GLI3 Is Required for M2 Macrophage Polarization and M2-Mediated Waldenström Macroglobulinemia Growth and Survival
Abstract
1. Introduction
2. Results
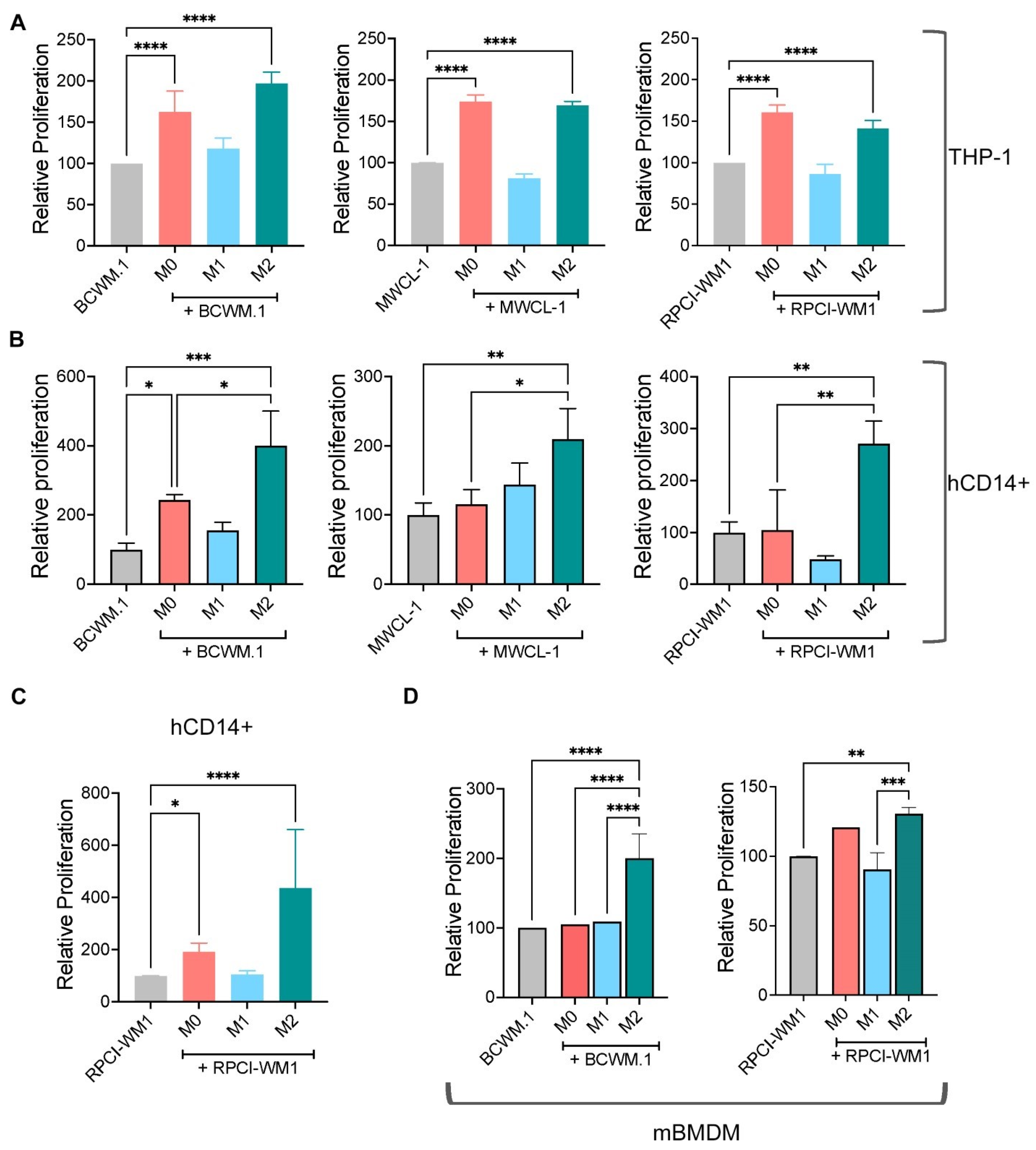
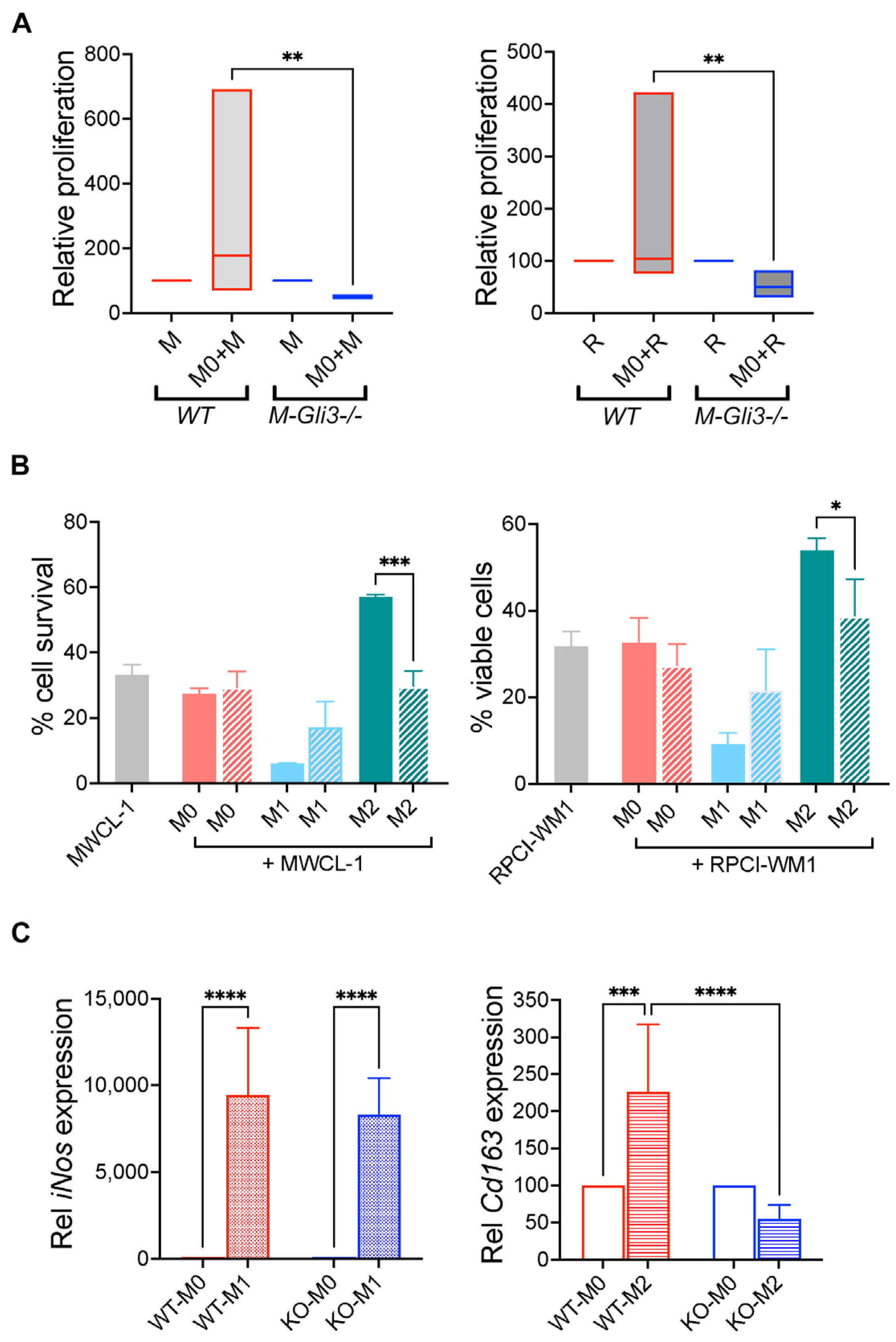
2.1. M0 and M2 Macrophages Increase WM Cell Growth and Survival
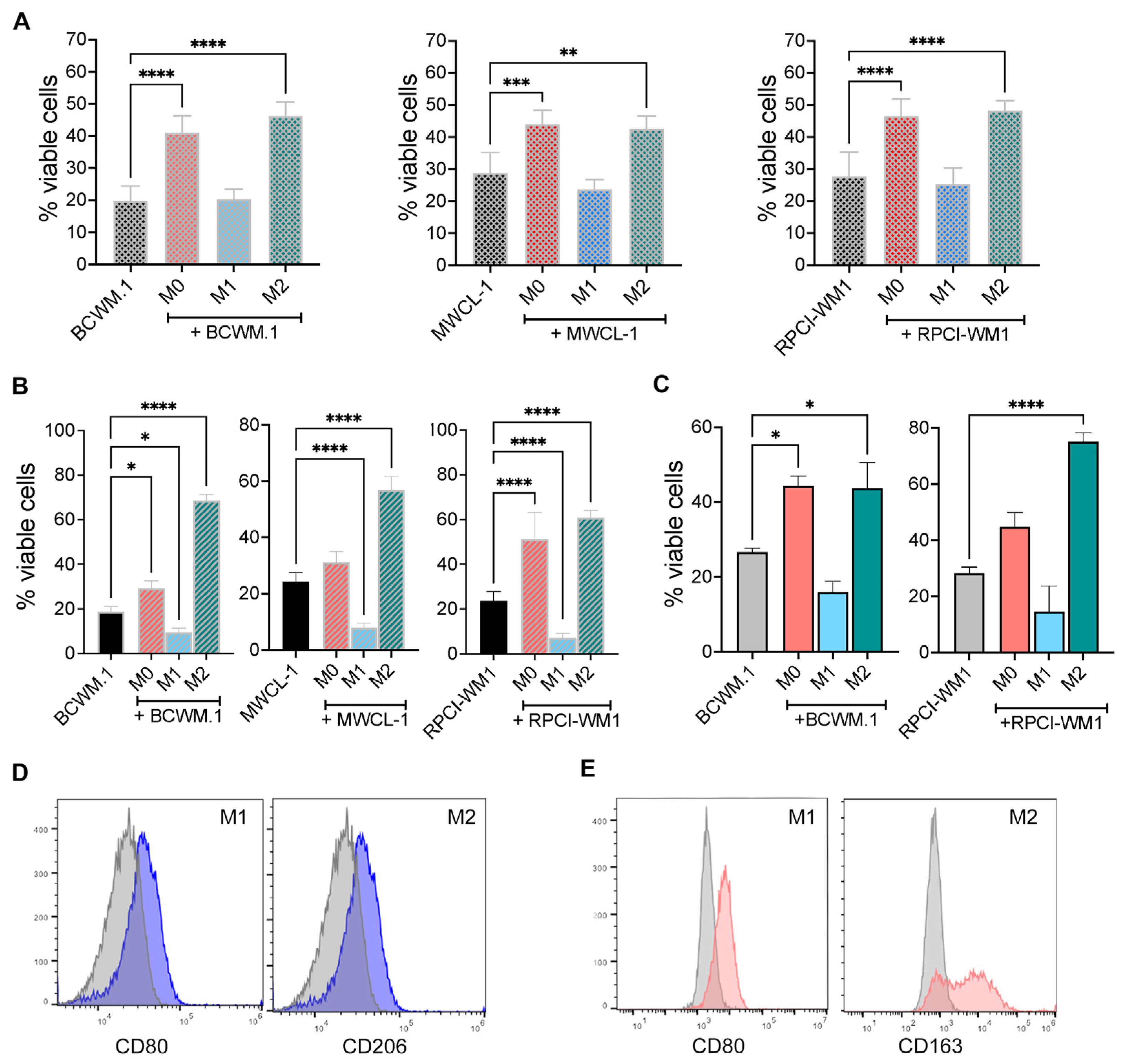
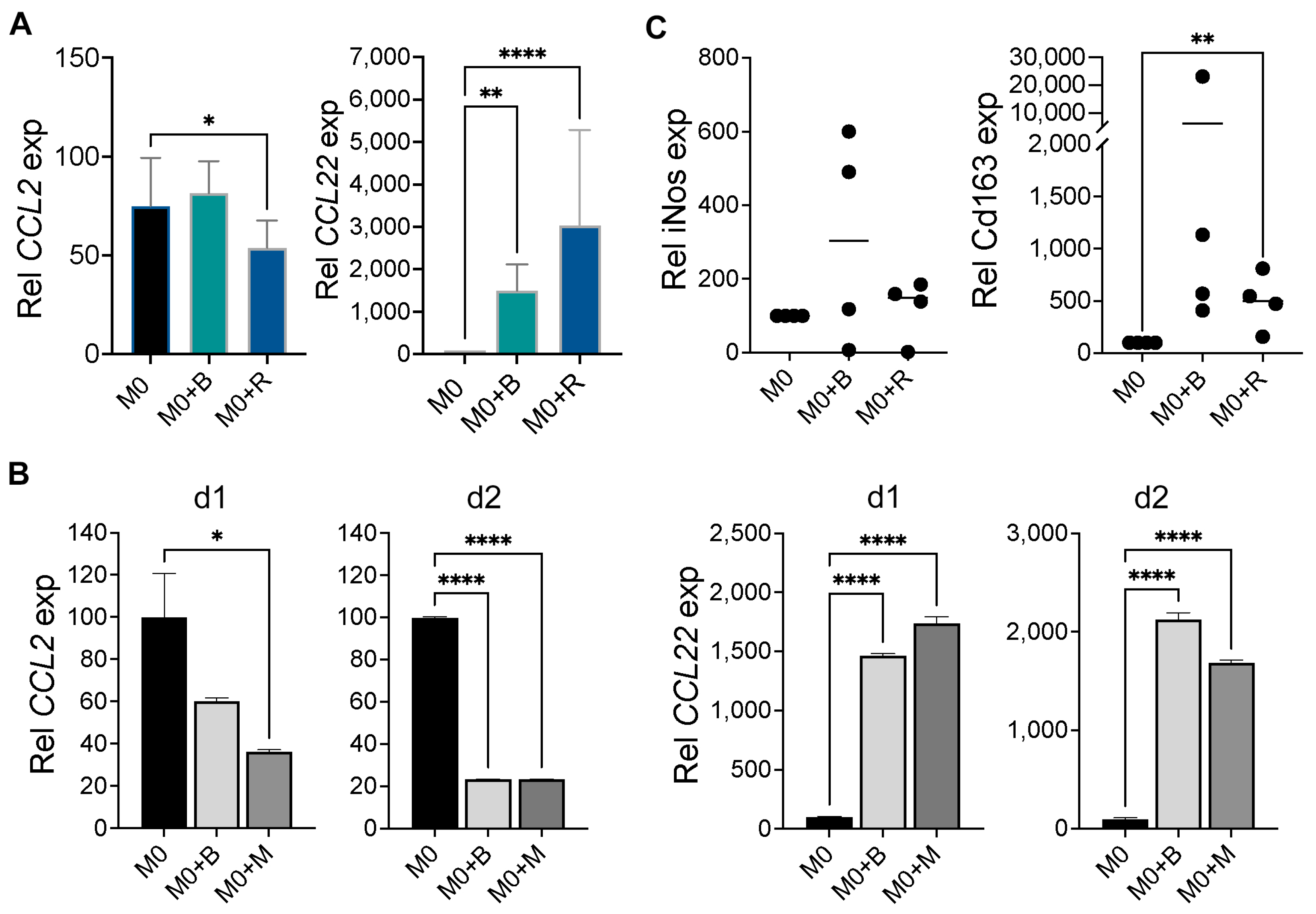
2.2. WM Cells Promote an Inhibitory M2 Macrophage Phenotype
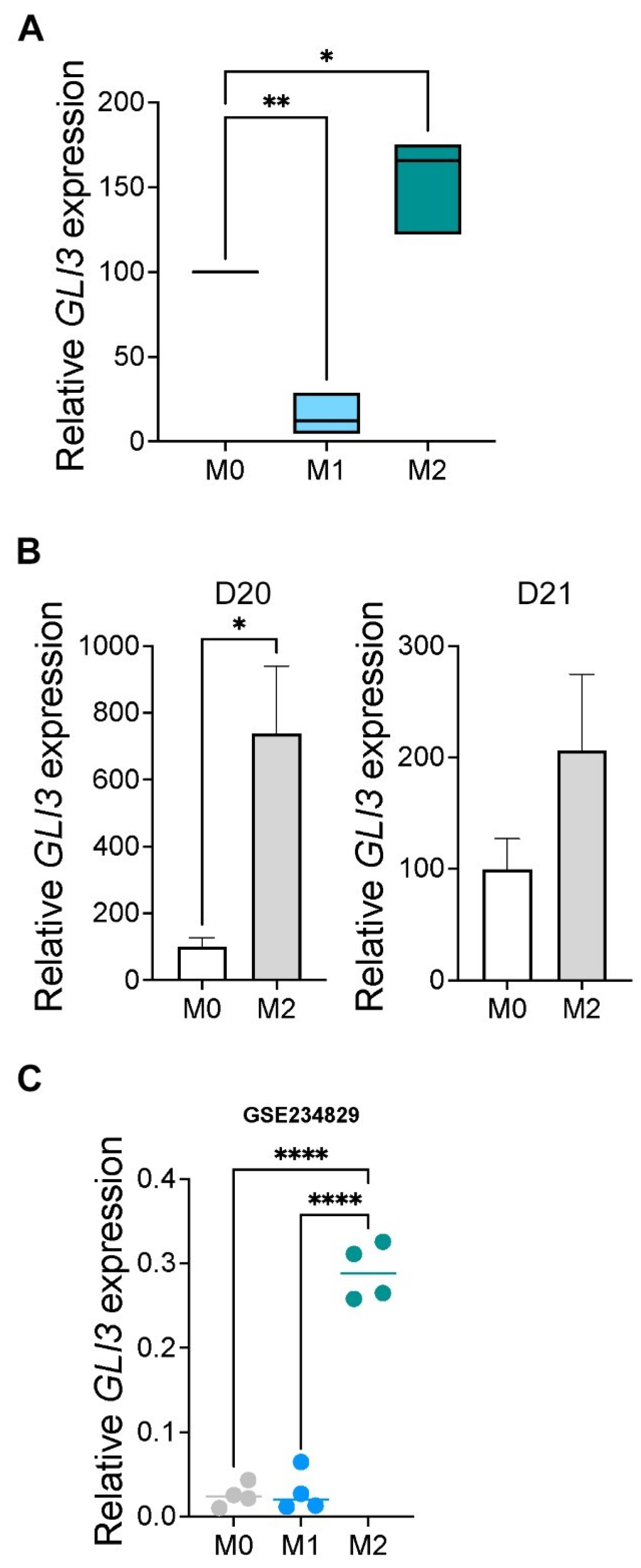
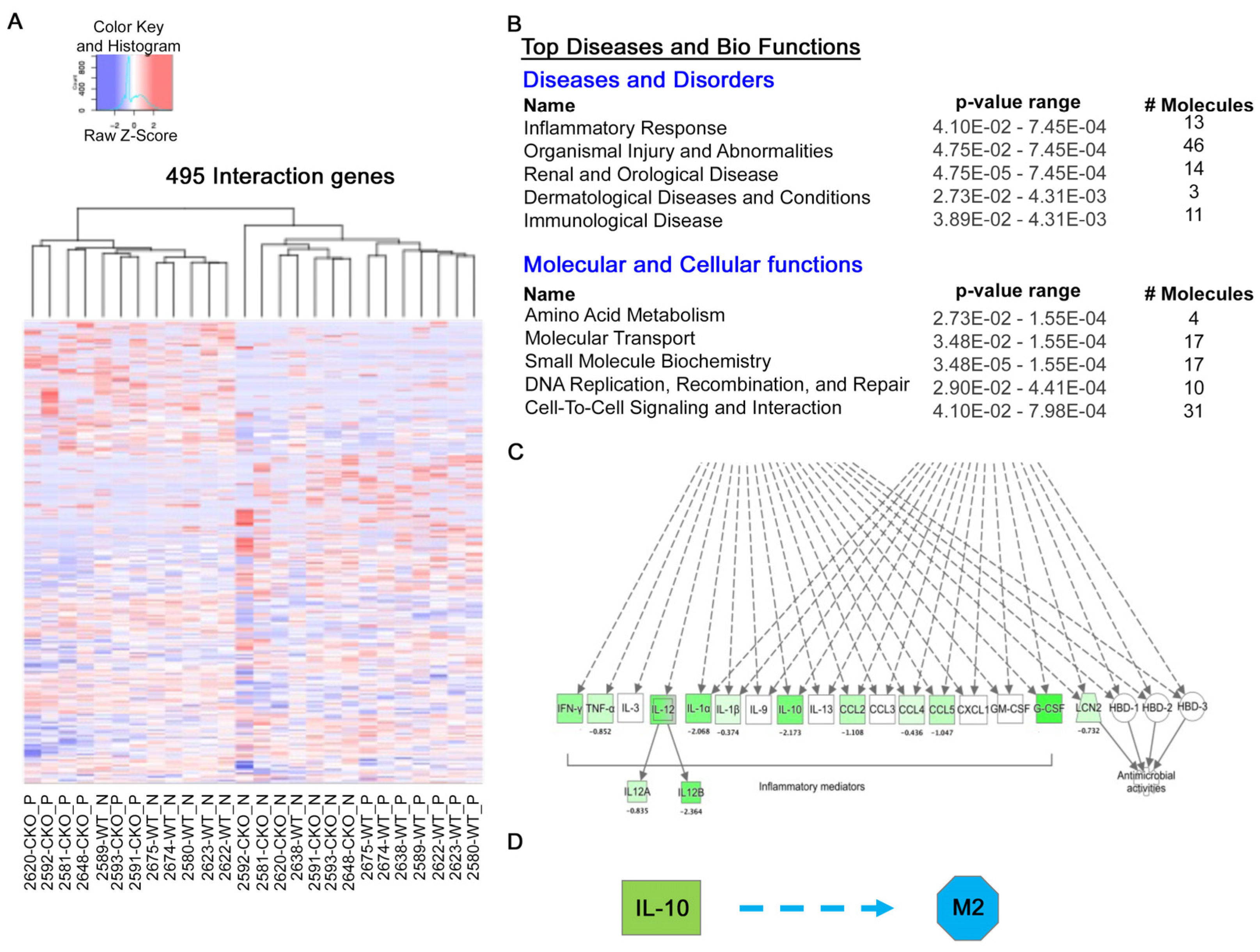
2.3. GLI3 Expression Is Induced in M2 Macrophages
2.4. GLI3 Promotes M2 Macrophage Polarization
2.5. GLI3 Is Required for Macrophage-Mediated Effects on WM Cells
3. Discussion
4. Materials and Methods
4.1. Cell Lines and Primary Cells
4.2. Mouse Bone Marrow-Derived Macrophages (BMDMs)
4.3. Macrophage Differentiation/Polarization
4.4. RNA Isolation and Reverse Transcription Quantitative PCR (RT-qPCR)
4.5. Assessment of Cell Proliferation
4.6. Assessment of Cell Viability
4.7. Flow Cytometry
4.8. RNA-Sequencing
4.9. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ansell, S.M.; Kyle, R.A.; Reeder, C.B.; Fonseca, R.; Mikhael, J.R.; Morice, W.G.; Bergsagel, P.L.; Buadi, F.K.; Colgan, J.P.; Dingli, D.; et al. Diagnosis and Management of Waldenström Macroglobulinemia: Mayo Stratification of Macroglobulinemia and Risk-Adapted Therapy (mSMART) Guidelines. Mayo Clin. Proc. 2010, 85, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Boutilier, A.J.; Huang, L.; Elsawa, S.F. Waldenström Macroglobulinemia: Mechanisms of Disease Progression and Current Therapies. Int. J. Mol. Sci. 2022, 23, 11145. [Google Scholar] [CrossRef] [PubMed]
