I. Carbohydrate metabolism. Preliminary paper. Biochem. J. 1925, 19, 492–499. [Google Scholar] [CrossRef]
- Holmes, E.G.; Holmes, B.E. Contributions to the study of brain metabolism. II. Carbohydrate metabolism. Biochem. J. 1925, 19, 836–839. [Google Scholar] [CrossRef]
- Holmes, E.G.; Holmes, B.E. Contributions to the study of brain metabolism. III. Carbohydrate metabolism relationship of glycogen and lactic acid. Biochem. J. 1926, 20, 1196–1203. [Google Scholar] [CrossRef]
- Holmes, E.G.; Holmes, B.E. Contributions to the study of brain metabolism. IV. Carbohydrate metabolism of the brain tissue of depancreatised cats. Biochem. J. 1927, 21, 412–418. [Google Scholar] [CrossRef]
- Ashford, C.A.; Holmes, E.G. Contributions to the study of brain metabolism. V. Role of phosphates in lactic acid production. Biochem. J. 1929, 23, 748–759. [Google Scholar] [CrossRef]
- Holmes, E.G. Oxidations in central and peripheral nervous tissue. Biochem. J. 1930, 24, 914–925. [Google Scholar] [CrossRef]
- Holmes, E.G.; Ashford, C.A. (Lactic acid oxidation in brain with reference to the “Meyerfof cycle”. Biochem. J. 1930, 24, 1119–1127. [Google Scholar] [CrossRef]
- Ashford, C.A.; Holmes, E.G. Further observations on the oxidation of lactic acid by brain tissue. Biochem. J. 1931, 25, 2028–2049. [Google Scholar] [CrossRef]
- Holmes, E.G. The metabolism of brain and nerve. Ann. Rev. Biochem. 1932, 1, 487–506. [Google Scholar] [CrossRef]
- Holmes, E.G. The relation between carbohydrate metabolism and the function of the grey matter of the central nervous system. Biochem. J. 1933, 27, 523–536. [Google Scholar] [PubMed]
- Schurr, A. Lactate: The ultimate cerebral oxidative energy substrate? J. Cereb. Blood Flow Metab. 2006, 26, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Margolis, H. Paradigms and Barriers: How Habits of Mind Govern Scientific Beliefs; The University of Chicago Press, Ltd.: London, UK, 1993. [Google Scholar]
- Schurr, A. Cerebral glycolysis: A century of persistent misunderstanding and misconception. Front. Neurosci. 2014, 8, 360. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. Lactate: Glycolytic product and oxidative substrate during sustained exercise in mammals—‘The lactate shuttle’. In Comparative Physiology and Biochemistry—Currecnt Topics and Trends Vol A. Respiration-Metabolism-Circulation; Gilles, R., Ed.; Springer: Berlin, Germany, 1985; pp. 208–218. [Google Scholar]
- Brooks, G.A.; Dubouchaud, H.; Brown, M.; Sicurello, J.P.; Butz, C.E. Role of mitochondrial lactate dehydrogenase and lactate oxidation in the intracellular lactate shuttle. Proc. Natl. Acad. Sci. USA 1999, 96, 1129–1134. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. Mammalian fuel utilization during sustained exercise. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 1998, 120, 89–107. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A. Intra- and extra-cellular lactate shuttles. Med. Sci. Sports Exerc. 2000, 32, 790–799. [Google Scholar] [CrossRef] [PubMed]
- Schurr, A.; West, C.A.; Rigor, B.M. Lactate-Supported Synaptic Function in the Rat Hippocampal Slice Preparation. Science 1988, 240, 1326–1328. [Google Scholar] [CrossRef]
- Schurr, A.; Payne, R.S.; Miller, J.J.; Rigor, B.M. Brain lactate, not glucose, fuels the recovery of synaptic function from hypoxia upon reoxygenation: An in vitro study. Brain Res. 1997, 744, 105–111. [Google Scholar] [CrossRef]
