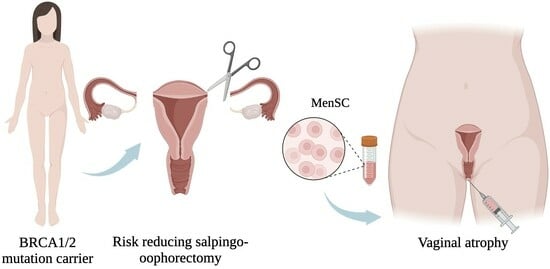
The Concept behind the Suitability of Menstrual Blood-Derived Stem Cells for the Management of Vaginal Atrophy among BRCA Mutation Carriers after RRSO
Abstract
1. Introduction
1.1. Risk-Reducing Bilateral Salpingo-Oophorectomy in BRCA Mutation Carriers
1.2. Gynecologic Consequences of RRSO
1.3. Treatment Options for Vaginal Atrophy after RRSO
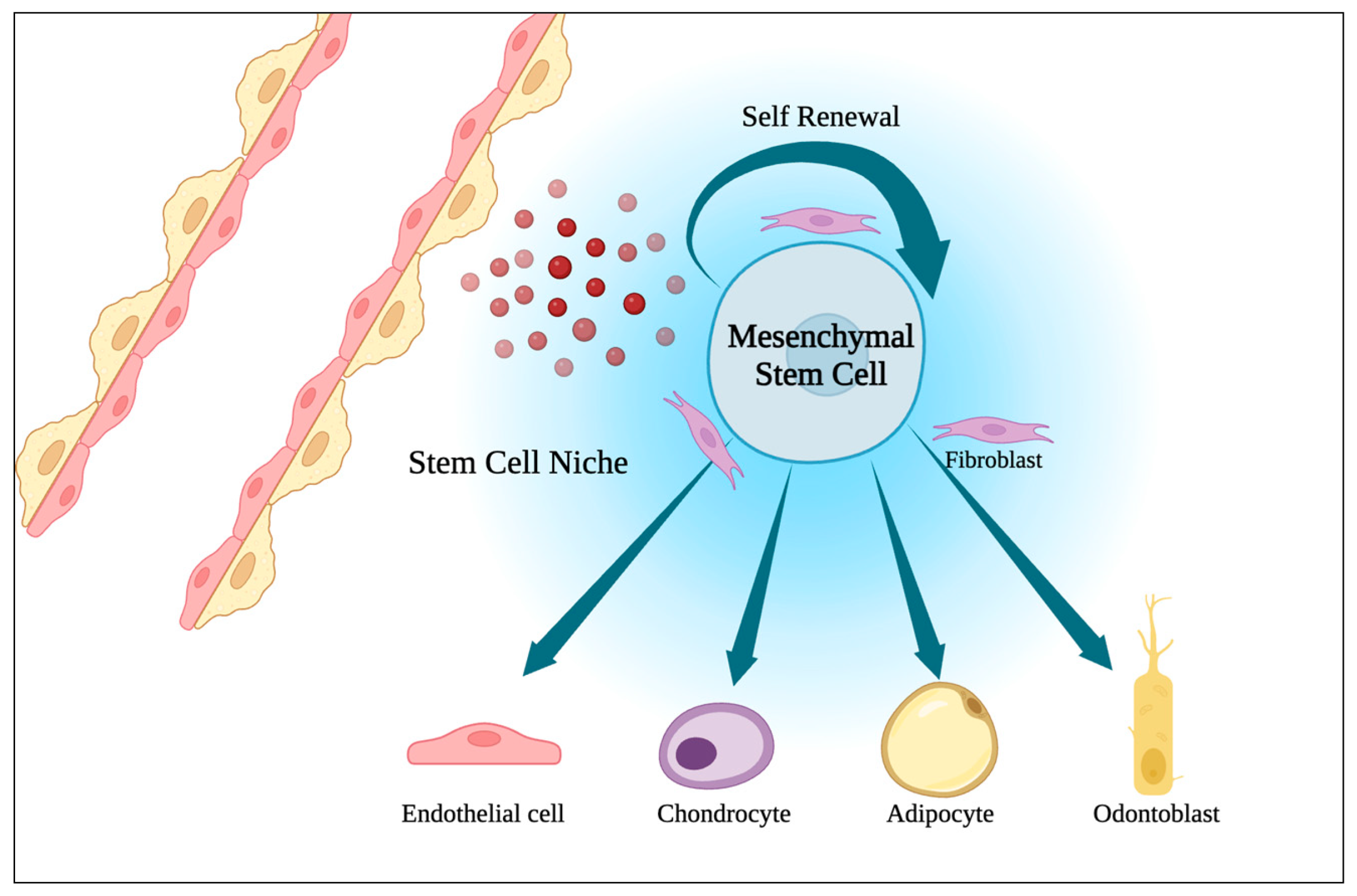
1.4. Menstrual Blood-Derived Stem Cells
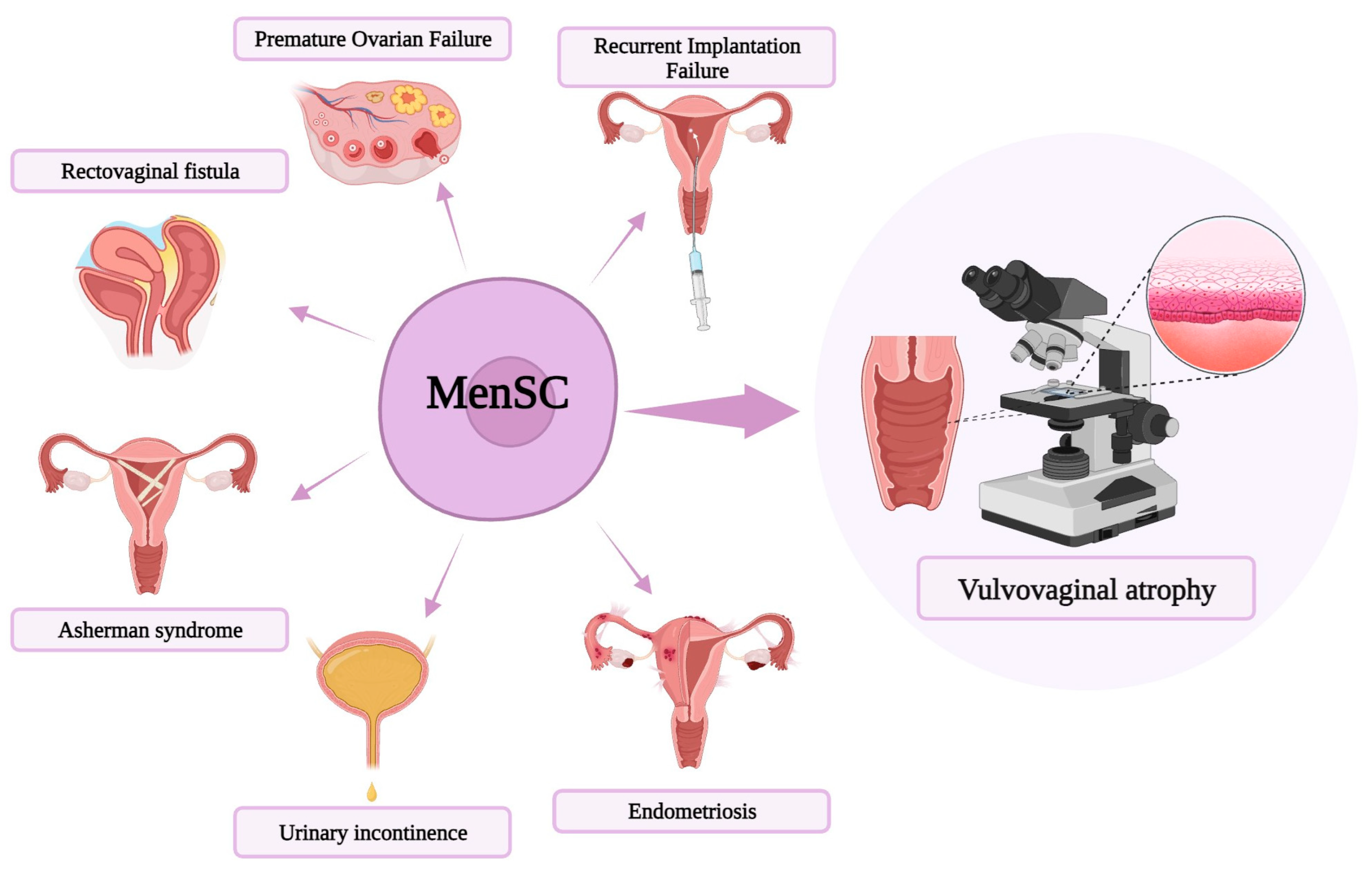
1.5. MenSC-Based Therapies Novel Approaches
1.6. MenSC-Based Therapy for Vaginal Atrophy
2. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eleje, G.U.; Eke, A.C.; Ezebialu, I.U.; Ikechebelu, J.I.; Ugwu, E.O.; Okonkwo, O.O. Risk-Reducing Bilateral Salpingo-Oophorectomy in Women with BRCA1 or BRCA2 Mutations; Cochrane Database of Systematic Reviews; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 2018; Volume 2018. [Google Scholar]
- Liu, Y.L.; Breen, K.; Catchings, A.; Ranganathan, M.; Latham, A.; Goldfrank, D.J.; Grisham, R.N.; Roche, K.L.; Frey, M.K.; Chi, D.S.; et al. Risk-Reducing Bilateral Salpingo-Oophorectomy for Ovarian Cancer: A Review and Clinical Guide for Hereditary Predisposition Genes. JCO Oncol. Pr. 2021, 18, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Finch, A.; Evans, G.; A Narod, S. BRCACarriers, Prophylactic Salpingo-Oophorectomy and Menopause: Clinical Management Considerations and Recommendations. Women’s Health 2012, 8, 543–555. [Google Scholar] [CrossRef] [PubMed]
- Loizzi, V.; Cicinelli, E.; Del Vecchio, V.; Arezzo, F.; Deromemaj, X.; Kardhashi, A.; Paradiso, A.; Legge, F.; Natalicchio, M.I.; Resta, L.; et al. A prospective multicentric study of risk-reducing salpingo-oophorectomy in BRCA mutation patients. Acta Biomed. 2022, 93, e2022051. [Google Scholar] [CrossRef]
- Sessa, C.; Balmaña, J.; Bober, S.; Cardoso, M.; Colombo, N.; Curigliano, G.; Domchek, S.; Evans, D.; Fischerova, D.; Harbeck, N.; et al. Risk reduction and screening of cancer in hereditary breast-ovarian cancer syndromes: ESMO Clinical Practice Guideline. Ann. Oncol. 2022, 34, 33–47. [Google Scholar] [CrossRef]
