Combined Use of Tocilizumab and Mesenchymal Stem Cells Attenuate the Development of an Anti-HLA-A2.1 Antibody in a Highly Sensitized Mouse Model
Abstract
1. Introduction
2. Results
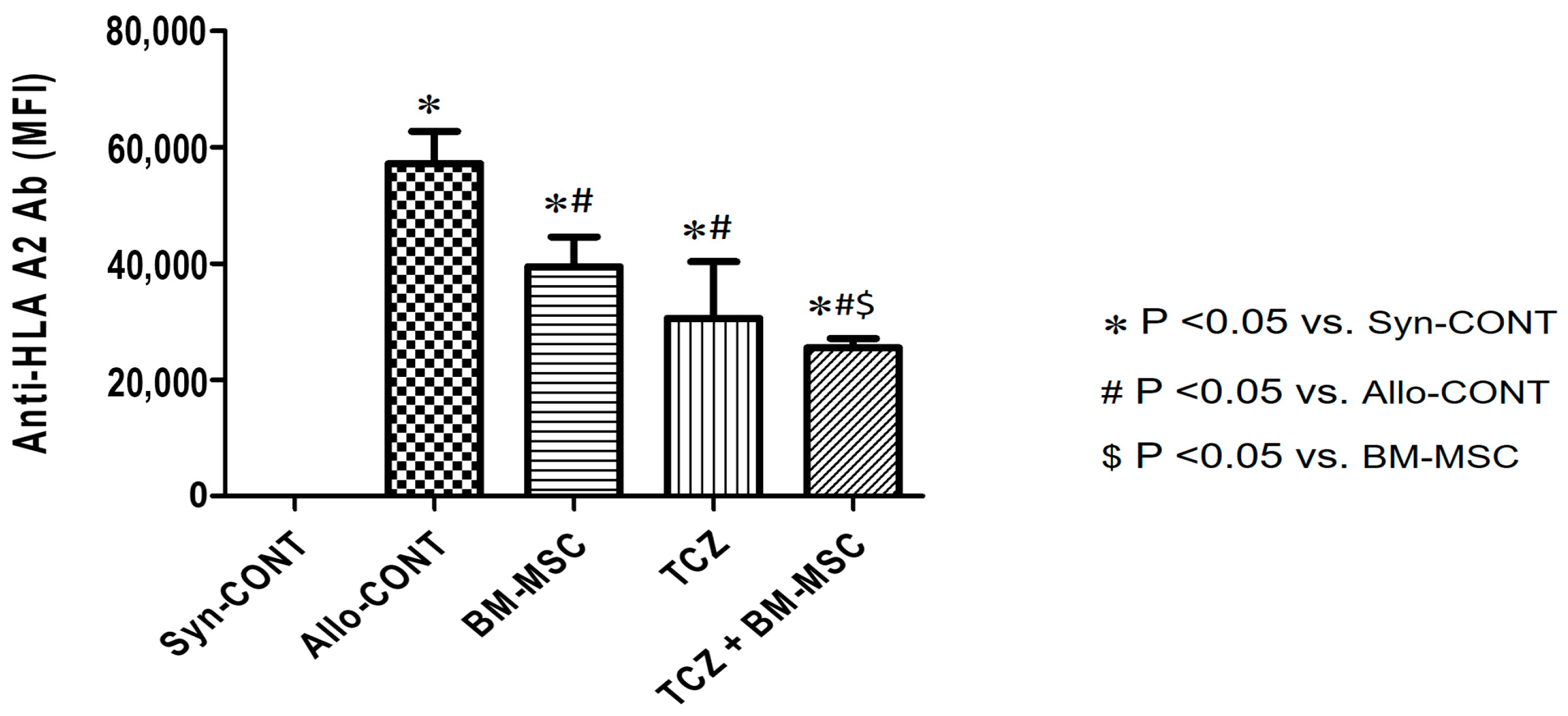
2.1. Comparison of Anti-HLA.A2 Antibody Responses in Sensitized Mice after Treatment
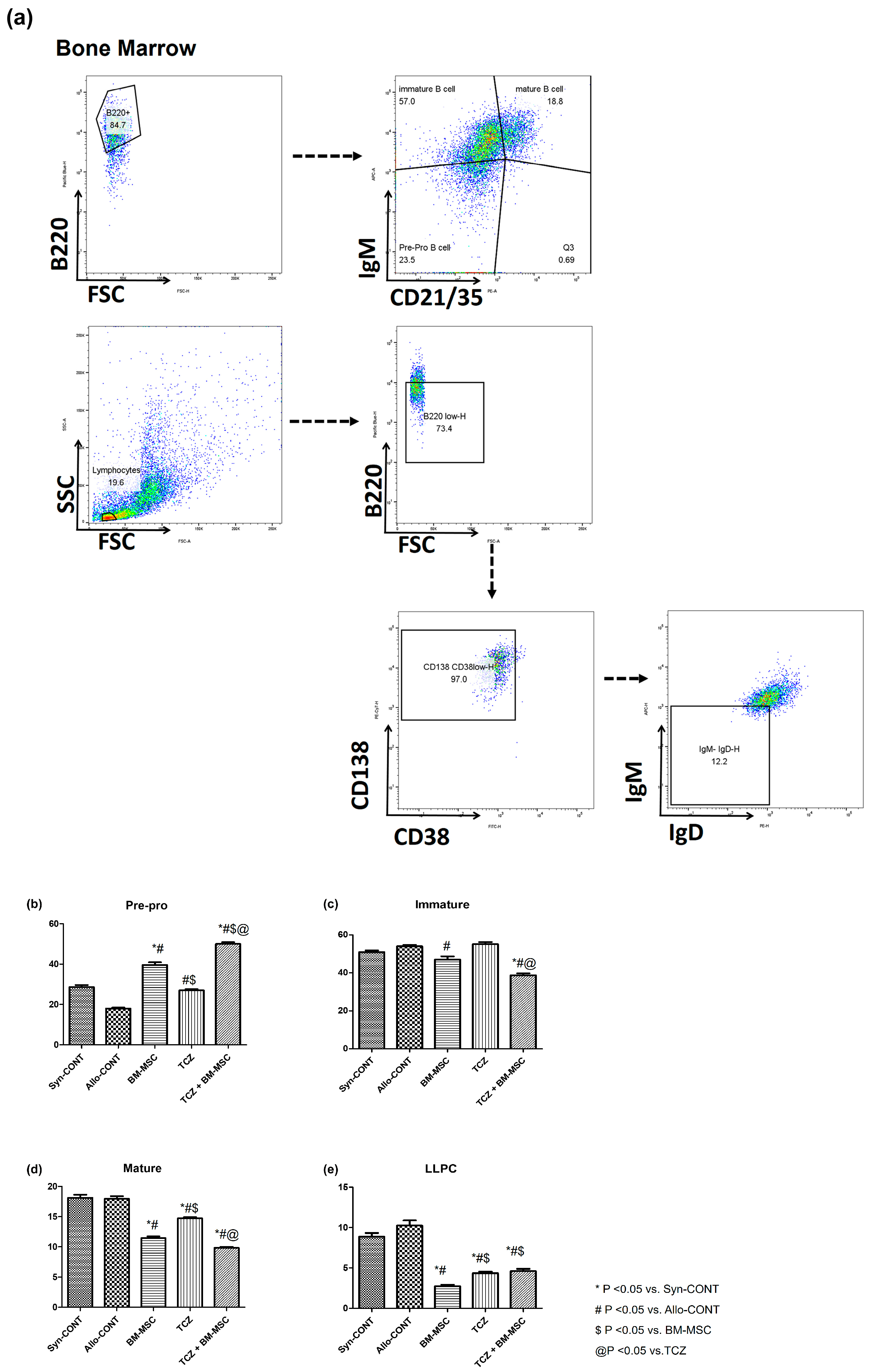
2.2. Comparison of B Cell Fractions in Bone Marrow
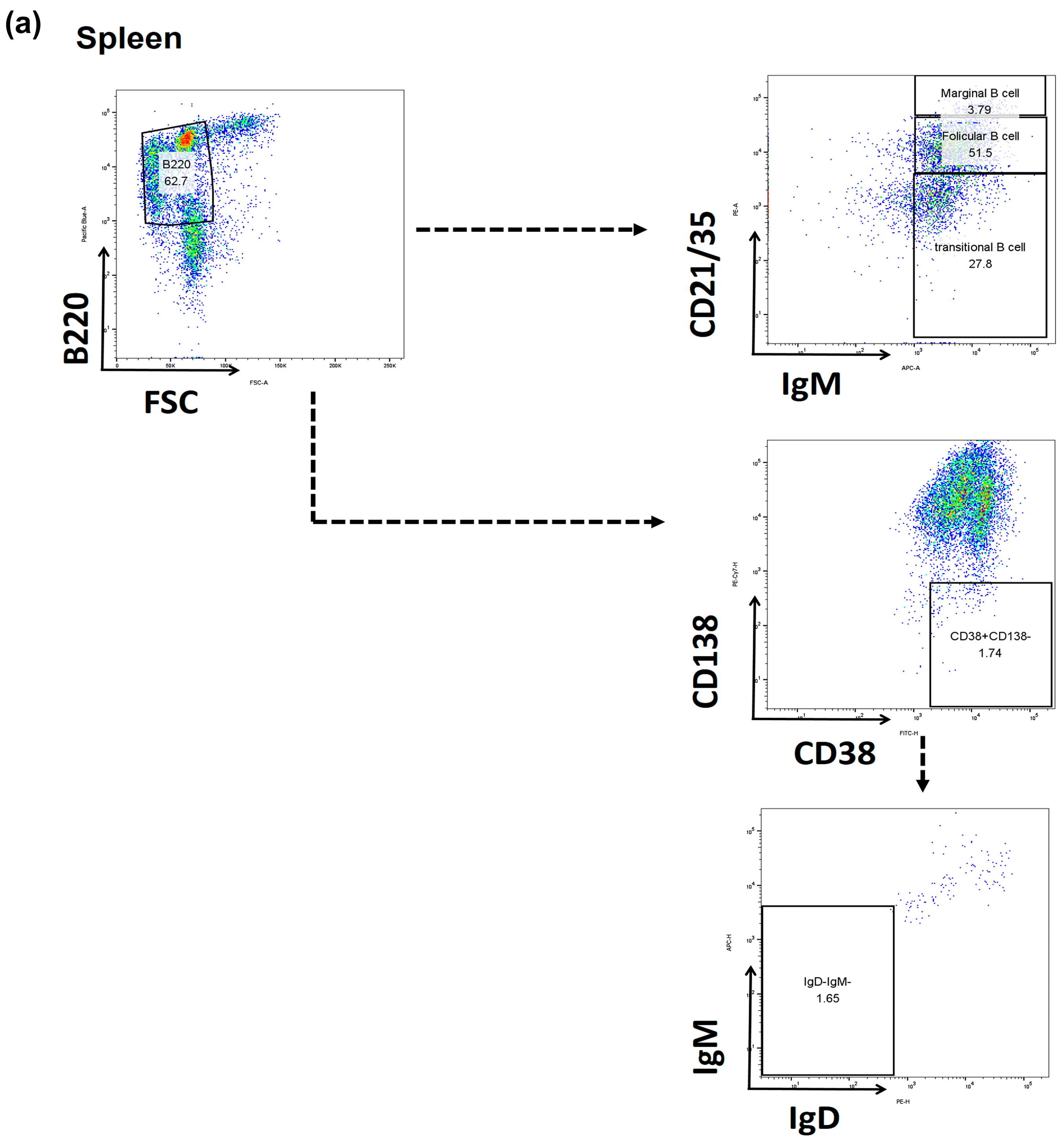
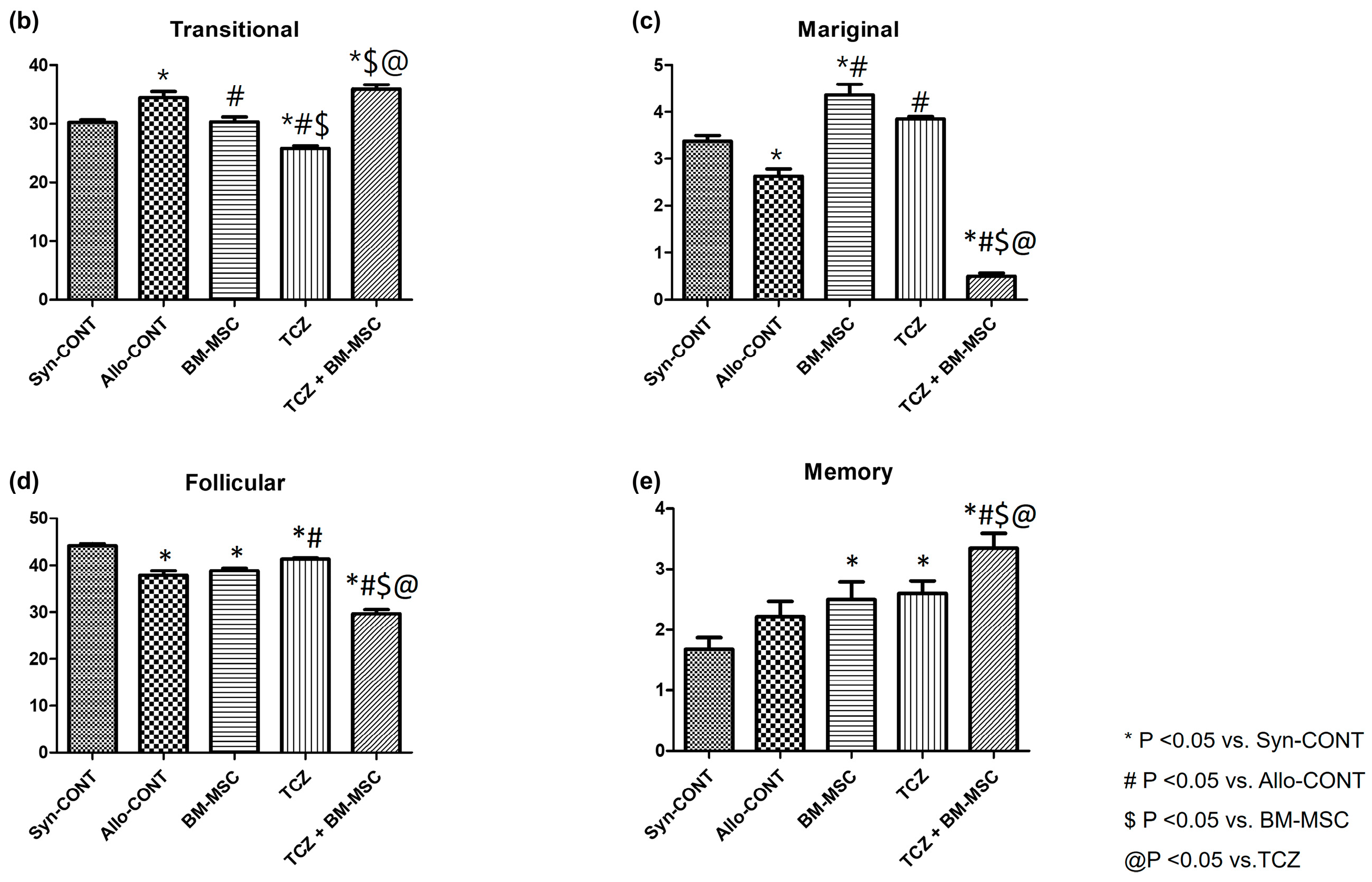
2.3. Comparison of B Cell Fractions in the Spleen
2.4. Comparison of T Cell Fractions in the Spleen
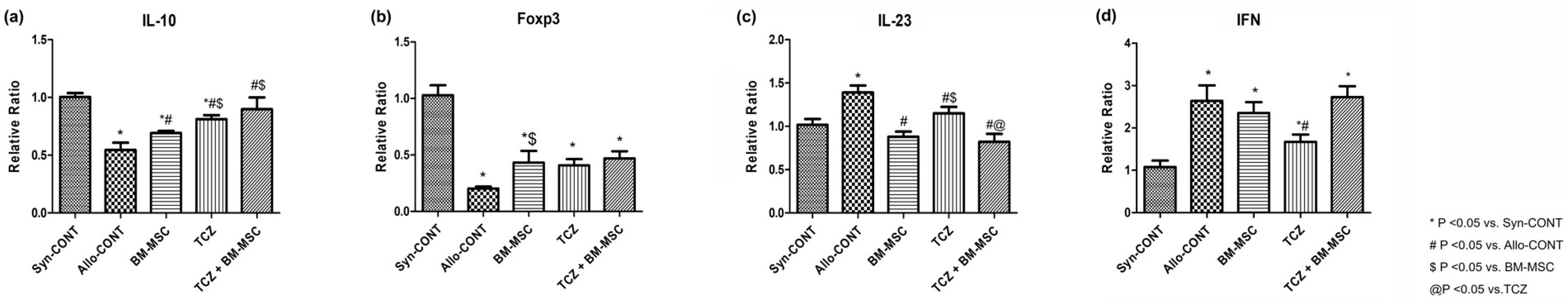
2.5. mRNA Expression of Cytokines after Treatment with BM-MSC and TCZ in a Highly Sensitized Mouse Model
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Skin Allograft Transplant Procedure
4.3. Preparation of Human Bone Marrow-Derived Mesenchymal Stem Cells
4.4. Experimental Design
4.5. Measurement of Serum Donor-Specific Anti-HLA.A2 Antibodies
4.6. Flow Cytometry Analysis
4.7. Quantitative Real-Time PCR (qRT-PCR)
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Min, J.W.; Shin, Y.J.; Lee, H.; Kim, B.M.; Park, K.H.; Doh, K.C.; Kim, T.M.; Lim, S.W.; Yang, C.W.; Oh, E.J.; et al. BAFF Inhibition Effectively Suppresses the Development of Anti-HLA.A2 Antibody in the Highly Sensitized Mouse Model. Int. J. Mol. Sci. 2021, 22, 861. [Google Scholar] [CrossRef]
