Osseointegration of Implants Through Ti Biofunctionalization with Biomass from Chlorella sorokiniana UTEX 1230 and Synechococcus sp. PCC 7002
Abstract
1. Introduction
2. Results and Discussion
2.1. Biomass Production of 1230 and 7002
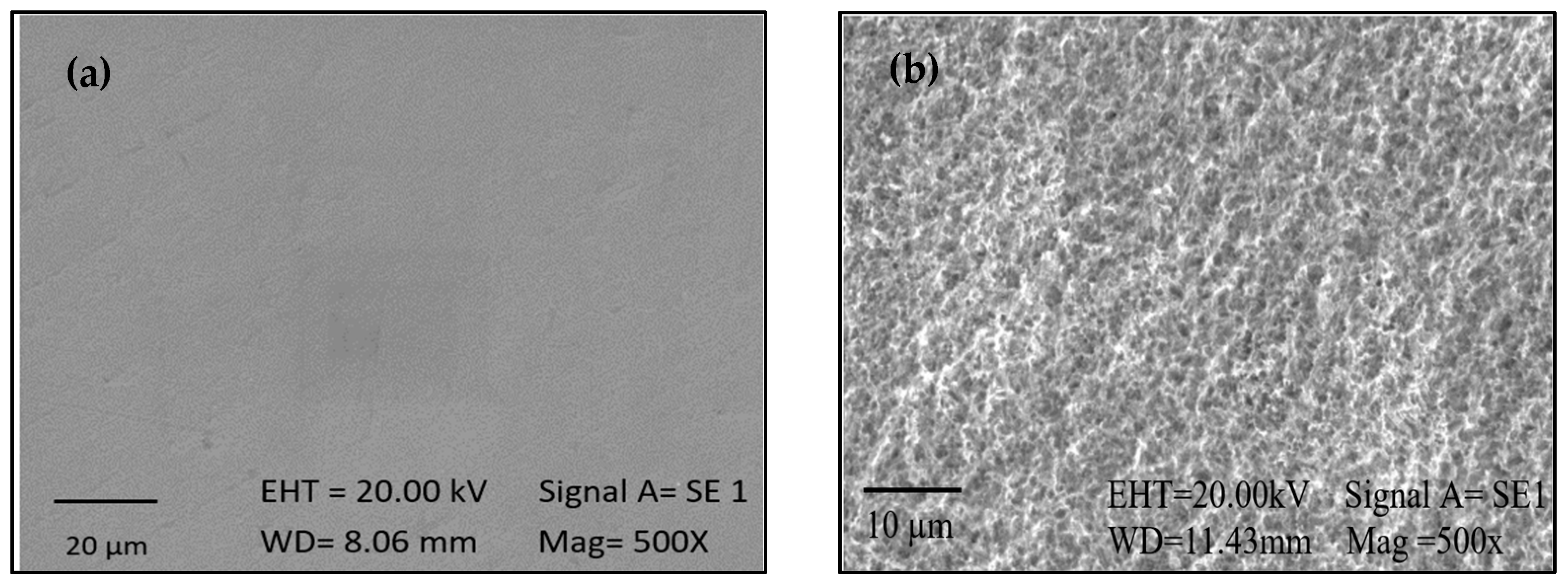
2.2. Activation
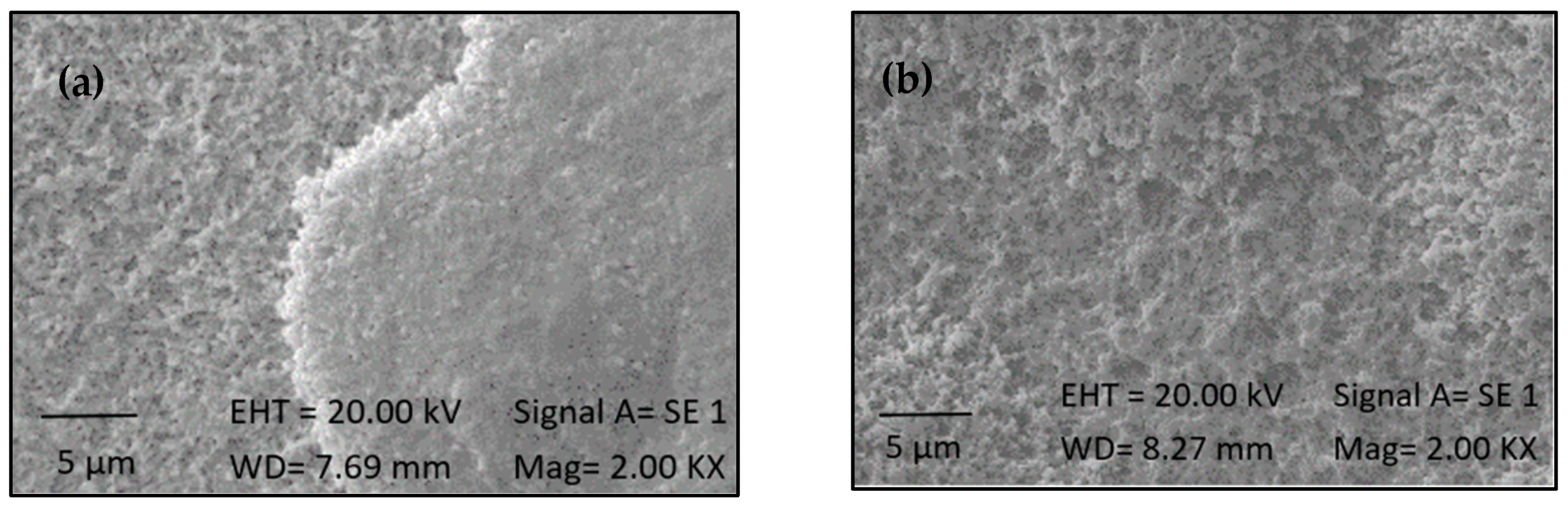
2.3. Silanization
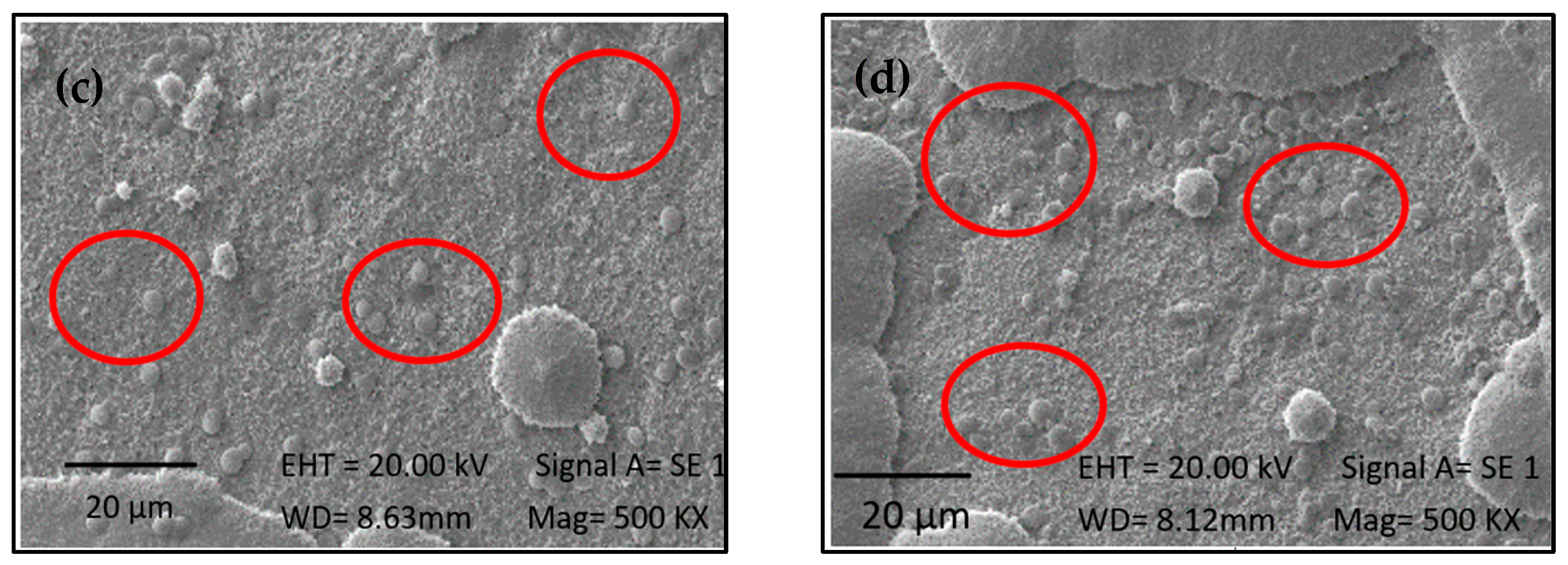
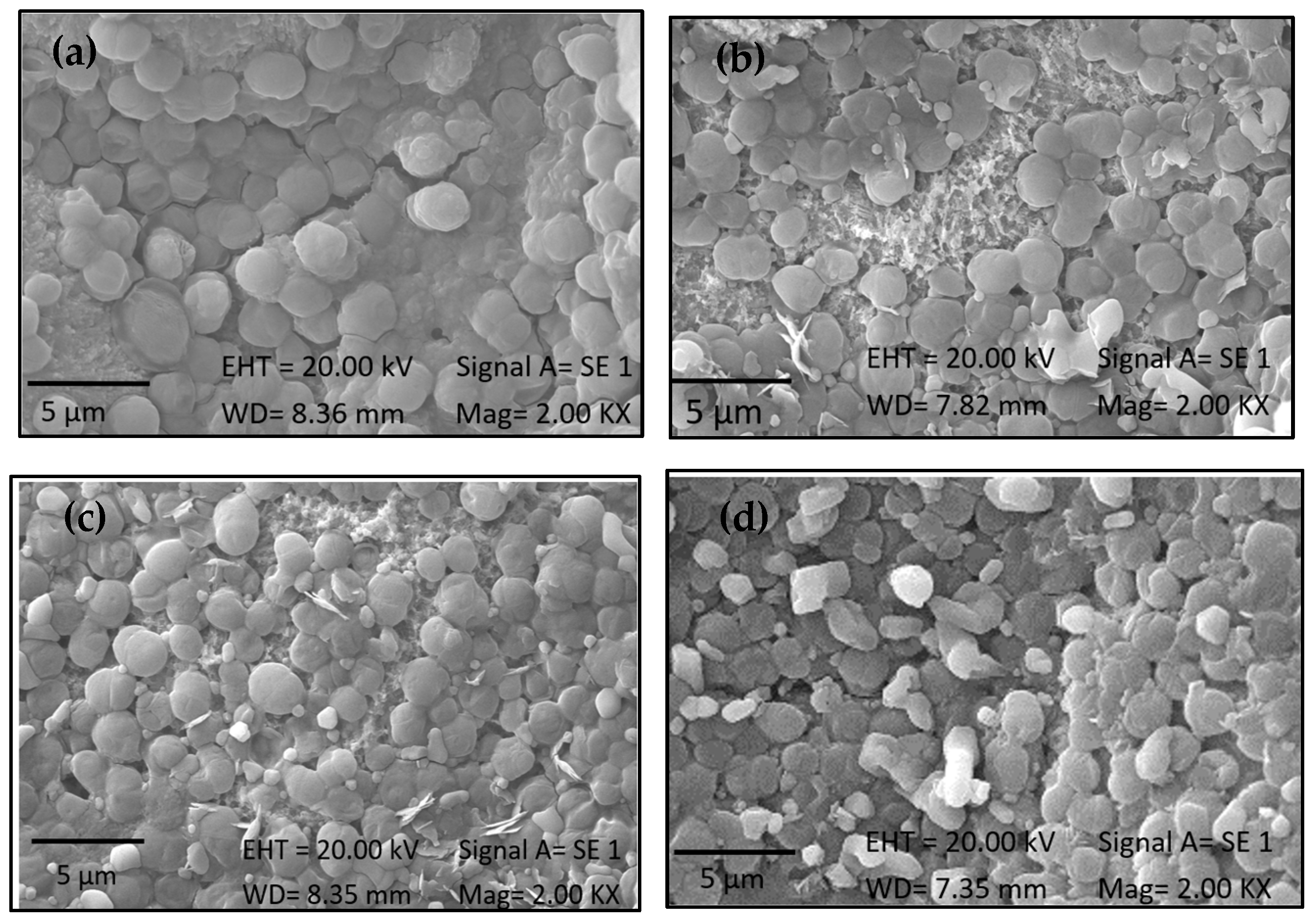
2.4. Immobilization of Biomass from 1230 and 7002
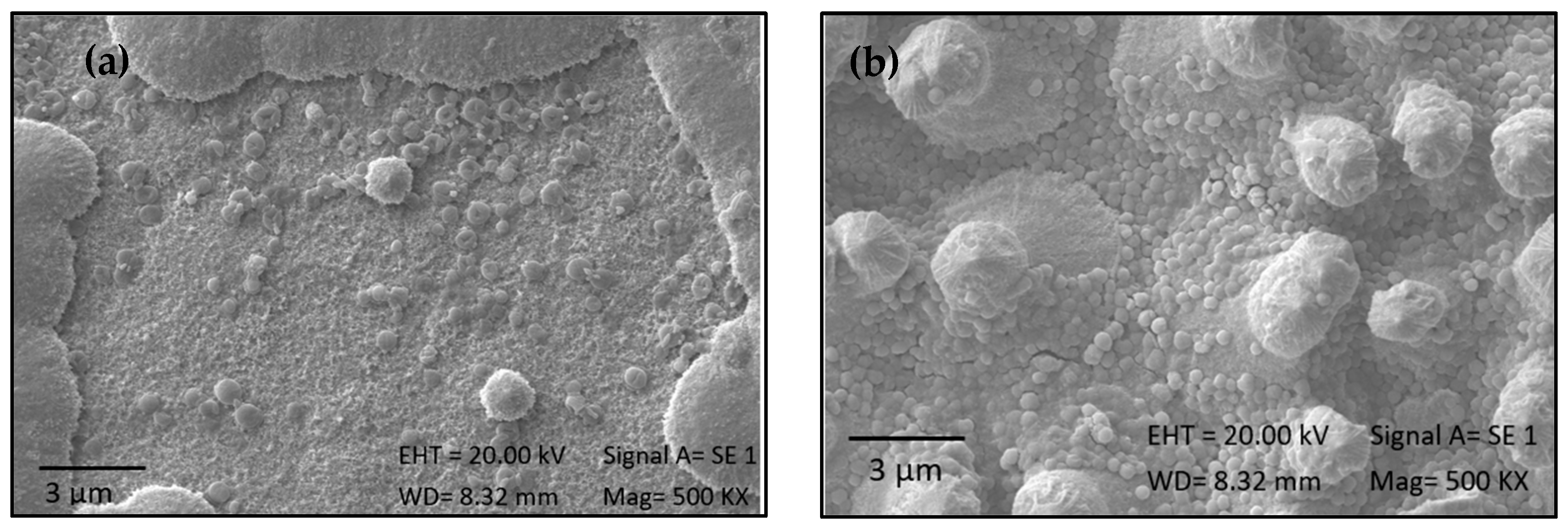
2.5. Effectiveness of Immobilization
2.6. Cellular Assays
2.6.1. Cytotoxicity and Cell Viability
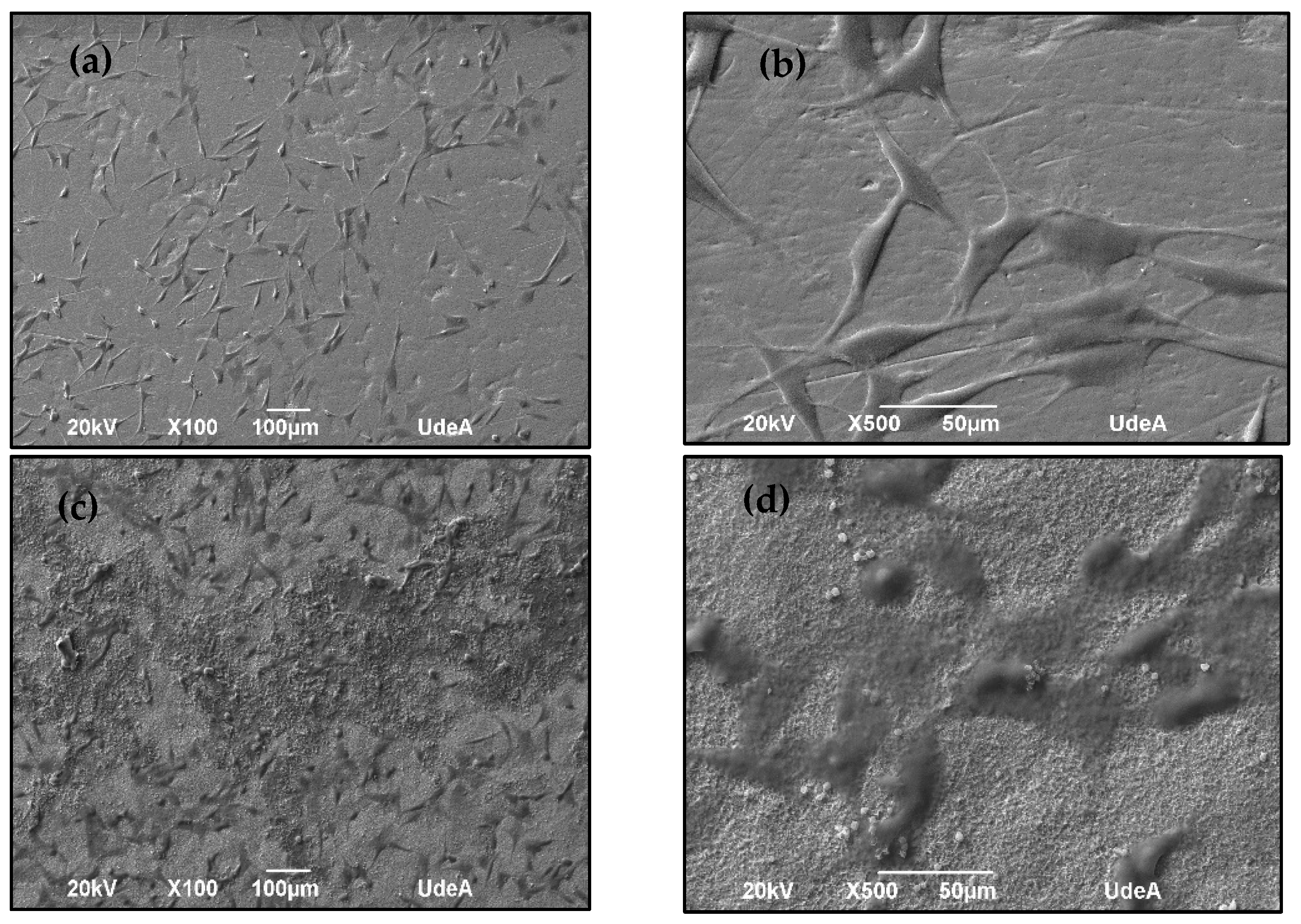
2.6.2. Cell Adhesion
3. Materials and Methods
3.1. Materials
3.2. Activation and Silanization
3.3. Microalgal Biomass
3.4. Immobilization
3.5. Surface Characterization
3.5.1. Contact Angle Measurement
3.5.2. Scanning Electron Microscopy (SEM)
3.6. Cell Assays
3.6.1. Viability and Cytotoxicity
3.6.2. Cell Adhesion
3.7. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quinn, J.; McFadden, R.; Chan, C.W.; Carson, L. Titanium for Orthopedic Applications: An Overview of Surface Modification to Improve Biocompatibility and Prevent Bacterial Biofilm Formation. iScience 2020, 23, 101745. [Google Scholar] [CrossRef]
- Rappe, K.S.; Ortiz-Hernandez, M.; Punset, M.; Molmeneu, M.; Barba, A.; Mas-Moruno, C.; Guillem-Marti, J.; Caparrós, C.; Rupérez, E.; Calero, J.; et al. On-Growth and In-Growth Osseointegration Enhancement in PM Porous Ti-Scaffolds by Two Different Bioactivation Strategies: Alkali Thermochemical Treatment and RGD Peptide Coating. Int. J. Mol. Sci. 2022, 23, 1750. [Google Scholar] [CrossRef]
- Gutiérrez Púa, L.d.C. Biofuncionalización de Magnesio Biodegradable Con Moléculas Osteoinductoras Para Mejorar Su Resistencia a La Corrosión y Propiciar Su Uso Como Elemento de Fijación Ortopédica. Repos. Univ. Norte 2022. [Google Scholar]