- Owen, R.G.; Treon, S.P.; Al-Katib, A.; Fonseca, R.; Greipp, P.R.; McMaster, M.L.; Morra, E.; Pangalis, G.A.; San Miguel, J.F.; Branagan, A.R.; et al. Clinicopathological definition of Waldenstrom’s macroglobulinemia: Consensus panel recommendations from the Second International Workshop on Waldenstrom’s Macroglobulinemia. Semin. Oncol. 2003, 30, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Buske, C.; Leblond, V. How to manage Waldenstrom’s macroglobulinemia. Leukemia 2013, 27, 762–772. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood 2016, 127, 2375–2390. [Google Scholar] [CrossRef]
- Raedler, L.A. Imbruvica (Ibrutinib): First Drug Approved for the Treatment of Patients with Waldenström’s Macroglobulinemia. Am. Health Drug Benefits 2016, 9, 89–92. [Google Scholar]
- Kapoor, P.; Ansell, S.M.; Fonseca, R.; Chanan-Khan, A.; Kyle, R.A.; Kumar, S.K.; Mikhael, J.R.; Witzig, T.E.; Mauermann, M.; Dispenzieri, A.; et al. Diagnosis and Management of Waldenström Macroglobulinemia. JAMA Oncol. 2017, 3, 1257–1265. [Google Scholar] [CrossRef]
- Han, W.; Jackson, D.A.; Matissek, S.J.; Misurelli, J.A.; Neil, M.S.; Sklavanitis, B.; Amarsaikhan, N.; Elsawa, S.F. Novel Molecular Mechanism of Regulation of CD40 Ligand by the Transcription Factor GLI2. J. Immunol. 2017, 198, 4481–4489. [Google Scholar] [CrossRef]
- Elsawa, S.F.; Almada, L.L.; Ziesmer, S.C.; Novak, A.J.; Witzig, T.E.; Ansell, S.M.; Fernandez-Zapico, M.E. GLI2 transcription factor mediates cytokine cross-talk in the tumor microenvironment. J. Biol. Chem. 2011, 286, 21524–21534. [Google Scholar] [CrossRef]
- Elsawa, S.F.; Novak, A.J.; Grote, D.M.; Ziesmer, S.C.; Witzig, T.E.; Kyle, R.A.; Dillon, S.R.; Harder, B.; Gross, J.A.; Ansell, S.M. B-lymphocyte stimulator (BLyS) stimulates immunoglobulin production and malignant B-cell growth in Waldenstrom macroglobulinemia. Blood 2006, 107, 2882–2888. [Google Scholar] [CrossRef]
- Elsawa, S.F.; Novak, A.J.; Grote, D.M.; Zeismer, S.C.; Witzig, T.E.; Kyle, R.A.; Ansell, S.M. Role of B-Lymphocyte Stimulator (BLyS) in Waldenstrom’s Macroglobulinemia. Blood 2005, 106, 601. [Google Scholar] [CrossRef]