- Kim, I.; Wu, G.; Chai, N.N.; Klein, A.S.; Jordan, S. Anti-interleukin 6 receptor antibodies attenuate antibody recall responses in a mouse model of allosensitization. Transplantation 2014, 98, 1262–1270. [Google Scholar] [CrossRef]
- Wolfe, R.A.; Ashby, V.B.; Milford, E.L.; Ojo, A.O.; Ettenger, R.E.; Agodoa, L.Y.C.; Held, P.J.; Port, F.K. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N. Engl. J. Med. 1999, 341, 1725–1730. [Google Scholar] [CrossRef]
- Terasaki, P.I. Humoral theory of transplantation. Am. J. Transpl. 2003, 3, 665–673. [Google Scholar] [CrossRef]
- Angaswamy, N.; Tiriveedhi, V.; Sarma, N.J.; Subramanian, V.; Klein, C.; Wellen, J.; Shenoy, S.; Chapman, W.C.; Mohanakumar, T. Interplay between immune responses to HLA and non-HLA self-antigens in allograft rejection. Hum. Immunol. 2013, 74, 1478–1485. [Google Scholar] [CrossRef]
- Kim, K.W.; Kim, B.M.; Doh, K.C.; Kim, C.D.; Jeong, K.H.; Lee, S.H.; Yang, C.W.; Chung, B.H. Clinical significance of CD161+CD4+ T cells in the development of chronic antibody-mediated rejection in kidney transplant recipients. PLoS ONE 2018, 13, e0200631. [Google Scholar] [CrossRef]
- Patel, R.; Terasaki, P.I. Significance of the positive crossmatch test in kidney transplantation. N. Engl. J. Med. 1969, 280, 735–739. [Google Scholar] [CrossRef]
- Stiller, C.R.; Sinclair, N.R.; Abrahams, S.; Ulan, R.A.; Fung, M.; Wallace, A.C. Lymphocyte-dependent antibody and renal graft rejection. Lancet 1975, 1, 953–954. [Google Scholar] [CrossRef]