- Vermeulen, R.F.M.; Van Beurden, M.; Korse, C.M.; Kenter, G.G. Impact of risk-reducing salpingo-oophorectomy in premenopausal women. Climacteric 2017, 20, 212–221. [Google Scholar] [CrossRef]
- Nebgen, D.R.; Domchek, S.M.; Kotsopoulos, J.; de Hullu, J.A.; Crosbie, E.J.; Paramanandam, V.S.; van Zanten, M.M.B.; Norquist, B.M.; Guise, T.; Rozenberg, S.; et al. Care after premenopausal risk-reducing salpingo-oophorectomy in high-risk women: Scoping review and international consensus recommendations. BJOG Int. J. Obstet. Gynaecol. 2023, 130, 1437–1450. [Google Scholar] [CrossRef]
- Marchetti, C.; De Felice, F.; Boccia, S.; Sassu, C.; Di Donato, V.; Perniola, G.; Palaia, I.; Monti, M.; Muzii, L.; Tombolini, V.; et al. Hormone replacement therapy after prophylactic risk-reducing salpingo-oophorectomy and breast cancer risk in BRCA1 and BRCA2 mutation carriers: A meta-analysis. Crit. Rev. Oncol. 2018, 132, 111–115. [Google Scholar] [CrossRef]
- Loizzi, V.; Dellino, M.; Cerbone, M.; Arezzo, F.; Cazzato, G.; Damiani, G.R.; Pinto, V.; Silvestris, E.; Kardhashi, A.; Cicinelli, E.; et al. The Role of Hormonal Replacement Therapy in BRCA Mutated Patients: Lights and Shadows. Int. J. Mol. Sci. 2023, 24, 764. [Google Scholar] [CrossRef]
- Benini, V.; Ruffolo, A.F.; Casiraghi, A.; Degliuomini, R.S.; Frigerio, M.; Braga, A.; Serati, M.; Torella, M.; Candiani, M.; Salvatore, S. New Innovations for the Treatment of Vulvovaginal Atrophy: An Up-to-Date Review. Medicina 2022, 58, 770. [Google Scholar] [CrossRef]
- Casarotti, G.; Tremolada, C. A new treatment of genito-urinary post-menopausal atrophy with autologous micro-fragmented fat tissue: A thirty-six months follow up case series. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7420–7426. [Google Scholar] [CrossRef]
- Kang, B.; Cai, Y.; Jia, Z.; Chen, C.; Deng, M.; Zhang, W.; Li, W. Cell-Free Fat Extract Prevents Vaginal Atrophy in an Ovariectomized Model by Promoting Proliferation of Vaginal Keratinocytes and Neovascularization. Aesthetic Surg. J. 2021, 42, NP55–NP68. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qu, J.; Xiang, C. The multi-functional roles of menstrual blood-derived stem cells in regenerative medicine. Stem Cell Res. Ther. 2019, 10, 1. [Google Scholar] [CrossRef]
