Novel Dithiocarbamic Flavanones with Antioxidant Properties—A Structure–Activity Relationship Study
Abstract
1. Introduction
2. Results
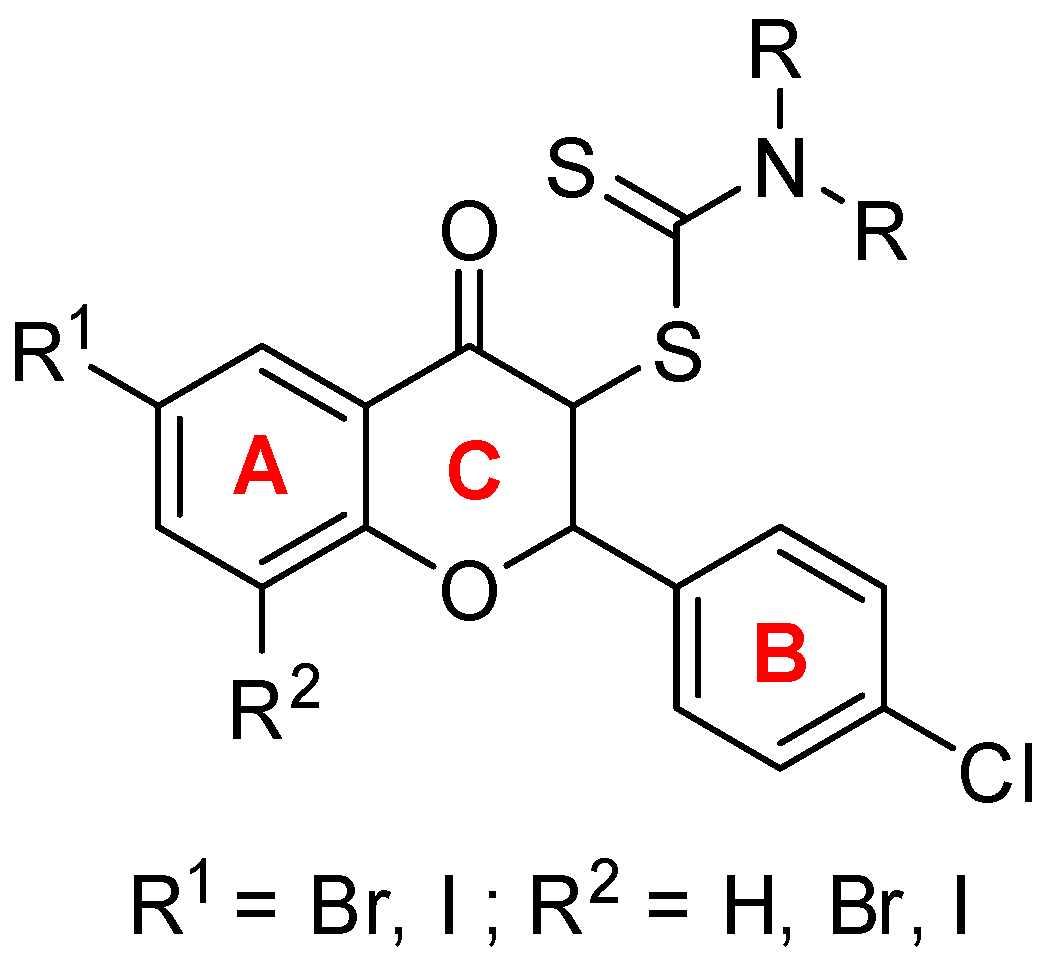
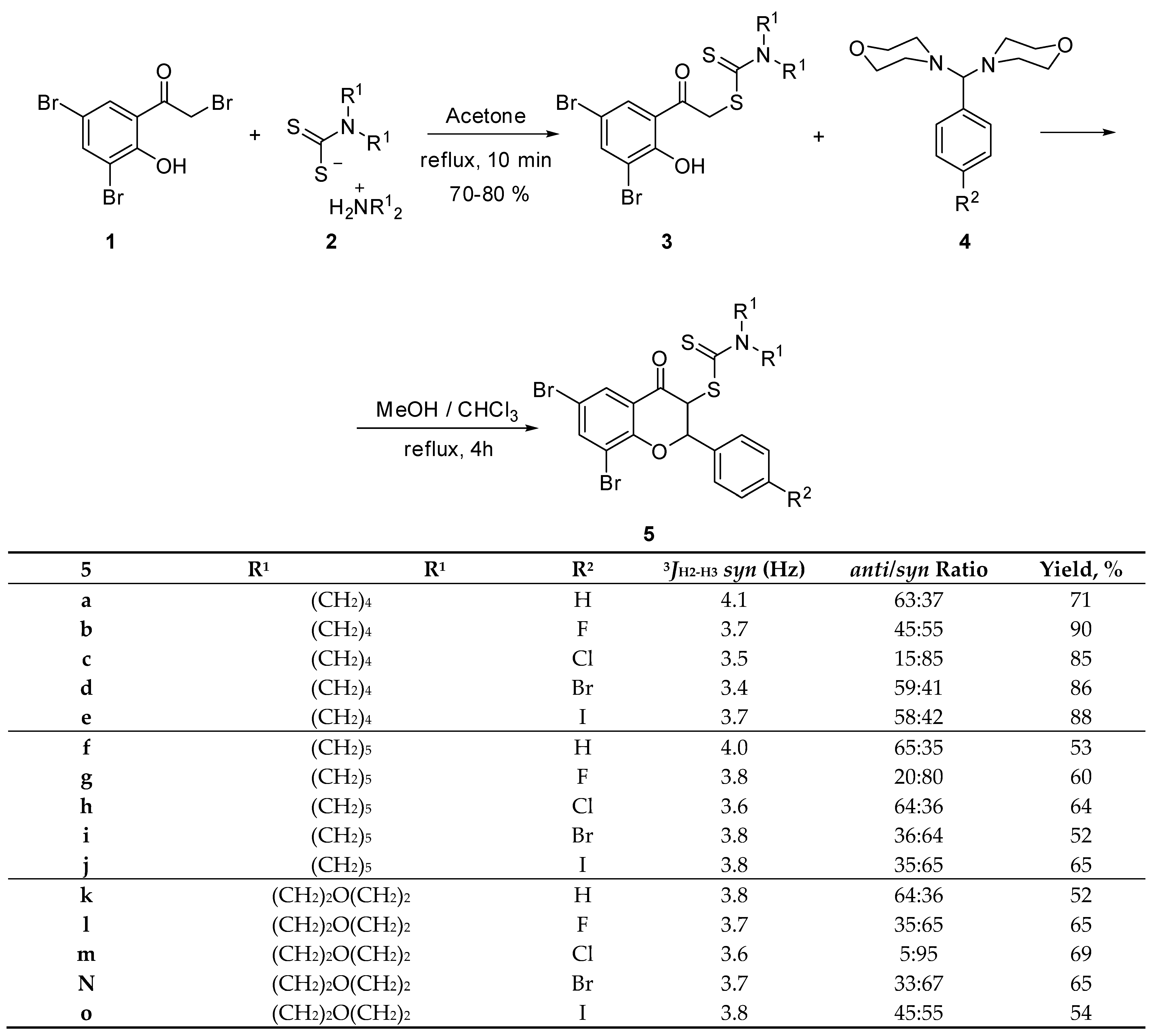
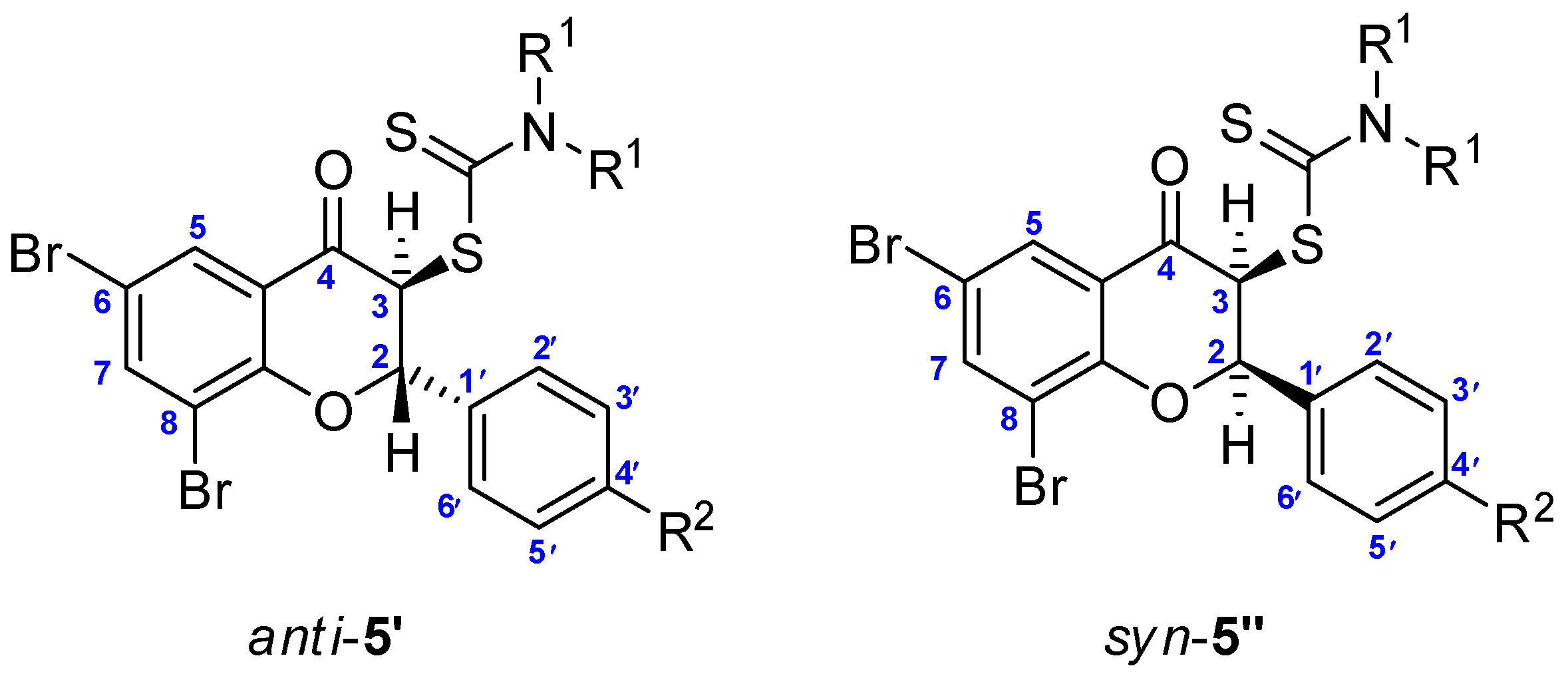
2.1. The Synthesis of Dithiocarbamic Flavanones
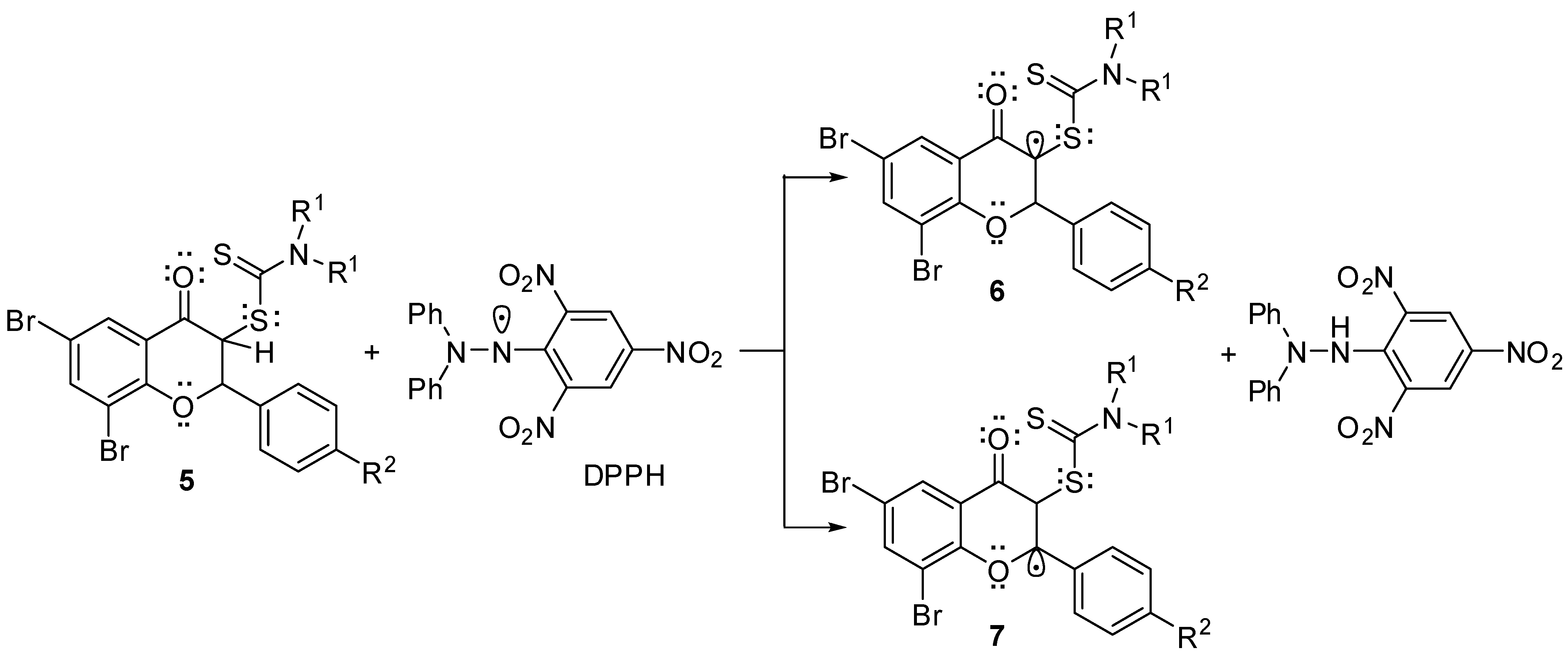
2.2. Antioxidant Activities of Flavanones 5a–o
3. Discussion
4. Materials and Methods
4.1. Chemistry
4.1.1. General Procedure for 6,8-Dibromo-2-phenyl-4-oxochroman-3-yl-pyrrolidine-1-carbodithioate (5a)
4.1.2. 6,8-Dibromo-2-(4-fluorophenyl)-4-oxochroman-3-yl-pyrrolidine-1-carbodithioate (5b)
4.1.3. 6,8-Dibromo-2-(4-bromophenyl)-4-oxochroman-3-yl-pyrrolidine-1-carbodithioate (5d)
4.1.4. 6,8-Dibromo-2-(4-iodophenyl)-4-oxochroman-3-yl-pyrrolidine-1-carbodithioate (5e)
4.1.5. 6,8-Dibromo-2-phenyl-4-oxochroman-3-yl-piperidine-1-carbodithioate (5f)
4.1.6. 6,8-Dibromo-2-(4-fluorophenyl)-4-oxochroman-3-yl-piperidine-1-carbodithioate (5g)
4.1.7. 6,8-Dibromo-2-(4-bromophenyl)-4-oxochroman-3-yl-piperidine-1-carbodithioate (5i)
4.1.8. 6,8-Dibromo-2-(4-iodophenyl)-4-oxochroman-3-yl-piperidine-1-carbodithioate (5j)
4.1.9. 6,8-Dibromo-2-phenyl-4-oxochroman-3-yl-morpholine-4-carbodithioate (5k)
4.1.10. 6,8-Dibromo-2-(4-fluorophenyl)-4-oxochroman-3-yl-morpholine-4-carbodithioate (5l)
4.1.11. 6,8-Dibromo-2-(4-bromophenyl)-4-oxochroman-3-yl-morpholine-4-carbodithioate (5n)
4.1.12. 6,8-Dibromo-2-(4-iodophenyl)-4-oxochroman-3-yl-morpholine-4-carbodithioate (5o)
4.2. In Vitro Antioxidant Activities
4.2.1. 2,2-Diphenyl-1-picrylhydrazyl Radical (DPPH) Assay
4.2.2. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic Acid) (ABTS) Assay