- Ghilini, F. Multifuncionalización de Superficies de Titanio Con Nanopartículas de Plata y Biomoleculas Para Mejorar El Desempeño de Dispositivos Implantables. Ph.D. Dissertation, Universidad Nacional de La Plata, La Plata, Argentina, 2020. [Google Scholar]
- Lario-Femenía, J.; Amigó-Mata, A.; Vicente-Escuder, Á.; Segovia-López, F.; Amigó-Borrás, V. Desarrollo de Las Aleaciones de Titanio y Tratamientos Superficiales Para Incrementar La Vida Útil de Los Implantes. Rev. Metal. 2016, 52, e084–e096. [Google Scholar] [CrossRef]
- Mohammadi, M.; Alibolandi, M.; Abnous, K.; Salmasi, Z.; Jaafari, M.R.; Ramezani, M. Comparison of Liposomal Formulations Incorporating BMP-2 Peptide to Induce Bone Tissue Engineering. Nanomed. J. 2020, 7, 225–230. [Google Scholar] [CrossRef]
- Godoy, M.; Sevilla, P.; Gil, F.J.; Rodriguez, D. Unión de Péptidos a Superficies de Titanio Para La Mejora de La Osteointegración. Biomecánica 2009, 17, 60–68. [Google Scholar] [CrossRef]
- Park, B.H.; Jeong, E.S.; Lee, S.; Jang, J.H. Bio-Functionalization and in-Vitro Evaluation of Titanium Surface with Recombinant Fibronectin and Elastin Fragment in Human Mesenchymal Stem Cell. PLoS ONE 2021, 16, e0260760. [Google Scholar] [CrossRef]
- Morra, M. Biochemical Modification of Titanium Surfaces: Peptides and ECM Proteins. Eur. Cell Mater. 2006, 12, 1–15. [Google Scholar] [CrossRef]
- Fang, K.; Song, W.; Wang, L.; Jia, S.; Wei, H.; Ren, S.; Xu, X.; Song, Y. Immobilization of Chitosan Film Containing Semaphorin 3A onto a Microarc Oxidized Titanium Implant Surface via Silane Reaction to Improve MG63 Osteogenic Differentiation. Int. J. Nanomed. 2014, 9, 4649–4657. [Google Scholar] [CrossRef]
- Arrieta Payares, L.M.; Del Carmen Gutiérrez Púa, L.; Di Mare Pareja, L.A.; Paredes Méndez, S.C.; Paredes Méndez, V.N. Microalgae Applications to Bone Repairing Processes: A Review. ACS Biomater. Sci. Eng. 2023, 9, 2991–3009. [Google Scholar] [CrossRef]
- Gurzawska, K.; Svava, R.; Jørgensen, N.R.; Gotfredsen, K. Nanocoating of Titanium Implant Surfaces with Organic Molecules. Polysaccharides Including Glycosaminoglycans. J. Biomed. Nanotechnol. 2012, 8, 1012–1024. [Google Scholar] [CrossRef]