- Kuchenbaecker, K.B.; Hopper, J.L.; Barnes, D.R.; Phillips, K.A.; Mooij, T.M.; Roos-Blom, M.J.; Jervis, S.; van Leeuwen, F.E.; Milne, R.L.; Andrieu, N. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA-J. Am. Med. Assoc. 2017, 317, 2402–2416. [Google Scholar] [CrossRef]
- Domchek, S.M.; Friebel, T.M.; Singer, C.F.; Evans, D.G.; Lynch, H.T.; Isaacs, C.; Garber, J.E.; Neuhausen, S.L.; Matloff, E.; Eeles, R.; et al. Association of Risk-Reducing Surgery in BRCA1 or BRCA2 Mutation Carriers With Cancer Risk and Mortality. JAMA 2010, 304, 967–975. [Google Scholar] [CrossRef] [PubMed]
- Gaba, F.; Blyuss, O.; Tan, A.; Munblit, D.; Oxley, S.; Khan, K.; Legood, R.; Manchanda, R. Breast Cancer Risk and Breast-Cancer-Specific Mortality following Risk-Reducing Salpingo-Oophorectomy in BRCA Carriers: A Systematic Review and Meta-Analysis. Cancers 2023, 15, 1625. [Google Scholar] [CrossRef] [PubMed]
- Stuursma, A.; van der Vegt, B.; Jansen, L.; Berger, L.P.V.; Mourits, M.J.E.; de Bock, G.H. The Effect of Risk-Reducing Salpingo-Oophorectomy on Breast Cancer Incidence and Histopathological Features in Women with a BRCA1 or BRCA2 Germline Pathogenic Variant. Cancers 2023, 15, 2095. [Google Scholar] [CrossRef]
- Steenbeek, M.P.; Harmsen, M.G.; Hoogerbrugge, N.; de Jong, M.A.; Maas, A.H.E.M.; Prins, J.B.; Bulten, J.; Teerenstra, S.; van Bommel, M.H.D.; van Doorn, H.C.; et al. Association of Salpingectomy With Delayed Oophorectomy Versus Salpingo-oophorectomy With Quality of Life in BRCA1/2 Pathogenic Variant Carriers. JAMA Oncol. 2021, 7, 1203–1212. [Google Scholar] [CrossRef]
- Alves-Nogueira, A.C.; Melo, D.; Carona, C.; Figueiredo-Dias, M. The Psychosocial Impact of the Decision to Undergo Risk-Reducing Salpingo-Oophorectomy Surgery in BRCA Mutation Carriers and the Role of Physician-Patient Communication. Curr. Oncol. 2023, 30, 2429–2440. [Google Scholar] [CrossRef]
- Kershaw, V.; Hickey, I.; Wyld, L.; Jha, S. The impact of risk reducing bilateral salpingo-oophorectomy on sexual function in BRCA1/2 mutation carriers and women with Lynch syndrome: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 265, 7–17. [Google Scholar] [CrossRef]
