The Diagnostic Value of Cerebrospinal Fluid Neurogranin in Neurodegenerative Diseases
Abstract
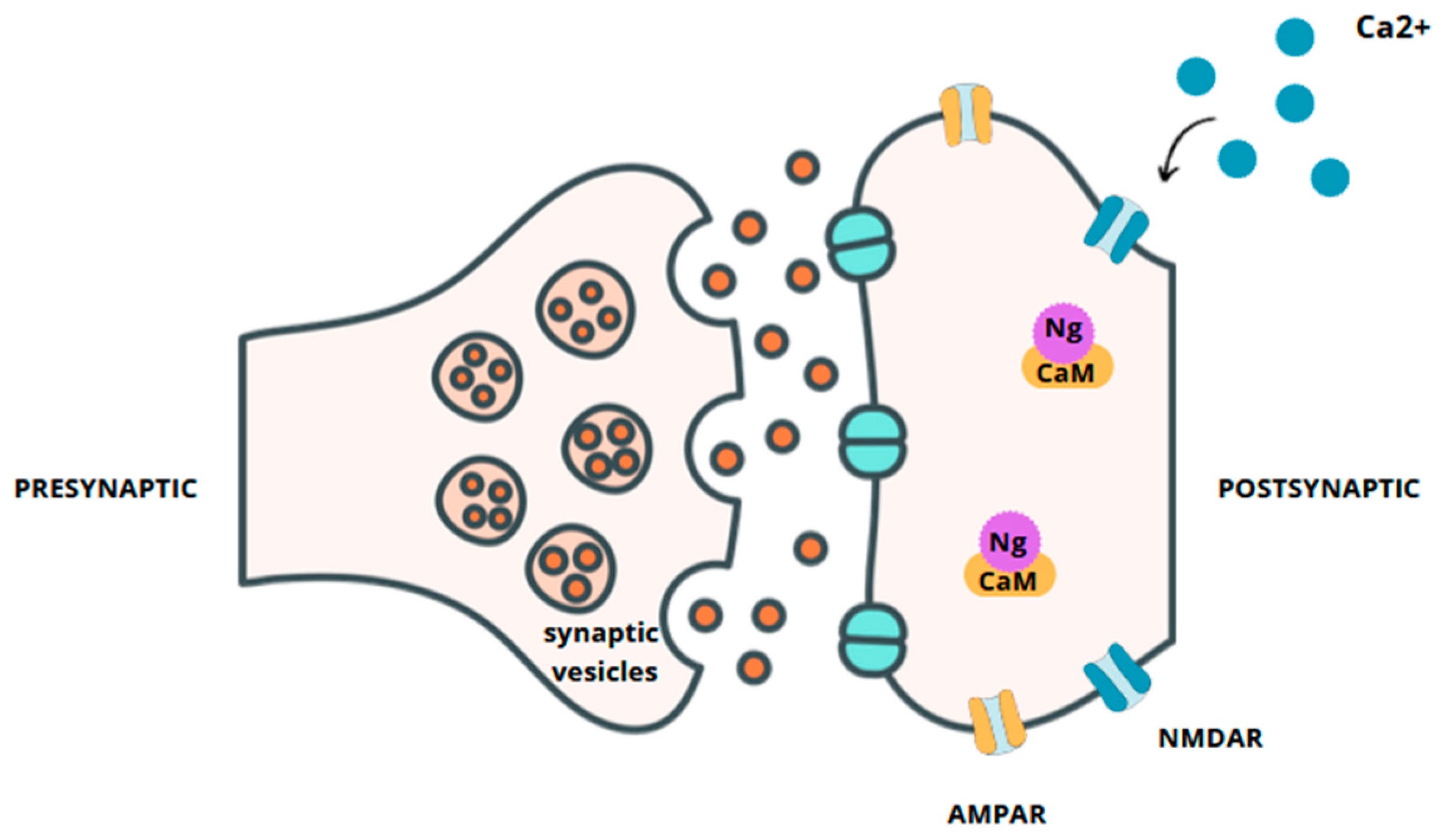
1. Introduction
2. Alzheimer’s Disease
Neurogranin in AD
3. Parkinson’s Disease
Neurogranin in PD
4. Creutzfeldt–Jacob’s Disease
Neurogranin in CJD
5. Legal Aspects of the Diagnostic Value of CSF Neurogranin in ND
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Li, H.Y.; Li, J.F.; Lu, G.W. Neurogranin: A brain-specific protein. Sheng Li Ke Xue Jin Zhan 2003, 34, 111–115. (In Chinese) [Google Scholar]
- Díez-Guerra, F.J. Neurogranin, a link between calcium/calmodulin and protein kinase C signaling in synaptic plasticity. IUBMB Life 2010, 62, 597–606. [Google Scholar] [CrossRef]
- Huang, K.P.; Huang, F.L.; Chen, H.C. Characterization of a 7.5-kDa protein kinase C substrate (RC3 protein, neurogranin) from rat brain. Arch. Biochem. Biophys. 1993, 305, 570–580. [Google Scholar] [CrossRef]
- Zhong, L.; Cherry, T.; Bies, C.E.; Florence, M.A.; Gerges, N.Z. Neurogranin enhances synaptic strength through its interaction with calmodulin. EMBO J. 2009, 28, 3027–3039. [Google Scholar] [CrossRef] [PubMed]
- Garrido-García, A.; de Andrés, R.; Jiménez-Pompa, A.; Soriano, P.; Sanz-Fuentes, D.; Martínez-Blanco, E.; Díez-Guerra, F.J. Neurogranin Expression Is Regulated by Synaptic Activity and Promotes Synaptogenesis in Cultured Hippocampal Neurons. Mol. Neurobiol. 2019, 56, 7321–7337. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.H.; Kelly, P.T. Postsynaptic injection of Ca2+/CaM induces synaptic potentiation requiring CaMKII and PKC activity. Neuron 1995, 15, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Ramakers, G.M.; Gerendasy, D.D.; de Graan, P.N. Substrate phosphorylation in the protein kinase Cgamma knockout mouse. J. Biol. Chem. 1999, 274, 1873–1874. [Google Scholar] [CrossRef] [PubMed]
- Mons, N.; Enderlin, V.; Jaffard, R.; Higueret, P. Selective age-related changes in the PKC-sensitive, calmodulin-binding protein, neurogranin, in the mouse brain. J. Neurochem. 2001, 79, 859–867. [Google Scholar] [CrossRef]
- Xiang, Y.; Xin, J.; Le, W.; Yang, Y. Neurogranin: A Potential Biomarker of Neurological and Mental Diseases. Front. Aging Neurosci. 2020, 12, 584743. [Google Scholar] [CrossRef]
- 2024 Alzheimer’s disease facts and figures. Alzheimers Dement 2024, 20, 3708–3821. [CrossRef]
- Duan, J.; Liu, Y.; Wu, H.; Wang, J.; Chen, L.; Chen, C.L.P. Broad learning for early diagnosis of alzheimer’s disease using fdg-pet of the brain. Front. Neurosci. 2023, 17, 1137567. [Google Scholar] [CrossRef]