- Jalali, S.; Price-Troska, T.; Paludo, J.; Villasboas, J.; Kim, H.-J.; Yang, Z.-Z.; Novak, A.J.; Ansell, S.M. Soluble PD-1 ligands regulate T-cell function in Waldenstrom macroglobulinemia. Blood Adv. 2018, 2, 1985–1997. [Google Scholar] [CrossRef] [PubMed]
- Nguyen-Khac, F.; Lambert, J.; Chapiro, E.; Grelier, A.; Mould, S.; Barin, C.; Daudignon, A.; Gachard, N.; Struski, S.; Henry, C.; et al. Chromosomal aberrations and their prognostic value in a series of 174 untreated patients with Waldenstrom’s macroglobulinemia. Haematologica 2013, 98, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Roccaro, A.M.; Sacco, A.; Chen, C.; Runnels, J.; Leleu, X.; Azab, F.; Azab, A.K.; Jia, X.; Ngo, H.T.; Melhem, M.R.; et al. microRNA expression in the biology, prognosis, and therapy of Waldenstrom macroglobulinemia. Blood 2009, 113, 4391–4402. [Google Scholar] [CrossRef]
- Tournilhac, O.; Santos, D.D.; Xu, L.; Kutok, J.; Tai, Y.T.; Le Gouill, S.; Catley, L.; Hunter, Z.; Branagan, A.R.; Boyce, J.A.; et al. Mast cells in Waldenstrom’s macroglobulinemia support lymphoplasmacytic cell growth through CD154/CD40 signaling. Ann. Oncol. 2006, 17, 1275–1282. [Google Scholar] [CrossRef]
- Agarwal, A.; Ghobrial, I.M. The bone marrow microenvironment in Waldenstrom macroglobulinemia. Clin. Lymphoma Myeloma Leuk. 2013, 13, 218–221. [Google Scholar] [CrossRef]
- Boutilier, A.; Elsawa, S. Macrophage Polarization States in the Tumor Microenvironment. Int. J. Mol. Sci. 2021, 22, 6995. [Google Scholar] [CrossRef]
- Leblebjian, H.; Agarwal, A.; Ghobrial, I. Novel Treatment Options for Waldenström Macroglobulinemia. Clin. Lymphoma Myeloma Leuk. 2013, 13, S310–S316. [Google Scholar] [CrossRef][Green Version]
- Azab, F.; Azab, A.K.; Maiso, P.; Calimeri, T.; Flores, L.; Liu, Y.; Quang, P.; Roccaro, A.M.; Sacco, A.; Ngo, H.T.; et al. Eph-B2/Ephrin-B2 Interaction Plays a Major Role in the Adhesion and Proliferation of Waldenstrom’s Macroglobulinemia. Clin. Cancer Res. 2012, 18, 91–104. [Google Scholar] [CrossRef]
