The Vestibular Nuclei: A Cerebral Reservoir of Stem Cells Involved in Balance Function in Normal and Pathological Conditions
Abstract
1. Introduction
2. Search Strategy and Selection Criteria
3. Neural Stem Cells and Neurogenic Niche
- –
- Neural stem cells.
- –
- Support cells that interact directly with stem cells and with each other via membrane receptors, gap junctions and soluble factors (growth factors, cytokines and various proteins; for review, see [11]). For neurogenic niches, the supporting cells are vascular cells, astrocytes, microglial cells, pericytes and ependymal cells.
- –
- Blood vessels that transport oxygen and nutrients. Vascularization also enables the recruitment of inflammatory cells and other circulating cells into the niche, as well as the entry and exit of stem cells. We can also highlight the role of blood vessels in the choroid plexus in regulating cerebrospinal fluid production and composition (for review, see Karakatsani et al., 2023 [13]).
- –
- An extracellular matrix that provides structure, organization and mechanical signals to the niche.
- –
- Nerve fibers that may also communicate physiological signals to the niche.
4. Spontaneous Neurogenesis
5. Reactive Neurogenesis
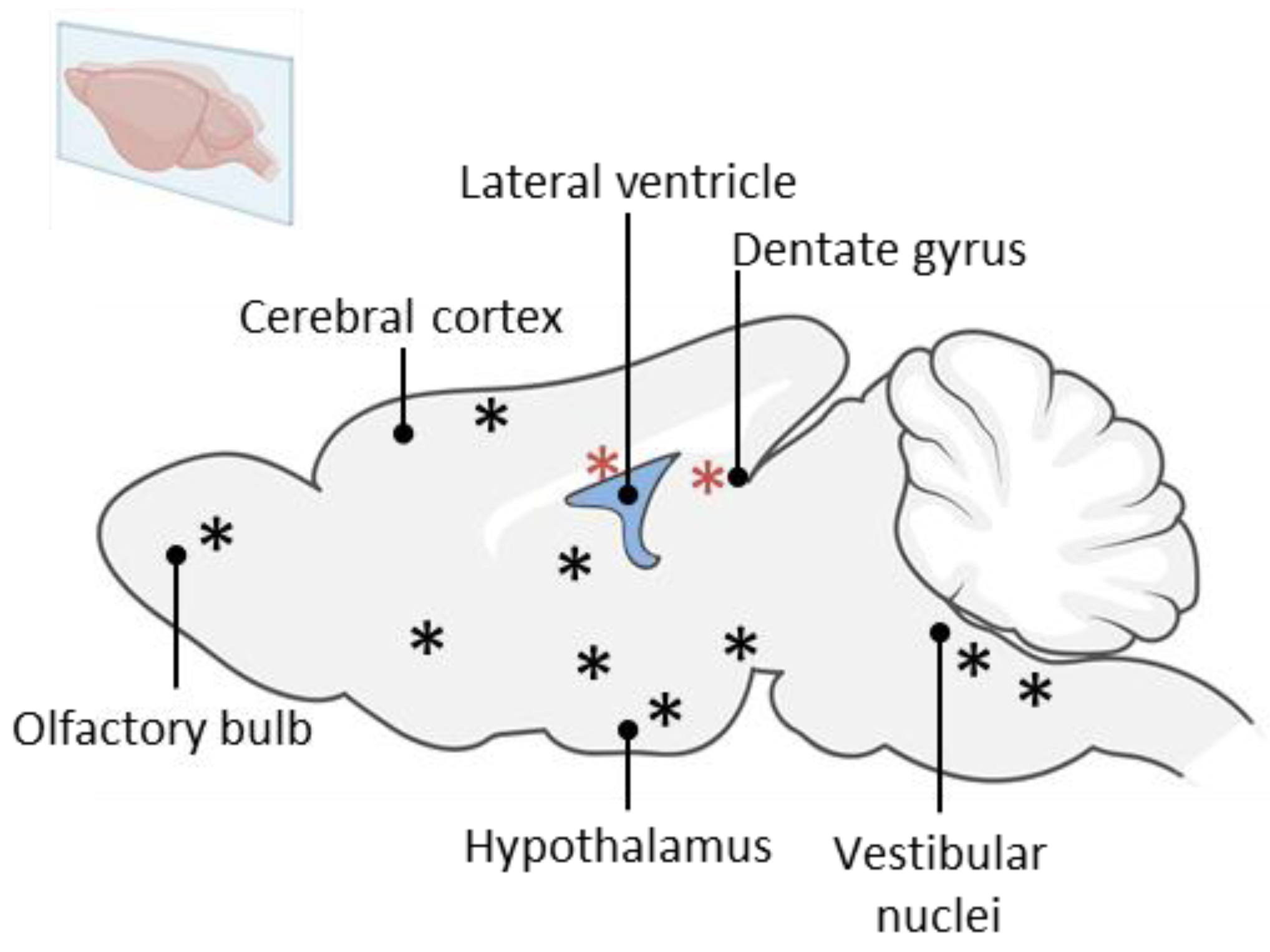
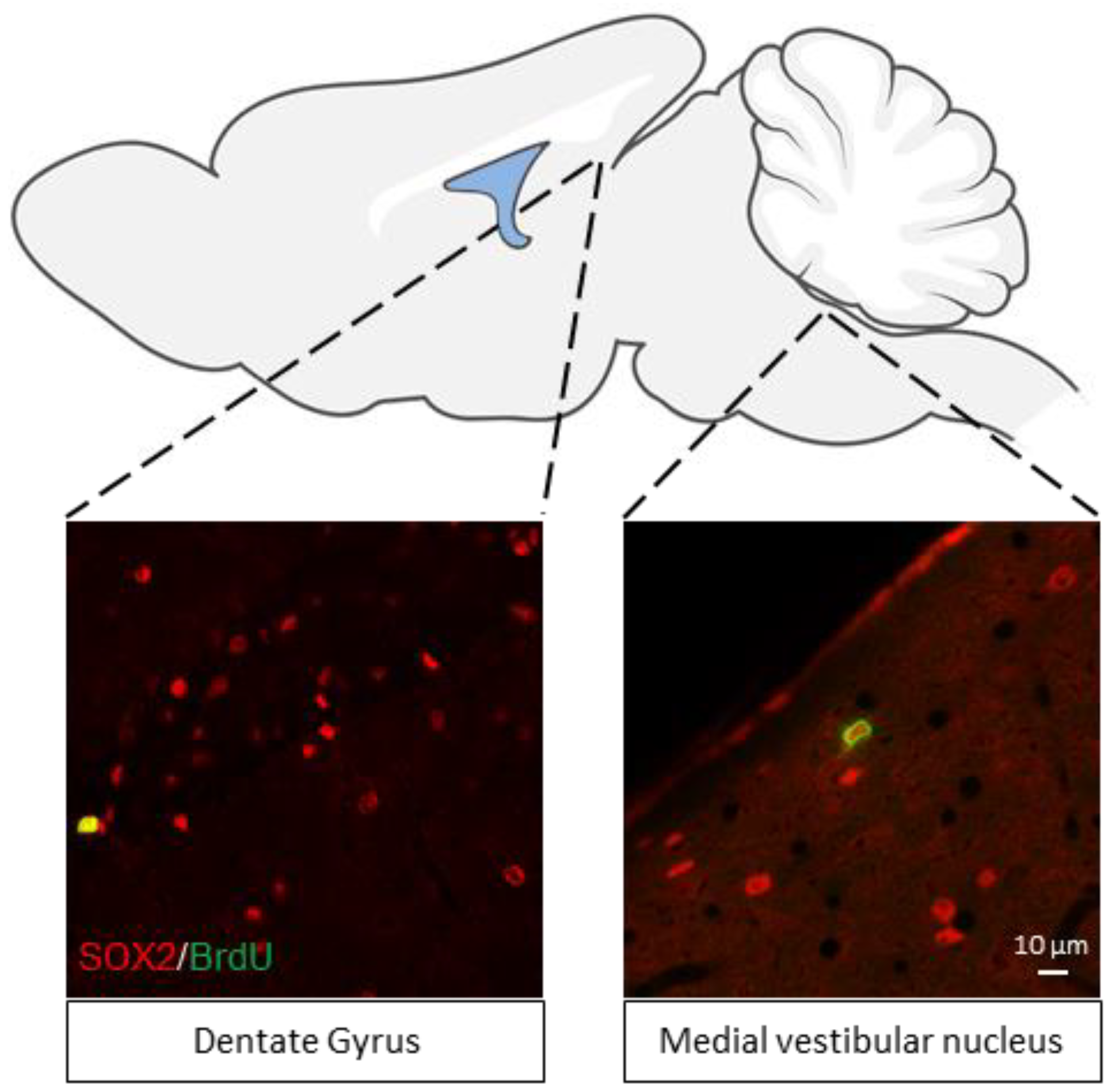
6. Vestibular Neurogenesis
7. A New Neurogenic Niche in the Brainstem: The Vestibular Nuclei
8. Why Neurogenesis Is Required in the Vestibular Nuclei
9. What Conditions Are Necessary to Activate the Vestibular Niche?
10. Neurogenesis or Gliogenesis to Promote Vestibular Compensation?
11. What about Oligodendrogenesis Playing a Role in Vestibular Compensation?
12. What Contribution Does Reactive Neurogenesis Exert on Vestibular Compensation?
13. Conclusions
Funding
Conflicts of Interest
References
- Gross, C.G. Neurogenesis in the adult brain: Death of a dogma. Nat. Rev. Neurosci. 2000, 1, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Cattaneo, E. Neural stem cell therapy for neurological diseases: Dreams and reality. Nat. Rev. Neurosci. 2002, 3, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, J.-W.; Shi, H.-Y.; Ma, Y.-M. Neural stem cell therapy for brain disease. World J. Stem Cells 2021, 13, 1278–1292. [Google Scholar] [CrossRef] [PubMed]
- Nie, L.; Yao, D.; Chen, S.; Wang, J.; Pan, C.; Wu, D.; Liu, N.; Tang, Z. Directional induction of neural stem cells, a new therapy for neurodegenerative diseases and ischemic stroke. Cell Death Discov. 2023, 9, 215. [Google Scholar] [CrossRef]
- Xu, Y.; Tamamaki, N.; Noda, T.; Kimura, K.; Itokazu, Y.; Matsumoto, N.; Dezawa, M.; Ide, C. Neurogenesis in the ependymal layer of the adult rat 3rd ventricle. Exp. Neurol. 2005, 192, 251–264. [Google Scholar] [CrossRef]
- Bolborea, M.; Dale, N. Hypothalamic tanycytes: Potential roles in the control of feeding and energy balance. Trends Neurosci. 2013, 36, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, G.; Trubert, C.; Terrien, J.; Pifferi, F.; Leroy, D.; Loyens, A.; Migaud, M.; Baroncini, M.; Maurage, C.-A.; Fontaine, C.; et al. A comparative study of the neural stem cell niche in the adult hypothalamus of human, mouse, rat and gray mouse lemur (Microcebus murinus). J. Comp. Neurol. 2018, 526, 1419–1443. [Google Scholar] [CrossRef]
- Rastoldo, G.; Watabe, I.; Lapotre, A.; Tonetto, A.; López-Juárez, A.; Tighilet, B. Vestibular Nuclei: A New Neural Stem Cell Niche? Cells 2022, 11, 3598. [Google Scholar] [CrossRef]
- Andreotti, J.P.; Silva, W.N.; Costa, A.C.; Picoli, C.C.; Bitencourt, F.C.O.; Coimbra-Campos, L.M.C.; Resende, R.R.; Magno, L.A.V.; Romano-Silva, M.A.; Mintz, A.; et al. Neural stem cell niche heterogeneity. In Seminars in Cell & Developmental Biology; Academic Press: Cambridge, MA, USA, 2019. [Google Scholar] [CrossRef]
- Scadden, D.T. The stem-cell niche as an entity of action. Nature 2006, 441, 1075–1079. [Google Scholar] [CrossRef]
- Bjornsson, C.S.; Apostolopoulou, M.; Tian, Y.; Temple, S. It Takes a Village: Constructing the Neurogenic Niche. Dev. Cell 2015, 32, 435–446. [Google Scholar] [CrossRef]