- Chételat, G.; Arbizu, J.; Barthel, H.; Garibotto, V.; Law, I.; Morbelli, S.; van de Giessen, E.; Agosta, F.; Barkhof, F.; Brooks, D.J.; et al. Amyloid-PET and 18F-FDG-PET in the diagnostic investigation of Alzheimer’s disease and other dementias. Lancet Neurol. 2020, 19, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Scheff, S.W.; Price, D.A.; Schmitt, F.A.; DeKosky, S.T.; Mufson, E.J. Synaptic alterations in CA1 in mild Alzheimer disease and mild cognitive impairment. Neurology 2007, 68, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef] [PubMed]
- Davidsson, P.; Blennow, K. Neurochemical dissection of synaptic pathology in Alzheimer’s disease. Int. Psychogeriatr. 1998, 10, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. Contributors. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Agnello, L.; Gambino, C.M.; Lo Sasso, B.; Bivona, G.; Milano, S.; Ciaccio, A.M.; Piccoli, T.; La Bella, V.; Ciaccio, M. Neurogranin as a Novel Biomarker in Alzheimer’s Disease. Lab. Med. 2021, 52, 188–196. [Google Scholar] [CrossRef]
- De Vos, A.; Jacobs, D.; Struyfs, H.; Fransen, E.; Andersson, K.; Portelius, E.; Andreasson, U.; De Surgeloose, D.; Hernalsteen, D.; Sleegers, K.; et al. C-terminal neurogranin is increased in cerebrospinal fluid but unchanged in plasma in Alzheimer’s disease. Alzheimer’s Dement. 2015, 11, 1461–1469. [Google Scholar] [CrossRef] [PubMed]
- Casaletto, K.B.; Elahi, F.M.; Bettcher, B.M.; Neuhaus, J.; Bendlin, B.B.; Asthana, S.; Johnson, S.C.; Yaffe, K.; Carlsson, C.; Blennow, K.; et al. Neurogranin, a synaptic protein, is associated with memory independent of Alzheimer biomarkers. Neurology 2017, 89, 1782–1788. [Google Scholar] [CrossRef] [PubMed]
- Liss, J.L.; Seleri Assunção, S.; Cummings, J.; Atri, A.; Geldmacher, D.S.; Candela, S.F.; Devanand, D.P.; Fillit, H.M.; Susman, J.; Mintzer, J.; et al. Practical recommendations for timely, accurate diagnosis of symptomatic Alzheimer’s disease (MCI and dementia) in primary care: A review and synthesis. J. Intern. Med. 2021, 290, 310–334. [Google Scholar] [CrossRef]
- Hellwig, K.; Kvartsberg, H.; Portelius, E.; Andreasson, U.; Oberstein, T.J.; Lewczuk, P.; Blennow, K.; Kornhuber, J.; Maler, J.M.; Zetterberg, H.; et al. Neurogranin and YKL-40: Independent markers of synaptic degeneration and neuroinflammation in Alzheimer’s disease. Alzheimer’s Res. Ther. 2015, 7, 74. [Google Scholar] [CrossRef]
- Wang, L. Alzheimer’s Disease Neuroimaging Initiative. Association of cerebrospinal fluid Neurogranin with Alzheimer’s disease. Aging Clin. Exp. Res. 2019, 31, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, C.; Forlenza, O.; Zetterberg, H.; Blennow, K. Increased neurogranin concentrations in cerebrospinal fluid of Alzheimer’s disease and in mild cognitive impairment due to AD. J. Neural Transm. 2016, 123, 1443–1447. [Google Scholar] [CrossRef] [PubMed]
- Portelius, E.; Zetterberg, H.; Skillbäck, T.; Törnqvist, U.; Andreasson, U.; Trojanowski, J.Q.; Weiner, M.W.; Shaw, L.M.; Mattsson, N.; Blennow, K. Alzheimer’s Disease Neuroimaging Initiative. Cerebrospinal fluid neurogranin: Relation to cognition and neurodegeneration in Alzheimer’s disease. Brain 2015, 138, 3373–3385. [Google Scholar] [CrossRef]
