Repetitive Overuse Injury Causes Entheseal Damage and Palmar Muscle Fibrosis in Older Rats
Abstract
1. Introduction
2. Results
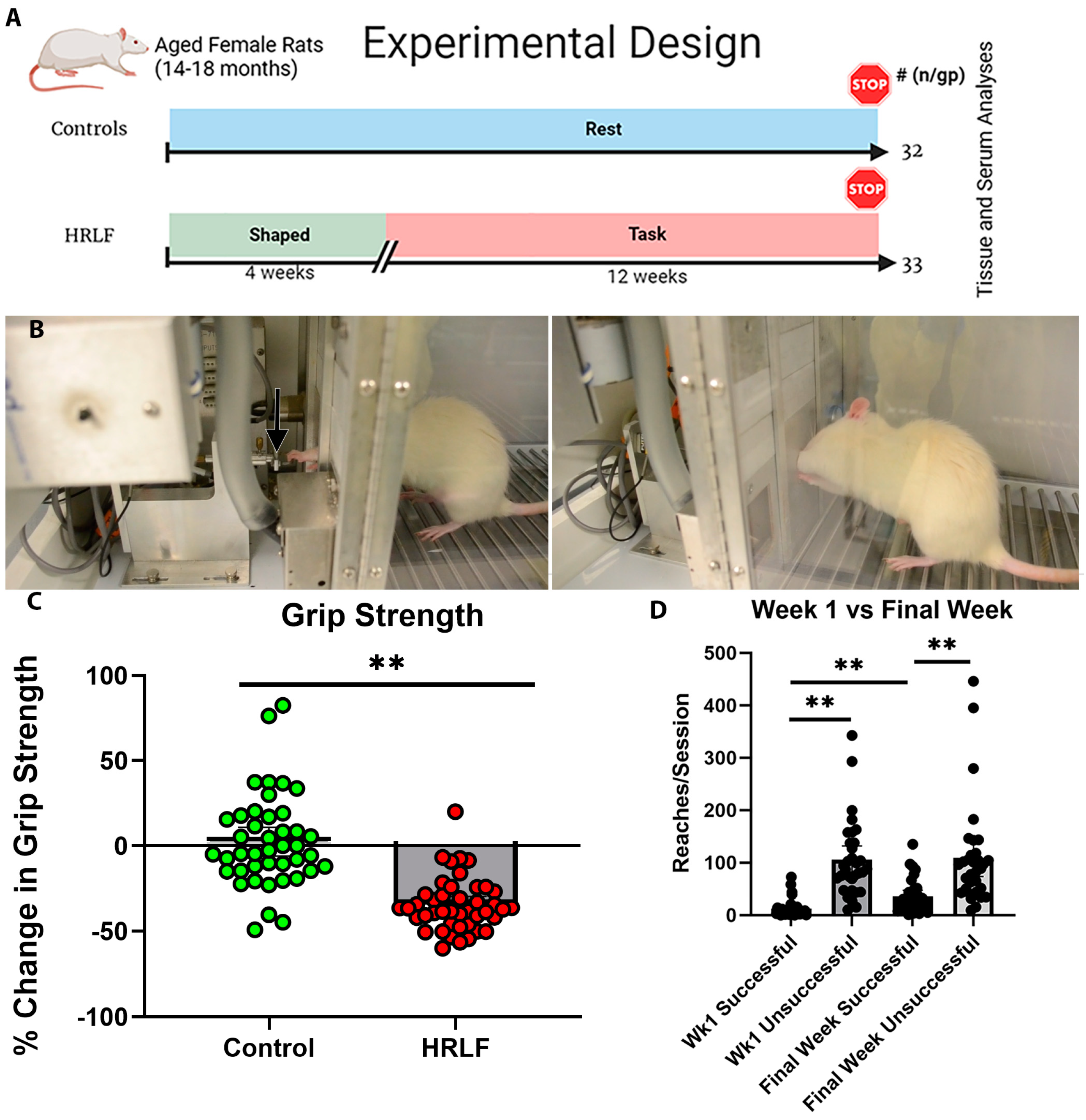
2.1. Functional Performance over Time
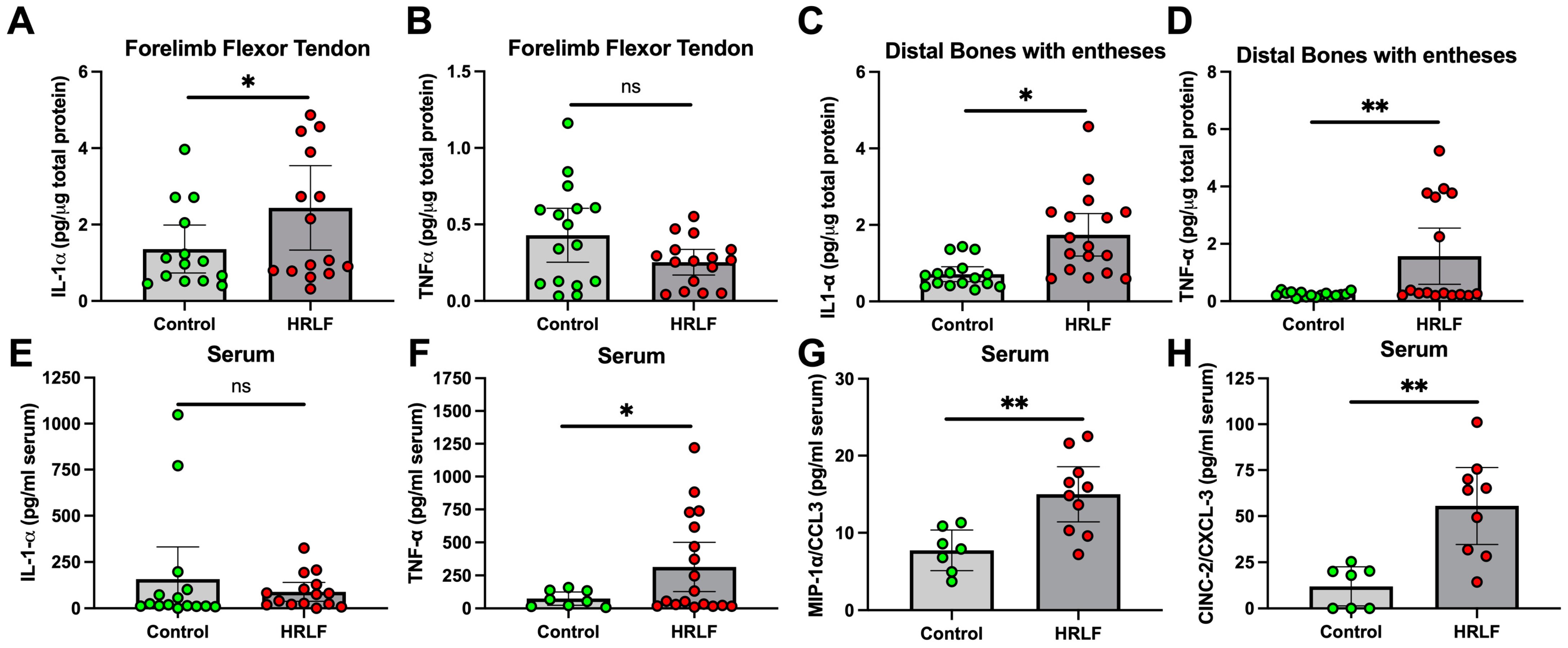
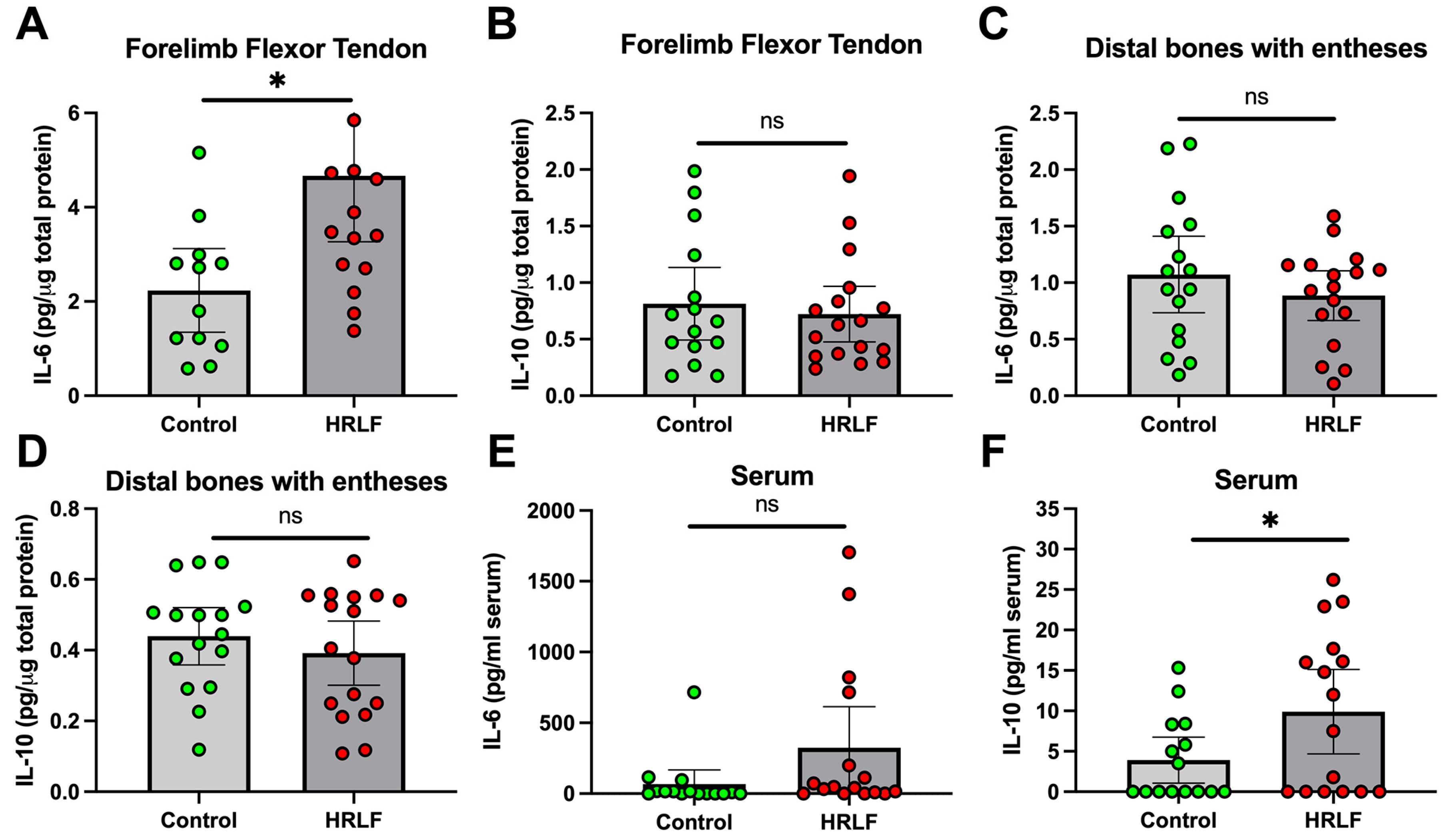
2.2. Several Pro-Inflammatory and Anti-Inflammatory Cytokines Changed in Tissues and Serum of Mature HRLF Rats
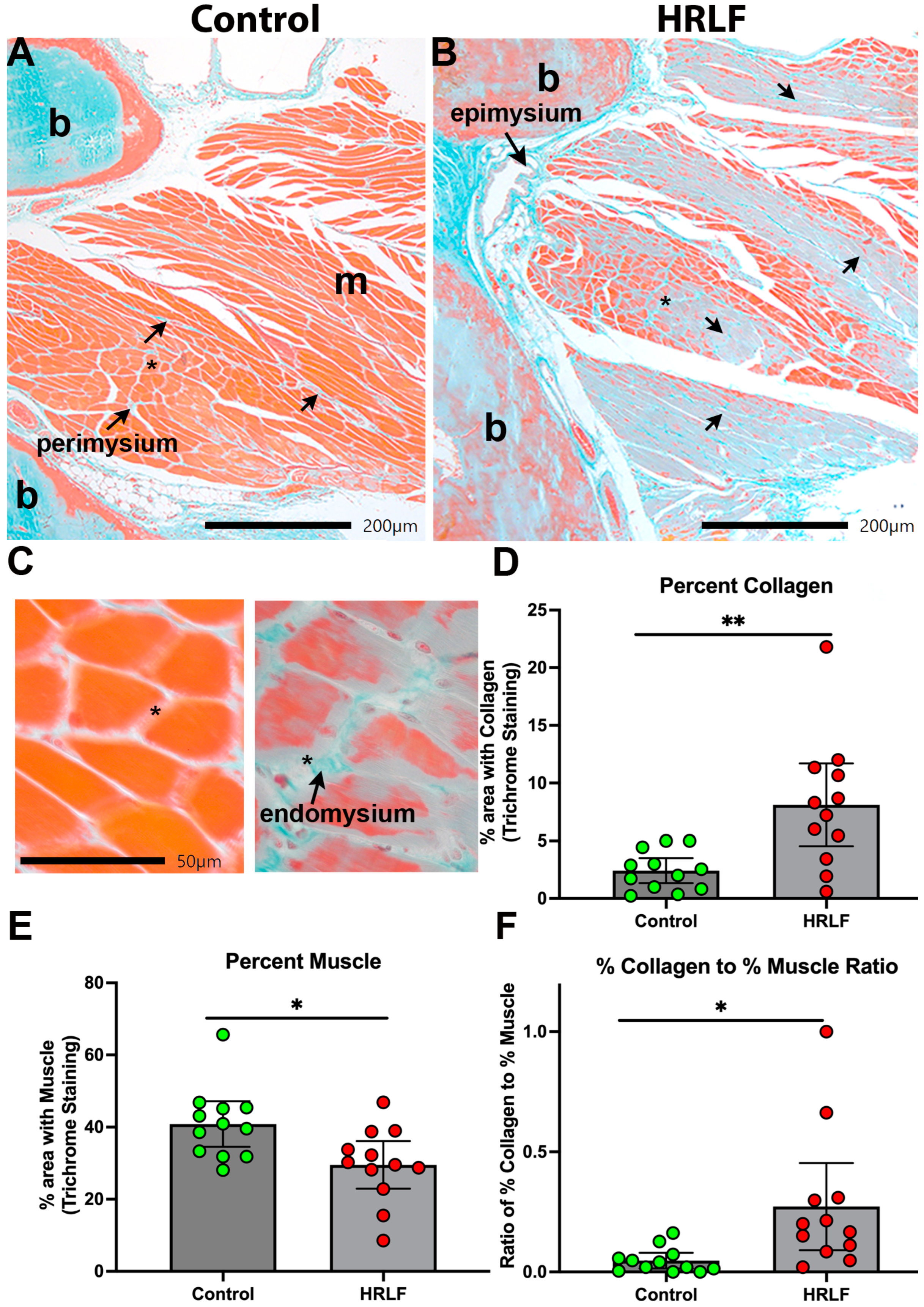
2.3. Collagen Was Elevated and Muscle Reduced in Mature HRLF Forepaw Muscles
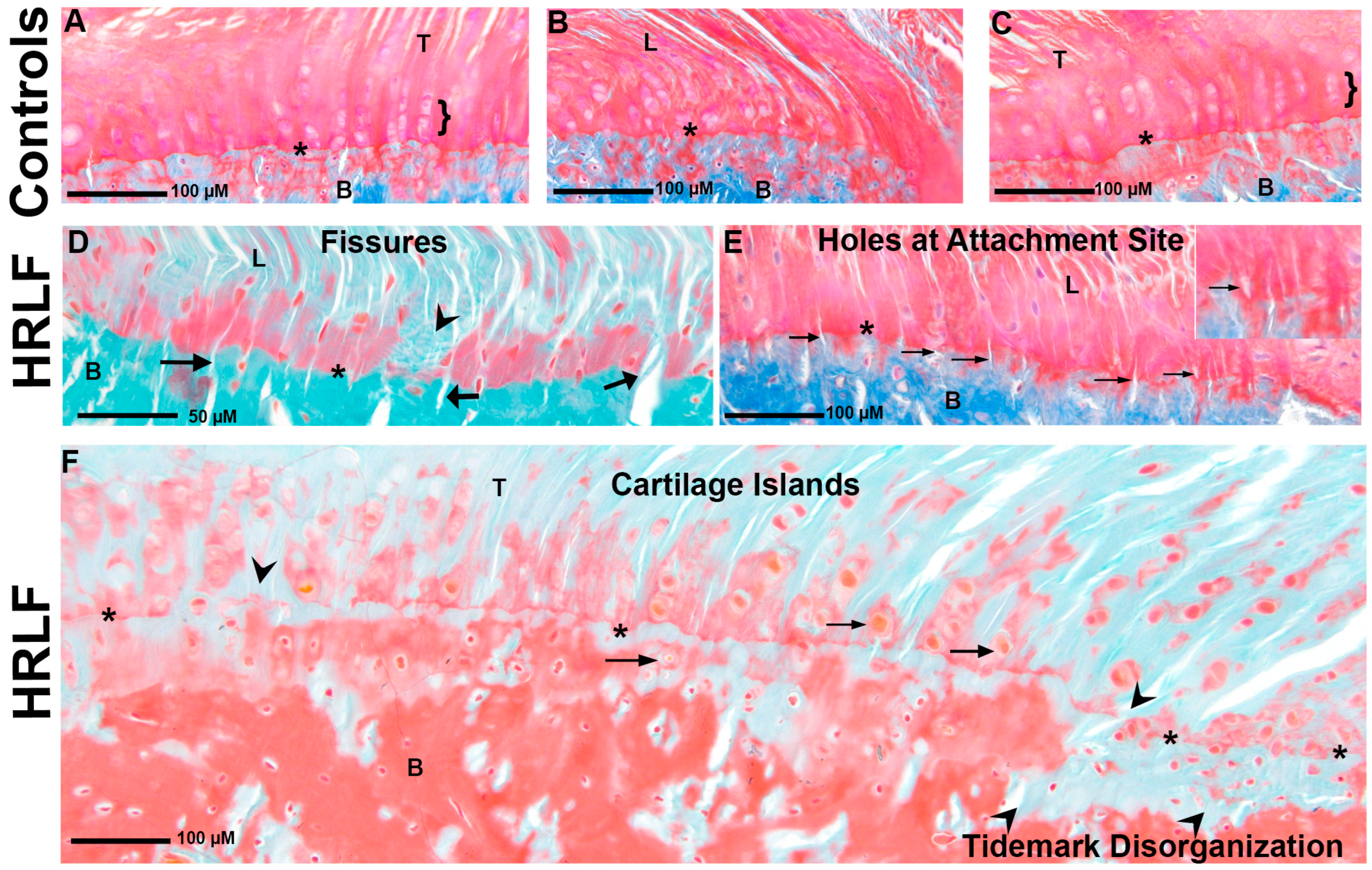
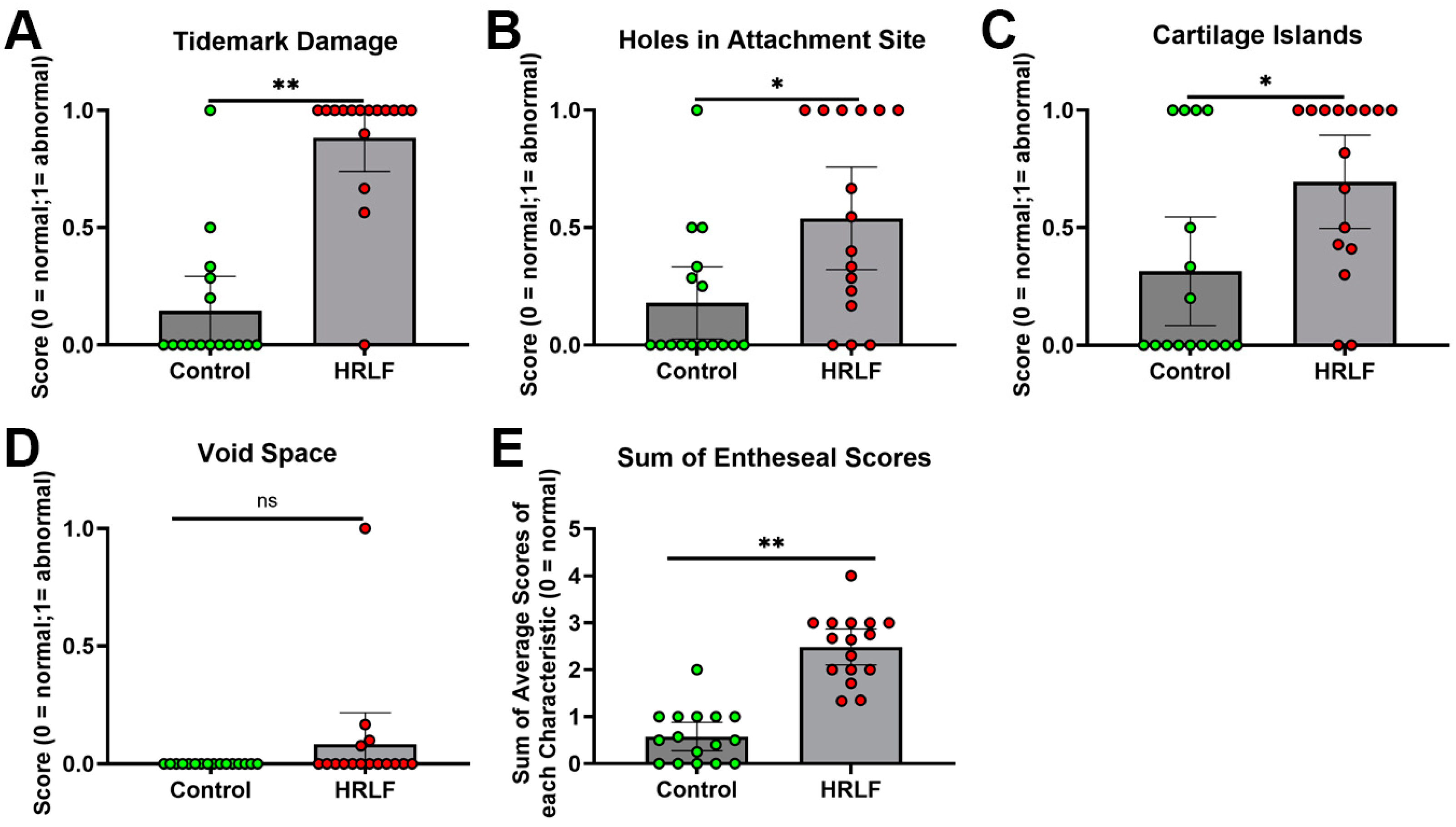
2.4. Evidence of Entheseal Histopathology in Mature HRLF Rats
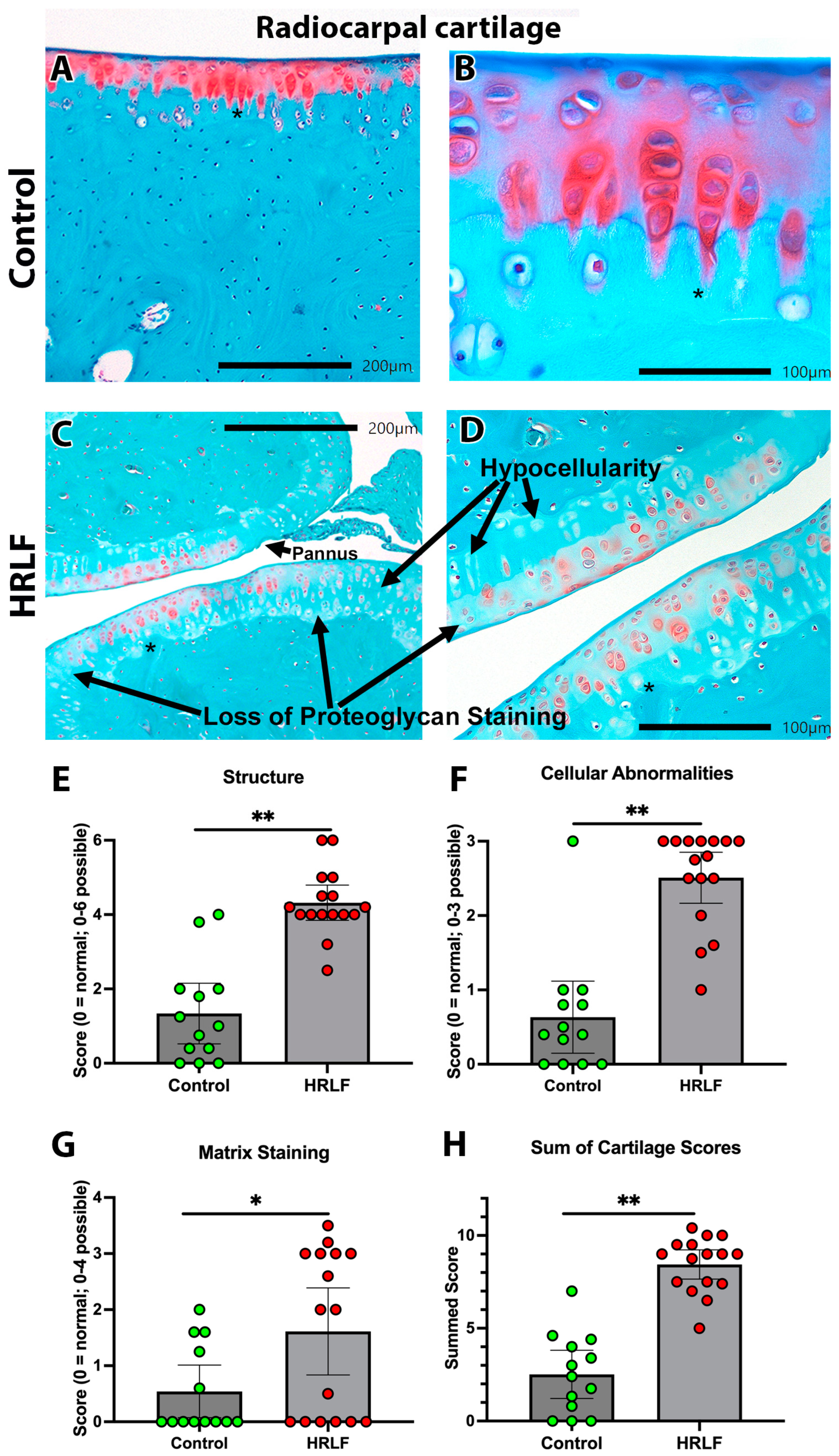
2.5. Evidence of Radiocarpal Articular Cartilage Microdamage with HRLF Task
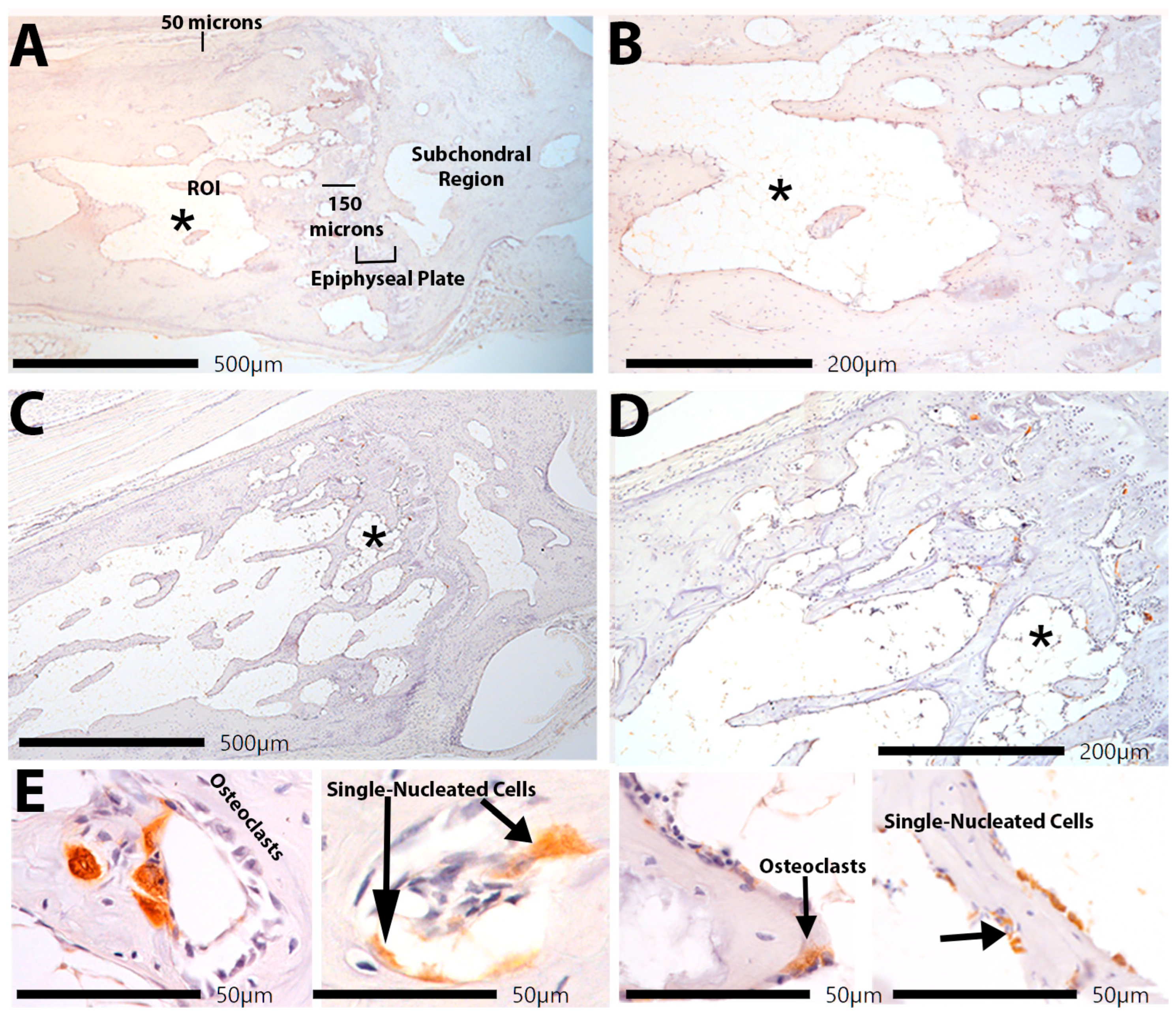
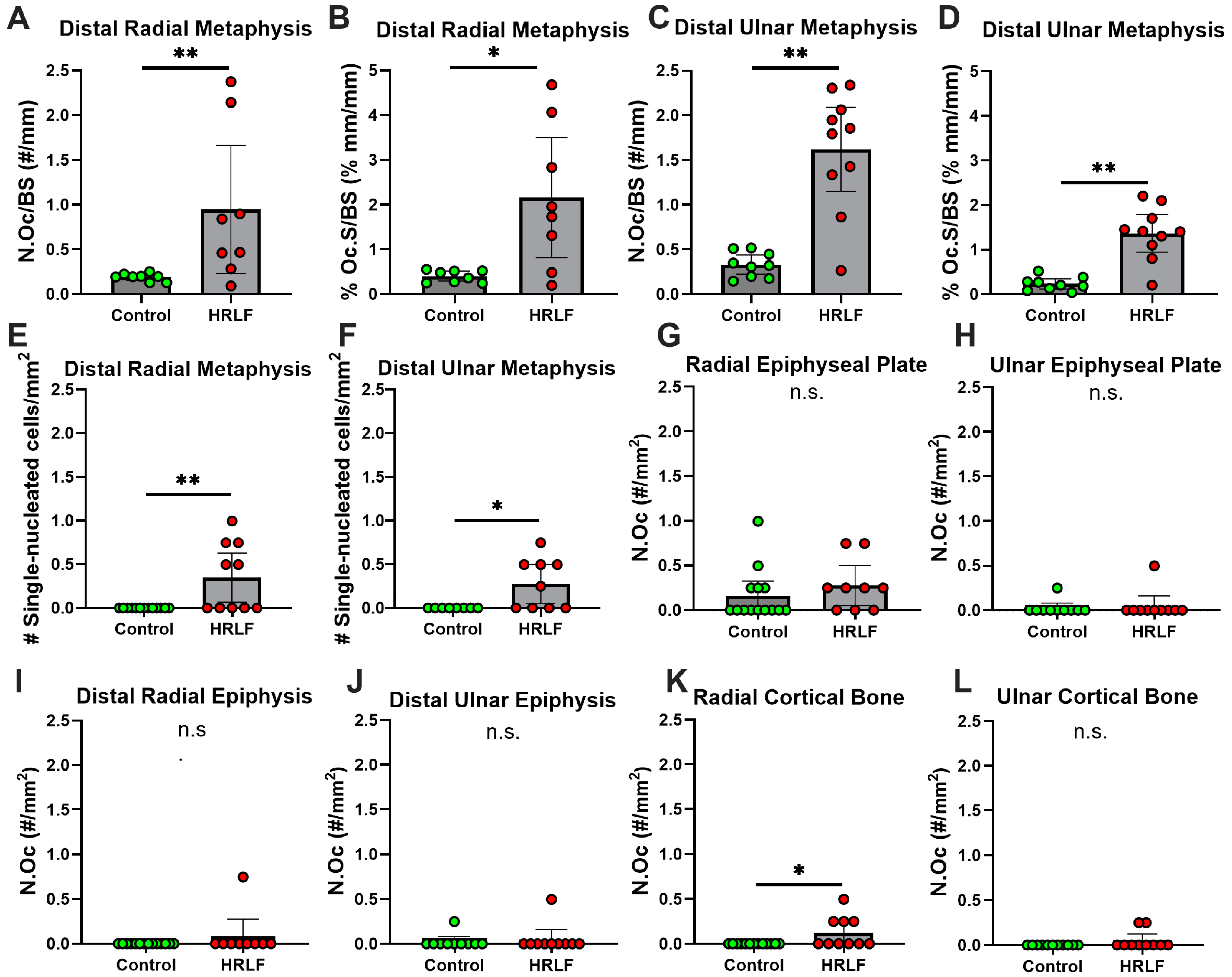
2.6. Osteoclast Surface and Count at Trabecular Bone Below the Epiphyseal Plate
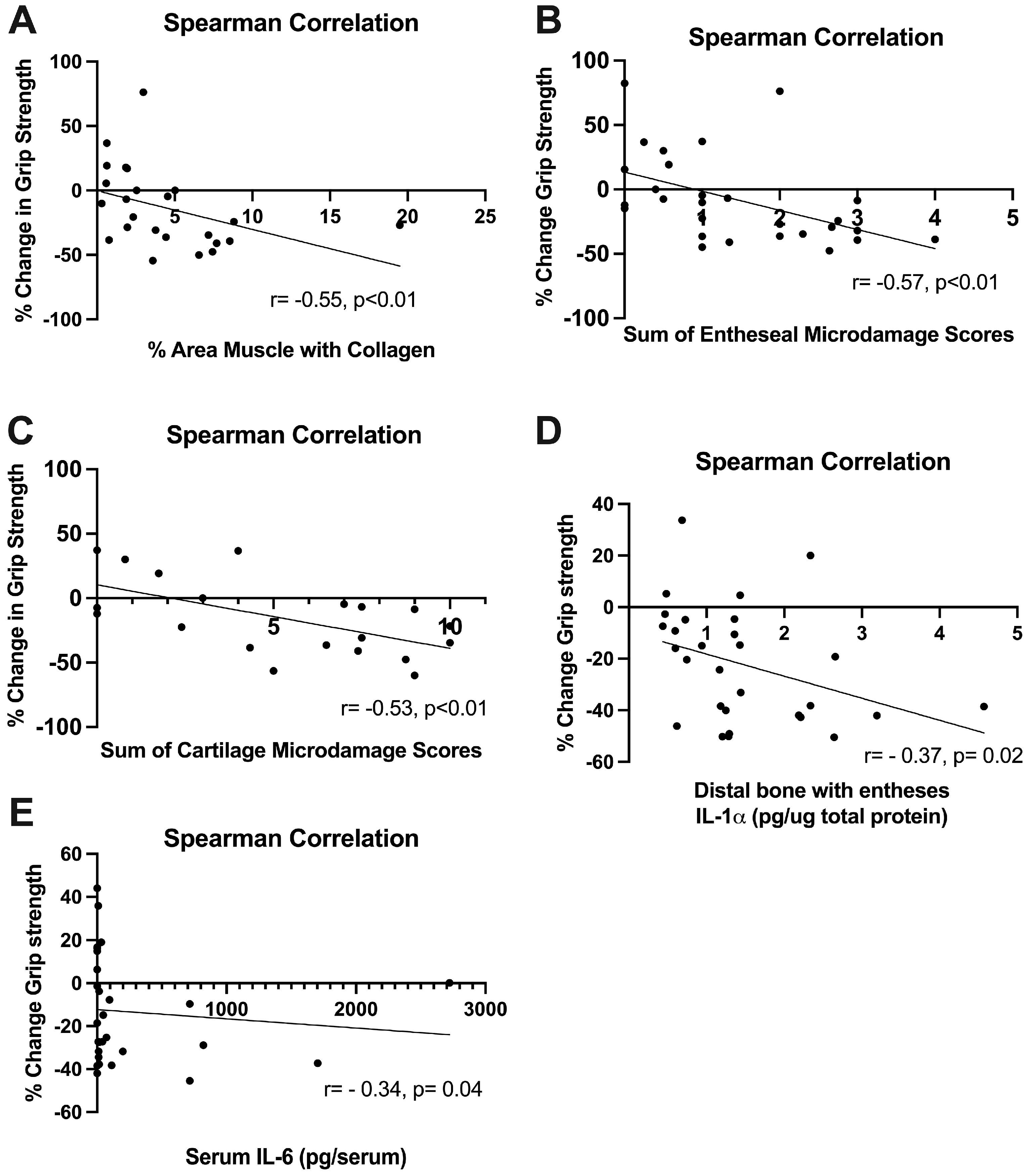
2.7. Grip Strength Correlated Inversely with Several Histopathological Changes
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Repetitive Overuse Task (High Repetition Low Force Task)
4.3. Grip Strength Behavioral Assay
4.4. Tissue Collection
4.5. Biochemical Assays
4.6. Histological Staining and Analyses
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Occupational Safety and Health Administration. Ergonomics Program. Available online: https://www.osha.gov/laws-regs/federalregister/1999-11-23 (accessed on 14 March 2023).
- Bureau of Labor Statistics. Civilian Labor Force, by Age, Sex, Race, and Ethnicity. Available online: https://www.bls.gov/emp/tables/civilian-labor-force-summary.htm (accessed on 14 March 2023).
- Hassard, J.; Teoh, K.R.H.; Visockaite, G.; Dewe, P.; Cox, T. The cost of work-related stress to society: A systematic review. J. Occup. Health Psychol. 2018, 23, 1–17. [Google Scholar] [CrossRef]
- Bureau of Labor Statistics. Nonfatal Occupational Injuries and Illnesses Involving Days Away from Work for Musculoskeletal Disorders by Parts of Body and Ownership. Available online: https://www.bls.gov/iif/nonfatal-injuries-and-illnesses-tables.htm (accessed on 22 June 2023).
- Algarni, F.S.; Gross, D.P.; Senthilselvan, A.; Battié, M.C. Ageing workers with work-related musculoskeletal injuries. Occup. Med. 2015, 65, 229–237. [Google Scholar] [CrossRef]
- Piligian, G.; Herbert, R.; Hearns, M.; Dropkin, J.; Landsbergis, P.; Cherniack, M. Evaluation and management of chronic work-related musculoskeletal disorders of the distal upper extremity. Am. J. Ind. Med. 2000, 37, 75–93. [Google Scholar] [CrossRef]
- Dennett, X.; Fry, H.J. Overuse syndrome: A muscle biopsy study. Lancet 1988, 1, 905–908. [Google Scholar] [CrossRef] [PubMed]
- Lluch, A.L. Thickening of the synovium of the digital flexor tendons: Cause or consequence of the carpal tunnel syndrome? J. Hand Surg. Br. 1992, 17, 209–212. [Google Scholar] [CrossRef] [PubMed]
- Descatha, A.; Dale, A.M.; Silverstein, B.A.; Roquelaure, Y.; Rempel, D. Lateral epicondylitis: New evidence for work relatedness. Jt. Bone Spine 2015, 82, 5–7. [Google Scholar] [CrossRef][Green Version]
- Harris-Adamson, C.; Eisen, E.A.; Kapellusch, J.; Hegmann, K.T.; Thiese, M.S.; Dale, A.M.; Evanoff, B.; Meyers, A.R.; Bao, S.; Gerr, F.; et al. Occupational risk factors for work disability following carpal tunnel syndrome: A pooled prospective study. Occup. Environ. Med. 2022, 79, 442–451. [Google Scholar] [CrossRef]