- Jones, D.L.; Wagers, A.J. No place like home: Anatomy and function of the stem cell niche. Nat. Rev. Mol. Cell Biol. 2008, 9, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Karakatsani, A.; Álvarez-Vergara, M.I.; Ruiz de Almodóvar, C. The vasculature of neurogenic niches: Properties and function. Cells Dev. 2023, 174, 203841. [Google Scholar] [CrossRef]
- Llorente, V.; Velarde, P.; Desco, M.; Gómez-Gaviro, M.V. Current Understanding of the Neural Stem Cell Niches. Cells 2022, 11, 3002. [Google Scholar] [CrossRef] [PubMed]
- Ming, G.; Song, H. Adult Neurogenesis in the Mammalian Brain: Significant Answers and Significant Questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Hardy, D.; Saghatelyan, A. Different forms of structural plasticity in the adult olfactory bulb. Neurogenesis 2017, 4, e1301850. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sun, W.; Kim, H.; Moon, Y. Control of neuronal migration through rostral migratory stream in mice. Anat. Cell Biol. 2010, 43, 269–279. [Google Scholar] [CrossRef]
- Ge, S.; Sailor, K.A.; Ming, G.; Song, H. Synaptic integration and plasticity of new neurons in the adult hippocampus. J. Physiol. 2008, 586, 3759–3765. [Google Scholar] [CrossRef]
- Fares, J.; Bou Diab, Z.; Nabha, S.; Fares, Y. Neurogenesis in the adult hippocampus: History, regulation, and prospective roles. Int. J. Neurosci. 2019, 129, 598–611. [Google Scholar] [CrossRef]
- Gould, E. How widespread is adult neurogenesis in mammals? Nat. Rev. Neurosci. 2007, 8, 481. [Google Scholar] [CrossRef]
- Migaud, M.; Batailler, M.; Segura, S.; Duittoz, A.; Franceschini, I.; Pillon, D. Emerging new sites for adult neurogenesis in the mammalian brain: A comparative study between the hypothalamus and the classical neurogenic zones: Neurogenesis in adult hypothalamus. Eur. J. Neurosci. 2010, 32, 2042–2052. [Google Scholar] [CrossRef]
- Pino, A.; Fumagalli, G.; Bifari, F.; Decimo, I. New neurons in adult brain: Distribution, molecular mechanisms and therapies. Biochem. Pharmacol. 2017, 141, 4–22. [Google Scholar] [CrossRef] [PubMed]
- Bédard, A.; Cossette, M.; Lévesque, M.; Parent, A. Proliferating cells can differentiate into neurons in the striatum of normal adult monkey. Neurosci. Lett. 2002, 328, 213–216. [Google Scholar] [CrossRef] [PubMed]
- Dayer, A.G.; Cleaver, K.M.; Abouantoun, T.; Cameron, H.A. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J. Cell Biol. 2005, 168, 415–427. [Google Scholar] [CrossRef]
- Gould, E.; Reeves, A.J.; Graziano, M.S.; Gross, C.G. Neurogenesis in the neocortex of adult primates. Science 1999, 286, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Momma, S.; Delfani, K.; Carlen, M.; Cassidy, R.M.; Johansson, C.B.; Brismar, H.; Shupliakov, O.; Frisen, J.; Janson, A.M. Evidence for neurogenesis in the adult mammalian substantia nigra. Proc. Natl. Acad. Sci. USA 2003, 100, 7925–7930. [Google Scholar] [CrossRef] [PubMed]
- Bernier, P.J.; Bedard, A.; Vinet, J.; Levesque, M.; Parent, A. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc. Natl. Acad. Sci. USA 2002, 99, 11464–11469. [Google Scholar] [CrossRef] [PubMed]
- Bauer, S.; Hay, M.; Amilhon, B.; Jean, A.; Moyse, E. In vivo neurogenesis in the dorsal vagal complex of the adult rat brainstem. Neuroscience 2005, 130, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Luzzati, F. Neurogenesis in the Caudate Nucleus of the Adult Rabbit. J. Neurosci. 2006, 26, 609–621. [Google Scholar] [CrossRef]
- Bédard, A.; Lévesque, M.; Bernier, P.J.; Parent, A. The rostral migratory stream in adult squirrel monkeys: Contribution of new neurons to the olfactory tubercle and involvement of the antiapoptotic protein Bcl-2: The rostral migratory stream in adult primates. Eur. J. Neurosci. 2002, 16, 1917–1924. [Google Scholar] [CrossRef]
- Bennett, L.; Yang, M.; Enikolopov, G.; Iacovitti, L. Circumventricular organs: A novel site of neural stem cells in the adult brain. Mol. Cell. Neurosci. 2009, 41, 337–347. [Google Scholar] [CrossRef]
- Chaker, Z.; George, C.; Petrovska, M.; Caron, J.-B.; Lacube, P.; Caillé, I.; Holzenberger, M. Hypothalamic neurogenesis persists in the aging brain and is controlled by energy-sensing IGF-I pathway. Neurobiol. Aging 2016, 41, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Kokoeva, M.V. Neurogenesis in the Hypothalamus of Adult Mice: Potential Role in Energy Balance. Science 2005, 310, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Cayre, M.; Canoll, P.; Goldman, J.E. Cell migration in the normal and pathological postnatal mammalian brain. Prog. Neurobiol. 2009, 88, 41–63. [Google Scholar] [CrossRef] [PubMed]