- Sarmento, A.C.A.; Costa, A.P.F.; Lírio, J.; Jr, J.E.; Baptista, P.V.; Gonçalves, A.K. Efficacy of Hormonal and Nonhormonal Approaches to Vaginal Atrophy and Sexual Dysfunctions in Postmenopausal Women: A Systematic Review. Rev. Bras. de Hematol. e Hemoter. 2022, 44, 986–994. [Google Scholar] [CrossRef]
- Palacios, S.; Nappi, R.E.; Cancelo, M.J.; Sánchez, S.; Simoncini, T. Sequential treatment in vulvovaginal atrophy. Climacteric 2023, 26, 292–295. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, O.E.; Christensen, S.E.; Løkkegaard, E. The evidence behind the use of LASER for genitourinary syndrome of menopause, vulvovaginal atrophy, urinary incontinence and lichen sclerosus: A state-of-the-art review. Acta Obstet. Gynecol. Scand. 2022, 101, 657–692. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.; Park, K.-M.; Chung, Y.-J.; Kim, M.-R. Updates on Therapeutic Alternatives for Genitourinary Syndrome of Menopause: Hormonal and Non-Hormonal Managements. J. Menopausal Med. 2021, 27, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Faubion, S.S.M.C.; Crandall, C.J.M.; Davis, L.D.; El Khoudary, S.R.P.; Hodis, H.N.; Lobo, R.A.; Maki, P.M.; Manson, J.E.M.; Pinkerton, J.V.M.; Santoro, N.F.; et al. The 2022 hormone therapy position statement of The North American Menopause Society. Menopause 2022, 29, 767–794. [Google Scholar] [CrossRef]
- Gordhandas, S.; Norquist, B.M.; Pennington, K.P.; Yung, R.L.; Laya, M.B.; Swisher, E.M. Hormone replacement therapy after risk reducing salpingo-oophorectomy in patients with BRCA1 or BRCA2 mutations; a systematic review of risks and benefits. Gynecol. Oncol. 2019, 153, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, R.; Gaba, F.; Talaulikar, V.; Pundir, J.; Gessler, S.; Davies, M.; Menon, U.; Royal College of Obstetricians and Gynaecologists. Risk-Reducing Salpingo-Oophorectomy and the Use of Hormone Replacement Therapy Below the Age of Natural Menopause: Scientific Impact Paper No. 66. BJOG 2022, 129, e16–e34. [Google Scholar] [CrossRef]