- Richmond, A. Handbook of Microalgal Culture: Biotechnology and Applied Phycology; Wiley-Blackwell: Oxford, UK, 2013. [Google Scholar]
- Bertasini, D.; Binati, R.L.; Bolzonella, D.; Battista, F. Single Cell Proteins Production from Food Processing Effluents and Digestate. Chemosphere 2022, 296, 134076. [Google Scholar] [CrossRef]
- Lizzul, A.M.; Lekuona-Amundarain, A.; Purton, S.; Campos, L.C. Characterization of Chlorella sorokiniana, UTEX 1230. Biology 2018, 7, 25. [Google Scholar] [CrossRef]
- Poveda, G.A.; Bonilla, V.P.; Rivera, Q.J.; Santamaría Aguirre, J.R.; Ron Garrido, L.J.; Mosquera Carrera, P.M. Activación y Producción de Superficies Silanizadas. infoANALÍTICA 2021, 9, 89–101. [Google Scholar] [CrossRef]
- Shimabukuro, M. Surface Functionalization of Titanium for the Control and Treatment of Infections. Springer Ser. Biomater. Sci. Eng. 2022, 17, 195–212. [Google Scholar] [CrossRef]
- Bhushan, S.; Eshkabilov, S.; Jayakrishnan, U.; Prajapati, S.K.; Simsek, H. A Comparative Analysis of Growth Kinetics, Image Analysis, and Biofuel Potential of Different Algal Strains. Chemosphere 2023, 336, 139196. [Google Scholar] [CrossRef]
- Padil, P.; Putra, M.D.; Nata, I.F.; Wicakso, D.R.; Zulfarina, Z.; Irawan, C.; Sunarno, S. Microalgae Growth Kinetic Study with Logistic and Monod Models. AIP Conf. Proc. 2023, 2818, 030005. [Google Scholar] [CrossRef]
- Hasan, A.; Saxena, V.; Pandey, L.M. Surface Functionalization of Ti6Al4V via Self-Assembled Monolayers for Improved Protein Adsorption and Fibroblast Adhesion. Langmuir 2018, 34, 3494–3506. [Google Scholar] [CrossRef]
- Safafar, H.; Nørregaard, P.U.; Ljubic, A.; Møller, P.; Holdt, S.L.; Jacobsen, C. Enhancement of Protein and Pigment Content in Two Chlorella Species Cultivated on Industrial Process Water. J. Mar. Sci. Eng. 2016, 4, 84. [Google Scholar] [CrossRef]
- Xie, T.; Xia, Y.; Zeng, Y.; Li, X.; Zhang, Y. Nitrate Concentration-Shift Cultivation to Enhance Protein Content of Heterotrophic Microalga Chlorella Vulgaris: Over-Compensation Strategy. Bioresour. Technol. 2017, 233, 247–255. [Google Scholar] [CrossRef]