- Nemirovich-Danchenko, N.M.; Khodanovich, M.Y. New Neurons in the Post-ischemic and Injured Brain: Migrating or Resident? Front. Neurosci. 2019, 13, 588. [Google Scholar] [CrossRef] [PubMed]
- Tighilet, B.; Brezun, J.M.; Sylvie, G.D.D.; Gaubert, C.; Lacour, M. New neurons in the vestibular nuclei complex after unilateral vestibular neurectomy in the adult cat. Eur. J. Neurosci. 2007, 25, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Dutheil, S.; Brezun, J.M.; Leonard, J.; Lacour, M.; Tighilet, B. Neurogenesis and astrogenesis contribution to recovery of vestibular functions in the adult cat following unilateral vestibular neurectomy: Cellular and behavioral evidence. Neuroscience 2009, 164, 1444–1456. [Google Scholar] [CrossRef] [PubMed]
- Dutheil, S.; Watabe, I.; Sadlaoud, K.; Tonetto, A.; Tighilet, B. BDNF signaling promotes vestibular compensation by increasing neurogenesis and remodeling the expression of potassium-chloride cotransporter KCC2 and GABAA receptor in the vestibular nuclei. J. Neurosci. 2016, 36, 6199–6212. [Google Scholar] [CrossRef] [PubMed]
- Rastoldo, G.; El Mahmoudi, N.; Marouane, E.; Pericat, D.; Watabe, I.; Toneto, A.; López-Juárez, A.; Chabbert, C.; Tighilet, B. Adult and endemic neurogenesis in the vestibular nuclei after unilateral vestibular neurectomy. Prog. Neurobiol. 2021, 196, 101899. [Google Scholar] [CrossRef]
- Cope, E.C.; Gould, E. Adult Neurogenesis, Glia, and the Extracellular Matrix. Cell Stem Cell 2019, 24, 690–705. [Google Scholar] [CrossRef]
- Mercier, F. Fractones: Extracellular matrix niche controlling stem cell fate and growth factor activity in the brain in health and disease. Cell. Mol. Life Sci. 2016, 73, 4661–4674. [Google Scholar] [CrossRef]
- Mercier, F.; Schnack, J.; Chaumet, M.S.G. Fractones: Home and Conductors of the Neural Stem Cell Niche. In Neurogenesis in the Adult Brain I; Springer: Berlin/Heidelberg, Germany, 2011; pp. 109–133. [Google Scholar] [CrossRef]
- Preston, M.; Sherman, L.S. Neural Stem Cell Niches: Critical Roles for the Hyaluronan- Based Extracellular Matrix in Neural Stem Cell Proliferation and Differentiation. Front. Biosci. 2012, 22, 1165. [Google Scholar]
- Gaal, B.; Jóhannesson, E.; Dattani, A.; Magyar, A.; Wéber, I.; Matesz, C. Modification of tenascin-R expression following unilateral labyrinthectomy in rats indicates its possible role in neural plasticity of the vestibular neural circuit. Neural Regen. Res. 2015, 10, 1463. [Google Scholar] [CrossRef] [PubMed]
- Rácz, E.; Gaál, B.; Kecskes, S.; Matesz, C. Molecular composition of extracellular matrix in the vestibular nuclei of the rat. Brain Struct. Funct. 2014, 219, 1385–1403. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, M.A.; Sorokin, L.; Coelho-Sampaio, T. Fractone Bulbs Derive from Ependymal Cells and Their Laminin Composition Influence the Stem Cell Niche in the Subventricular Zone. J. Neurosci. 2018, 38, 3880–3889. [Google Scholar] [CrossRef]
- Karakatsani, A.; Shah, B.; Ruiz de Almodovar, C. Blood Vessels as Regulators of Neural Stem Cell Properties. Front. Mol. Neurosci. 2019, 12, 85. [Google Scholar] [CrossRef]
- Shen, Q.; Wang, Y.; Kokovay, E.; Lin, G.; Chuang, S.-M.; Goderie, S.K.; Roysam, B.; Temple, S. Adult SVZ stem cells lie in a vascular niche: A quantitative analysis of niche cell-cell interactions. Cell Stem Cell 2008, 3, 289–300. [Google Scholar] [CrossRef]
- Tavazoie, M.; Van der Veken, L.; Silva-Vargas, V.; Louissaint, M.; Colonna, L.; Zaidi, B.; Garcia-Verdugo, J.M.; Doetsch, F. A specialized vascular niche for adult neural stem cells. Cell Stem Cell 2008, 3, 279–288. [Google Scholar] [CrossRef]
- Lee, J.O.; Park, S.-H.; Kim, H.J.; Kim, M.S.; Park, B.R.; Kim, J.-S. Vulnerability of the vestibular organs to transient ischemia: Implications for isolated vascular vertigo. Neurosci. Lett. 2014, 558, 180–185. [Google Scholar] [CrossRef]
- Smith, P.F.; Horii, A.; Russell, N.; Bilkey, D.K.; Zheng, Y.; Liu, P.; Kerr, D.S.; Darlington, C.L. The effects of vestibular lesions on hippocampal function in rats. Prog. Neurobiol. 2005, 75, 391–405. [Google Scholar] [CrossRef]
