The mRNA-Binding Protein KSRP Limits the Inflammatory Response of Macrophages
Abstract
1. Introduction
2. Results
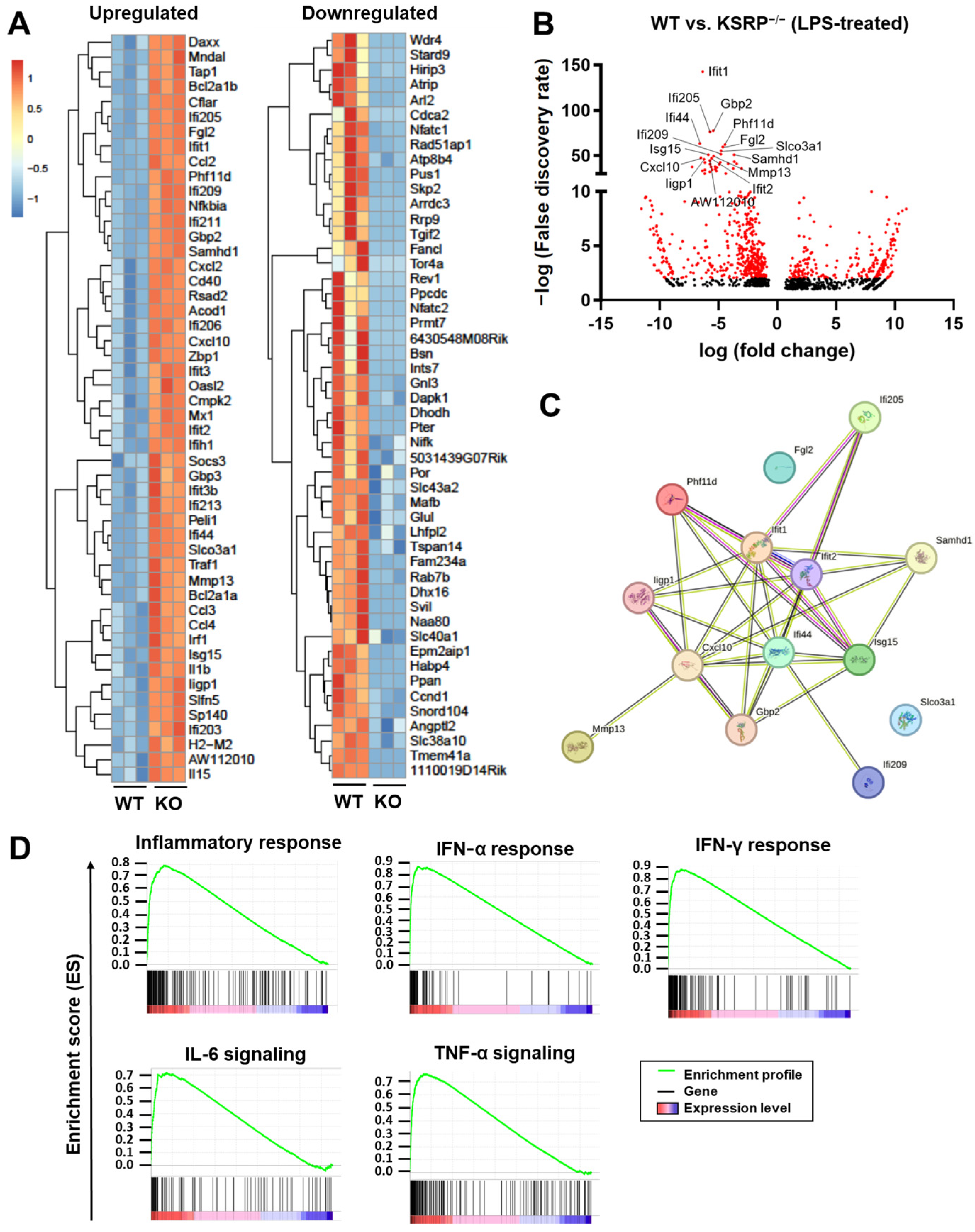
2.1. KSRP−/− Mice Displayed Higher mRNA Expression Levels of Stimulation-Induced Cytokines
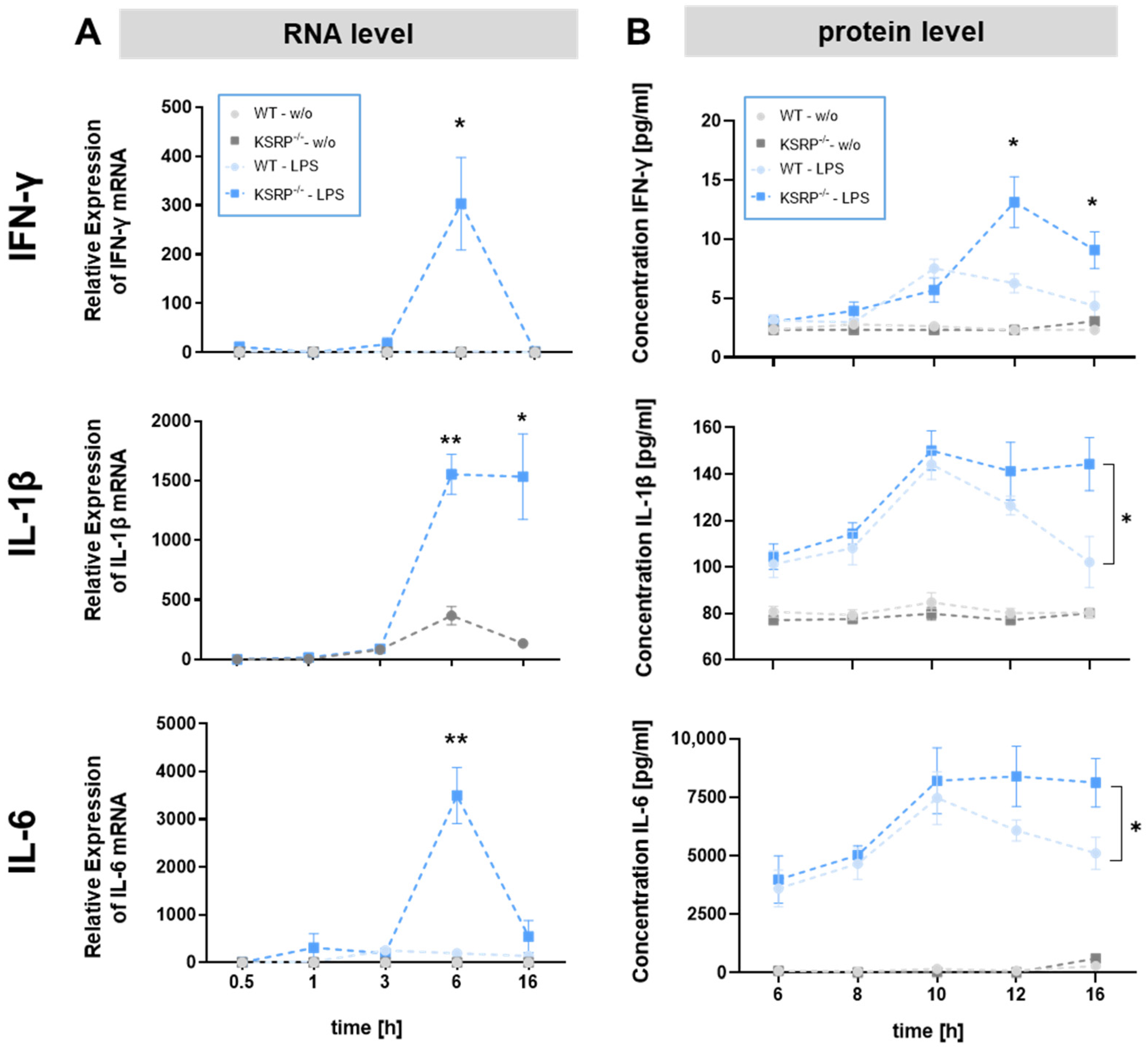
2.2. KSRP−/− Mice Show Higher Protein Expression of IFN-γ, IL-1β and IL-6 in Response to Stimulation
2.3. KSRP Binds Directly to IL-1β mRNA
2.4. KSRP−/− Primary Macrophages from the Peritoneum Produce Higher Levels of Pro-Inflammatory Cytokines in Response to Stimulation
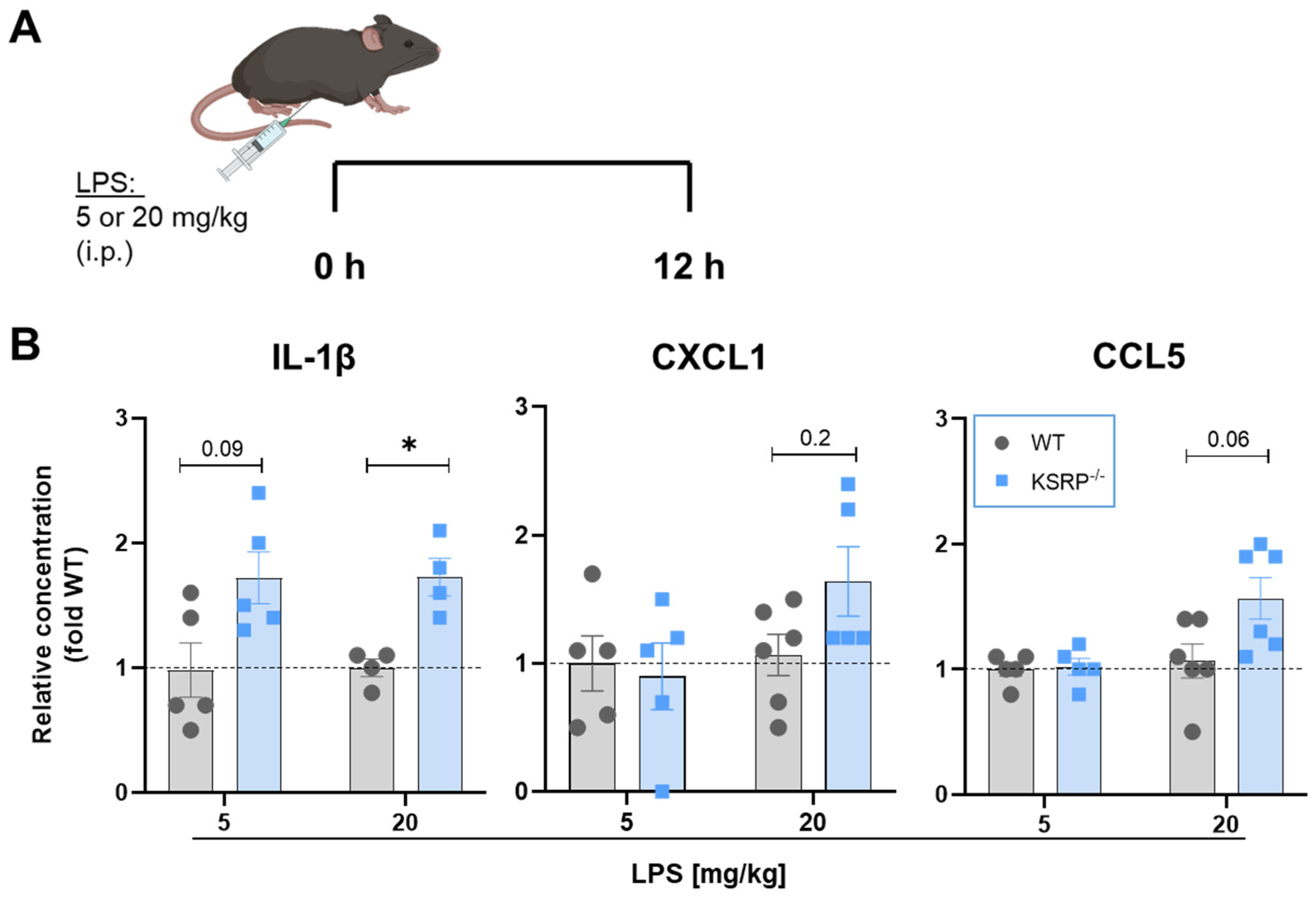
2.5. KSRP−/− Display Tendencies to Higher Proinflammatory Cytokine Production in LPS-Induced Sepsis
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Bone Marrow-Derived Macrophages (BMDM)