- Moriya, T.; Zhao, C.; Cha, S.S.; Schmelzer, J.D.; Low, P.A.; An, K.N.; Amadio, P.C. Tendon injury produces changes in SSCT and nerve physiology similar to carpal tunnel syndrome in an in vivo rabbit model. Hand 2011, 6, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Zambelis, T.; Tsivgoulis, G.; Karandreas, N. Carpal tunnel syndrome: Associations between risk factors and laterality. Eur. Neurol. 2010, 63, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Sjogaard, G.; Zebis, M.K.; Kiilerich, K.; Saltin, B.; Pilegaard, H. Exercise training and work task induced metabolic and stress-related mRNA and protein responses in myalgic muscles. Biomed. Res. Int. 2013, 2013, 984523. [Google Scholar] [CrossRef]
- Kadi, F.; Hagg, G.; Hakansson, R.; Holmner, S.; Butler-Browne, G.S.; Thornell, L.E. Structural changes in male trapezius muscle with work-related myalgia. Acta Neuropathol. 1998, 95, 352–360. [Google Scholar] [CrossRef]
- You, D.; Smith, A.H.; Rempel, D. Meta-analysis: Association between wrist posture and carpal tunnel syndrome among workers. Saf. Health Work 2014, 5, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Murinova, L.; Perecinsky, S.; Jancova, A.; Murin, P.; Legath, L. Is Dupuytren’s disease an occupational illness? Occup. Med. 2021, 71, 28–33. [Google Scholar] [CrossRef]
- Fadel, M.; Leclerc, A.; Evanoff, B.; Dale, A.M.; Ngabirano, L.; Roquelaure, Y.; Descatha, A. Association between occupational exposure and Dupuytren’s contracture using a job-exposure matrix and self-reported exposure in the CONSTANCES cohort. Occup. Environ. Med. 2019, 76, 845–848. [Google Scholar] [CrossRef] [PubMed]
- van den Berge, B.A.; Wiberg, A.; Werker, P.M.N.; Broekstra, D.C.; Furniss, D. Dupuytren’s disease is a work-related disorder: Results of a population-based cohort study. Occup. Environ. Med. 2023, 80, 137–145. [Google Scholar] [CrossRef]
- Silverstein, M. Meeting the challenges of an aging workforce. Am. J. Ind. Med. 2008, 51, 269–280. [Google Scholar] [CrossRef]
- Berecki-Gisolf, J.; Clay, F.J.; Collie, A.; McClure, R.J. The impact of aging on work disability and return to work: Insights from workers’ compensation claim records. J. Occup. Environ. Med. 2012, 54, 318–327. [Google Scholar] [CrossRef]
- Palmer, K.T.; Goodson, N. Ageing, musculoskeletal health and work. Best Pract. Res. Clin. Rheumatol. 2015, 29, 391–404. [Google Scholar] [CrossRef] [PubMed]
- Barbe, M.F.; Gallagher, S.; Massicotte, V.S.; Tytell, M.; Popoff, S.N.; Barr-Gillespie, A.E. The interaction of force and repetition on musculoskeletal and neural tissue responses and sensorimotor behavior in a rat model of work-related musculoskeletal disorders. BMC Musculoskelet. Disord. 2013, 14, 303. [Google Scholar] [CrossRef] [PubMed]
- Xin, D.L.; Harris, M.Y.; Wade, C.K.; Amin, M.; Barr, A.E.; Barbe, M.F. Aging enhances serum cytokine response but not task-induced grip strength declines in a rat model of work-related musculoskeletal disorders. BMC Musculoskelet. Disord. 2011, 12, 63. [Google Scholar] [CrossRef] [PubMed]
- Kietrys, D.M.; Barr-Gillespie, A.E.; Amin, M.; Wade, C.K.; Popoff, S.N.; Barbe, M.F. Aging contributes to inflammation in upper extremity tendons and declines in forelimb agility in a rat model of upper extremity overuse. PLoS ONE 2012, 7, e46954. [Google Scholar] [CrossRef]
- Massicotte, V.S.; Frara, N.; Harris, M.Y.; Amin, M.; Wade, C.K.; Popoff, S.N.; Barbe, M.F. Prolonged performance of a high repetition low force task induces bone adaptation in young adult rats, but loss in mature rats. Exp. Gerontol. 2015, 72, 204–217. [Google Scholar] [CrossRef] [PubMed]