- El Mahmoudi, N.; Laurent, C.; Péricat, D.; Watabe, I.; Lapotre, A.; Jacob, P.-Y.; Tonetto, A.; Tighilet, B.; Sargolini, F. Long-lasting spatial memory deficits and impaired hippocampal plasticity following unilateral vestibular loss. Prog. Neurobiol. 2023, 223, 102403. [Google Scholar] [CrossRef]
- Pettorossi, V.E.; Dieni, C.V.; Scarduzio, M.; Grassi, S. Long-term potentiation of synaptic response and intrinsic excitability in neurons of the rat medial vestibular nuclei. Neuroscience 2011, 187, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Pettorossi, V.E.; Dutia, M.; Frondaroli, A.; Dieni, C.; Grassi, S. Long-term Potentiation and Depression after Unilateral Labyrinthectomy in the Medial Vestibular Nucleus of Rats. Acta Oto-Laryngol. 2003, 123, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Rastoldo, G.; Marouane, E.; El Mahmoudi, N.; Péricat, D.; Bourdet, A.; Timon-David, E.; Dumas, O.; Chabbert, C.; Tighilet, B. Quantitative Evaluation of a New Posturo-Locomotor Phenotype in a Rodent Model of Acute Unilateral Vestibulopathy. Front. Neurol. 2020, 11, 505. [Google Scholar] [CrossRef] [PubMed]
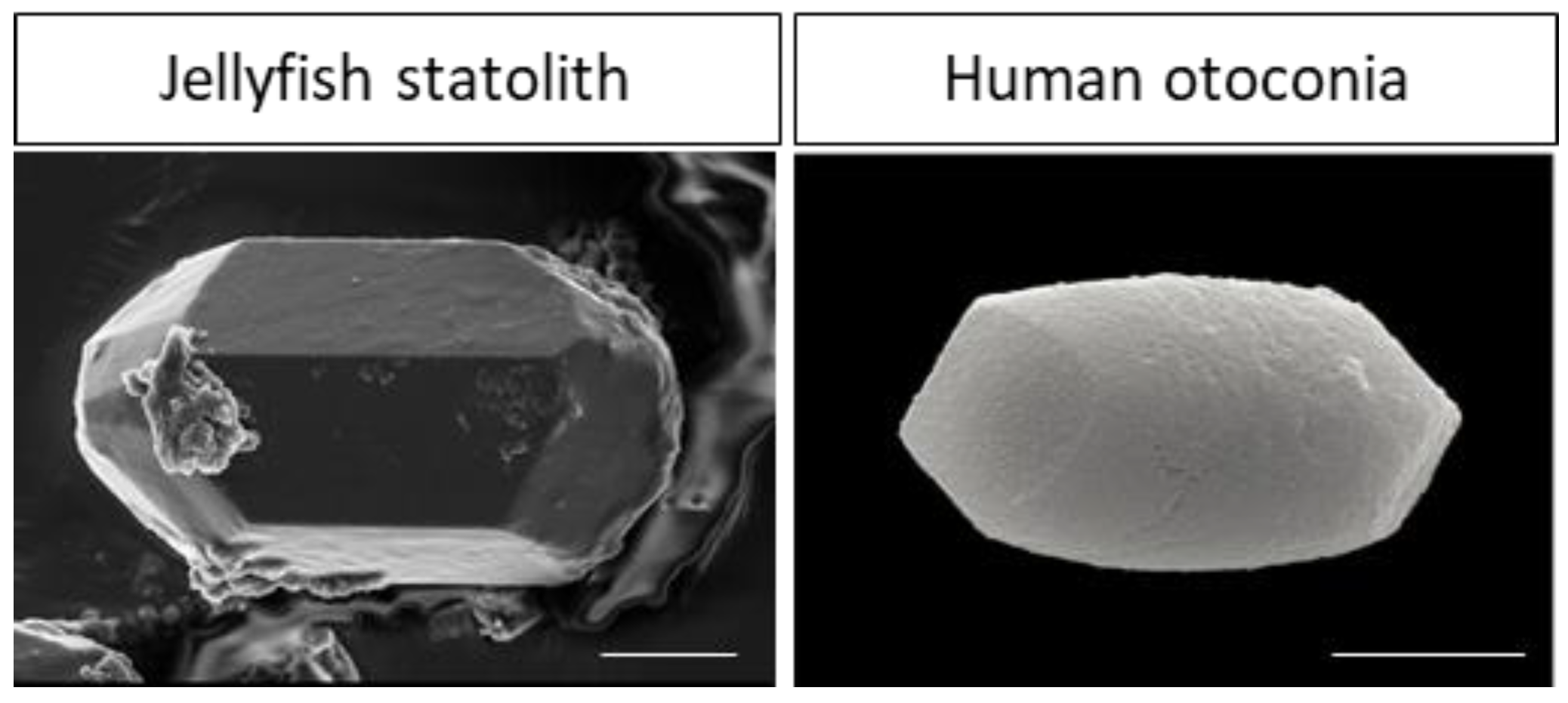
- Ramos de Miguel, A.; Zarowski, A.; Sluydts, M.; Ramos Macias, A.; Wuyts, F.L. The Superiority of the Otolith System. Audiol. Neurootol. 2020, 25, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Heins, A.; Sötje, I.; Holst, S. Assessment of investigation techniques for scyphozoan statoliths, with focus on early development of the jellyfish Sanderia malayensis. Mar. Ecol. Prog. Ser. 2018, 591, 37–56. [Google Scholar] [CrossRef]
- Sötje, I.; Dishon, T.; Hoffmann, F.; Holst, S. New Methods of Morphometric Analyses on Scyphozoan Jellyfish Statoliths Including the first Direct Evidence for Statolith Growth Using Calcein as a Fluorescent Marker. Microsc. Microanal. 2017, 23, 553–568. [Google Scholar] [CrossRef] [PubMed]
- Kniep, R.; Zahn, D.; Wulfes, J.; Walther, L.E. The sense of balance in humans: Structural features of otoconia and their response to linear acceleration. PLoS ONE 2017, 12, e0175769. [Google Scholar] [CrossRef] [PubMed]
- Toyokawa, M.; Shirai, K.; Takahata, N.; Ishida, A.; Sano, Y. Growth and formation of statoliths in Aurelia coerulea examined by using 34S- and Sr-labels. J. Plankton Res. 2018, 40, 619–626. [Google Scholar] [CrossRef]
- Daynac, M.; Chicheportiche, A.; Pineda, J.R.; Gauthier, L.R.; Boussin, F.D.; Mouthon, M.-A. Quiescent neural stem cells exit dormancy upon alteration of GABAAR signaling following radiation damage. Stem. Cell Res. 2013, 11, 516–528. [Google Scholar] [CrossRef]
- Moss, J.; Toni, N. A circuit-based gatekeeper for adult neural stem cell proliferation: Parvalbumin-expressing interneurons of the dentate gyrus control the activation and proliferation of quiescent adult neural stem cells. BioEssays News Rev. Mol. Cell. Dev. Biol. 2013, 35, 28–33. [Google Scholar] [CrossRef]
- Pontes, A.; Zhang, Y.; Hu, W. Novel functions of GABA signaling in adult neurogenesis. Front. Biol. 2013, 8, 496–507. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Zhong, C.; Bonaguidi, M.A.; Sun, G.J.; Hsu, D.; Gu, Y.; Meletis, K.; Huang, Z.J.; Ge, S.; Enikolopov, G.; et al. Neuronal circuitry mechanism regulating adult quiescent neural stem-cell fate decision. Nature 2012, 489, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Barmack, N.H. Vestibular Nuclei and Their Cerebellar Connections. In Essentials of Cerebellum and Cerebellar Disorders: A Primer for Graduate Students; Gruol, D.L., Koibuchi, N., Manto, M., Molinari, M., Schmahmann, J.D., Shen, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 69–78. [Google Scholar] [CrossRef]
- Malinvaud, D.; Vassias, I.; Reichenberger, I.; Rossert, C.; Straka, H. Functional Organization of Vestibular Commissural Connections. Frog. J. Neurosci. 2010, 30, 3310–3325. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.Y.; Huang, J.; Wang, H. Waking up quiescent neural stem cells: Molecular mechanisms and implications in neurodevelopmental disorders. PLoS Genet. 2020, 16, e1008653. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Aparicio, I.; Paris, I.; Sierra-Torre, V.; Plaza-Zabala, A.; Rodríguez-Iglesias, N.; Márquez-Ropero, M.; Beccari, S.; Huguet, P.; Abiega, O.; Alberdi, E.; et al. Microglia Actively Remodel Adult Hippocampal Neurogenesis through the Phagocytosis Secretome. J. Neurosci. 2020, 40, 1453–1482. [Google Scholar] [CrossRef] [PubMed]
