Sexual Dimorphism of Ethanol-Induced Mitochondrial Dynamics in Purkinje Cells
Abstract
1. Introduction
2. Results
2.1. Distribution of Mitochondria in Purkinje Cells
2.2. Acute Effect of Ethanol Intake on Purkinje Cell Mitochondrial Dynamics
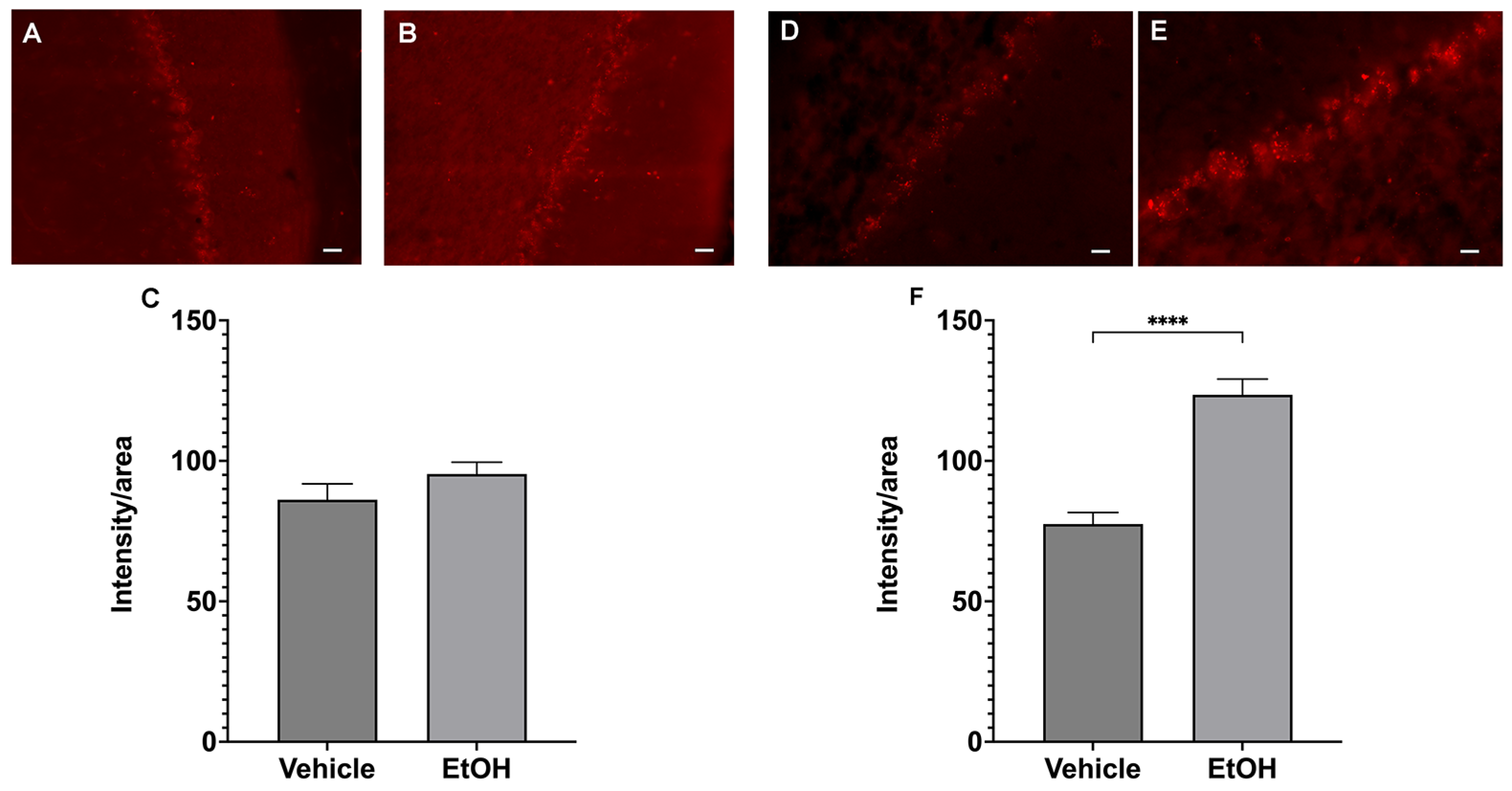
2.3. Ethanol Intake Leads to Increased Production of ROS in Female Purkinje Cells
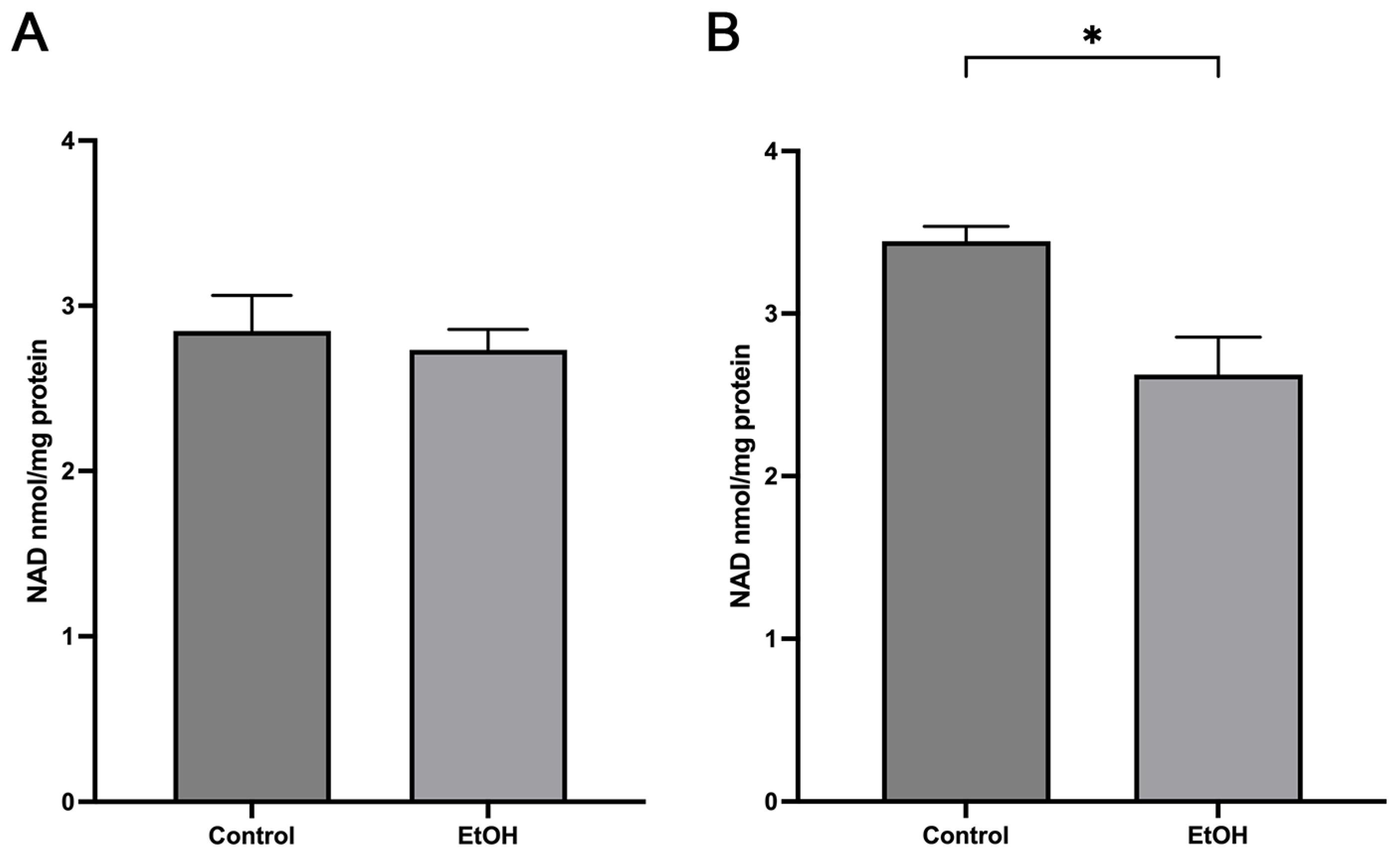
2.4. Acute Ethanol Effect on Mitochondrial NAD Levels
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Measurements of Serum Ethanol Concentration
4.3. Isolation of Mitochondria from Cerebellum
Measurement of Mitochondrial NAD Levels by HPLC
4.4. Immunohistochemistry
4.5. Superoxide Detection In Vivo
4.6. Western Blots
4.7. Mitochondrial Dynamics Quantification
4.8. Laser Scanning Confocal Microscopy
4.9. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wallgren, B.H. (Ed.) Ataxia and sleeping time. In Actions of Alcohol; Elsevier: New York, NY, USA, 1970; pp. 367–376. [Google Scholar]
- Watanabe, M. Molecular mechanisms governing competitive synaptic wiring in cerebellar Purkinje cells. Tohoku J. Exp. Med. 2008, 214, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Okamoto, K.; Hayashi, Y.; Sheng, M. The importance of dendritic mitochondria in the morphogenesis and plasticity of spines and synapses. Cell 2004, 119, 873–887. [Google Scholar] [CrossRef] [PubMed]
- Waddell, J.; McKenna, M.C.; Kristian, T. Brain ethanol metabolism and mitochondria. Curr. Top. Biochem. Res. 2022, 23, 1–13. [Google Scholar]
- Waddell, J.; Banerjee, A.; Kristian, T. Acetylation in Mitochondria Dynamics and Neurodegeneration. Cells 2021, 10, 3031. [Google Scholar] [CrossRef] [PubMed]
- Balog, J.; Mehta, S.L.; Vemuganti, R. Mitochondrial fission and fusion in secondary brain damage after CNS insults. J. Cereb. Blood Flow. Metab. 2016, 36, 2022–2033. [Google Scholar] [CrossRef] [PubMed]
- Bonet-Ponce, L.; Saez-Atienzar, S.; da Casa, C.; Flores-Bellver, M.; Barcia, J.M.; Sancho-Pelluz, J.; Romero, F.J.; Jordan, J.; Galindo, M.F. On the mechanism underlying ethanol-induced mitochondrial dynamic disruption and autophagy response. Biochim. Biophys. Acta 2015, 1852, 1400–1409. [Google Scholar] [CrossRef]
