CCAAT/Enhancer-Binding Protein β (C/EBPβ) Regulates Calcium Deposition in Smooth Muscle Cells
Abstract
1. Introduction
2. Results
2.1. C/EBPβ Is Upregulated in Inorganic Phosphate with VSMCs
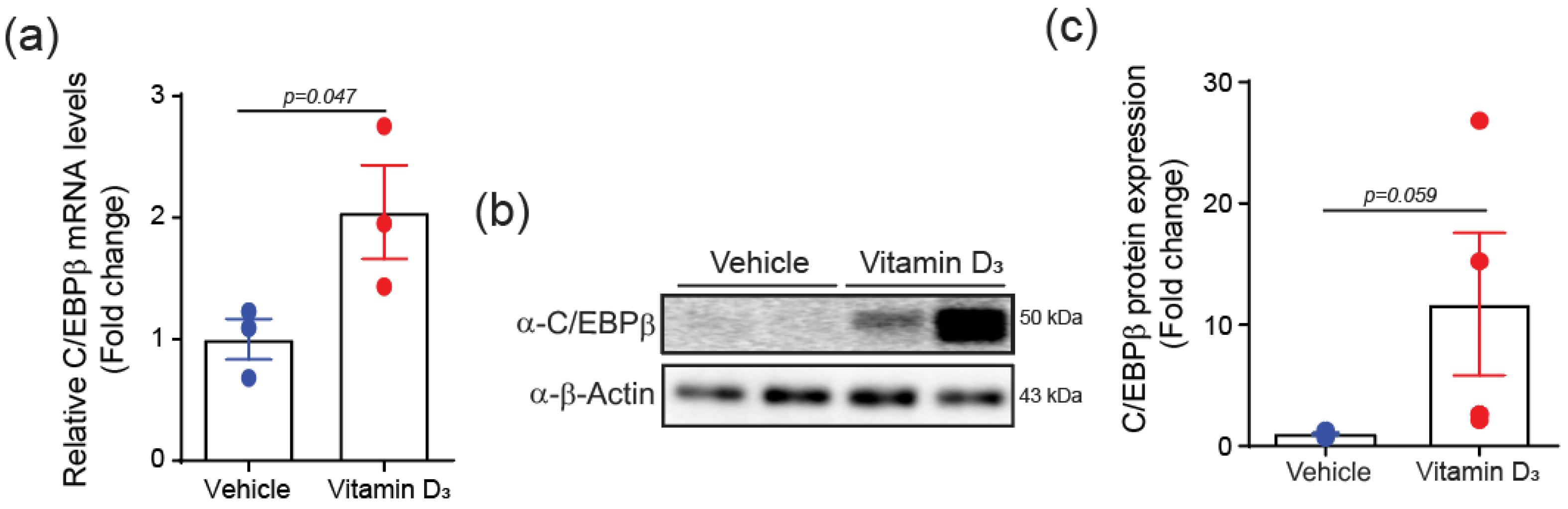
2.2. C/EBPβ Is Increased in Vitamin D3-Induced VC Mice
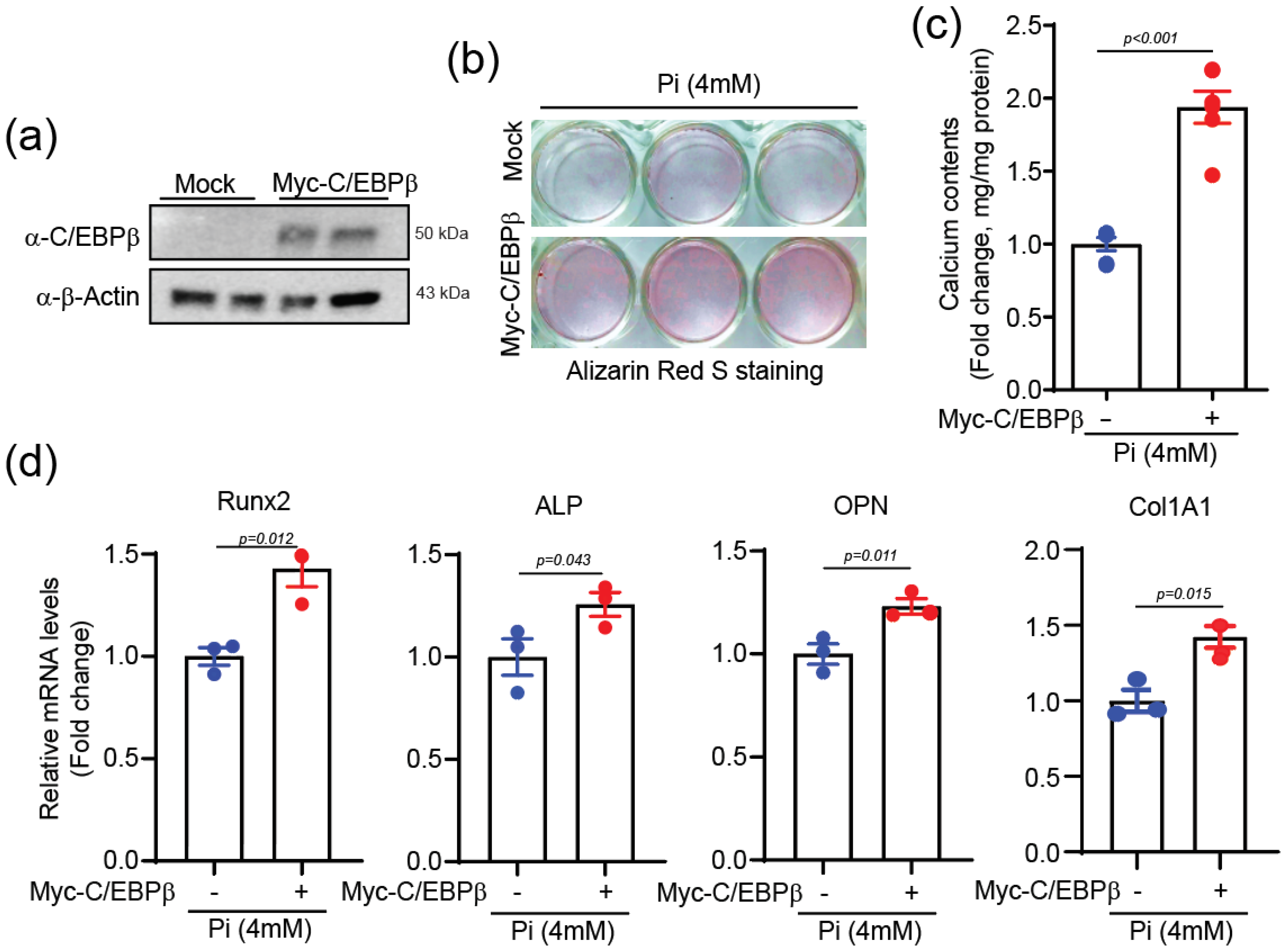
2.3. C/EBPβ Overexpression Elevates Calcium Deposition
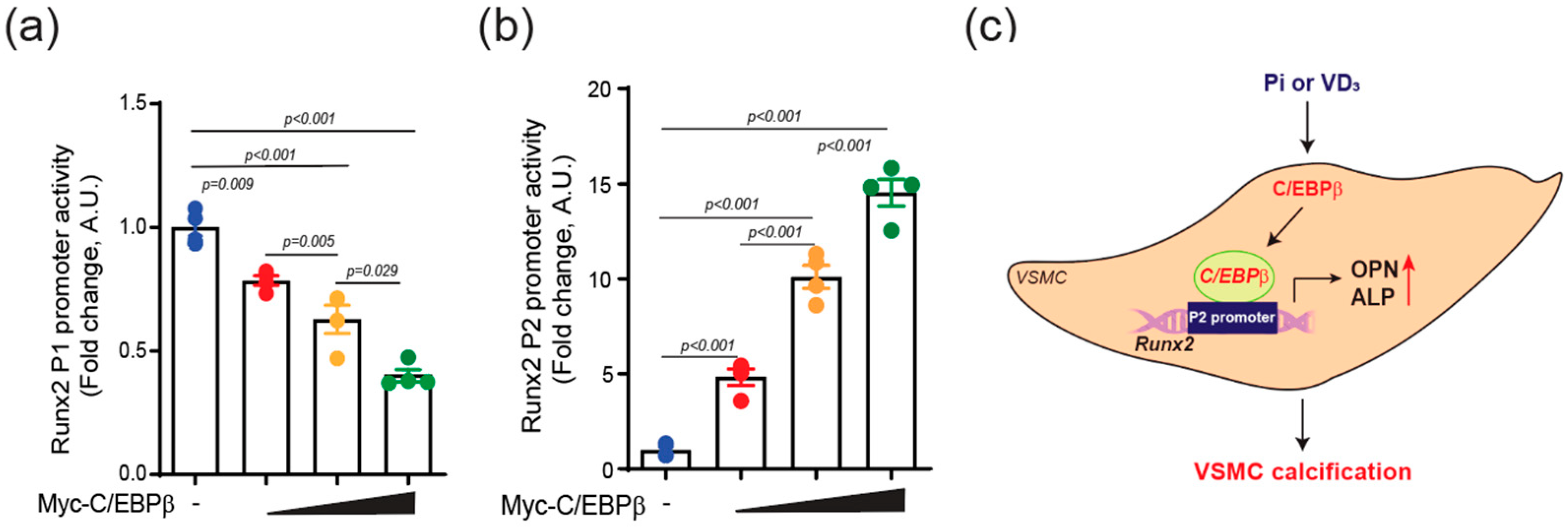
2.4. C/EBPβ Regulates Runx2 P2 Promoter Activity in VSMCs
3. Discussion
4. Materials and Methods
4.1. Small Interfering RNA (siRNA), and Antibodies
4.2. Cell Cultures
4.3. Induction of Vascular Calcification
4.4. mRNA Microarray and RNA Seq Analysis
4.5. Quantification of Calcium Deposition
4.6. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.7. Western Blot Analysis
4.8. Cloning
4.9. Luciferase Assay
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| VSMCs | Vascular smooth muscle cells |
| VC | Vascular calcification |
| C/EBPβ | CCAAT/enhancer-binding protein beta |
| Pi | Inorganic phosphate |
| Runx2 | Runt-related transcription factor 2 |
| ALP | Alkaline phosphatase |
| OPN | Osteopontin |
| Col1a1 | Collagen type 1 alpha 1 |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| GEO | Gene Expression Omnibus |
References
- Demer, L.L.; Tintut, Y. Vascular calcification: Pathobiology of a multifaceted disease. Circulation 2008, 117, 2938–2948. [Google Scholar] [CrossRef] [PubMed]
- Lanzer, P.; Boehm, M.; Sorribas, V.; Thiriet, M.; Janzen, J.; Zeller, T.; St Hilaire, C.; Shanahan, C. Medial vascular calcification revisited: Review and perspectives. Eur. Heart J. 2014, 35, 1515–1525. [Google Scholar] [CrossRef]
- Vervloet, M.; Cozzolino, M. Vascular calcification in chronic kidney disease: Different bricks in the wall? Kidney Int. 2017, 91, 808–817. [Google Scholar] [CrossRef]