- Gadducci, A.; Biglia, N.; Cosio, S.; Sismondi, P.; Genazzani, A.R. Gynaecologic challenging issues in the management of BRCA mutation carriers: Oral contraceptives, prophylactic salpingo-oophorectomy and hormone replacement therapy. Gynecol. Endocrinol. 2010, 26, 568–577. [Google Scholar] [CrossRef]
- Guidozzi, F. Hormone therapy after prophylactic risk-reducing bilateral salpingo-oophorectomy in women who have BRCA gene mutation. Climacteric 2016, 19, 419–422. [Google Scholar] [CrossRef]
- Comini, A.C.M.; Carvalho, B.M.; Moreira, M.J.B.; Reis, P.C.A.; Colapietro, L.; Northern, J.; Batalini, F. Safety and Serum Estradiol Levels in Hormonal Treatments for Vulvovaginal Atrophy in Breast Cancer Survivors: A Systematic Review and Meta-Analysis. Clin. Breast Cancer 2023, 23, 835–846. [Google Scholar] [CrossRef]
- Nappi, R.E.; Palacios, S.; Panay, N.; Particco, M.; Krychman, M.L. Vulvar and vaginal atrophy in four European countries: Evidence from the European REVIVE Survey. Climacteric 2015, 19, 188–197. [Google Scholar] [CrossRef]
- Kingsberg, S.A.; Wysocki, S.; Magnus, L.; Krychman, M.L. Vulvar and Vaginal Atrophy in Postmenopausal Women: Findings from the REVIVE (REal Women’s VIews of Treatment Options for Menopausal Vaginal ChangEs) Survey. J. Sex. Med. 2013, 10, 1790–1799. [Google Scholar] [CrossRef]
- A Freedman, M. Perceptions of Dyspareunia in Postmenopausal Women with Vulvar and Vaginal Atrophy: Findings from the Revive Survey. Women’s Health 2014, 10, 445–454. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, F.; Lo Celso, C.; Scadden, D. Adult stem cells and their niches. Adv. Exp. Med. Biol. 2010, 695, 155–168. [Google Scholar] [PubMed]
- Romito, A.; Cobellis, G. Pluripotent Stem Cells: Current Understanding and Future Directions. Stem Cells Int. 2015, 2016, 9451492. [Google Scholar] [CrossRef]
- Sanchez-Mata, A.; Gonzalez-Muñ Oz, E. iScience Understanding menstrual blood-derived stromal/stem cells: Definition and properties. Are we rushing into their therapeutic applications? iScience 2021, 24, 103501. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Qu, J.; Cheng, T.; Chen, X.; Xiang, C. Menstrual blood-derived stem cells: Toward therapeutic mechanisms, novel strategies, and future perspectives in the treatment of diseases. Stem Cell Res. Ther. 2019, 10, 406. [Google Scholar] [CrossRef]