- Klimova, N.; Long, A.; Kristian, T. Significance of Mitochondrial Protein Post-translational Modifications in Pathophysiology of Brain Injury. Transl. Stroke Res. 2018, 9, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.R.; Blackstone, C. Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Ann. N. Y. Acad. Sci. 2010, 1201, 34–39. [Google Scholar] [CrossRef]
- Chen, H.; Chan, D.C. Mitochondrial dynamics--fusion, fission, movement, and mitophagy--in neurodegenerative diseases. Hum. Mol. Genet. 2009, 18, R169–R176. [Google Scholar] [CrossRef]
- Forster, M.J.; Dubey, A.; Dawson, K.M.; Stutts, W.A.; Lal, H.; Sohal, R.S. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc. Natl. Acad. Sci. USA 1996, 93, 4765–4769. [Google Scholar] [CrossRef]
- Rewal, M.; Wen, Y.; Simpkins, J.W.; Jung, M.E. Ethanol withdrawal reduces cerebellar parvalbumin expression in a manner reversed by estrogens. Neurosci. Lett. 2005, 377, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.E.; Agarwal, R.; Simpkins, J.W. Ethanol withdrawal posttranslationally decreases the activity of cytochrome c oxidase in an estrogen reversible manner. Neurosci. Lett. 2007, 416, 160–164. [Google Scholar] [CrossRef]
- Chandrasekaran, K.; Hazelton, J.L.; Wang, Y.; Fiskum, G.; Kristian, T. Neuron-specific conditional expression of a mitochondrially targeted fluorescent protein in mice. J. Neurosci. 2006, 26, 13123–13127. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Owens, K.; Park, J.H.; Gourley, S.; Jones, H.; Kristian, T. Mitochondrial dynamics: Cell-type and hippocampal region specific changes following global cerebral ischemia. J. Bioenerg. Biomembr. 2015, 47, 13–31. [Google Scholar] [CrossRef] [PubMed]
- Erondu, N.E.; Kennedy, M.B. Regional distribution of type II Ca2+/calmodulin-dependent protein kinase in rat brain. J. Neurosci. 1985, 5, 3270–3277. [Google Scholar] [CrossRef]
- Walaas, S.I.; Lai, Y.; Gorelick, F.S.; DeCamilli, P.; Moretti, M.; Greengard, P. Cell-specific localization of the alpha-subunit of calcium/calmodulin-dependent protein kinase II in Purkinje cells in rodent cerebellum. Brain Res. 1988, 464, 233–242. [Google Scholar] [CrossRef]