4.2.3. Ferric Reducing Antioxidant Power (FRAP) Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Checa, J.; Aran, J.M. Reactive Oxygen Species: Drivers of Physiological and Pathological Processes. J. Inflamm. Res. 2020, 13, 1057–1073. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B. Antioxidants and Human Disease: A General Introduction. Nutr. Rev. 1997, 55, S44–S49. [Google Scholar] [CrossRef] [PubMed]
- Arteel, G.E. Leveraging oxidative stress questions in vivo: Implications and limitations. Arch. Biochem. Biophys. 2016, 595, 40–45. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative stress: Harms and benefits for human health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef] [PubMed]
- Sies, H. Oxidative stress: Concept and some practical aspects. Antioxidants 2020, 9, 852. [Google Scholar] [CrossRef]
- Mirończuk-Chodakowska, I.; Witkowska, A.M.; Zujko, M.E. Endogenous non-enzymatic antioxidants in the human body. Adv. Med. Sci. 2018, 63, 68–78. [Google Scholar] [CrossRef]
- Williamson, G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017, 42, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Du, M.; Wei, Y.; Wang, C.; Shen, L. A review on the structure-activity relationship of dietary flavonoids for protecting vascular endothelial function: Current understanding and future issues. J. Food Biochem. 2018, 42, e12557. [Google Scholar] [CrossRef]
- Chang, H.; Lei, L.; Zhou, Y.; Ye, F.; Zhao, G. Dietary flavonoids and the risk of colorectal cancer: An updated meta-analysis of epidemiological studies. Nutrients 2018, 10, 950. [Google Scholar] [CrossRef]
- Shahidi, F.; Yeo, J. Bioactivities of phenolics by focusing on suppression of chronic diseases: A review. Int. J. Mol. Sci. 2018, 19, 1573. [Google Scholar] [CrossRef]