- Mantovani, M.; Gennai, A.; Russo, P.R. A new approach to regenerative medicine in gynecology. Int. J. Gynecol. Obstet. 2021, 157, 536–543. [Google Scholar] [CrossRef]
- Chen, L.; Qu, J.; Mei, Q.; Chen, X.; Fang, Y.; Chen, L.; Li, Y.; Xiang, C. Small extracellular vesicles from menstrual blood-derived mesenchymal stem cells (MenSCs) as a novel therapeutic impetus in regenerative medicine. Stem Cell Res. Ther. 2021, 12, 433. [Google Scholar] [CrossRef]
- Stem Cell Therapies in Obstetrics and Gynaecology: The Female Urogenital Tract and the Fetus as Sources and Targets for Molecular and Regenerative Medicine. 2013. Available online: https://www.rcog.org.uk/media/31fkqxtt/sip_38.pdf (accessed on 28 September 2023).
- He, Y.; Han, Y.; Ye, Y. Therapeutic Potential of Menstrual Blood-Derived Stem Cell Transplantation for Intrauterine Adhesions. Front. Surg. 2022, 9, 847213. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Yang, T.; Li, J.; Yang, X. Study of the reparative effects of menstrual-derived stem cells on premature ovarian failure in mice. Stem Cell Res. Ther. 2017, 8, 11. [Google Scholar] [CrossRef]
- Liu, T.; Huang, Y.; Zhang, J.; Qin, W.; Chi, H.; Chen, J.; Yu, Z.; Chen, C.; Zuo, W.; Xie, B.; et al. Transplantation of Human Menstrual Blood Stem Cells to Treat Premature Ovarian Failure in Mouse Model. Stem Cells Dev. 2014, 23, 1548–1557. [Google Scholar] [CrossRef]
- Erceg Ivkošić, I.; Fureš, R.; Ćosić, V.; Mikelin, N.; Bulić, L.; Dobranić, D.; Brlek, P.; Primorac, D. Unlocking the Potential of Mesenchymal Stem Cells in Gynecology: Where Are We Now? J. Pers. Med. 2023, 13, 1253. [Google Scholar] [CrossRef]
- Marx, V. Guide RNAs: It’s good to be choosy. Nat. Methods Nat. Res. 2020, 17, 1179–1182. [Google Scholar] [CrossRef]