- Murakami, K.; Kondo, T.; Kawase, M.; Chan, P.H. The development of a new mouse model of global ischemia: Focus on the relationships between ischemia duration, anesthesia, cerebral vasculature, and neuronal injury following global ischemia in mice. Brain Res. 1998, 780, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Suh, S.W.; Shin, B.S.; Ma, H.; Van Hoecke, M.; Brennan, A.M.; Yenari, M.A.; Swanson, R.A. Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann. Neurol. 2008, 64, 654–663. [Google Scholar] [CrossRef]
- Cheng, A.; Yang, Y.; Zhou, Y.; Maharana, C.; Lu, D.; Peng, W.; Liu, Y.; Wan, R.; Marosi, K.; Misiak, M.; et al. Mitochondrial SIRT3 Mediates Adaptive Responses of Neurons to Exercise and Metabolic and Excitatory Challenges. Cell Metab. 2016, 23, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Klimova, N.; Fearnow, A.; Long, A.; Kristian, T. NAD(+) precursor modulates post-ischemic mitochondrial fragmentation and reactive oxygen species generation via SIRT3 dependent mechanisms. Exp. Neurol. 2020, 325, 113144. [Google Scholar] [CrossRef] [PubMed]
- Li, S.J.; Liu, H.; Wu, F.F.; Feng, D.Y.; Zhang, S.; Zheng, J.; Wang, L.; Tian, F.; Yang, Y.L.; Wang, Y.Y. Meshed neuronal mitochondrial networks empowered by AI-powered classifiers and immersive VR reconstruction. Front. Neurosci. 2023, 17, 1059965. [Google Scholar] [CrossRef] [PubMed]
- Pham, A.H.; McCaffery, J.M.; Chan, D.C. Mouse lines with photo-activatable mitochondria to study mitochondrial dynamics. Genesis 2012, 50, 833–843. [Google Scholar] [CrossRef]
- Fukumitsu, K.; Fujishima, K.; Yoshimura, A.; Wu, Y.K.; Heuser, J.; Kengaku, M. Synergistic action of dendritic mitochondria and creatine kinase maintains ATP homeostasis and actin dynamics in growing neuronal dendrites. J. Neurosci. 2015, 35, 5707–5723. [Google Scholar] [CrossRef] [PubMed]
- Landis, S.C. Ultrastructural changes in the mitochondria of cerebellar Purkinje cells of nervous mutant mice. J. Cell Biol. 1973, 57, 782–797. [Google Scholar] [CrossRef] [PubMed]