- Taylor, L.P.; Grotewold, E. Flavonoids as developmental regulators. Curr. Opin. Plant Biol. 2005, 8, 317–323. [Google Scholar] [CrossRef]
- Peer, W.A.; Murphy, A.S. Flavonoids and auxin transport: Modulators or regulators? Trends Plant Sci. 2007, 12, 556–563. [Google Scholar] [CrossRef]
- Buer, C.S.; Imin, N.; Djordjevic, M.A. Flavonoids: New roles for old molecules. J. Integr. Plant Biol. 2010, 52, 98–111. [Google Scholar] [CrossRef] [PubMed]
- Cushnie, T.P.T.; Lamb, A.J. Antimicrobial activity of flavonoids. Int. J. Antimicrob. Agents 2005, 26, 343–356. [Google Scholar] [CrossRef]
- Hwang, S.L.; Shih, P.H.; Yen, G.C. Neuroprotective Effects of Citrus Flavonoids. J. Agric. Food Chem. 2012, 60, 877–885. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Qiao, Z.; Wang, H.; Zhu, L.; Zhang, L. Flavonoids: Promising anticancer agents. Med. Res. Rev. 2003, 23, 519–534. [Google Scholar] [CrossRef]
- Ninfali, P.; Antonelli, A.; Magnani, M.; Scarpa, E.S. Antiviral Properties of Flavonoids and Delivery Strategies. Nutrients 2020, 12, 2534. [Google Scholar] [CrossRef]
- Badshah, S.L.; Faisal, S.; Muhammad, A.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Antiviral activities of flavonoids. Biomed. Pharmacother. 2021, 140, 111596. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Wang, Y.; Tian, W.; Cui, X.; Tu, P.; Li, J.; Shi, S.; Liu, X. Biosynthesis Investigations of Terpenoid, Alkaloid, and Flavonoid Antimicrobial Agents Derived from Medicinal Plants. Antibiotics 2022, 11, 1380. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.A.; Grayer, J. Anthocyanins and other flavonoids. Nat. Prod. Rep. 2004, 21, 539–573. [Google Scholar] [CrossRef]
- Speisky, H.; Shahidi, F.; Costa de Camargo, A.; Fuentes, J. Revisiting the Oxidation of Flavonoids: Loss, Conservation or Enhancement of Their Antioxidant Properties. Antioxidants 2022, 11, 133. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [PubMed]