- Lacolley, P.; Regnault, V.; Nicoletti, A.; Li, Z.; Michel, J.B. The vascular smooth muscle cell in arterial pathology: A cell that can take on multiple roles. Cardiovasc. Res. 2012, 95, 194–204. [Google Scholar] [CrossRef]
- Durham, A.L.; Speer, M.Y.; Scatena, M.; Giachelli, C.M.; Shanahan, C.M. Role of smooth muscle cells in vascular calcification: Implications in atherosclerosis and arterial stiffness. Cardiovasc. Res. 2018, 114, 590–600. [Google Scholar] [CrossRef] [PubMed]
- Giachelli, C.M. The emerging role of phosphate in vascular calcification. Kidney Int. 2009, 75, 890–897. [Google Scholar] [CrossRef]
- Ewing, S.J.; Zhu, S.; Zhu, F.; House, J.S.; Smart, R.C. C/EBPβ represses p53 to promote cell survival downstream of DNA damage independent of oncogenic Ras and p19Arf. Cell Death Differ. 2008, 15, 1734–1744. [Google Scholar] [CrossRef]
- Messenger, Z.J.; Hall, J.R.; Jima, D.D.; House, J.S.; Tam, H.W.; Tokarz, D.A.; Smart, R.C. C/EBPβ deletion in oncogenic Ras skin tumors is a synthetic lethal event. Cell Death Dis. 2018, 9, 1054. [Google Scholar] [CrossRef]
- Ramji, D.P.; Foka, P. CCAAT/enhancer-binding proteins: Structure, function and regulation. Biochem. J. 2002, 365 Pt 3, 561–575. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Liu, Z.; Wu, L.; Yin, G.; Xie, X.; Kong, W.; Zhou, J.; Liu, S. C/EBPβ: The structure, regulation, and its roles in inflammation-related diseases. Biomed. Pharmacother. 2023, 169, 115938. [Google Scholar] [CrossRef]
- Bostrom, P.; Mann, N.; Wu, J.; Quintero, P.A.; Plovie, E.R.; Panakova, D.; Gupta, R.K.; Xiao, C.; MacRae, C.A.; Rosenzweig, A.; et al. C/EBPβ controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell 2010, 143, 1072–1083. [Google Scholar] [CrossRef]
- Cortes-Canteli, M.; Aguilar-Morante, D.; Sanz-Sancristobal, M.; Megias, D.; Santos, A.; Perez-Castillo, A. Role of C/EBPβ transcription factor in adult hippocampal neurogenesis. PLoS ONE 2011, 6, e24842. [Google Scholar] [CrossRef] [PubMed]