- Wang, Y.; Tibbetts, S.M.; McGinn, P.J. Microalgae as Sources of High-Quality Protein for Human Food and Protein Supplements. Foods 2021, 10, 3002. [Google Scholar] [CrossRef]
- Paredes, V.; Salvagni, E.; Rodríguez-Castellón, E.; Manero, J.M. Comparative Study of Surface Chemical Composition and Oxide Layer Modification upon Oxygen Plasma Cleaning and Piranha Etching on a Novel Low Elastic Modulus Ti25Nb21Hf Alloy. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2017, 48, 3770–3776. [Google Scholar] [CrossRef]
- Chouirfa, H.; Bouloussa, H.; Migonney, V.; Falentin-Daudré, C. Review of Titanium Surface Modification Techniques and Coatings for Antibacterial Applications. Acta Biomater. 2019, 83, 37–54. [Google Scholar] [CrossRef]
- Paredes, V.; Salvagni, E.; Manero, J.M. Assessment of Biomimetic Modification on a Novel Low Elastic Modulus TiNbHf Alloy for Dental Implants. J. Mater. Sci. Eng. 2015, 4, 193. [Google Scholar] [CrossRef]
- Ratner, B.D. Advances in the Analysis of Surfaces of Biomedical Interest. Surf. Interface Anal. 1995, 23, 521–528. [Google Scholar] [CrossRef]
- Manjaiah, M.; Laubscher, R.F. A review of the surface modifications of titanium alloys for biomedical applications. Mater. Tehnol. 2017, 51, 181–193. [Google Scholar] [CrossRef]
- Senna, P.M.; de Almeida Barros Mourão, C.F.; Mello-Machado, R.C.; Javid, K.; Montemezzi, P.; Cury, A.A.D.B.; Meirelles, L. Silane-Coating Strategy for Titanium Functionalization Does Not Impair Osteogenesis In Vivo. Materials 2021, 14, 1814. [Google Scholar] [CrossRef]
- Chen, R.; Willcox, M.D.P.; Ho, K.K.K.; Smyth, D.; Kumar, N. Antimicrobial Peptide Melimine Coating for Titanium and Its in Vivo Antibacterial Activity in Rodent Subcutaneous Infection Models. Biomaterials 2016, 85, 142–151. [Google Scholar] [CrossRef]