- Rice-Evans, C. Plant polyphenols: Free radical scavengers or chain-breaking antioxidants? Biochem. Soc. Symp. 1995, 61, 103–116. [Google Scholar] [CrossRef]
- Amić, A.; Marković, Z.; Marković, J.M.D.; Stepanić, V.; Lučić, B.; Amić, D. Towards an improved prediction of the free radical scavenging potency of flavonoids: The significance of double pcet mechanisms. Food Chem. 2014, 152, 578–585. [Google Scholar] [CrossRef]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary Flavonoids: Cardioprotective Potential with Antioxidant Effects and Their Pharmacokinetic, Toxicological and Therapeutic Concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, S.; Meganathan, K.; Wagh, V.; Winkler, J.; Hescheler, J.; Sachinidis, A. Chemoprotective Mechanism of the Natural Compounds, Epigallocatechin- 3-O-Gallate, Quercetin and Curcumin Against Cancer and Cardiovascular Diseases. Curr. Med. Chem. 2009, 16, 1451–1462. [Google Scholar] [CrossRef]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic biochemical mechanisms behind the health benefits of polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef]
- Franco, R.; Navarro, G.; Martínez-Pinilla, E. Hormetic and mitochondria-related mechanisms of antioxidant action of phytochemicals. Antioxidants 2019, 8, 373. [Google Scholar] [CrossRef] [PubMed]
- Hrelia, S.; Angeloni, C. New mechanisms of action of natural antioxidants in health and disease. Antioxidants 2020, 9, 344. [Google Scholar] [CrossRef]
- Kadam, R.S.; Bourne, D.W.A.; Kompella, U.B. Nano-advantage in enhanced drug delivery with biodegradable nanoparticles: Contribution of reduced clearance. Drug Metab. Dispos. 2012, 40, 1380–1388. [Google Scholar] [CrossRef] [PubMed]