- Dutheil, S.; Lacour, M.; Tighilet, B. Neurogenic Potential of the Vestibular Nuclei and Behavioural Recovery Time Course in the Adult Cat Are Governed by the Nature of the Vestibular Damage. PLoS ONE 2011, 6, e22262. [Google Scholar] [CrossRef]
- Shaabani, M.; Lotfi, Y.; Karimian, S.M.; Rahgozar, M.; Hooshmandi, M. Short-term galvanic vestibular stimulation promotes functional recovery and neurogenesis in unilaterally labyrinthectomized rats. Brain Res. 2016, 1648, 152–162. [Google Scholar] [CrossRef]
- Mao, D.; He, Z.; Xuan, W.; Deng, J.; Li, W.; Fang, X.; Li, L.; Zhang, F. Effect and mechanism of BDNF/TrkB signaling on vestibular compensation. Bioengineered 2021, 12, 11823–11836. [Google Scholar] [CrossRef]
- Crutcher, K.A.; Gendelman, H.E.; Kipnis, J.; Perez-Polo, J.R.; Perry, V.H.; Popovich, P.G.; Weaver, L.C. Debate: “is increasing neuroinflammation beneficial for neural repair?”. J. Neuroimmune Pharmacol. 2006, 1, 195–211. [Google Scholar] [CrossRef]
- Kyritsis, N.; Kizil, C.; Brand, M. Neuroinflammation and central nervous system regeneration in vertebrates. Trends Cell Biol. 2014, 24, 128–135. [Google Scholar] [CrossRef]
- Llorens-Bobadilla, E.; Zhao, S.; Baser, A.; Saiz-Castro, G.; Zwadlo, K.; Martin-Villalba, A. Single-Cell Transcriptomics Reveals a Population of Dormant Neural Stem Cells that Become Activated upon Brain Injury. Cell Stem Cell 2015, 17, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Liberge, M.; Manrique, C.; Bernard-Demanze, L.; Lacour, M. Changes in TNFα, NFκB and MnSOD protein in the vestibular nuclei after unilateral vestibular deafferentation. J. Neuroinflamm. 2010, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Campos-Torres, A.; Touret, M.; Vidal, P.P.; Barnum, S.; de Waele, C. The differential response of astrocytes within the vestibular and cochlear nuclei following unilateral labyrinthectomy or vestibular afferent activity blockade by transtympanic tetrodotoxin injection in the rat. Neuroscience 2005, 130, 853–865. [Google Scholar] [CrossRef] [PubMed]
- El Mahmoudi, N.; Marouane, E.; Rastoldo, G.; Pericat, D.; Watabe, I.; Lapotre, A.; Tonetto, A.; Chabbert, C.; Tighilet, B. Microglial Dynamics Modulate Vestibular Compensation in a Rodent Model of Vestibulopathy and Condition the Expression of Plasticity Mechanisms in the Deafferented Vestibular Nuclei. Cells 2022, 11, 2693. [Google Scholar] [CrossRef] [PubMed]
- El Mahmoudi, N.; Rastoldo, G.; Marouane, E.; Péricat, D.; Watabe, I.; Tonetto, A.; Hautefort, C.; Chabbert, C.; Sargolini, F.; Tighilet, B. Breaking a dogma: Acute anti-inflammatory treatment alters both post-lesional functional recovery and endogenous adaptive plasticity mechanisms in a rodent model of acute peripheral vestibulopathy. J. Neuroinflamm. 2021, 18, 183. [Google Scholar] [CrossRef]
- Lacour, M.; Tighilet, B. Plastic events in the vestibular nuclei during vestibular compensation: The brain orchestration of a “deafferentation” code. Restor. Neurol. Neurosci. 2010, 28, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Rastoldo, G.; Marouane, E.; El-Mahmoudi, N.; Péricat, D.; Watabe, I.; Lapotre, A.; Tonetto, A.; López-Juárez, A.; El-Ahmadi, A.; Caron, P.; et al. L-Thyroxine Improves Vestibular Compensation in a Rat Model of Acute Peripheral Vestibulopathy: Cellular and Behavioral Aspects. Cells 2022, 11, 684. [Google Scholar] [CrossRef]
- Marouane, E.; El Mahmoudi, N.; Rastoldo, G.; Péricat, D.; Watabe, I.; Lapôtre, A.; Tonetto, A.; Xavier, F.; Dumas, O.; Chabbert, C.; et al. Sensorimotor Rehabilitation Promotes Vestibular Compensation in a Rodent Model of Acute Peripheral Vestibulopathy by Promoting Microgliogenesis in the Deafferented Vestibular Nuclei. Cells 2021, 10, 3377. [Google Scholar] [CrossRef]
- Haruwaka, K.; Ying, Y.; Liang, Y.; Umpierre, A.D.; Yi, M.-H.; Kremen, V.; Chen, T.; Xie, T.; Dong, H.; Worrell, G.A.; et al. Microglial Process Dynamics Enhance Neuronal Activity by Shielding GABAergic Synaptic Inputs. bioRxiv 2022. [Google Scholar] [CrossRef]