- Schroeder-Gloeckler, J.M.; Rahman, S.M.; Janssen, R.C.; Qiao, L.; Shao, J.; Roper, M.; Fischer, S.J.; Lowe, E.; Orlicky, D.J.; McManaman, J.L.; et al. CCAAT/enhancer-binding protein β deletion reduces adiposity, hepatic steatosis, and diabetes in Leprdb/db mice. J. Biol. Chem. 2007, 282, 15717–15729. [Google Scholar] [CrossRef]
- Rahman, S.M.; Baquero, K.C.; Choudhury, M.; Janssen, R.C.; de la Houssaye, B.A.; Sun, M.; Miyazaki-Anzai, S.; Wang, S.; Moustaid-Moussa, N.; Miyazaki, M.; et al. C/EBPβ in bone marrow is essential for diet induced inflammation, cholesterol balance, and atherosclerosis. Atherosclerosis 2016, 250, 172–179. [Google Scholar] [CrossRef]
- Huber, R.; Pietsch, D.; Panterodt, T.; Brand, K. Regulation of C/EBPβ and resulting functions in cells of the monocytic lineage. Cell. Signal. 2012, 24, 1287–1296. [Google Scholar] [CrossRef]
- Hirata, M.; Kugimiya, F.; Fukai, A.; Ohba, S.; Kawamura, N.; Ogasawara, T.; Kawasaki, Y.; Saito, T.; Yano, F.; Ikeda, T.; et al. C/EBPβ Promotes transition from proliferation to hypertrophic differentiation of chondrocytes through transactivation of p57Kip2. PLoS ONE 2009, 4, e4543. [Google Scholar] [CrossRef]
- Hata, K.; Nishimura, R.; Ueda, M.; Ikeda, F.; Matsubara, T.; Ichida, F.; Hisada, K.; Nokubi, T.; Yamaguchi, A.; Yoneda, T. A CCAAT/enhancer binding protein β isoform, liver-enriched inhibitory protein, regulates commitment of osteoblasts and adipocytes. Mol. Cell. Biol. 2005, 25, 1971–1979. [Google Scholar] [CrossRef]
- Gutierrez, S.; Javed, A.; Tennant, D.K.; van Rees, M.; Montecino, M.; Stein, G.S.; Stein, J.L.; Lian, J.B. CCAAT/enhancer-binding proteins (C/EBP) β and δ activate osteocalcin gene transcription and synergize with Runx2 at the C/EBP element to regulate bone-specific expression. J. Biol. Chem. 2002, 277, 1316–1323. [Google Scholar] [CrossRef] [PubMed]