- Pereira, J.B.; Janelidze, S.; Ossenkoppele, R.; Kvartsberg, H.; Brinkmalm, A.; Mattsson-Carlgren, N.; Stomrud, E.; Smith, R.; Zetterberg, H.; Blennow, K.; et al. Untangling the association of amyloid-β and tau with synaptic and axonal loss in Alzheimer’s disease. Brain 2021, 144, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Portelius, E.; Olsson, B.; Höglund, K.; Cullen, N.C.; Kvartsberg, H.; Andreasson, U.; Zetterberg, H.; Sandelius, Å.; Shaw, L.M.; Lee, V.M.Y.; et al. Cerebrospinal fluid neurogranin concentration in neurodegeneration: Relation to clinical phenotypes and neuropathology. Acta Neuropathol. 2018, 136, 363–376. [Google Scholar] [CrossRef] [PubMed]
- Janelidze, S.; Hertze, J.; Zetterberg, H.; Landqvist Waldö, M.; Santillo, A.; Blennow, K.; Hansson, O. Cerebrospinal fluid neurogranin and YKL-40 as biomarkers of Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2015, 3, 12–20. [Google Scholar] [CrossRef]
- Agnello, L.; Lo Sasso, B.; Vidali, M.; Scazzone, C.; Piccoli, T.; Gambino, C.M.; Bivona, G.; Giglio, R.V.; Ciaccio, A.M.; La Bella, V.; et al. Neurogranin as a Reliable Biomarker for Synaptic Dysfunction in Alzheimer’s Disease. Diagnostics 2021, 11, 2339. [Google Scholar] [CrossRef] [PubMed]
- Antonell, A.; Tort-Merino, A.; Ríos, J.; Balasa, M.; Borrego-Écija, S.; Auge, J.M.; Muñoz-García, C.; Bosch, B.; Falgàs, N.; Rami, L.; et al. Synaptic, axonal damage and inflammatory cerebrospinal fluid biomarkers in neurodegenerative dementias. Alzheimer’s Dement. 2020, 16, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, N.; Insel, P.S.; Palmqvist, S.; Portelius, E.; Zetterberg, H.; Weiner, M.; Blennow, K.; Hansson, O. Alzheimer’s Disease Neuroimaging Initiative. Cerebrospinal fluid tau, neurogranin, and neurofilament light in Alzheimer’s disease. EMBO Mol. Med. 2016, 8, 1184–1196. [Google Scholar] [CrossRef] [PubMed]
- Piccoli, T.; Blandino, V.; Maniscalco, L.; Matranga, D.; Graziano, F.; Guajana, F.; Agnello, L.; Lo Sasso, B.; Gambino, C.M.; Giglio, R.V.; et al. Biomarkers Related to Synaptic Dysfunction to Discriminate Alzheimer’s Disease from Other Neurological Disorders. Int. J. Mol. Sci. 2022, 23, 10831. [Google Scholar] [CrossRef]
- Jurasova, V.; Andel, R.; Katonova, A.; Veverova, K.; Zuntychova, T.; Horakova, H.; Vyhnalek, M.; Kolarova, T.; Matoska, V.; Blennow, K.; et al. CSF neurogranin levels as a biomarker in Alzheimer’s disease and frontotemporal lobar degeneration: A cross-sectional analysis. Alzheimer’s Res. Ther. 2024, 16, 199. [Google Scholar] [CrossRef] [PubMed]