- Mughal, M.P.; Farooq, M.U.; Mumtaz, J.; Mia, M.; Shareef, M.; Javed, M.; Jamil, M.; Pruncu, C.I. Surface Modification for Osseointegration of Ti6Al4V ELI Using Powder Mixed Sinking EDM. J. Mech. Behav. Biomed. Mater. 2021, 113, 104145. [Google Scholar] [CrossRef]
- Baldin, E.K.K.; Garcia, C.; Henriques, J.A.P.; Ely, M.R.; Birriel, E.J.; Brandalise, R.N.; Malfatti, C.D.F. Effect of Sterilization Processes on the Properties of a Silane Hybrid Coating Applied to Ti6Al4V Alloy. J. Mater. Res. 2018, 33, 161–177. [Google Scholar] [CrossRef]
- Misiura, A.; Dutta, C.; Leung, W.; Zepeda, O.J.; Terlier, T.; Landes, C.F. The Competing Influence of Surface Roughness, Hydrophobicity, and Electrostatics on Protein Dynamics on a Self-Assembled Monolayer. J. Chem. Phys. 2022, 156, 094707. [Google Scholar] [CrossRef]
- Sánchez-Bodón, J.; Del Olmo, J.A.; Alonso, J.M.; Moreno-Benítez, I.; Vilas-Vilela, J.L.; Pérez-Álvarez, L. Bioactive Coatings on Titanium: A Review on Hydroxylation, Self-Assembled Monolayers (SAMs) and Surface Modification Strategies. Polymers 2021, 14, 165. [Google Scholar] [CrossRef]
- Kashin, A.D.; Sedelnikova, M.B.; Uvarkin, P.V.; Ugodchikova, A.V.; Luginin, N.A.; Sharkeev, Y.P.; Khimich, M.A.; Bakina, O.V. Functionalizing Diatomite-Based Micro-Arc Coatings for Orthopedic Implants: Influence of TiO2 Addition. Biomimetics 2023, 8, 280. [Google Scholar] [CrossRef]
- Arrieta Payares, L.M.; Gutierrez Pua, L.D.C.; Rincon Montenegro, J.C.; Fonseca Reyes, A.; Paredes Mendez, V.N. Influence of the Activation Time of Magnesium Surfaces on the Concentration of Active Hydroxyl Groups and Corrosion Resistance. Heliyon 2024, 10, e34772. [Google Scholar] [CrossRef]
- Felgueiras, H.P.; Antunes, J.C.; Martins, M.C.L.; Barbosa, M.A. Fundamentals of Protein and Cell Interactions in Biomaterials. In Peptides and Proteins as Biomaterials for Tissue Regeneration and Repair; Elsevier Ltd.: Amsterdam, The Netherlands, 2018; pp. 1–27. [Google Scholar] [CrossRef]
- Colorado, S. Determinación de Parámetros Cinéticos de Synechococcus sp. PCC 7002 En Cultivo Sumergido; Universidad EAFIT: Medellín, Colombia, 2018. [Google Scholar]