- Brooks, G.A. Lactate shuttle—Between but not within cells? J. Physiol. 2002, 541, 333. [Google Scholar] [CrossRef]
- Brooks, G.A. Lactate shuttles in nature. Biochem. Soc. Trans. 2002, 30, 258–264. [Google Scholar] [CrossRef]
- Sahlin, K.; Fernstrom, M.; Tonkonogi, M. No evidence of an intracellular lactate shuttle in rat skeletal muscle. J. Physiol. 2002, 541, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, H.N.; van Hall, G.; Rasmussen, U.F. Lactate dehydrogenase is not a mitochondrial enzyme in human and mouse vastus lateralis muscle. J. Physiol. 2002, 541, 575–580. [Google Scholar] [CrossRef] [PubMed]
- Rehncrona, S.; Rosen, I.; Seisö, B.K. Brain lactic acidosis and ischemic cell-damage. 1. Biochemistry and neurophysiology. J. Cereb. Blood Flow Metab. 1981, 1, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Kalimo, H.; Rehncrona, S.; Soderfeldt, B.; Olsson, Y.; Siejö, B.K. Brain lactic acidosis and ischemic cell-damage. 2. Histopathology. J. Cereb. Blood Flow Metab. 1981, 1, 313–327. [Google Scholar] [CrossRef]
- Siesjö, B.K. Cell-damage in the brain—A speculative synthesis. J. Cereb. Blood Flow Metab. 1981, 1, 155–185. [Google Scholar] [CrossRef]
- Lluis, C. Lactate dehydrogenase associated with the mitochondrial fraction and with a mitochondrial inhibitor--II. Enzyme in-teraction with a mitochondrial inhibitor. Int. J. Biochem. 1984, 16, 1005–1013. [Google Scholar] [CrossRef]
- Lluis, C. Lactate dehydrogenase binding to the mitochondrial fraction and to a mitochondrial inhibitor as a function of the isoenzymatic composition. Int. J. Biochem. 1985, 17, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Kline, E.S.; Brandt, R.B.; Laux, J.E.; Spainhour, S.E.; Higgins, E.S.; Rogers, K.S.; Tinsley, S.B.; Waters, M. G Localization of l-lactate dehydrogenase in mitochondri. Arch. Biochem. Biophys. 1986, 246, 673–680. [Google Scholar] [CrossRef]
- Brandt, R.B.; Laux, J.E.; Spainhour, S.E.; Kline, E.S. Lactate dehydrogenase in rat mitochondria. Arch. Biochem. Biophys. 1987, 259, 412–422. [Google Scholar] [CrossRef]
- Schurr, A.; Dong, W.; Reid, K.H.; West, C.A.; Rigor, B.M. Lactic acidosis and recovery of neuronal function following cerebral hypoxia in vitro. Brain Res. 1988, 438, 311–314. [Google Scholar] [CrossRef]
- Tombaugh, G.C.; Sapolsky, R.M. Mild acidosis protects hippocampal neurons from injury induced by oxygen and glucose deprivation. Brain Res. 1990, 506, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Myers, R.E.; Yamaguchi, S. Nervous system effects of cardiac arrest in monkeys. Preservation of vision. Arch. Neurol. 1977, 34, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Schurr, A.; Payne, R.S.; Tseng, M.T.; Miller, J.J.; Rigor, B.M. The glucose paradox in cerebral ischemia. New insights. Ann. N. Y. Acad. Sci. 1999, 893, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Schurr, A.; Payne, R.S.; Miller, J.J.; Tseng, M.T. Preischemic hyperglycemia-aggravated damage: Evidence that lactate utilization is beneficial and glucose-induced corticosterone release is detrimental. J. Neurosci. Res. 2001, 66, 782–789. [Google Scholar] [CrossRef] [PubMed]
- Clarke, D.D.; Sokoloff, L. Ciculation and energy metabolism of the brain (Chap. 31). In Basic Neurochemistry, 5th ed.; Siegle, G.J., Agranoff, B.W., Albers, R.W., Molinoff, P.B., Eds.; Raven Press: New York, NY, USA, 1994; pp. 645–680. [Google Scholar]
- Chih, C.-P.; Lipton, P.; Roberts, E.L., Jr. Do active cerebral neurons really use lactate rather than glucose? Trends Neurosci. 2001, 24, 573–578. [Google Scholar] [CrossRef]