- Fulop, T.; Larbi, A.; Pawelec, G.; Khalil, A.; Cohen, A.A.; Hirokawa, K.; Witkowski, J.M.; Franceschi, C. Immunology of Aging: The Birth of Inflammaging. Clin. Rev. Allergy Immunol. 2023, 64, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Hilliard, B.A.; Amin, M.; Popoff, S.N.; Barbe, M.F. Force dependent effects of chronic overuse on fibrosis-related genes and proteins in skeletal muscles. Connect. Tissue. Res. 2021, 62, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Driban, J.B.; Barr, A.E.; Amin, M.; Sitler, M.R.; Barbe, M.F. Joint inflammation and early degeneration induced by high-force reaching are attenuated by ibuprofen in an animal model of work-related musculoskeletal disorder. J. Biomed. Biotechnol. 2011, 2011, 691412. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.T.G.; Barr-Gillespie, A.E.; Klyne, D.M.; Harris, M.Y.; Amin, M.; Paul, R.W.; Cruz, G.E.; Zhao, H.; Gallagher, S.; Barbe, M.F. Forced treadmill running reduces systemic inflammation yet worsens upper limb discomfort in a rat model of work-related musculoskeletal disorders. BMC Musculoskelet. Disord. 2020, 21, 57. [Google Scholar] [CrossRef] [PubMed]
- Lambi, A.G.; DeSante, R.J.; Patel, P.R.; Hilliard, B.A.; Popoff, S.N.; Barbe, M.F. Blocking CCN2 Reduces Established Palmar Neuromuscular Fibrosis and Improves Function Following Repetitive Overuse Injury. Int. J. Mol. Sci. 2023, 24, 13866. [Google Scholar] [CrossRef]
- Anstey, K.J.; Smith, G.A. Interrelationships among biological markers of aging, health, activity, acculturation, and cognitive performance in late adulthood. Psychol. Aging 1999, 14, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Anstey, K.J.; Smith, G.A.; Lord, S. Test-retest reliability of a battery of sensory motor and physiological measures of aging. Percept. Mot. Ski. 1997, 84, 831–834. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, B.; Mahabadi, N.; Varacallo, M. Anatomy, Fascia; StatPearls: Treasure Island, FL, USA, 2024. [Google Scholar]
- Turrina, A.; Martinez-Gonzalez, M.A.; Stecco, C. The muscular force transmission system: Role of the intramuscular connective tissue. J. Bodyw. Mov. Ther. 2013, 17, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.; Toumi, H.; Ralphs, J.R.; Bydder, G.; Best, T.M.; Milz, S. Where tendons and ligaments meet bone: Attachment sites (‘entheses’) in relation to exercise and/or mechanical load. J. Anat. 2006, 208, 471–490. [Google Scholar] [CrossRef]
- Shaw, H.M.; Benjamin, M. Structure-function relationships of entheses in relation to mechanical load and exercise. Scand. J. Med. Sci. Sports 2007, 17, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Bordoni, B.; Escher, A.R.; Tobbi, F.; Pianese, L.; Ciardo, A.; Yamahata, J.; Hernandez, S.; Sanchez, O. Fascial Nomenclature: Update 2022. Cureus 2022, 14, e25904. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Xu, B.; Liu, N.; Wang, L. Histopathological changes in patellar tendon enthesis of rabbit induced by electrical stimulation intensity. J. Orthop. Sci. 2020, 25, 344–348. [Google Scholar] [CrossRef] [PubMed]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.P.; Foster, R.J.; Mow, V.C. Composition and dynamics of articular cartilage: Structure, function, and maintaining healthy state. J. Orthop. Sports Phys. Ther. 1998, 28, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Elliott, M.B.; Barr, A.E.; Clark, B.D.; Wade, C.K.; Barbe, M.F. Performance of a repetitive task by aged rats leads to median neuropathy and spinal cord inflammation with associated sensorimotor declines. Neuroscience 2010, 170, 929–941. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, M.; Ralphs, J.R. Fibrocartilage in tendons and ligaments--an adaptation to compressive load. J. Anat. 1998, 193, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Min. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef]