- Xu, Y.; Li, Z. CRISPR-Cas systems: Overview, innovations and applications in human disease research and gene therapy. Comput. Struct. Biotechnol. J. 2020, 18, 2401–2415. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.-H.; Kim, J.-H. A Comprehensive Review on Factors Influences Biogenesis, Functions, Therapeutic and Clinical Implications of Exosomes. Int. J. Nanomed. 2021, 16, 1281–1312. [Google Scholar] [CrossRef] [PubMed]
- Wazer, D.E.; Gage, I.; Homer, M.J.; Krosnick, S.H.; Schmid, C. Age-related differences in patients with nonpalpable breast carcinomas. Cancer 1996, 78, 1432–1437. [Google Scholar] [CrossRef]
- Jovic, D.; Liang, X.; Zeng, H.; Lin, L.; Xu, F.; Luo, Y. Single-cell RNA sequencing technologies and applications: A brief overview. Clin. Transl. Med. 2022, 12, e694. [Google Scholar] [CrossRef]
- Kasap, B.; Kasap, Ş.; Vatansever, S.; Kendirci, R.; Yılmaz, O.; Çalışır, M.; Edgünlü, T.; Akın, M.N. Effects of adipose and bone marrow-derived mesenchymal stem cells on vaginal atrophy in a rat menopause model. Gene 2019, 711, 143937. [Google Scholar] [CrossRef]
- Tremolada, C. Mesenchymal Stromal Cells and Micro Fragmented Adipose Tissue: New Horizons of Effectiveness of Lipogems. J. Stem Cells Res. Dev. Ther. 2019, 5, 1–7. [Google Scholar] [CrossRef]
- Onesti, M.G.; Carella, S.; Ceccarelli, S.; Marchese, C.; Scuderi, N. The Use of Human Adipose-Derived Stem Cells in the Treatment of Physiological and Pathological Vulvar Dystrophies. Stem Cells Int. 2016, 2016, 2561461. [Google Scholar] [CrossRef]
- Mohamed Faruk, E. New Possible Approach in Treatment of Experimental Induced Vaginal Atrophy by Bone Marrow-derived Mesenchymal Stem Cells in Treatment of Induced Vaginal Atrophy in Adult Female Albino Rats (Histological, Immunohistochemical and Biochemical Study). J. Cytol. Histol. 2019, 10, 1–9. [Google Scholar]
- Michaellweinfeld, F.B. Advances in Experimental Medicine and Biology 951 Biobanking and Cryopreservation of Stem Cells. Available online: http://www.springer.com/series/5584 (accessed on 22 September 2023).
- Hoang, V.T.; Nguyen, H.-P.; Nguyen, V.N.; Hoang, D.M.; Nguyen, T.-S.T.; Thanh, L.N. Adipose-derived mesenchymal stem cell therapy for the management of female sexual dysfunction: Literature reviews and study design of a clinical trial. Front. Cell Dev. Biol. 2022, 10, 956274. [Google Scholar] [CrossRef] [PubMed]