- Schurr, A.; Gozal, E. Aerobic production and utilization of lactate satisfy increased energy demands upon neuronal activation in hippocampal slices and provide neuroprotection against oxidative stress. Front. Pharmacol. 2012, 2, 96. [Google Scholar] [CrossRef]
- Pellerin, L.; Magistretti, P.J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA 1994, 91, 10625–10629. [Google Scholar] [CrossRef]
- Dienel, G.A. Brain Lactate Metabolism: The Discoveries and the Controversies. J. Cereb. Blood Flow Metab. 2012, 32, 1107–1138. [Google Scholar] [CrossRef]
- Dienel, G.A. Brain Glucose Metabolism: Integration of Energetics with Function. Physiol. Rev. 2019, 99, 949–1045. [Google Scholar] [CrossRef]
- Schurr, A. Glycolysis Paradigm Shift Dictates a Reevaluation of Glucose and Oxygen Metabolic Rates of Activated Neural Tissue. Front. Neurosci. 2018, 12, 700. [Google Scholar] [CrossRef]
- Bak, L.K.; Schousboe, A. Misconceptions regarding basic thermodynamics and enzyme kinetics have led to erroneous conclusions regarding the metabolic importance of lactate dehydrogenase isoenzyme expression. J. Neurosci. Res. 2017, 95, 2098–2102. [Google Scholar] [CrossRef] [PubMed]
- Atlante, A.; de Bari, L.; Bobba, A.; Marra, E.; Passarella, S. Transport and metabolism of L-lactate occur in mitochondria from cerebellar granule cells and are modified in cells undergoing low potassium dependent apoptosis. Biochim. Biophys. Acta 2007, 1767, 1285–1299. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Passarella, S.; de Bari, L.; Valenti, D.; Pizzuto, R.; Paventi, G.; Altane, A. Miochondria and l-lactate metabolism. FEBS Lett. 2008, 582, 3569–3576. [Google Scholar] [CrossRef] [PubMed]
- Kane, D.A. Lactate oxidation at the mitochondria: A lactate-malate-aspartate shuttle at work. Front. Neurosci. 2014, 8, 366. [Google Scholar] [CrossRef] [PubMed]
- Rogatzki, M.J.; Ferguson, B.; Goodwin, M.L.; Gladden, L.B. Lactate is always the end product of glycolysis. Front. Neurosci. 2015, 9, 22. [Google Scholar] [CrossRef]
- Altinok, O.; Poggio, J.L.; Stein, D.E.; Bowne, W.B.; Shieh, A.C.; Snyder, N.W.; Orynbayeva, Z. Malate-aspartate shuttle promotes l-lactate oxidation in mitochondria. J. Cell. Physiol. 2020, 235, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Rabinowitz, J.D.; Enerbäck, S. Lactate: The ugly duckling of energy metabolism. Nat. Metab. 2020, 2, 566–571. [Google Scholar] [CrossRef] [PubMed]
- Brooks, G.A.; Curl, C.C.; Leija, R.G.; Osmond, A.D.; Duong, J.J.; Arevalo, J.A. Tracing the lactate shuttle to the mito-chondrial reticulum. Exp. Mol. Med. 2022, 54, 1332–1347. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Xu, L.; Wang, A.; Zou, Y.; Li, T.; Huang, L.; Chen, W.; Liu, S.; Jiang, K.; et al. Ultrasensitive sensors reveal the spatiotemporal landscape of lactate metabolism in physiology and disease. Cell Metab. 2022, 34, 1–12. [Google Scholar] [CrossRef]
- John, S.; Calmettes, G.; Xu, S.; Ribalet, B. Real-time resolution studies of the regulation of pyruvate-dependent lactate metabolism by hexokinases in single cells. PLoS ONE 2023, 18, e0286660. [Google Scholar] [CrossRef]
- Passarella, S.; Paventi, G.; Pizzuto, R. The mitochondrial L-lactate dehydrogenase affair. Front. Neurosci. 2014, 8, 407. [Google Scholar] [CrossRef] [PubMed]