- Yeo, J.; Lee, J.; Lee, S.; Kim, W.J. Polymeric Antioxidant Materials for Treatment of Inflammatory Disorders. Adv. Therap. 2021, 4, 2000270. [Google Scholar] [CrossRef]
- Singh, A.; Kaushik, A.; Dhau, J.S.; Kumar, R. Exploring coordination preferences and biological applications of pyridyl-based organochalcogen (Se, Te) ligands. Coord. Chem. Rev. 2022, 450, 214254. [Google Scholar] [CrossRef]
- Bahrin, L.G.; Jones, P.G.; Hopf, H. Tricyclic flavonoids with 1,3-dithiolium substructure. Beilstein J. Org. Chem. 2012, 8, 1999–2003. [Google Scholar] [CrossRef] [PubMed]
- Bahrin, L.G.; Sarbu, L.G.; Hopf, H.; Jones, P.G.; Babii, C.; Stefan, M.; Birsa, M.L. The influence of halogen substituents on the biological properties of sulfur-containing flavonoids. Bioorg. Med. Chem. 2016, 24, 3166–3173. [Google Scholar] [CrossRef]
- Birsa, M.L.; Sarbu, L.G. A Structure–Activity Relationship Study on the Antioxidant Properties of Dithiocarbamic Flavanones. Antioxidants 2024, 13, 963. [Google Scholar] [CrossRef]
- Buu-Hoi, N.P.; Lavit, D. The bromination of o- and p-hydroxyaryl ketones. J. Chem. Soc. 1955, 18–20. [Google Scholar] [CrossRef]
- Bahrin, L.G.; Asaftei, I.V.; Sandu, I.; Sarbu, L.G. Synthesis of (4-Methylpiperazin-1-yl)carbodithioates and of their 1,3-Dithiolium Derivatives. Rev. Chim. 2014, 65, 1046–1048. [Google Scholar]
- Bahrin, L.G.; Sarbu, L.G.; Jones, P.G.; Birsa, L.M.; Hopf, H. [2.2]Paracyclophane-Bis(triazole) Systems: Synthesis and Photochemical Behavior. Chem. Eur. J. 2017, 23, 12338–12345. [Google Scholar] [CrossRef]
- Seliger, H.; Happ, E.; Cascaval, A.; Birsa, M.L.; Nicolaescu, T.; Poinescu, I.; Cojocariu, C. Synthesis and characterization of new photostabilizers from 2,4-dihydroxybenzophenone. Eur. Polym. J. 1999, 35, 827–833. [Google Scholar] [CrossRef]