- Andrews, P.W.; Ben-David, U.; Benvenisty, N.; Coffey, P.; Eggan, K.; Knowles, B.B.; Nagy, A.; Pera, M.; Reubinoff, B.; Rugg-Gunn, P.J.; et al. Assessing the Safety of Human Pluripotent Stem Cells and Their Derivatives for Clinical Applications. Stem Cell Rep. 2017, 9, 1–4. [Google Scholar] [CrossRef] [PubMed]
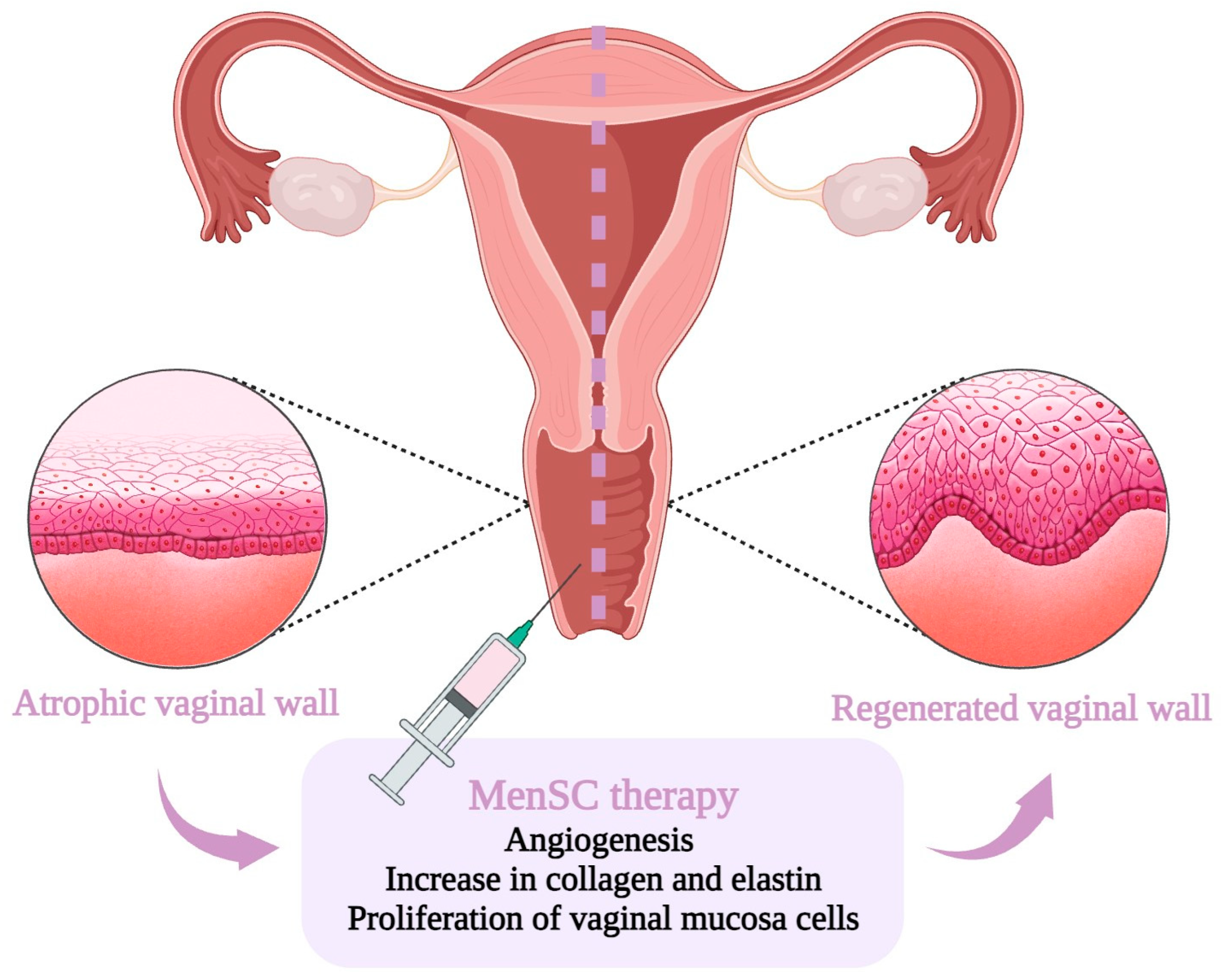
| MenSC 1 Property | Therapeutical Assumption |
|---|---|
| Regenerative | Help repair and regenerate the vaginal lining, increasing its thickness and reducing dryness. |
| Anti-inflammatory | Minimize the discomfort and pain associated with vaginal atrophy. |
| Pro-angiogenic | Improve the overall health of the vaginal tissue, leading to increased lubrication and elasticity. |
| Collagen production | Enhance the strength and elasticity of the vaginal walls. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cordeiro, M.R.; Laranjeiro, B.; Figueiredo-Dias, M. The Concept behind the Suitability of Menstrual Blood-Derived Stem Cells for the Management of Vaginal Atrophy among BRCA Mutation Carriers after RRSO. Int. J. Mol. Sci. 2024, 25, 1025. https://doi.org/10.3390/ijms25021025
Cordeiro MR, Laranjeiro B, Figueiredo-Dias M. The Concept behind the Suitability of Menstrual Blood-Derived Stem Cells for the Management of Vaginal Atrophy among BRCA Mutation Carriers after RRSO. International Journal of Molecular Sciences. 2024; 25(2):1025. https://doi.org/10.3390/ijms25021025
Chicago/Turabian StyleCordeiro, Mariana Robalo, Bárbara Laranjeiro, and Margarida Figueiredo-Dias. 2024. "The Concept behind the Suitability of Menstrual Blood-Derived Stem Cells for the Management of Vaginal Atrophy among BRCA Mutation Carriers after RRSO" International Journal of Molecular Sciences 25, no. 2: 1025. https://doi.org/10.3390/ijms25021025
APA StyleCordeiro, M. R., Laranjeiro, B., & Figueiredo-Dias, M. (2024). The Concept behind the Suitability of Menstrual Blood-Derived Stem Cells for the Management of Vaginal Atrophy among BRCA Mutation Carriers after RRSO. International Journal of Molecular Sciences, 25(2), 1025. https://doi.org/10.3390/ijms25021025