- Tarawneh, R.; D’Angelo, G.; Crimmins, D.; Herries, E.; Griest, T.; Fagan, A.M.; Zipfel, G.J.; Ladenson, J.H.; Morris, J.C.; Holtzman, D.M. Diagnostic and Prognostic Utility of the Synaptic Marker Neurogranin in Alzheimer Disease. JAMA Neurol. 2016, 73, 561–571. [Google Scholar] [CrossRef] [PubMed]
- Kvartsberg, H.; Duits, F.H.; Ingelsson, M.; Andreasen, N.; Öhrfelt, A.; Andersson, K.; Brinkmalm, G.; Lannfelt, L.; Minthon, L.; Hansson, O.; et al. Cerebrospinal fluid levels of the synaptic protein neurogranin correlates with cognitive decline in prodromal Alzheimer’s disease. Alzheimer’s Dement. 2015, 11, 1180–1190. [Google Scholar] [CrossRef]
- Kester, M.I.; Teunissen, C.E.; Crimmins, D.L.; Herries, E.M.; Ladenson, J.H.; Scheltens, P.; van der Flier, W.M.; Morris, J.C.; Holtzman, D.M.; Fagan, A.M. Neurogranin as a Cerebrospinal Fluid Biomarker for Synaptic Loss in Symptomatic Alzheimer Disease. JAMA Neurol. 2015, 72, 1275–1280. [Google Scholar] [CrossRef]
- Scheff, S.W.; Price, D.A.; Ansari, M.A.; Roberts, K.N.; Schmitt, F.A.; Ikonomovic, M.D.; Mufson, E.J. Synaptic change in the posterior cingulate gyrus in the progression of Alzheimer’s disease. J. Alzheimer’s Dis. 2015, 43, 1073–1090. [Google Scholar] [CrossRef] [PubMed]
- Headley, A.; De Leon-Benedetti, A.; Dong, C.; Levin, B.; Loewenstein, D.; Camargo, C.; Rundek, T.; Zetterberg, H.; Blennow, K.; Wright, C.B.; et al. Alzheimer’s Disease Neuroimaging Initiative. Neurogranin as a predictor of memory and executive function decline in MCI patients. Neurology 2018, 90, e887–e895. [Google Scholar] [CrossRef]
- Galasko, D.; Xiao, M.; Xu, D.; Smirnov, D.; Salmon, D.P.; Dewit, N.; Vanbrabant, J.; Jacobs, D.; Vanderstichele, H.; Eugeen, V.; et al. Synaptic biomarkers in CSF aid in diagnosis, correlate with cognition and predict progression in MCI and Alzheimer’s disease. Alzheimer’s Dement. 2019, 5, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Farotti, L.; Paolini Paoletti, F.; Simoni, S.; Parnetti, L. Unraveling Pathophysiological Mechanisms of Parkinson’s Disease: Contribution of CSF Biomarkers. Biomark. Insights 2020, 15, 1177271920964077. [Google Scholar] [CrossRef]
- Ingelsson, M. Alpha-Synuclein Oligomers-Neurotoxic Molecules in Parkinson’s Disease and Other Lewy Body Disorders. Front. Neurosci. 2016, 10, 408. [Google Scholar] [CrossRef]
- Morris, H.R.; Spillantini, M.G.; Sue, C.M.; Williams-Gray, C.H. The pathogenesis of Parkinson’s disease. Lancet 2024, 403, 293–304. [Google Scholar] [CrossRef]
- Koob, A.O.; Shaked, G.M.; Bender, A.; Bisquertt, A.; Rockenstein, E.; Masliah, E. Neurogranin binds α-synuclein in the human superior temporal cortex and interaction is decreased in Parkinson’s disease. Brain Res. 2014, 1591, 102–110. [Google Scholar] [CrossRef] [PubMed]