- Tangye, S.G.; Avery, D.T.; Deenick, E.K.; Hodgkin, P.D. Intrinsic differences in the proliferation of naive and memory human B cells as a mechanism for enhanced secondary immune responses. J. Immunol. 2003, 170, 686–694. [Google Scholar] [CrossRef]
- Lion, J.; Taflin, C.; Cross, A.R.; Robledo-Sarmiento, M.; Mariotto, E.; Savenay, A.; Carmagnat, M.; Suberbielle, C.; Charron, D.; Haziot, A.; et al. HLA Class II Antibody Activation of Endothelial Cells Promotes Th17 and Disrupts Regulatory T Lymphocyte Expansion. Am. J. Transpl. 2016, 16, 1408–1420. [Google Scholar] [CrossRef]
- Ban, T.H.; Lee, S.; Kim, H.D.; Ko, E.; Kim, B.-M.; Kim, K.-W.; Chung, B.; Yang, C. Clinical Trial of Allogeneic Mesenchymal Stem Cell Therapy for Chronic Active Antibody-Mediated Rejection in Kidney Transplant Recipients Unresponsive to Rituximab and Intravenous Immunoglobulin. Stem Cells Int. 2021, 2021, 6672644. [Google Scholar] [CrossRef]
- Loverre, A.; Tataranni, T.; Castellano, G.; Divella, C.; Battaglia, M.; Ditonno, P.; Corcelli, M.; Mangino, M.; Gesualdo, L.; Schena, F.P.; et al. IL-17 expression by tubular epithelial cells in renal transplant recipients with acute antibody-mediated rejection. Am. J. Transpl. 2011, 11, 1248–1259. [Google Scholar] [CrossRef]
- Chung, B.H.; Oh, H.J.; Piao, S.G.; Hwang, H.S.; Sun, I.O.; Choi, S.R.; Park, H.S.; Choi, B.S.; Choi, Y.J.; Park, C.W.; et al. Clinical significance of the ratio between FOXP3 positive regulatory T cell and interleukin-17 secreting cell in renal allograft biopsies with acute T-cell-mediated rejection. Immunology 2012, 136, 344–351. [Google Scholar] [CrossRef]