- Selão, T.; Włodarczyk, A.; Nixon, P.; Norling, B. Growth and Selection of the Cyanobacterium Synechococcus sp. PCC 7002 Using Alternative Nitrogen and Phosphorus Sources. Metab. Eng. 2019, 54, 255–263. [Google Scholar] [CrossRef]
- Majhy, B.; Priyadarshini, P.; Sen, A.K. Effect of Surface Energy and Roughness on Cell Adhesion and Growth—Facile Surface Modification for Enhanced Cell Culture. RSC Adv. 2021, 11, 15467–15476. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C.W. The Effect of Nanoparticle Size, Shape, and Surface Chemistry on Biological Systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef]
- Arboleya, L.; Castañeda, S. Osteoclastos: Mucho Más Que Células Remodeladoras Del Hueso. Rev. Osteoporos. Metab. Miner. 2014, 6, 109–121. [Google Scholar] [CrossRef]
- López-González, I.; Zamora-Ledezma, C.; Sanchez-Lorencio, M.I.; Barrenechea, E.T.; Gabaldón-Hernández, J.A.; Meseguer-olmo, L. Modifications in Gene Expression in the Process of Osteoblastic Differentiation of Multipotent Bone Marrow-Derived Human Mesenchymal Stem Cells Induced by a Novel Osteoinductive Porous Medical-Grade 3D-Printed Poly(ε-Caprolactone)/β-Tricalcium Phosphate Composite. Int. J. Mol. Sci. 2021, 22, 11216. [Google Scholar] [CrossRef] [PubMed]
- International Organization for Standardization (ISO). Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity; International Organization for Standardization (ISO): Geneva, Switzerland, 2009. [Google Scholar]
- Talha, M.; Ma, Y.; Kumar, P.; Lin, Y.; Singh, A. Role of Protein Adsorption in the Bio Corrosion of Metallic Implants—A Review. Colloids Surf. B Biointerfaces 2019, 176, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chen, J.; Scheideler, L.; Reichl, R.; Geis-Gerstorfer, J. Effects of Topography and Composition of Titanium Surface Oxides on Osteoblast Responses. Biomaterials 2004, 25, 4087–4103. [Google Scholar] [CrossRef] [PubMed]