- Le, K.; Sun, J.; Khawaja, H.; Shibata, M.; Maggirwar, S.B.; Smith, M.R.; Gupta, M. Mantle cell lymphoma polarizes tumor-associated macrophages into M2-like macrophages, which in turn promote tumorigenesis. Blood Adv. 2021, 5, 2863–2878. [Google Scholar] [CrossRef]
- Le, K.; Sun, J.; Ghaemmaghami, J.; Smith, M.R.; Ip, W.K.E.; Phillips, T.J.; Gupta, M. Blockade of CCR1 induces a phenotypic shift in macrophages and triggers a favorable antilymphoma activity. Blood Adv. 2023, 7, 3952–3967. [Google Scholar] [CrossRef] [PubMed]
- Matissek, S.J.; Karbalivand, M.; Han, W.; Boutilier, A.; Yzar-Garcia, E.; Kehoe, L.L.; Gardner, D.S.; Hage, A.; Fleck, K.; Jeffers, V.; et al. A novel mechanism of regulation of the oncogenic transcription factor GLI3 by toll-like receptor signaling. Oncotarget 2022, 13, 944–959. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Babar, Q.; Saeed, A.; Tabish, T.A.; Sarwar, M.; Thorat, N.D. Targeting the tumor microenvironment: Potential strategy for cancer therapeutics. Biochim. Biophys. Acta Mol. Basis Dis. 2023, 1869, 166746. [Google Scholar] [CrossRef] [PubMed]
- Baghban, R.; Roshangar, L.; Jahanban-Esfahlan, R.; Seidi, K.; Ebrahimi-Kalan, A.; Jaymand, M.; Kolahian, S.; Javaheri, T.; Zare, P. Tumor microenvironment complexity and therapeutic implications at a glance. Cell Commun. Signal. 2020, 18, 59. [Google Scholar] [CrossRef]
- Feig, C.; Gopinathan, A.; Neesse, A.; Chan, D.S.; Cook, N.; Tuveson, D.A. The pancreas cancer microenvironment. Clin. Cancer Res. 2012, 18, 4266–4276. [Google Scholar] [CrossRef]
- Hanahan, D.; Coussens, L.M. Accessories to the crime: Functions of cells recruited to the tumor microenvironment. Cancer Cell 2012, 21, 309–322. [Google Scholar] [CrossRef]