- McKeith, I.G.; Boeve, B.F.; Dickson, D.W.; Halliday, G.; Taylor, J.P.; Weintraub, D.; Aarsland, D.; Galvin, J.; Attems, J.; Ballard, C.G.; et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 2017, 89, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Sancesario, G.M.; Di Lazzaro, G.; Alwardat, M.; Biticchi, B.; Basile, V.; Salimei, C.; Colona, V.L.; Sinibaldi Salimei, P.; Bernardini, S.; Mercuri, N.B.; et al. Amyloid-β42/Neurogranin Ratio as a Potential Index for Cognitive Impairment in Parkinson’s Disease. J. Alzheimer’s Dis. 2020, 76, 1171–1178. [Google Scholar] [CrossRef] [PubMed]
- Selnes, P.; Stav, A.L.; Johansen, K.K.; Bjørnerud, A.; Coello, C.; Auning, E.; Kalheim, L.; Almdahl, I.S.; Hessen, E.; Zetterberg, H.; et al. Impaired synaptic function is linked to cognition in Parkinson’s disease. Ann. Clin. Transl. Neurol. 2017, 4, 700–713. [Google Scholar] [CrossRef]
- Janelidze, S.; Zetterberg, H.; Brix, B.; Mattsson, N.; Surova, Y.; Blennow, K.; Hansson, O. Cerebrospinal fluid levels of neurogranin in Parkinsonian disorders. Mov. Disord. 2020, 35, 513–518. [Google Scholar] [CrossRef]
- Bereczki, E.; Bogstedt, A.; Höglund, K.; Tsitsi, P.; Brodin, L.; Ballard, C.; Svenningsson, P.; Aarsland, D. Synaptic proteins in CSF relate to Parkinson’s disease stage markers. NPJ Park. Dis. 2017, 3, 7. [Google Scholar] [CrossRef]
- Villar-Pique, A.; Zerr, I.; Llorens, F. Cerebrospinal fluid neurogranin as a new player in prion disease diagnosis and prognosis. Neural Regen. Res. 2020, 15, 861–862. [Google Scholar] [CrossRef] [PubMed]
- Gambetti, P.; Kong, Q.; Zou, W.; Parchi, P.; Chen, S.G. Sporadic and familial CJD: Classification and characterisation. Br. Med. Bull. 2003, 66, 213–239. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.S.; Wu, Y.T.; Hung, C.I.; Kwan, S.Y.; Teng, S.; Soong, B.W. Early detection of periodic sharp wave complexes on EEG by independent component analysis in patients with Creutzfeldt-Jakob disease. J. Clin. Neurophysiol. 2008, 25, 25–31. [Google Scholar] [CrossRef]
- Blennow, K.; Diaz-Lucena, D.; Zetterberg, H.; Villar-Pique, A.; Karch, A.; Vidal, E.; Hermann, P.; Schmitz, M.; Ferrer Abizanda, I.; Zerr, I. Llorens FCSF neurogranin as a neuronal damage marker in CJD: A comparative study with AD. J. Neurol. Neurosurg. Psychiatry 2019, 90, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Guzik-Makaruk, E.M.; Pływaczewski, E.W.; Laskowska, K.; Filipkowski, W.; Jurgielewicz-Delegacz, E.; Mroczko, P. A Comparative Analysis of the Treatment of Decision-Making by or for Patients with Neurodegenerative Diseases in Four Legal Jurisdictions. J. Alzheimer’s Dis. 2019, 70, 1–10. [Google Scholar] [CrossRef]
- Santos, A.N.; Ewers, M.; Minthon, L.; Simm, A.; Silber, R.-E.; Blennow, K.; Prvulovic, D.; Hansson, O.; Hampel, H. Amyloid-β oligomers in cerebrospinal fluid are associated with cognitive decline in patients with Alzheimer’s disease. J Alzheimers Dis 2012;29:171-6. and Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging 2006, 27, 1372–1384. [Google Scholar]