- Jono, S.; McKee, M.D.; Murry, C.E.; Shioi, A.; Nishizawa, Y.; Mori, K.; Morii, H.; Giachelli, C.M. Phosphate regulation of vascular smooth muscle cell calcification. Circ. Res. 2000, 87, E10–E17. [Google Scholar] [CrossRef]
- Price, P.A.; June, H.H.; Buckley, J.R.; Williamson, M.K. Osteoprotegerin inhibits artery calcification induced by warfarin and by vitamin D. Arterioscler. Thromb. Vasc. Biol. 2001, 21, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
- Zebger-Gong, H.; Muller, D.; Diercke, M.; Haffner, D.; Hocher, B.; Verberckmoes, S.; Schmidt, S.; D’Haese, P.C.; Querfeld, U. 1,25-Dihydroxyvitamin D3-induced aortic calcifications in experimental uremia: Up-regulation of osteoblast markers, calcium-transporting proteins and osterix. J. Hypertens. 2011, 29, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Komori, T. Regulation of osteoblast differentiation by transcription factors. J. Cell. Biochem. 2006, 99, 1233–1239. [Google Scholar] [CrossRef]
- Chen, Y.; Zhao, X.; Wu, H. Transcriptional Programming in Arteriosclerotic Disease: A Multifaceted Function of the Runx2 (Runt-Related Transcription Factor 2). Arterioscler. Thromb. Vasc. Biol. 2021, 41, 20–34. [Google Scholar] [CrossRef]
- Chang, D.F.; Belaguli, N.S.; Iyer, D.; Roberts, W.B.; Wu, S.P.; Dong, X.R.; Marx, J.G.; Moore, M.S.; Beckerle, M.C.; Majesky, M.W.; et al. Cysteine-rich LIM-only proteins CRP1 and CRP2 are potent smooth muscle differentiation cofactors. Dev. Cell 2003, 4, 107–118. [Google Scholar] [CrossRef]
- Lee, S.J.; Baek, S.E.; Jang, M.A.; Kim, C.D. Osteopontin plays a key role in vascular smooth muscle cell proliferation via EGFR-mediated activation of AP-1 and C/EBPβ pathways. Pharmacol. Res. 2016, 108, 1–8. [Google Scholar] [CrossRef]
- Yang, T.C.; Lu, M.H.; Wang, W.J.; Chen, J.Y. CEBPB/POU2F2 modulates endothelin 1 expression in prehypertensive SHR vascular smooth muscle cells. J. Mol. Endocrinol. 2023, 71, e220178. [Google Scholar] [CrossRef]