- Chung, B.H.; Oh, H.J.; Piao, S.G.; Sun, I.O.; Kang, S.H.; Choi, S.R.; Park, H.S.; Choi, B.S.; Choi, Y.J.; Park, C.W.; et al. Higher infiltration by Th17 cells compared with regulatory T cells is associated with severe acute T-cell-mediated graft rejection. Exp. Mol. Med. 2011, 43, 630–637. [Google Scholar] [CrossRef]
- Choi, J.; Aubert, O.; Vo, A.; Loupy, A.; Haas, M.; Puliyanda, D.; Kim, I.; Louie, S.; Kang, A.; Peng, A.; et al. Assessment of Tocilizumab (Anti-Interleukin-6 Receptor Monoclonal) as a Potential Treatment for Chronic Antibody-Mediated Rejection and Transplant Glomerulopathy in HLA-Sensitized Renal Allograft Recipients. Am. J. Transplant. 2017, 17, 2381–2389. [Google Scholar] [CrossRef]
- Vandenbroecke, C.; Caillat-Zucman, S.; Legendre, C.; Noel, L.H.; Kreis, H.; Woodrow, D.; Bach, J.F.; Tovey, M.G. Differential in situ expression of cytokines in renal allograft rejection. Transplantation 1991, 51, 602–609. [Google Scholar] [CrossRef]
- Sonkar, G.K.; Singh, S.; Sonkar, S.K.; Singh, U.; Singh, R.G. Evaluation of serum interleukin 6 and tumour necrosis factor alpha levels, and their association with various non-immunological parameters in renal transplant recipients. Singap. Med. J. 2013, 54, 511–515. [Google Scholar] [CrossRef]