- Fukumitsu, K.; Hatsukano, T.; Yoshimura, A.; Heuser, J.; Fujishima, K.; Kengaku, M. Mitochondrial fission protein Drp1 regulates mitochondrial transport and dendritic arborization in cerebellar Purkinje cells. Mol. Cell Neurosci. 2016, 71, 56–65. [Google Scholar] [CrossRef]
- Kageyama, Y.; Zhang, Z.; Roda, R.; Fukaya, M.; Wakabayashi, J.; Wakabayashi, N.; Kensler, T.W.; Reddy, P.H.; Iijima, M.; Sesaki, H. Mitochondrial division ensures the survival of postmitotic neurons by suppressing oxidative damage. J. Cell Biol. 2012, 197, 535–551. [Google Scholar] [CrossRef]
- Biser, P.S.; Thayne, K.A.; Kong, J.Q.; Fleming, W.W.; Taylor, D.A. Quantification of the alpha(3) subunit of the Na(+)/K(+)-ATPase in developing rat cerebellum. Brain Res. Dev. Brain Res. 2000, 123, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Marcos, D.; Sepulveda, M.R.; Berrocal, M.; Mata, A.M. Ontogeny of ATP hydrolysis and isoform expression of the plasma membrane Ca(2+)-ATPase in mouse brain. BMC Neurosci. 2009, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Howarth, C.; Gleeson, P.; Attwell, D. Updated energy budgets for neural computation in the neocortex and cerebellum. J. Cereb. Blood Flow. Metab. 2012, 32, 1222–1232. [Google Scholar] [CrossRef] [PubMed]
- Napper, R.M.; Harvey, R.J. Quantitative study of the Purkinje cell dendritic spines in the rat cerebellum. J. Comp. Neurol. 1988, 274, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Egervari, G.; Siciliano, C.A.; Whiteley, E.L.; Ron, D. Alcohol and the brain: From genes to circuits. Trends Neurosci. 2021, 44, 1004–1015. [Google Scholar] [CrossRef]
- Calabrese, V.; Renis, M.; Calderone, A.; Russo, A.; Reale, S.; Barcellona, M.L.; Rizza, V. Stress proteins and SH-groups in oxidant-induced cellular injury after chronic ethanol administration in rat. Free Radic. Biol. Med. 1998, 24, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Heaton, M.B.; Paiva, M.; Mayer, J.; Miller, R. Ethanol-mediated generation of reactive oxygen species in developing rat cerebellum. Neurosci. Lett. 2002, 334, 83–86. [Google Scholar] [CrossRef]