- Birsa, M.L. Synthesis of some new substituted flavanones and related 4-chromanones by a novel synthetic method. Synth. Commun. 2002, 32, 115–118. [Google Scholar] [CrossRef]
- Kano, M.; Takayanagi, T.; Harada, K.; Makino, K.; Ishikawa, F. Antioxidative activity of anthocyanins from purple sweet potato Ipomoera batatas cultivar Ayamurasaki. Biosci. Biotechnol. Biochem. 2005, 69, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Ricci, D.; Fraternale, D.; Giamperi, L.; Bucchini, A.; Epifano, F.; Burini, G.; Curini, M. Chemical composition, antimicrobial and antioxidant activity of the essential oil of Teucrium marum (Lamiaceae). J. Ethnopharmacol. 2005, 98, 195–200. [Google Scholar] [CrossRef]
- Mimica-Dukic, N.; Bozin, B.; Sokovic, M.; Simin, N. Antimicrobial and antioxidant activities of Melissa officinalis L. (Lamiaceae) essential oil. J. Agric. Food Chem. 2004, 52, 2485–2489. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Gursoy, N.; Sarikurkcu, C.; Cengiz, M.; Halil Solak, M. Antioxidant activities, metal contents, total phenolics and flavonoids of seven Morchella species. Food Chem. Toxicol. 2009, 47, 2381–2388. [Google Scholar] [CrossRef]
- Arts, M.J.T.J.; Haenen, G.R.M.M.; Voss, H.-P.; Bast, A. Antioxidant capacity of reaction products limits the applicability of the Trolox Equivalent Antioxidant Capacity (TEAC) assay. Food Chem. Toxicol. 2004, 42, 45–49. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef] [PubMed]
| R1 | R1 | Compound | DPPH | ABTS+ | FRAP |
|---|---|---|---|---|---|
| (CH2)4 | 5a | 179.2 ± 0.4 | 19.0 ± 0.6 | 35.2 ± 0.2 | |
| 5b | 91.7 ± 0.5 | 13.5 ± 0.4 | 19.0 ± 0.4 | ||
| 5c | 125.9 ± 0.2 | 14.1 ± 0.2 | 20.0 ± 0.2 | ||