- Umpierre, A.D.; Wu, L.-J. How Microglia Sense and Regulate Neuronal Activity. Glia 2021, 69, 1637–1653. [Google Scholar] [CrossRef]
- Bergquist, F.; Ludwig, M.; Dutia, M.B. Role of the commissural inhibitory system in vestibular compensation in the rat. J. Physiol. 2008, 586, 4441–4452. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari, Y. NKCC1 Chloride Importer Antagonists Attenuate Many Neurological and Psychiatric Disorders. Trends Neurosci. 2017, 40, 536–554. [Google Scholar] [CrossRef]
- Boulenguez, P.; Liabeuf, S.; Bos, R.; Bras, H.; Jean-Xavier, C.; Brocard, C.; Stil, A.; Darbon, P.; Cattaert, D.; Delpire, E.; et al. Down-regulation of the potassium-chloride cotransporter KCC2 contributes to spasticity after spinal cord injury. Nat. Med. 2010, 16, 302–307. [Google Scholar] [CrossRef]
- Dargaei, Z.; Bang, J.Y.; Mahadevan, V.; Khademullah, C.S.; Bedard, S.; Parfitt, G.M.; Kim, J.C.; Woodin, M.A. Restoring GABAergic inhibition rescues memory deficits in a Huntington’s disease mouse model. Proc. Natl. Acad. Sci. USA 2018, 115, E1618–E1626. [Google Scholar] [CrossRef] [PubMed]
- Gagnon, M.; Bergeron, M.J.; Lavertu, G.; Castonguay, A.; Tripathy, S.; Bonin, R.P.; Perez-Sanchez, J.; Boudreau, D.; Wang, B.; Dumas, L.; et al. Chloride extrusion enhancers as novel therapeutics for neurological diseases. Nat. Med. 2013, 19, 1524–1528. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yu, X.; Chen, P.; Jin, K.; Zhou, J.; Wang, G.; Yu, J.; Wu, T.; Wang, Y.; Lin, F.; et al. BDNF-TrkB signaling pathway-mediated microglial activation induces neuronal KCC2 downregulation contributing to dynamic allodynia following spared nerve injury. Mol. Pain 2023, 19, 17448069231185440. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zhou, W.; Zhang, S.; Liu, B.; Liang, P.; Zhou, Y.; Zhou, T.; Zhang, K.; Leng, Y.; Kong, W. BDNF signaling in the rat cerebello-vestibular pathway during vestibular compensation: BDNF signaling in vestibular compensation. FEBS J. 2015, 282, 3579–3591. [Google Scholar] [CrossRef]
- Kalambogias, J.; Chen, C.-C.; Khan, S.; Son, T.; Wercberger, R.; Headlam, C.; Lin, C.; Brumberg, J.C. Development and Sensory Experience Dependent Regulation of Microglia in Barrel Cortex. J. Comp. Neurol. 2020, 528, 559–573. [Google Scholar] [CrossRef]
- Cheadle, L.; Rivera, S.A.; Phelps, J.S.; Ennis, K.A.; Stevens, B.; Burkly, L.C.; Lee, W.-C.A.; Greenberg, M.E. Sensory Experience Engages Microglia to Shape Neural Connectivity through a Non-Phagocytic Mechanism. Neuron 2020, 108, 451–468.e9. [Google Scholar] [CrossRef]
- Tremblay, M.-È.; Lowery, R.L.; Majewska, A.K. Microglial Interactions with Synapses Are Modulated by Visual Experience. PLoS Biol. 2010, 8, e1000527. [Google Scholar] [CrossRef]
- Jamali, M.; Mitchell, D.E.; Dale, A.; Carriot, J.; Sadeghi, S.G.; Cullen, K.E. Neuronal detection thresholds during vestibular compensation: Contributions of response variability and sensory substitution. J. Physiol. 2014, 592, 1565–1580. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.G.; Minor, L.B.; Cullen, K.E. Neural correlates of sensory substitution in vestibular pathways following complete vestibular loss. J. Neurosci. 2012, 32, 14685–14695. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.G.; Minor, L.B.; Cullen, K.E. Multimodal integration after unilateral labyrinthine lesion: Single vestibular nuclei neuron responses and implications for postural compensation. J. Neurophysiol. 2011, 105, 661–673. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, S.G.; Minor, L.B.; Cullen, K.E. Neural correlates of motor learning in the vestibulo-ocular reflex: Dynamic regulation of multimodal integration in the macaque vestibular system. J. Neurosci. 2010, 30, 10158–10168. [Google Scholar] [CrossRef] [PubMed]
- Chang, M.Y.; Park, S.; Choi, J.J.; Kim, Y.-K.; Suh, M.-W.; Lee, J.H.; Oh, S.H.; Park, M.K. MicroRNAs 218a-5p, 219a-5p, and 221-3p regulate vestibular compensation. Sci. Rep. 2017, 7, 8701. [Google Scholar] [CrossRef]
- Dugas, J.C.; Cuellar, T.L.; Scholze, A.; Ason, B.; Ibrahim, A.; Emery, B.; Zamanian, J.L.; Foo, L.C.; McManus, M.T.; Barres, B.A. Dicer1 and miR-219 Are required for normal oligodendrocyte differentiation and myelination. Neuron 2010, 65, 597–611. [Google Scholar] [CrossRef]