- Comporti, M.; Signorini, C.; Leoncini, S.; Gardi, C.; Ciccoli, L.; Giardini, A.; Vecchio, D.; Arezzini, B. Ethanol-induced oxidative stress: Basic knowledge. Genes. Nutr. 2010, 5, 101–109. [Google Scholar] [CrossRef]
- Jung, M.E.; Metzger, D.B. Alcohol withdrawal and brain injuries: Beyond classical mechanisms. Molecules 2010, 15, 4984–5011. [Google Scholar] [CrossRef] [PubMed]
- Hoek, J.B.; Cahill, A.; Pastorino, J.G. Alcohol and mitochondria: A dysfunctional relationship. Gastroenterology 2002, 122, 2049–2063. [Google Scholar] [CrossRef] [PubMed]
- Jung, M.E.; Metzger, D.B. Aberrant histone acetylation promotes mitochondrial respiratory suppression in the brain of alcoholic rats. J. Pharmacol. Exp. Ther. 2015, 352, 258–266. [Google Scholar] [CrossRef]
- Mansouri, A.; Demeilliers, C.; Amsellem, S.; Pessayre, D.; Fromenty, B. Acute ethanol administration oxidatively damages and depletes mitochondrial dna in mouse liver, brain, heart, and skeletal muscles: Protective effects of antioxidants. J. Pharmacol. Exp. Ther. 2001, 298, 737–743. [Google Scholar] [PubMed]
- Klimova, N.; Fearnow, A.; Kristian, T. Role of NAD(+)-Modulated Mitochondrial Free Radical Generation in Mechanisms of Acute Brain Injury. Brain Sci. 2020, 10, 449. [Google Scholar] [CrossRef]
- Ren, T.; Zhang, H.; Wang, J.; Zhu, J.; Jin, M.; Wu, Y.; Guo, X.; Ji, L.; Huang, Q.; Zhang, H.; et al. MCU-dependent mitochondrial Ca(2+) inhibits NAD(+)/SIRT3/SOD2 pathway to promote ROS production and metastasis of HCC cells. Oncogene 2017, 36, 5897–5909. [Google Scholar] [CrossRef]
- Zheng, J.; Shi, L.; Liang, F.; Xu, W.; Li, T.; Gao, L.; Sun, Z.; Yu, J.; Zhang, J. Sirt3 Ameliorates Oxidative Stress and Mitochondrial Dysfunction After Intracerebral Hemorrhage in Diabetic Rats. Front. Neurosci. 2018, 12, 414. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, L.; Wang, P.; Li, X.; Qiu, D.; Li, L.; Zhang, J.; Hou, X.; Han, L.; Ge, J.; et al. Sirt3-dependent deacetylation of SOD2 plays a protective role against oxidative stress in oocytes from diabetic mice. Cell Cycle 2017, 16, 1302–1308. [Google Scholar] [CrossRef]
- Klimova, N.; Long, A.; Kristian, T. Nicotinamide mononucleotide alters mitochondrial dynamics by SIRT3-dependent mechanism in male mice. J. Neurosci. Res. 2019, 97, 975–990. [Google Scholar] [CrossRef] [PubMed]