- Chung, B.H.; Kim, K.W.; Kim, B.M.; Doh, K.C.; Cho, M.L.; Yang, C.W. Increase of Th17 Cell Phenotype in Kidney Transplant Recipients with Chronic Allograft Dysfunction. PLoS ONE 2015, 10, e0145258. [Google Scholar] [CrossRef]
- Jordan, S.C.; Ammerman, N.; Huang, E.; Vo, A. Importance of IL-6 inhibition in prevention and treatment of antibody-mediated rejection in kidney allografts. Am. J. Transpl. 2022, 22 (Suppl. S4), 28–37. [Google Scholar] [CrossRef]
- Ge, W.; Jiang, J.; Arp, J.; Liu, W.; Garcia, B.; Wang, H. Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation 2010, 90, 1312–1320. [Google Scholar] [CrossRef]
- Jordan, S.C.; Choi, J.; Kim, I.; Wu, G.; Toyoda, M.; Shin, B.; Vo, A. Interleukin-6, A Cytokine Critical to Mediation of Inflammation, Autoimmunity and Allograft Rejection: Therapeutic Implications of IL-6 Receptor Blockade. Transplantation 2017, 101, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, J.; Hashizume, M.; Kaneko, Y.; Yoshimoto, K.; Nishina, N.; Takeuchi, T. Peripheral blood CD4(+) CD25(+) CD127(low) regulatory T cells are significantly increased by tocilizumab treatment in patients with rheumatoid arthritis: Increase in regulatory T cells correlates with clinical response. Arthritis Res. Ther. 2015, 17, 10. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Boenisch, O.; Yeung, M.; Mfarrej, B.; Yang, S.M.; Turka, L.A.; Sayegh, M.H.; Iacomini, J.; Yuan, X. Critical Role of Proinflammatory Cytokine IL-6 in Allograft Rejection and Tolerance. Am. J. Transpl. 2012, 12, 90–101. [Google Scholar] [CrossRef] [PubMed]