| 5d | 130.6 ± 0.3 | 15.9 ± 0.2 | 21.9 ± 0.3 | ||
| 5e | 132.9 ± 0.4 | 17.4 ± 0.4 | 23.7 ± 0.4 | ||
| (CH2)5 | 5f | 250.4 ± 0.5 | 22.0 ± 0.5 | 33.1 ± 0.5 | |
| 5g | 215.6 ± 0.2 | 16.9 ± 0.2 | 22.1 ± 0.3 | ||
| 5h | 222.1 ± 0.4 | 17.4 ± 0.2 | 23.8 ± 0.4 | ||
| 5i | 233.7 ± 0.3 | 18.6 ± 0.5 | 25.5 ± 0.2 | ||
| 5j | 247.9 ± 0.2 | 21.1 ± 0.6 | 30.1 ± 0.4 | ||
| (CH2)2O(CH2)2 | 5k | 165.2 ± 0.1 | 14.9 ± 0.3 | 32.7 ± 0.3 | |
| 5l | 139.0 ± 0.5 | 10.1 ± 0.2 | 15.6 ± 0.5 | ||
| 5m | 147.4 ± 0.2 | 10.7 ± 0.2 | 17.7 ± 0.4 | ||
| 5n | 156.0 ± 0.4 | 11.8 ± 0.2 | 20.9 ± 0.2 | ||
| 5o | 157.0 ± 0.6 | 12.6 ± 0.3 | 24.1 ± 0.3 | ||
| Ascorbic acid | 23.4 ± 0.2 | 18.0 ± 0.2 | 29.7 ± 0.1 | ||
| BHT | 265.0 ± 0.1 | 14.6 ± 0.3 | 68.1 ± 0.2 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Birsa, M.L.; Sarbu, L.G. Novel Dithiocarbamic Flavanones with Antioxidant Properties—A Structure–Activity Relationship Study. Int. J. Mol. Sci. 2024, 25, 13698. https://doi.org/10.3390/ijms252413698
Birsa ML, Sarbu LG. Novel Dithiocarbamic Flavanones with Antioxidant Properties—A Structure–Activity Relationship Study. International Journal of Molecular Sciences. 2024; 25(24):13698. https://doi.org/10.3390/ijms252413698
Chicago/Turabian StyleBirsa, Mihail Lucian, and Laura Gabriela Sarbu. 2024. "Novel Dithiocarbamic Flavanones with Antioxidant Properties—A Structure–Activity Relationship Study" International Journal of Molecular Sciences 25, no. 24: 13698. https://doi.org/10.3390/ijms252413698
APA StyleBirsa, M. L., & Sarbu, L. G. (2024). Novel Dithiocarbamic Flavanones with Antioxidant Properties—A Structure–Activity Relationship Study. International Journal of Molecular Sciences, 25(24), 13698. https://doi.org/10.3390/ijms252413698