- Young, A.; Oldford, C.; Mailloux, R. Lactate dehydrogenase supports lactate oxidation in mitochondria isolated from different mouse tissues. Redox Biol. 2020, 28, 101339. [Google Scholar] [CrossRef] [PubMed]
- Bergersen, L.H. Lactate transport and signaling in the brain: Potential therapeutic targets and roles in body–brain interaction. J. Cereb. Blood Flow Metab. 2015, 35, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Schurr, A.; Passarella, S. Aerobic Glycolysis: A DeOxymoron of (Neuro)Biology. Metabolites 2022, 12, 72. [Google Scholar] [CrossRef]
- Dawkins, R. The Selfish Gene; Oxford University Press: Oxford, UK, 1976; pp. 245–260. [Google Scholar]
- Warburg, O.; Posener, K.; Negelein, E. Ueber den stoffwechsel der tumoren. Biochem. Z. 1924, 152, 319–344. [Google Scholar]
- Warburg, O. On the origin of cancer cells. Science 1956, 24, 309–314. [Google Scholar] [CrossRef]
- Fox, P.T.; Raichle, M.E. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc. Natl. Acad. Sci. USA 1986, 83, 1140–1144. [Google Scholar] [CrossRef]
- Fox, P.T.; Raichle, M.E.; Mintun, M.A.; Dence, C. Nonoxidative glucose consumption during focal physiologic neural activity. Science 1988, 241, 462–464. [Google Scholar] [CrossRef]
- Wiback, S.J.; Palsson, B.O. Extreme pathway analysis of human red blood cell metabolism. Biophys. J. 2000, 83, 808–818. [Google Scholar] [CrossRef]
- Hillman, E.M.C. Coupling mechanism and significance of the BOLD signal: A status report. Annu. Rev. Neurosci. 2014, 37, 161–181. [Google Scholar] [CrossRef]
- Hu, Y.; Wilson, G.S. Rapid changes in local extracellular rat brain glucose observed with an in vivo glucose sensor. J. Neurochem. 1997, 68, 1745–1752. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Wilson, G.S. A temporary local energy pool coupled to neuronal activity: Fluctuations of extracellular lactate levels in rat brain monitored with rapid-response enzyme-based sensor. J. Neurochem. 1997, 69, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Theriault, J.E.; Shaffer, C.; Dienel, G.A.; Sander, C.Y.; Hooker, J.M.; Dickerson, B.C.; Barrett, L.F.; Quigley, K.S. A func-tional account of stimulation-based aerobic glycolysis and its role in interpreting BOLD signal intensity increases in neuroim-aging experiments. Neurosci. Behav. Rev. 2023, 153, 105373. [Google Scholar] [CrossRef] [PubMed]
- Schurr, A. From rags to riches: Lactate ascension as a pivotal metabolite in neuroenergetics. Front. Neurosci. 2023, 17, 1145358. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schurr, A. How the ‘Aerobic/Anaerobic Glycolysis’ Meme Formed a ‘Habit of Mind’ Which Impedes Progress in the Field of Brain Energy Metabolism. Int. J. Mol. Sci. 2024, 25, 1433. https://doi.org/10.3390/ijms25031433
Schurr A. How the ‘Aerobic/Anaerobic Glycolysis’ Meme Formed a ‘Habit of Mind’ Which Impedes Progress in the Field of Brain Energy Metabolism. International Journal of Molecular Sciences. 2024; 25(3):1433. https://doi.org/10.3390/ijms25031433
Chicago/Turabian StyleSchurr, Avital. 2024. "How the ‘Aerobic/Anaerobic Glycolysis’ Meme Formed a ‘Habit of Mind’ Which Impedes Progress in the Field of Brain Energy Metabolism" International Journal of Molecular Sciences 25, no. 3: 1433. https://doi.org/10.3390/ijms25031433
APA StyleSchurr, A. (2024). How the ‘Aerobic/Anaerobic Glycolysis’ Meme Formed a ‘Habit of Mind’ Which Impedes Progress in the Field of Brain Energy Metabolism. International Journal of Molecular Sciences, 25(3), 1433. https://doi.org/10.3390/ijms25031433