- Tawara, I.; Koyama, M.; Liu, C.; Toubai, T.; Thomas, D.; Evers, R.; Chockley, P.; Nieves, E.; Sun, Y.P.; Lowler, K.P.; et al. Interleukin-6 Modulates Graft-versus-Host Responses after Experimental Allogeneic Bone Marrow Transplantation. Clin. Cancer Res. 2011, 17, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Fukasawa, T.; Yoshizaki, A.; Ebata, S.; Yoshizaki-Ogawa, A.; Asano, Y.; Enomoto, A.; Miyagawa, K.; Kazoe, Y.; Mawatari, K.; Kitamori, T.; et al. Single-cell-level protein analysis revealing the roles of autoantigen-reactive B lymphocytes in autoimmune disease and the murine model. Elife 2021, 10, e67209. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Yang, Q.; Lin, L.; Xu, C.; Zheng, C.; Chen, X.; Han, Y.; Li, M.; Cao, W.; Cao, K.; et al. Interleukin-17 enhances immunosuppression by mesenchymal stem cells. Cell Death Differ. 2014, 21, 1758–1768. [Google Scholar] [CrossRef]
- Maccario, R.; Podestà, M.; Moretta, A.; Cometa, A.; Comoli, P.; Montagna, D.; Daudt, L.; Ibatici, A.; Piaggio, G.; Pozzi, S.; et al. Interaction of human mesenchymal stem cells with cells involved in alloantigen-specific immune response favors the differentiation of CD4+ T-cell subsets expressing a regulatory/suppressive phenotype. Haematologica 2005, 90, 516–525. [Google Scholar]