- Jayaraman, M.; Meyer, U.; Bühner, M.; Joos, U.; Wiesmann, H.P. Influence of Titanium Surfaces on Attachment of Osteoblast-like Cells in Vitro. Biomaterials 2004, 25, 625–631. [Google Scholar] [CrossRef]
- Kurup, A.; Dhatrak, P.; Khasnis, N. Surface Modification Techniques of Titanium and Titanium Alloys for Biomedical Dental Applications: A Review. Mater. Today Proc. 2021, 39, 84–90. [Google Scholar] [CrossRef]



| Treatment | Protein (%) | |
|---|---|---|
| Strain 1230 | Strain 7002 | |
| T1 | 17.17 | 48.1 |
| T2 | 11.17 | 25.6 |
| T3 | 47.70 | 11.7 |
| T4 | 38.70 | 15.4 |
| Parameter (%) | Strain | |
|---|---|---|
| 1230 | 7002 | |
| Humidity | 8.19 | 9.52 |
| Ashes | 16.86 | 18.81 |
| Proteins | 47.70 | 48.10 |
| Lipids | 11.13 | 13.57 |
| Fiber | 0.54 | 0.41 |
| Carbohydrates | 15.58 | 9.61 |
| Atom | Control | Sil 3 h | Sil 14 h | |||
|---|---|---|---|---|---|---|
| Weight % | Atomic % | Weight % | Atomic % | Weight % | Atomic % | |
| Ti | 88.97 | 84.70 | 78.55 | 63.52 | 79.17 | 58.06 |
| Al | 6.82 | 84.70 | 4.20 | 5.65 | 1.70 | 2.22 |
| V | 4.22 | 3.77 | 3.77 | 5.89 | 4.52 | 3.12 |
| C | 0 | 0 | 10.97 | 25.84 | 6.97 | 20.26 |
| Si | 0 | 0 | 0.33 | 0.44 | 0.54 | 0.83 |
| Time | 3 h | 14 h | ||||||
|---|---|---|---|---|---|---|---|---|
| Atom | 3 g/L | 5 g/L | 3 g/L | 5 g/L | ||||
| Weight % | Atomic % | Weight % | Atomic % | Weight % | Atomic % | Weight % | Atomic % | |
| Ti | 43.11 | 35.26 | 43.29 | 34.82 | 46.91 | 42.01 | 53.23 | 44.22 |
| Al | 2.78 | 3.10 | 2.06 | 2.95 | 2.36 | 3.75 | 3.36 | 4.78 |
| V | 3.15 | 2.57 | 3.32 | 2.53 | 3.16 | 2.66 | 3.25 | 2.60 |
| Si | 0.43 | 0.56 | 0.32 | 0.44 | 0.54 | 0.83 | 0.43 | 0.59 |
| C | 3.45 | 10.39 | 3.39 | 10.86 | 3.23 | 11.55 | 3.94 | 12.18 |
| O | 15.67 | 35.32 | 16.99 | 40.92 | 11.24 | 30.14 | 12.05 | 27.94 |
| N | 2.10 | 3.87 | 0.81 | 2.23 | 2.03 | 6.10 | 2.15 | 7.51 |
| P | 1.45 | 1.35 | 1.15 | 1.41 | 1.11 | 1.43 | 1.20 | 1.53 |
| Time | 3 h | 14 h | ||||||
|---|---|---|---|---|---|---|---|---|
| Atom | 3 g/L | 5 g/L | 3 g/L | 5 g/L | ||||
| Weight % | Atomic % | Weight % | Atomic % | Weight % | Atomic % | Weight % | Atomic % | |