- Masuda, M.; Miyazaki-Anzai, S.; Keenan, A.L.; Shiozaki, Y.; Okamura, K.; Chick, W.S.; Williams, K.; Zhao, X.; Rahman, S.M.; Tintut, Y.; et al. Activating transcription factor-4 promotes mineralization in vascular smooth muscle cells. JCI Insight 2016, 1, e88646. [Google Scholar] [CrossRef]
- Dutta, P.; Kodigepalli, K.M.; LaHaye, S.; Thompson, J.W.; Rains, S.; Nagel, C.; Thatcher, K.; Hinton, R.B.; Lincoln, J. KPT-330 Prevents Aortic Valve Calcification via a Novel C/EBPβ Signaling Pathway. Circ. Res. 2021, 128, 1300–1316. [Google Scholar] [CrossRef]
- Liu, J.C.; Lengner, C.J.; Gaur, T.; Lou, Y.; Hussain, S.; Jones, M.D.; Borodic, B.; Colby, J.L.; Steinman, H.A.; van Wijnen, A.J.; et al. Runx2 protein expression utilizes the Runx2 P1 promoter to establish osteoprogenitor cell number for normal bone formation. J. Biol. Chem. 2011, 286, 30057–30070. [Google Scholar] [CrossRef] [PubMed]
- Harada, H.; Tagashira, S.; Fujiwara, M.; Ogawa, S.; Katsumata, T.; Yamaguchi, A.; Komori, T.; Nakatsuka, M. Cbfa1 isoforms exert functional differences in osteoblast differentiation. J. Biol. Chem. 1999, 274, 6972–6978. [Google Scholar] [CrossRef]
- Henriquez, B.; Hepp, M.; Merino, P.; Sepulveda, H.; van Wijnen, A.J.; Lian, J.B.; Stein, G.S.; Stein, J.L.; Montecino, M. C/EBPβ binds the P1 promoter of the Runx2 gene and up-regulates Runx2 transcription in osteoblastic cells. J. Cell. Physiol. 2011, 226, 3043–3052. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Hong, W.; Chen, Z.; Gordillo-Martinez, F.; Wang, S.; Fan, H.; Liu, Y.; Dai, Y.; Wang, B.; Jiang, L.; et al. CCAAT/Enhancer-Binding Protein Alpha Is a Novel Regulator of Vascular Smooth Muscle Cell Osteochondrogenic Transition and Vascular Calcification. Front. Physiol. 2022, 13, 755371. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.B.; Benkusky, N.A.; Pike, J.W. The RUNX2 cistrome in osteoblasts: Characterization, down-regulation following differentiation, and relationship to gene expression. J. Biol. Chem. 2014, 289, 16016–16031. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Xiao, Z.; Luo, J.; He, N.; Mahlios, J.; Quarles, L.D. Dose-dependent effects of Runx2 on bone development. J. Bone Miner. Res. 2009, 24, 1889–1904. [Google Scholar] [CrossRef] [PubMed]
- Kwon, D.H.; Eom, G.H.; Ko, J.H.; Shin, S.; Joung, H.; Choe, N.; Nam, Y.S.; Min, H.K.; Kook, T.; Yoon, S.; et al. MDM2 E3 ligase-mediated ubiquitination and degradation of HDAC1 in vascular calcification. Nat. Commun. 2016, 7, 10492. [Google Scholar] [CrossRef]
- Choe, N.; Kwon, D.H.; Ryu, J.; Shin, S.; Cho, H.J.; Joung, H.; Eom, G.H.; Ahn, Y.; Park, W.J.; Nam, K.I.; et al. miR-27a-3p Targets ATF3 to Reduce Calcium Deposition in Vascular Smooth Muscle Cells. Mol. Ther. Nucleic Acids 2020, 22, 627–639. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choe, N.; Shin, S.; Kim, Y.-K.; Kook, H.; Kwon, D.-H. CCAAT/Enhancer-Binding Protein β (C/EBPβ) Regulates Calcium Deposition in Smooth Muscle Cells. Int. J. Mol. Sci. 2024, 25, 13667. https://doi.org/10.3390/ijms252413667
Choe N, Shin S, Kim Y-K, Kook H, Kwon D-H. CCAAT/Enhancer-Binding Protein β (C/EBPβ) Regulates Calcium Deposition in Smooth Muscle Cells. International Journal of Molecular Sciences. 2024; 25(24):13667. https://doi.org/10.3390/ijms252413667
Chicago/Turabian StyleChoe, Nakwon, Sera Shin, Young-Kook Kim, Hyun Kook, and Duk-Hwa Kwon. 2024. "CCAAT/Enhancer-Binding Protein β (C/EBPβ) Regulates Calcium Deposition in Smooth Muscle Cells" International Journal of Molecular Sciences 25, no. 24: 13667. https://doi.org/10.3390/ijms252413667
APA StyleChoe, N., Shin, S., Kim, Y.-K., Kook, H., & Kwon, D.-H. (2024). CCAAT/Enhancer-Binding Protein β (C/EBPβ) Regulates Calcium Deposition in Smooth Muscle Cells. International Journal of Molecular Sciences, 25(24), 13667. https://doi.org/10.3390/ijms252413667