- Bagayogo, I.P.; Dreyfus, C.F. Regulated Release of BDNF by Cortical Oligodendrocytes is Mediated Through Metabotropic Glutamate Receptors and the PLC Pathway. ASN Neuro 2009, 1, AN20090006. [Google Scholar] [CrossRef]
- Narine, M.; Colognato, H. Current Insights Into Oligodendrocyte Metabolism and Its Power to Sculpt the Myelin Landscape. Front. Cell Neurosci. 2022, 16, 892968. [Google Scholar] [CrossRef]
- Wilkins, A.; Majed, H.; Layfield, R.; Compston, A.; Chandran, S. Oligodendrocytes Promote Neuronal Survival and Axonal Length by Distinct Intracellular Mechanisms: A Novel Role for Oligodendrocyte-Derived Glial Cell Line-Derived Neurotrophic Factor. J. Neurosci. 2003, 23, 4967–4974. [Google Scholar] [CrossRef]
- Fritzsch, B.; Tessarollo, L.; Coppola, E.; Reichardt, L.F. Neurotrophins in the ear: Their roles in sensory neuron survival and fiber guidance. Prog. Brain Res. 2004, 146, 265–278. [Google Scholar] [CrossRef]
- Johnson Chacko, L.; Blumer, M.J.F.; Pechriggl, E.; Rask-Andersen, H.; Dietl, W.; Haim, A.; Fritsch, H.; Glueckert, R.; Dudas, J.; Schrott-Fischer, A. Role of BDNF and neurotrophic receptors in human inner ear development. Cell Tissue Res. 2017, 370, 347–363. [Google Scholar] [CrossRef] [PubMed]
- Maingay, M.G.; Sansom, A.J.; Kerr, D.R.; Smith, P.F.; Darlington, C.L. The effects of intra-vestibular nucleus administration of brain-derived neurotrophic factor (BDNF) on recovery from peripheral vestibular damage in guinea pig. Neuroreport 2000, 11, 2429–2432. [Google Scholar] [CrossRef] [PubMed]
- Nutma, E.; van Gent, D.; Amor, S.; Peferoen, L.A.N. Astrocyte and Oligodendrocyte Cross-Talk in the Central Nervous System. Cells 2020, 9, 600. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E.M.; Purger, D.; Mount, C.W.; Goldstein, A.K.; Lin, G.L.; Wood, L.S.; Inema, I.; Miller, S.E.; Bieri, G.; Zuchero, J.B.; et al. Neuronal Activity Promotes Oligodendrogenesis and Adaptive Myelination in the Mammalian Brain. Science 2014, 344, 1252304. [Google Scholar] [CrossRef] [PubMed]
- McKenzie, I.A.; Ohayon, D.; Li, H.; Paes de Faria, J.; Emery, B.; Tohyama, K.; Richardson, W.D. Motor skill learning requires active central myelination. Science 2014, 346, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Teng, E.M.; Summers, R.G.; Ming, G.; Gage, F.H. Distinct Morphological Stages of Dentate Granule Neuron Maturation in the Adult Mouse Hippocampus. J. Neurosci. 2006, 26, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Iglesias, N.; Sierra, A.; Valero, J. Rewiring of Memory Circuits: Connecting Adult Newborn Neurons With the Help of Microglia. Front. Cell Dev. Biol. 2019, 7, 24. [Google Scholar] [CrossRef]
- Marouane, E.; Rastoldo, G.; El Mahmoudi, N.; Péricat, D.; Chabbert, C.; Artzner, V.; Tighilet, B. Identification of New Biomarkers of Posturo-Locomotor Instability in a Rodent Model of Vestibular Pathology. Front. Neurol. 2020, 11, 470. [Google Scholar] [CrossRef]
- Tighilet, B.; Péricat, D.; Frelat, A.; Cazals, Y.; Rastoldo, G.; Boyer, F.; Dumas, O.; Chabbert, C. Adjustment of the dynamic weight distribution as a sensitive parameter for diagnosis of postural alteration in a rodent model of vestibular deficit. PLoS ONE 2017, 12, e0187472. [Google Scholar] [CrossRef]
- Ris, L.; Hachemaoui, M.; Godaux, E. Effect of labyrinthectomy on the spike generator of vestibular neurons in the guinea pig. Neuroreport 2002, 13, 1875–1879. [Google Scholar] [CrossRef]
- Ris, L.; Wattiez, R.; de Waele, C.; Vidal, P.-P.; Godaux, E. Reappearance of activity in the vestibular neurones of labyrinthectomized guinea-pigs is not delayed by cycloheximide. J. Physiol. 1998, 512, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Ris, L.; Capron, B.; de Waele, C.; Vidal, P.P.; Godaux, E. Dissociations between behavioural recovery and restoration of vestibular activity in the unilabyrinthectomized guinea-pig. J. Physiol. 1997, 500, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Ris, L.; de Waele, C.; Serafin, M.; Vidal, P.P.; Godaux, E. Neuronal activity in the ipsilateral vestibular nucleus following unilateral labyrinthectomy in the alert guinea pig. J. Neurophysiol. 1995, 74, 2087–2099. [Google Scholar] [CrossRef] [PubMed]