| Ti | 36.31 | 28.50 | 48.80 | 37.62 | 42.33 | 52.23 | 45.40 | 32.75 |
| Al | 2.28 | 3.18 | 2.70 | 3.70 | 3.45 | 3.45 | 2.63 | 3.37 |
| V | 3.12 | 2.87 | 3.34 | 2.42 | 2.56 | 3.45 | 2.69 | 1.82 |
| Si | 0.41 | 0.65 | 0.34 | 0.55 | 0.57 | 0.84 | 0.44 | 0.60 |
| C | 4.27 | 13.36 | 6.12 | 16.21 | 4.55 | 13.78 | 4.94 | 14.23 |
| O | 17.34 | 40.76 | 13.13 | 30.30 | 12.05 | 30.12 | 15.29 | 36.23 |
| N | 2.57 | 6.05 | 3.53 | 9.32 | 2.15 | 6.13 | 2.54 | 6.27 |
| P | 1.35 | 1.45 | 1.05 | 1.51 | 1.23 | 1.53 | 1.20 | 1.63 |
| Treatment | % Viability Saos-2 X ± DS |
|---|---|
| Control Ti | 88.9 ± 6.8 a |
| 7002 | 86.8 ± 8.3 a |
| Untreated control | 100.0 ± 4.1 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bravo, Y.; Miranda, A.M.; Hernandez-Tenorio, F.; Sáez, A.A.; Paredes, V. Osseointegration of Implants Through Ti Biofunctionalization with Biomass from Chlorella sorokiniana UTEX 1230 and Synechococcus sp. PCC 7002. Int. J. Mol. Sci. 2024, 25, 13161. https://doi.org/10.3390/ijms252313161
Bravo Y, Miranda AM, Hernandez-Tenorio F, Sáez AA, Paredes V. Osseointegration of Implants Through Ti Biofunctionalization with Biomass from Chlorella sorokiniana UTEX 1230 and Synechococcus sp. PCC 7002. International Journal of Molecular Sciences. 2024; 25(23):13161. https://doi.org/10.3390/ijms252313161
Chicago/Turabian StyleBravo, Yarelis, Alejandra M. Miranda, Fabian Hernandez-Tenorio, Alex A. Sáez, and Virginia Paredes. 2024. "Osseointegration of Implants Through Ti Biofunctionalization with Biomass from Chlorella sorokiniana UTEX 1230 and Synechococcus sp. PCC 7002" International Journal of Molecular Sciences 25, no. 23: 13161. https://doi.org/10.3390/ijms252313161
APA StyleBravo, Y., Miranda, A. M., Hernandez-Tenorio, F., Sáez, A. A., & Paredes, V. (2024). Osseointegration of Implants Through Ti Biofunctionalization with Biomass from Chlorella sorokiniana UTEX 1230 and Synechococcus sp. PCC 7002. International Journal of Molecular Sciences, 25(23), 13161. https://doi.org/10.3390/ijms252313161