| Cut-Off Value | Diagnostic Performance | Group | Method | References |
|---|---|---|---|---|
| 306 pg/mL | 0.84% sensitivity, 78% specificity, Youden index 0.621 | AD vs. controls | ELISA | [17] |
| 319 pg/mL | 73% sensitivity, 73% specificity, AUC 0.78 | AD vs. controls | ELISA | [28] |
| 167.78 pg/mL | 20% sensitivity, 85.4% specificity, AUC 0.504 | AD vs. controls | ELISA | [38] |
| 250 pg/mL | 48% sensitivity, 96% specificity, AUC 0.73 | controls vs. MCI+AD+FTD+CJD | ELISA | [29] |
| 230 pg/mL | 64% sensitivity, 88% specificity, AUC 0.85 | healthy controls vs. AD | ELISA | [29] |
| 293 pg/mL | 68% sensitivity, 78% specificity, AUC 0.77 | AD vs. non-AD disorders | ELISA | [31] |
| 165.5 pg/mL | 93% sensitivity, 41% specificity, AUC 0.699 | AD vs. non-neurodegenerative disorders | ELISA | [31] |
| Mean Value | Group | Method | References |
|---|---|---|---|
| 215.07 pg/mL vs. 336.53 pg/mL | PD vs. controls | ELISA | [44] |
| 251 pg/mL vs. 397 pg/mL | PD vs. controls | ELISA | [45] |
| 242.2 pg/mL vs. 311.7 pg/mL | PD vs. controls | ELISA | [46] |
| 242.2 pg/mL vs. 480.4 pg/mL | PD vs. AD | ELISA | [46] |
| 242.2 pg/mL vs. 339.8 pg/mL | PD vs. DLB | ELISA | [46] |
| 359 pg/mL vs. 338 pg/mL | PD vs. controls | ELISA | [47] |
| Mean Value | Group | Method | References |
|---|---|---|---|
| 587.84 pg/mL vs. 173 pg/mL | CJD vs. controls | ELISA | [29] |
| 587.54 pg/mL vs. 252.4 pg/mL | CJD vs. AD | ELISA | [29] |
| 587.54 pg/mL vs. 135.5 pg/mL | CJD vs. FTD | ELISA | [29] |
| 571 pg/mL vs. 120 pg/mL | CJD vs. controls | ELISA | [51] |
| 571 pg/mL vs. 233 pg/mL | CJD vs. AD | ELISA | [51] |
| 384 pg/mL vs. 630 pg/mL | CJDVV vs. CJDMM | ELISA | [51] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krawczuk, D.; Mroczko, P.; Winkel, I.; Mroczko, B. The Diagnostic Value of Cerebrospinal Fluid Neurogranin in Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 13578. https://doi.org/10.3390/ijms252413578
Krawczuk D, Mroczko P, Winkel I, Mroczko B. The Diagnostic Value of Cerebrospinal Fluid Neurogranin in Neurodegenerative Diseases. International Journal of Molecular Sciences. 2024; 25(24):13578. https://doi.org/10.3390/ijms252413578
Chicago/Turabian StyleKrawczuk, Daria, Piotr Mroczko, Izabela Winkel, and Barbara Mroczko. 2024. "The Diagnostic Value of Cerebrospinal Fluid Neurogranin in Neurodegenerative Diseases" International Journal of Molecular Sciences 25, no. 24: 13578. https://doi.org/10.3390/ijms252413578
APA StyleKrawczuk, D., Mroczko, P., Winkel, I., & Mroczko, B. (2024). The Diagnostic Value of Cerebrospinal Fluid Neurogranin in Neurodegenerative Diseases. International Journal of Molecular Sciences, 25(24), 13578. https://doi.org/10.3390/ijms252413578