- Curthoys, I.S.; Halmagyi, G.M. Vestibular compensation: A review of the oculomotor, neural, and clinical consequences of unilateral vestibular loss. J. Vestib. Res. Equilib. Orientat. 1995, 5, 67–107. [Google Scholar] [CrossRef]
- Smith, P.F.; Curthoys, I.S. Mechanisms of recovery following unilateral labyrinthectomy: A review. Brain Res. Rev. 1989, 14, 155–180. [Google Scholar] [CrossRef] [PubMed]
- Bushong, E.A.; Martone, M.E.; Ellisman, M.H. Maturation of astrocyte morphology and the establishment of astrocyte domains during postnatal hippocampal development. Int. J. Dev. Neurosci. 2004, 22, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Lankford, K.; Radtke, C.; Greer, C.A.; Kocsis, J.D. Remyelination of spinal cord axons by olfactory ensheathing cells and Schwann cells derived from a transgenic rat expressing alkaline phosphatase marker gene. Neuron Glia Biol. 2004, 1, 47–55. [Google Scholar] [CrossRef] [PubMed]
- Chanoumidou, K.; Mozafari, S.; Evercooren, A.B.-V.; Kuhlmann, T. Stem cell derived oligodendrocytes to study myelin diseases. Glia 2020, 68, 705–720. [Google Scholar] [CrossRef]
- Beraneck, M.; Idoux, E. Reconsidering the Role of Neuronal Intrinsic Properties and Neuromodulation in Vestibular Homeostasis. Front. Neurol. 2012, 3, 25. [Google Scholar] [CrossRef]
- Cameron, S.A.; Dutia, M.B. Cellular basis of vestibular compensation: Changes in intrinsic excitability of MVN neurones. Neuroreport 1997, 8, 2595–2599. [Google Scholar] [CrossRef]
- Chen, Z.-P.; Zhang, X.-Y.; Peng, S.-Y.; Yang, Z.-Q.; Wang, Y.-B.; Zhang, Y.-X.; Chen, X.; Wang, J.-J.; Zhu, J.-N. Histamine H1 Receptor Contributes to Vestibular Compensation. J. Neurosci. 2019, 39, 420–433. [Google Scholar] [CrossRef]
- Eugène, D.; Idoux, E.; Beraneck, M.; Moore, L.E.; Vidal, P.-P. Intrinsic membrane properties of central vestibular neurons in rodents. Exp. Brain Res. 2011, 210, 423–436. [Google Scholar] [CrossRef]
- Lim, R.; Callister, R.J.; Brichta, A.M. An increase in glycinergic quantal amplitude and frequency during early vestibular compensation in mouse. J. Neurophysiol. 2010, 103, 16–24. [Google Scholar] [CrossRef]
- Shao, M.; Reddaway, R.; Hirsch, J.C.; Peusner, K.D. Presynaptic GABAB Receptors Decrease Neurotransmitter Release in Vestibular Nuclei Neurons During Vestibular Compensation. Neuroscience 2012, 223, 333–354. [Google Scholar] [CrossRef][Green Version]
- Yamanaka, T.; Him, A.; Cameron, S.A.; Dutia, M.B. Rapid compensatory changes in GABA receptor efficacy in rat vestibular neurones after unilateral labyrinthectomy. J. Physiol. 2000, 523, 413–424. [Google Scholar] [CrossRef]
- Zwergal, A.; Lindner, M.; Grosch, M.; Dieterich, M. In vivo neuroplasticity in vestibular animal models. Mol. Cell Neurosci. 2022, 120, 103721. [Google Scholar] [CrossRef]
- Zwergal, A.; Schlichtiger, J.; Xiong, G.; Beck, R.; Günther, L.; Schniepp, R.; Schöberl, F.; Jahn, K.; Brandt, T.; Strupp, M.; et al. Sequential [(18)F]FDG µPET whole-brain imaging of central vestibular compensation: A model of deafferentation-induced brain plasticity. Brain Struct. Funct. 2016, 221, 159–170. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rastoldo, G.; Tighilet, B. The Vestibular Nuclei: A Cerebral Reservoir of Stem Cells Involved in Balance Function in Normal and Pathological Conditions. Int. J. Mol. Sci. 2024, 25, 1422. https://doi.org/10.3390/ijms25031422
Rastoldo G, Tighilet B. The Vestibular Nuclei: A Cerebral Reservoir of Stem Cells Involved in Balance Function in Normal and Pathological Conditions. International Journal of Molecular Sciences. 2024; 25(3):1422. https://doi.org/10.3390/ijms25031422
Chicago/Turabian StyleRastoldo, Guillaume, and Brahim Tighilet. 2024. "The Vestibular Nuclei: A Cerebral Reservoir of Stem Cells Involved in Balance Function in Normal and Pathological Conditions" International Journal of Molecular Sciences 25, no. 3: 1422. https://doi.org/10.3390/ijms25031422
APA StyleRastoldo, G., & Tighilet, B. (2024). The Vestibular Nuclei: A Cerebral Reservoir of Stem Cells Involved in Balance Function in Normal and Pathological Conditions. International Journal of Molecular Sciences, 25(3), 1422. https://doi.org/10.3390/ijms25031422