- Barbe, M.F.; Massicotte, V.S.; Assari, S.; Monroy, M.A.; Frara, N.; Harris, M.Y.; Amin, M.; King, T.; Cruz, G.E.; Popoff, S.N. Prolonged high force high repetition pulling induces osteocyte apoptosis and trabecular bone loss in distal radius, while low force high repetition pulling induces bone anabolism. Bone 2018, 110, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Orejel Bustos, A.; Belluscio, V.; Camomilla, V.; Lucangeli, L.; Rizzo, F.; Sciarra, T.; Martelli, F.; Giacomozzi, C. Overuse-Related Injuries of the Musculoskeletal System: Systematic Review and Quantitative Synthesis of Injuries, Locations, Risk Factors and Assessment Techniques. Sensors 2021, 21, 2438. [Google Scholar] [CrossRef]
- Gerr, F.; Marcus, M.; Ensor, C.; Kleinbaum, D.; Cohen, S.; Edwards, A.; Gentry, E.; Ortiz, D.J.; Monteilh, C. A prospective study of computer users: I. Study design and incidence of musculoskeletal symptoms and disorders. Am. J. Ind. Med. 2002, 41, 221–235. [Google Scholar] [CrossRef]
- Gardner, T.; Kenter, K.; Li, Y. Fibrosis following Acute Skeletal Muscle Injury: Mitigation and Reversal Potential in the Clinic. J. Sports Med. (Hindawi. Publ. Corp.) 2020, 2020, 7059057. [Google Scholar] [CrossRef]
- Aicale, R.; Tarantino, D.; Maffulli, N. Overuse injuries in sport: A comprehensive overview. J. Orthop. Surg. Res. 2018, 13, 309. [Google Scholar] [CrossRef] [PubMed]
- Andarawis-Puri, N.; Flatow, E.L. Tendon fatigue in response to mechanical loading. J. Musculoskelet. Neuronal. Interact. 2011, 11, 106–114. [Google Scholar] [PubMed]
- Battery, L.; Maffulli, N. Inflammation in overuse tendon injuries. Sports Med. Arthrosc. Rev. 2011, 19, 213–217. [Google Scholar] [CrossRef] [PubMed]
- Delloiacono, N. Musculoskeletal safety for older adults in the workplace: Review of current best practice evidence. Workplace Health Saf. 2015, 63, 48–53. [Google Scholar] [CrossRef] [PubMed]
- Committee on the Health and Safety Needs of Older Workers. Effects of Workplace Exposures on Older Workers; National Academies Press: Washington, DC, USA, 2004; Volume 6. [Google Scholar]
- Chau, N.; Bhattacherjee, A.; Kunar, B.M. Relationship between job, lifestyle, age and occupational injuries. Occup. Med. 2009, 59, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F. Aging and osteoarthritis: The role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthr. Cartil. 2009, 17, 971–979. [Google Scholar] [CrossRef]
- Stowe, E.J.; Keller, M.R.; Connizzo, B.K. Cellular senescence impairs tendon extracellular matrix remodeling in response to mechanical unloading. Aging Cell 2024, 23, e14278. [Google Scholar] [CrossRef]
- Long, Z.; Nakagawa, K.; Wang, Z.; Amadio, P.C.; Zhao, C.; Gingery, A. Age-related cellular and microstructural changes in the rotator cuff enthesis. J. Orthop. Res. 2022, 40, 1883–1895. [Google Scholar] [CrossRef] [PubMed]
- Fede, C.; Fan, C.; Pirri, C.; Petrelli, L.; Biz, C.; Porzionato, A.; Macchi, V.; De Caro, R.; Stecco, C. The Effects of Aging on the Intramuscular Connective Tissue. Int. J. Mol. Sci. 2022, 23, 11061. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Pirri, C.; Fede, C.; Guidolin, D.; Biz, C.; Petrelli, L.; Porzionato, A.; Macchi, V.; De Caro, R.; Stecco, C. Age-Related Alterations of Hyaluronan and Collagen in Extracellular Matrix of the Muscle Spindles. J. Clin. Med. 2021, 11, 86. [Google Scholar] [CrossRef]