- Simsek, H.; Klotzsch, E. The solid tumor microenvironment-Breaking the barrier for T cells: How the solid tumor microenvironment influences T cells: How the solid tumor microenvironment influences T cells. Bioessays 2022, 44, e2100285. [Google Scholar] [CrossRef]
- Jalali, S.; Ansell, S.M. Bone marrow microenvironment in Waldenstrom’s Macroglobulinemia. Best Pract. Res. Clin. Haematol. 2016, 29, 148–155. [Google Scholar] [CrossRef]
- Jalali, S.; Ansell, S.M. The Bone Marrow Microenvironment in Waldenstrom Macroglobulinemia. Hematol. Oncol. Clin. N. Am. 2018, 32, 777–786. [Google Scholar] [CrossRef]
- Santos, D.D.; Hatjiharissi, E.; Tournilhac, O.; Chemaly, M.Z.; Leleu, X.; Xu, L.; Patterson, C.; Branagan, A.R.; Manning, R.J.; Ho, A.W.; et al. CD52 is expressed on human mast cells and is a potential therapeutic target in Waldenstrom’s Macroglobulinemia and mast cell disorders. Clin. Lymphoma Myeloma 2006, 6, 478–483. [Google Scholar] [CrossRef] [PubMed]
- Jalali, S.; Ansell, S.M. Role of the Bone Marrow Niche in Supporting the Pathogenesis of Lymphoid Malignancies. Front. Cell Dev. Biol. 2021, 9, 692320. [Google Scholar] [CrossRef] [PubMed]
- Tabata, R.; Yasumizu, R.; Tabata, C.; Kojima, M. Bone marrow macrophages in Waldenstrom’s macroglobulinemia: A report of four cases. J. Clin. Exp. Hematop. 2014, 54, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Allavena, P.; Sica, A.; Solinas, G.; Porta, C.; Mantovani, A. The inflammatory micro-environment in tumor progression: The role of tumor-associated macrophages. Crit. Rev. Oncol. Hematol. 2008, 66, 1–9. [Google Scholar] [CrossRef]
- Ma, J.; Liu, L.; Che, G.; Yu, N.; Dai, F.; You, Z. The M1 form of tumor-associated macrophages in non-small cell lung cancer is positively associated with survival time. BMC Cancer 2010, 10, 112. [Google Scholar] [CrossRef]