- Siegel, C.S.; McCullough, L.D. NAD+ and nicotinamide: Sex differences in cerebral ischemia. Neuroscience 2013, 237, 223–231. [Google Scholar] [CrossRef]
- Kristian, T. Isolation of Mitochondria from the CNS; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; Chapter 7, Unit 7 22. [Google Scholar] [CrossRef]
- Broetto-Biazon, A.C.; Bracht, F.; Bracht, L.; Kelmer-Bracht, A.M.; Bracht, A. Transformation and action of extracellular NAD+ in perfused rat and mouse livers. Acta Pharmacol. Sin. 2009, 30, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Long, A.; Park, J.H.; Klimova, N.; Fowler, C.; Loane, D.J.; Kristian, T. CD38 Knockout Mice Show Significant Protection Against Ischemic Brain Damage Despite High Level Poly-ADP-Ribosylation. Neurochem. Res. 2017, 42, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Zielonka, J.; Vasquez-Vivar, J.; Kalyanaraman, B. Detection of 2-hydroxyethidium in cellular systems: A unique marker product of superoxide and hydroethidine. Nat. Protoc. 2008, 3, 8–21. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khatoon, R.; Fick, J.; Elesinnla, A.; Waddell, J.; Kristian, T. Sexual Dimorphism of Ethanol-Induced Mitochondrial Dynamics in Purkinje Cells. Int. J. Mol. Sci. 2024, 25, 13714. https://doi.org/10.3390/ijms252413714
Khatoon R, Fick J, Elesinnla A, Waddell J, Kristian T. Sexual Dimorphism of Ethanol-Induced Mitochondrial Dynamics in Purkinje Cells. International Journal of Molecular Sciences. 2024; 25(24):13714. https://doi.org/10.3390/ijms252413714
Chicago/Turabian StyleKhatoon, Rehana, Jordan Fick, Abosede Elesinnla, Jaylyn Waddell, and Tibor Kristian. 2024. "Sexual Dimorphism of Ethanol-Induced Mitochondrial Dynamics in Purkinje Cells" International Journal of Molecular Sciences 25, no. 24: 13714. https://doi.org/10.3390/ijms252413714
APA StyleKhatoon, R., Fick, J., Elesinnla, A., Waddell, J., & Kristian, T. (2024). Sexual Dimorphism of Ethanol-Induced Mitochondrial Dynamics in Purkinje Cells. International Journal of Molecular Sciences, 25(24), 13714. https://doi.org/10.3390/ijms252413714