- Pavan, P.; Monti, E.; Bondí, M.; Fan, C.; Stecco, C.; Narici, M.; Reggiani, C.; Marcucci, L. Alterations of Extracellular Matrix Mechanical Properties Contribute to Age-Related Functional Impairment of Human Skeletal Muscles. Int. J. Mol. Sci. 2020, 21, 3992. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune-metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef] [PubMed]
- Rezuș, E.; Cardoneanu, A.; Burlui, A.; Luca, A.; Codreanu, C.; Tamba, B.I.; Stanciu, G.D.; Dima, N.; Bădescu, C.; Rezuș, C. The Link Between Inflammaging and Degenerative Joint Diseases. Int. J. Mol. Sci. 2019, 20, 614. [Google Scholar] [CrossRef]
- Rea, I.M.; Gibson, D.S.; McGilligan, V.; McNerlan, S.E.; Alexander, H.D.; Ross, O.A. Age and Age-Related Diseases: Role of Inflammation Triggers and Cytokines. Front. Immunol. 2018, 9, 586. [Google Scholar] [CrossRef] [PubMed]
- Chisari, E.; Rehak, L.; Khan, W.S.; Maffulli, N. Tendon healing is adversely affected by low-grade inflammation. J. Orthop. Surg. Res. 2021, 16, 700. [Google Scholar] [CrossRef] [PubMed]
- Nichols, A.E.C.; Best, K.T.; Loiselle, A.E. The cellular basis of fibrotic tendon healing: Challenges and opportunities. Transl. Res. 2019, 209, 156–168. [Google Scholar] [CrossRef]
- Arvind, V.; Huang, A.H. Reparative and Maladaptive Inflammation in Tendon Healing. Front. Bioeng. Biotechnol. 2021, 9, 719047. [Google Scholar] [CrossRef] [PubMed]
- Xin, D.L.; Hadrevi, J.; Elliott, M.E.; Amin, M.; Harris, M.Y.; Barr-Gillespie, A.E.; Barbe, M.F. Effectiveness of conservative interventions for sickness and pain behaviors induced by a high repetition high force upper extremity task. BMC Neurosci. 2017, 18, 36. [Google Scholar] [CrossRef] [PubMed]
- Takano, K.; Nakagawa, H. Contribution of cytokine-induced neutrophil chemoattractant CINC-2 and CINC-3 to neutrophil recruitment in lipopolysaccharide-induced inflammation in rats. Inflamm. Res. 2001, 50, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Cook, D.N. The role of MIP-1 alpha in inflammation and hematopoiesis. J. Leukoc. Biol. 1996, 59, 61–66. [Google Scholar] [CrossRef]
- Antoniv, T.T.; Ivashkiv, L.B. Dysregulation of interleukin-10-dependent gene expression in rheumatoid arthritis synovial macrophages. Arthritis Rheum. 2006, 54, 2711–2721. [Google Scholar] [CrossRef]
- Fujiwara, M.; Iwata, M.; Inoue, T.; Aizawa, Y.; Yoshito, N.; Hayashi, K.; Suzuki, S. Decreased grip strength, muscle pain, and atrophy occur in rats following long-term exposure to excessive repetitive motion. FEBS Open Bio. 2017, 7, 1737–1749. [Google Scholar] [CrossRef]
- Sommerich, C.M.; Lavender, S.A.; Buford, J.A.; Jacob, J.B.; Korkmaz, S.V.; Pease, W.S. Towards development of a nonhuman primate model of carpal tunnel syndrome: Performance of a voluntary, repetitive pinching task induces median mononeuropathy in Macaca fascicularis. J. Orthop. Res. 2007, 25, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Stauber, W.T. Factors involved in strain-induced injury in skeletal muscles and outcomes of prolonged exposures. J. Electromyogr. Kinesiol. 2004, 14, 61–70. [Google Scholar] [CrossRef]
- Mann, C.J.; Perdiguero, E.; Kharraz, Y.; Aguilar, S.; Pessina, P.; Serrano, A.L.; Muñoz-Cánoves, P. Aberrant repair and fibrosis development in skeletal muscle. Skelet. Muscle 2011, 1, 21. [Google Scholar] [CrossRef] [PubMed]
- Barbe, M.F.; Hilliard, B.A.; Delany, S.P.; Iannarone, V.J.; Harris, M.Y.; Amin, M.; Cruz, G.E.; Barreto-Cruz, Y.; Tran, N.; Day, E.P.; et al. Blocking CCN2 Reduces Progression of Sensorimotor Declines and Fibrosis in a Rat Model of Chronic Repetitive Overuse. J. Orthop. Res. 2019, 37, 2004–2018. [Google Scholar] [CrossRef] [PubMed]