- Jackute, J.; Zemaitis, M.; Pranys, D.; Sitkauskiene, B.; Miliauskas, S.; Vaitkiene, S.; Sakalauskas, R. Distribution of M1 and M2 macrophages in tumor islets and stroma in relation to prognosis of non-small cell lung cancer. BMC Immunol. 2018, 19, 3. [Google Scholar] [CrossRef]
- Jackute, J.; Zemaitis, M.; Pranys, D.; Sitkauskiene, B.; Miliauskas, S.; Vaitkiene, S.; Sakalauskas, R. A high M1/M2 ratio of tumor-associated macrophages is associated with extended survival in ovarian cancer patients. J. Ovarian Res. 2014, 7, 19. [Google Scholar]
- Alves, A.M.; Diel, L.F.; Lamers, M.L. Macrophages and prognosis of oral squamous cell carcinoma: A systematic review. J. Oral Pathol. Med. 2018, 47, 460–467. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage plasticity and polarization: In vivo veritas. J. Clin. Invest. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Yeung, O.W.; Lo, C.-M.; Ling, C.-C.; Qi, X.; Geng, W.; Li, C.-X.; Ng, K.T.; Forbes, S.J.; Guan, X.-Y.; Poon, R.T.; et al. Alternatively activated (M2) macrophages promote tumour growth and invasiveness in hepatocellular carcinoma. J. Hepatol. 2015, 62, 607–616. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Sozzani, S.; Locati, M.; Allavena, P.; Sica, A. Macrophage polarization: Tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002, 23, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Park, C.; Guenthner, N.; Gurley, S.; Zhang, L.; Lubben, B.; Adebayo, O.; Bash, H.; Chen, Y.; Maksimos, M.; et al. Tumor-associated macrophages in multiple myeloma: Advances in biology and therapy. J. Immunother Cancer 2022, 10, e003975. [Google Scholar] [CrossRef] [PubMed]
- Chitta, K.S.; Paulus, A.; Ailawadhi, S.; Foster, B.A.; Moser, M.T.; Starostik, P.; Masood, A.; Sher, T.; Miller, K.C.; Iancu, D.M.; et al. Development and characterization of a novel human Waldenstrom macroglobulinemia cell line: RPCI-WM1, Roswell Park Cancer Institute—Waldenstrom Macroglobulinemia 1. Leuk. Lymphoma 2013, 54, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Santos, D.D.; Ho, A.W.; Tournilhac, O.; Hatjiharissi, E.; Leleu, X.; Xu, L.; Tassone, P.; Neri, P.; Hunter, Z.R.; Chemaly, M.A.; et al. Establishment of BCWM.1 cell line for Waldenstrom’s macroglobulinemia with productive in vivo engraftment in SCID-hu mice. Exp. Hematol. 