- Perico, N.; Casiraghi, F.; Todeschini, M.; Cortinovis, M.; Gotti, E.; Portalupi, V.; Mister, M.; Gaspari, F.; Villa, A.; Fiori, S.; et al. Long-Term Clinical and Immunological Profile of Kidney Transplant Patients Given Mesenchymal Stromal Cell Immunotherapy. Front. Immunol. 2018, 9, 1359. [Google Scholar] [CrossRef]
- Lo, H.Y.; Cheng, S.P.; Huang, J.L.; Chang, K.T.; Chang, Y.L.; Huang, C.H.; Chang, C.J.; Chiu, C.H.; Chen-Yang, Y.W.; Chan, C.K. High Induction of IL-6 Secretion From hUCMSCs Optimize the Potential of hUCMSCs and TCZ as Therapy for COVID-19-Related ARDS. Cell Transpl. 2021, 30, 9636897211054481. [Google Scholar] [CrossRef]
- Senegaglia, A.C.; Rebelatto, C.L.K.; Franck, C.L.; Lima, J.S.; Boldrini-Leite, L.M.; Daga, D.R.; Leitao, C.A.; Shigunov, P.; de Azambuja, A.P.; Bana, E.; et al. Combined Use of Tocilizumab and Mesenchymal Stromal Cells in the Treatment of Severe COVID-19: Case Report. Cell Transpl. 2021, 30, 9636897211021008. [Google Scholar] [CrossRef]
- Benson, M.J.; Dillon, S.R.; Castigli, E.; Geha, R.S.; Xu, S.; Lam, K.P.; Noelle, R.J. Cutting edge: The dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J. Immunol. 2008, 180, 3655–3659. [Google Scholar] [CrossRef] [PubMed]
- Nandiwada, S.L. Overview of human B-cell development and antibody deficiencies. J. Immunol. Methods 2023, 519, 113485. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.H.; Kim, S.M.; Lim, J.Y.; Ryu, C.H.; Jun, J.A.; Jeun, S.S. Mesenchymal stem cells expressing brain-derived neurotrophic factor enhance endogenous neurogenesis in an ischemic stroke model. Biomed. Res. Int. 2014, 2014, 129145. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, X.; Cui, S.; Lee, H.; Min, J.W.; Lim, S.W.; Oh, E.-J.; Yang, C.W.; Shin, Y.J.; Chung, B.H. Combined Use of Tocilizumab and Mesenchymal Stem Cells Attenuate the Development of an Anti-HLA-A2.1 Antibody in a Highly Sensitized Mouse Model. Int. J. Mol. Sci. 2024, 25, 1378. https://doi.org/10.3390/ijms25031378
Fang X, Cui S, Lee H, Min JW, Lim SW, Oh E-J, Yang CW, Shin YJ, Chung BH. Combined Use of Tocilizumab and Mesenchymal Stem Cells Attenuate the Development of an Anti-HLA-A2.1 Antibody in a Highly Sensitized Mouse Model. International Journal of Molecular Sciences. 2024; 25(3):1378. https://doi.org/10.3390/ijms25031378
Chicago/Turabian StyleFang, Xianying, Sheng Cui, Hanbi Lee, Ji Won Min, Sun Woo Lim, Eun-Jee Oh, Chul Woo Yang, Yoo Jin Shin, and Byung Ha Chung. 2024. "Combined Use of Tocilizumab and Mesenchymal Stem Cells Attenuate the Development of an Anti-HLA-A2.1 Antibody in a Highly Sensitized Mouse Model" International Journal of Molecular Sciences 25, no. 3: 1378. https://doi.org/10.3390/ijms25031378
APA StyleFang, X., Cui, S., Lee, H., Min, J. W., Lim, S. W., Oh, E.-J., Yang, C. W., Shin, Y. J., & Chung, B. H. (2024). Combined Use of Tocilizumab and Mesenchymal Stem Cells Attenuate the Development of an Anti-HLA-A2.1 Antibody in a Highly Sensitized Mouse Model. International Journal of Molecular Sciences, 25(3), 1378. https://doi.org/10.3390/ijms25031378