- Fisher, P.W.; Zhao, Y.; Rico, M.C.; Massicotte, V.S.; Wade, C.K.; Litvin, J.; Bove, G.M.; Popoff, S.N.; Barbe, M.F. Increased CCN2, substance P and tissue fibrosis are associated with sensorimotor declines in a rat model of repetitive overuse injury. J. Cell Commun. Signal. 2015, 9, 37–54. [Google Scholar] [CrossRef] [PubMed]
- Abdelmagid, S.M.; Barr, A.E.; Rico, M.; Amin, M.; Litvin, J.; Popoff, S.N.; Safadi, F.F.; Barbe, M.F. Performance of repetitive tasks induces decreased grip strength and increased fibrogenic proteins in skeletal muscle: Role of force and inflammation. PLoS ONE 2012, 7, e38359. [Google Scholar] [CrossRef]
- Besen, E.; Young, A.E.; Gaines, B.; Pransky, G. Relationship Between Age, Tenure, and Disability Duration in Persons With Compensated Work-Related Conditions. J. Occup. Environ. Med. 2016, 58, 140–147. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gustafsson, T.; Ulfhake, B. Aging Skeletal Muscles: What Are the Mechanisms of Age-Related Loss of Strength and Muscle Mass, and Can We Impede Its Development and Progression? Int. J. Mol. Sci. 2024, 25, 10932. [Google Scholar] [CrossRef] [PubMed]
- Contreras, O.; Rebolledo, D.L.; Oyarzún, J.E.; Olguín, H.C.; Brandan, E. Connective tissue cells expressing fibro/adipogenic progenitor markers increase under chronic damage: Relevance in fibroblast-myofibroblast differentiation and skeletal muscle fibrosis. Cell Tissue Res. 2016, 364, 647–660. [Google Scholar] [CrossRef] [PubMed]
- Chapman, M.A.; Meza, R.; Lieber, R.L. Skeletal muscle fibroblasts in health and disease. Differentiation 2016, 92, 108–115. [Google Scholar] [CrossRef]
- Apostolakos, J.; Durant, T.J.; Dwyer, C.R.; Russell, R.P.; Weinreb, J.H.; Alaee, F.; Beitzel, K.; McCarthy, M.B.; Cote, M.P.; Mazzocca, A.D. The enthesis: A review of the tendon-to-bone insertion. Muscles Ligaments Tendons J. 2014, 4, 333–342. [Google Scholar] [CrossRef]
- Killian, M.L. Growth and mechanobiology of the tendon-bone enthesis. Semin. Cell Dev. Biol. 2022, 123, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Macía-Villa, C.; Cruz Valenciano, A.; De Miguel, E. Enthesis lesions are associated with X-ray progression in psoriatic arthritis. Int. J. Rheum. Dis. 2021, 24, 828–833. [Google Scholar] [CrossRef]
- Zhang, H.; Liang, J.; Qiu, J.; Wang, F.; Sun, L. Ultrasonographic evaluation of enthesitis in patients with ankylosing spondylitis. J. Biomed. Res. 2017, 31, 162–169. [Google Scholar] [CrossRef]
- Putera, K.H.; Kim, J.; Baek, S.Y.; Schlecht, S.H.; Beaulieu, M.L.; Haritos, V.; Arruda, E.M.; Ashton-Miller, J.A.; Wojtys, E.M.; Banaszak Holl, M.M. Fatigue-driven compliance increase and collagen unravelling in mechanically tested anterior cruciate ligament. Commun. Biol. 2023, 6, 564. [Google Scholar] [CrossRef] [PubMed]
- Epsley, S.; Tadros, S.; Farid, A.; Kargilis, D.; Mehta, S.; Rajapakse, C.S. The Effect of Inflammation on Bone. Front. Physiol. 2020, 11, 511799. [Google Scholar] [CrossRef]
- Kehl, A.S.; Corr, M.; Weisman, M.H. Review: Enthesitis: New Insights Into Pathogenesis, Diagnostic Modalities, and Treatment. Arthritis Rheumatol. 2016, 68, 312–322. [Google Scholar] [CrossRef] [PubMed]
- Dilley, J.E.; Bello, M.A.; Roman, N.; McKinley, T.; Sankar, U. Post-traumatic osteoarthritis: A review of pathogenic mechanisms and novel targets for mitigation. Bone Rep. 2023, 18, 101658. [Google Scholar] [CrossRef] [PubMed]