2007, 35, 1366–1375. [Google Scholar] [CrossRef]
- Hodge, L.S.; Novak, A.J.; Grote, D.M.; Braggio, E.; Ketterling, R.P.; Manske, M.K.; Troska, T.L.P.; Ziesmer, S.C.; Fonseca, R.; Witzig, T.E.; et al. Establishment and characterization of a novel Waldenstrom macroglobulinemia cell line, MWCL-1. Blood 2011, 117, e190–e197. [Google Scholar] [CrossRef]
- Jackson, D.A.; Smith, T.D.; Amarsaikhan, N.; Han, W.; Neil, M.S.; Boi, S.K.; Vrabel, A.M.; Tolosa, E.J.; Almada, L.L.; Fernandez-Zapico, M.E.; et al. Modulation of the IL-6 Receptor alpha Underlies GLI2-Mediated Regulation of Ig Secretion in Waldenstrom Macroglobulinemia Cells. J. Immunol. 2015, 195, 2908–2916. [Google Scholar] [CrossRef]
- Karbalivand, M.; Almada, L.L.; Ansell, S.M.; Fernandez-Zapico, M.E.; Elsawa, S.F. MLL1 inhibition reduces IgM levels in Waldenstrom macroglobulinemia. Leuk. Res. 2022, 116, 106841. [Google Scholar] [CrossRef]
- Andrews, S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2010. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/ (accessed on 22 October 2024).
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020. [Google Scholar]
| Target | Species | Sense (5′-3′) | Antisense (5′-3′) |
|---|---|---|---|
| CD206 | Human | CTACAAGGGATCGGGTTTATGGA | TTGGCATTGCCTAGTAGCGTA |
| CCL2 | Human | GCCACCTTCATTCCCCAAGGG | GCTTCTTTGGGACACTTGCTGC |
| CCL22 | Human | ATTACGTCCGTTACCGTCTG | TAGGCTCTTCATTGGCTCAG |
| IL-1b | Human | GGACAGGATATGGAGCAACAA | CCCAAGGCCACAGGTATTT |
| GAPDH | Human | CTCGACTTCAACAGCGACA | GTAGCCAAATTCGTTGTCATACC |
| Cd163 | Mouse | GCAAAAACTGGCAGTGGG | GTCAAAATCACAGACGGAG |
| Arg2 | Mouse | GAAGTGGTTAGTAGAGCTGTGTC | GGTGAGAGGTGTATTAATGTCCG |
| Tnf-a | Mouse | CTTCTGTCTACTGAACTTCGGG | CACTTGGTGGTTTGCTACGAC |
| iNos | Mouse | CAGCACAGGAAATGTTTCAGC | TAGCCAGCGTACCGGATGA |
| Gapdh | Mouse | CGTCCCGTAGACAAAATGGT | TTGATGGCAACAATCTCCAC |
| GLI3 | Human | AGGGTGAATGGTATCAAGATGG | CCCACGGTTTGGTCATAGAA |
| Gli3 | Mouse | CACTGGTGACCCTATGCATAATA | CTTGACTAGGGTTGTTCCTTCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boutilier, A.J.; Raad, M.; Paar, K.E.; Matissek, S.J.; Banks, C.E.; Carl, A.L.; Murray, J.M.; Metzler, A.D.; Koeppen, K.U.; Gupta, M.; et al. GLI3 Is Required for M2 Macrophage Polarization and M2-Mediated Waldenström Macroglobulinemia Growth and Survival. Int. J. Mol. Sci. 2024, 25, 13120. https://doi.org/10.3390/ijms252313120
Boutilier AJ, Raad M, Paar KE, Matissek SJ, Banks CE, Carl AL, Murray JM, Metzler AD, Koeppen KU, Gupta M, et al. GLI3 Is Required for M2 Macrophage Polarization and M2-Mediated Waldenström Macroglobulinemia Growth and Survival. International Journal of Molecular Sciences. 2024; 25(23):13120. https://doi.org/10.3390/ijms252313120
Chicago/Turabian StyleBoutilier, Ava J., Mohammad Raad, Kailey E. Paar, Stephan J. Matissek, Cameron E. Banks, Allison L. Carl, Jenna M. Murray, Anna D. Metzler, Katja U. Koeppen, Mamta Gupta, and et al. 2024. "GLI3 Is Required for M2 Macrophage Polarization and M2-Mediated Waldenström Macroglobulinemia Growth and Survival" International Journal of Molecular Sciences 25, no. 23: 13120. https://doi.org/10.3390/ijms252313120
APA StyleBoutilier, A. J., Raad, M., Paar, K. E., Matissek, S. J., Banks, C. E., Carl, A. L., Murray, J. M., Metzler, A. D., Koeppen, K. U., Gupta, M., & Elsawa, S. F. (2024). GLI3 Is Required for M2 Macrophage Polarization and M2-Mediated Waldenström Macroglobulinemia Growth and Survival. International Journal of Molecular Sciences, 25(23), 13120. https://doi.org/10.3390/ijms252313120