- Sengprasert, P.; Kamenkit, O.; Tanavalee, A.; Reantragoon, R. The Immunological Facets Of Chondrocytes In Osteoarthritis: A Narrative Review. J. Rheumatol. 2024, 51, 13–24. [Google Scholar] [CrossRef]
- Moody, H.R.; Heard, B.J.; Frank, C.B.; Shrive, N.G.; Oloyede, A.O. Investigating the potential value of individual parameters of histological grading systems in a sheep model of cartilage damage: The Modified Mankin method. J. Anat. 2012, 221, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Henao-Murillo, L.; Pastrama, M.I.; Ito, K.; van Donkelaar, C.C. The Relationship Between Proteoglycan Loss, Overloading-Induced Collagen Damage, and Cyclic Loading in Articular Cartilage. Cartilage 2021, 13, 1501s–1512s. [Google Scholar] [CrossRef]
- Zhang, Y.; Pizzute, T.; Pei, M. Anti-inflammatory strategies in cartilage repair. Tissue Eng. Part B Rev. 2014, 20, 655–668. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, A.; Murugesh, D.K.; Mendez, M.E.; Hum, N.R.; Rios-Arce, N.D.; McCool, J.L.; Christiansen, B.A.; Loots, G.G. Global Gene Expression Analysis Identifies Age-Related Differences in Knee Joint Transcriptome during the Development of Post-Traumatic Osteoarthritis in Mice. Int. J. Mol. Sci. 2020, 21, 364. [Google Scholar] [CrossRef]
- Pulford, K.A.; Sipos, A.; Cordell, J.L.; Stross, W.P.; Mason, D.Y. Distribution of the CD68 macrophage/myeloid associated antigen. Int. Immunol. 1990, 2, 973–980. [Google Scholar] [CrossRef]
- Hume, D.A. Applications of myeloid-specific promoters in transgenic mice support in vivo imaging and functional genomics but do not support the concept of distinct macrophage and dendritic cell lineages or roles in immunity. J. Leukoc. Biol. 2011, 89, 525–538. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, J.; Xie, X.; Gu, F.; Sui, Z.; Zhang, K.; Yu, T. Macrophage-Osteoclast Associations: Origin, Polarization, and Subgroups. Front. Immunol. 2021, 12, 778078. [Google Scholar] [CrossRef]
- Sandberg, O.; Aspenberg, P. Different effects of indomethacin on healing of shaft and metaphyseal fractures. Acta Orthop. 2015, 86, 243–247. [Google Scholar] [CrossRef]
- Sandberg, O.H.; Aspenberg, P. Glucocorticoids inhibit shaft fracture healing but not metaphyseal bone regeneration under stable mechanical conditions. Bone Jt. Res. 2015, 4, 170–175. [Google Scholar] [CrossRef]
- Beyreuther, B.K.; Geis, C.; Stöhr, T.; Sommer, C. Antihyperalgesic efficacy of lacosamide in a rat model for muscle pain induced by TNF. Neuropharmacology 2007, 52, 1312–1317. [Google Scholar] [CrossRef] [PubMed]
- Neogi, T. Structural correlates of pain in osteoarthritis. Clin. Exp. Rheumatol. 2017, 35 (Suppl. S107), 75–78. [Google Scholar] [PubMed]
- Strazdins, L.; Bammer, G. Women, work and musculoskeletal health. Soc. Sci. Med. 2004, 58, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Okezue, O.C.; Anamezie, T.H.; Nene, J.J.; Okwudili, J.D. Work-Related Musculoskeletal Disorders among Office Workers in Higher Education Institutions: A Cross-Sectional Study. Ethiop. J. Health Sci. 2020, 30, 715–724. [Google Scholar] [CrossRef]
- Chan, X.W.; Hutchings, K. Inequalities, barriers, intersectionality, and facilitators of careers of women with disabilities: Themes and future research agenda from a scoping review. Front. Psychol. 2023, 14, 1104784. [Google Scholar] [CrossRef]
- Jetha, A.; Gignac, M.A.M.; Ibrahim, S.; Martin Ginis, K.A. Disability and sex/gender intersections in unmet workplace support needs: Findings from a large Canadian survey of workers. Am. J. Ind. Med. 2021, 64, 149–161. [Google Scholar] [CrossRef]
- Fede, C.; Albertin, G.; Petrelli, L.; Sfriso, M.M.; Biz, C.; De Caro, R.; Stecco, C. Hormone receptor expression in human fascial tissue. Eur. J. Histochem. 2016, 60, 2710. [Google Scholar] [CrossRef]
- Talwar, R.M.; Wong, B.S.; Svoboda, K.; Harper, R.P. Effects of estrogen on chondrocyte proliferation and collagen synthesis in skeletally mature articular cartilage. J. Oral Maxillofac. Surg. 2006, 64, 600–609. [Google Scholar] [CrossRef]
- Ling, F.; Chen, Y.; Li, J.; Xu, M.; Song, G.; Tu, L.; Wang, H.; Li, S.; Zhu, L. Estrogen Receptor β Activation Mitigates Colitis-associated Intestinal Fibrosis via Inhibition of TGF-β/Smad and TLR4/MyD88/NF-κB Signaling Pathways. Inflamm. Bowel. Dis. 2024, 30, izae156. [Google Scholar] [CrossRef]
- Beynnon, B.D.; Bernstein, I.M.; Belisle, A.; Brattbakk, B.; Devanny, P.; Risinger, R.; Durant, D. The effect of estradiol and progesterone on knee and ankle joint laxity. Am. J. Sports Med. 2005, 33, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.K.; Srivastava, S. Grip strength of occupational workers in relation to carpal tunnel syndrome and individual factors. Int. J. Occup. Saf. Erg. 2020, 26, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Golberg, M.; Kobos, J.; Clarke, E.; Bajaka, A.; Smędra, A.; Balawender, K.; Wawrzyniak, A.; Seneczko, M.; Orkisz, S.; Żytkowski, A. Application of histochemical stains in anatomical research: A brief overview of the methods. Transl. Res. Anat. 2024, 35, 100294. [Google Scholar] [CrossRef]
- Bove, G.M.; Harris, M.Y.; Zhao, H.; Barbe, M.F. Manual therapy as an effective treatment for fibrosis in a rat model of upper extremity overuse injury. J. Neurol. Sci. 2016, 361, 168–180. [Google Scholar] [CrossRef]
- Mishra, P.; Pandey, C.M.; Singh, U.; Gupta, A.; Sahu, C.; Keshri, A. Descriptive statistics and normality tests for statistical data. Ann. Card. Anaesth. 2019, 22, 67–72. [Google Scholar] [CrossRef]
- Swinscow, T.D.V. Statistics at Square One, 9th ed.; BMJ Publishing Group: London, UK, 1997; Revised by Campbell, M.J., University of Southampton. [Google Scholar]
- LaMorte, W.W. The Correlation Coefficient (r). Available online: https://sphweb.bumc.bu.edu/otlt/MPH-Modules/PH717-QuantCore/PH717-Module9-Correlation-Regression/PH717-Module9-Correlation-Regression4.html (accessed on 4 March 2023).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, P.R.; Tamas, I.P.; Van Der Bas, M.; Kegg, A.; Hilliard, B.A.; Lambi, A.G.; Popoff, S.N.; Barbe, M.F. Repetitive Overuse Injury Causes Entheseal Damage and Palmar Muscle Fibrosis in Older Rats. Int. J. Mol. Sci. 2024, 25, 13546. https://doi.org/10.3390/ijms252413546
Patel PR, Tamas IP, Van Der Bas M, Kegg A, Hilliard BA, Lambi AG, Popoff SN, Barbe MF. Repetitive Overuse Injury Causes Entheseal Damage and Palmar Muscle Fibrosis in Older Rats. International Journal of Molecular Sciences. 2024; 25(24):13546. https://doi.org/10.3390/ijms252413546
Chicago/Turabian StylePatel, Parth R., Istvan P. Tamas, Megan Van Der Bas, Abby Kegg, Brendan A. Hilliard, Alex G. Lambi, Steven N. Popoff, and Mary F. Barbe. 2024. "Repetitive Overuse Injury Causes Entheseal Damage and Palmar Muscle Fibrosis in Older Rats" International Journal of Molecular Sciences 25, no. 24: 13546. https://doi.org/10.3390/ijms252413546
APA StylePatel, P. R., Tamas, I. P., Van Der Bas, M., Kegg, A., Hilliard, B. A., Lambi, A. G., Popoff, S. N., & Barbe, M. F. (2024). Repetitive Overuse Injury Causes Entheseal Damage and Palmar Muscle Fibrosis in Older Rats. International Journal of Molecular Sciences, 25(24), 13546. https://doi.org/10.3390/ijms252413546