4.3. Isolation of Peritoenal Cells
4.4. Cytometric Bead Array
4.5. Immunoprecipitation-qRT-PCR Assay
4.6. Analysis of mRNA Expression in Cells or Tissues of KSRP−/− or WT Animals
4.7. RNA-Sequencing and Bioinformatical Analysis
4.8. Real-Time PCR
4.9. The LPS-Induced Sepsis Model
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Lendeckel, U.; Venz, S.; Wolke, C. Macrophages: Shapes and functions. Chemtexts 2022, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in Inflammatory Disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed]
- Hirayama, D.; Iida, T.; Nakase, H. The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis. Int. J. Mol. Sci. 2018, 19, 92. [Google Scholar] [CrossRef]
- Rinn, J.L.; Chang, H.Y. Genome Regulation by Long Noncoding RNAs. Annu. Rev. Biochem. 2012, 81, 145–166. [Google Scholar] [CrossRef] [PubMed]
- García-Mauriño, S.M.; Rivero-Rodríguez, F.; Velázquez-Cruz, A.; Hernández-Vellisca, M.; Díaz-Quintana, A.; De la Rosa, M.A.; Díaz-Moreno, I. RNA Binding Protein Regulation and Cross-Talk in the Control of AU-rich mRNA Fate. Front. Mol. Biosci. 2017, 4, 71. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 388354. [Google Scholar] [CrossRef] [PubMed]
- Glisovic, T.; Bachorik, J.L.; Yong, J.; Dreyfuss, G. RNA-binding proteins and post-transcriptional gene regulation. FEBS Lett. 2008, 582, 1977–1986. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Maeda, K. Control of RNA Stability in Immunity. Annu. Rev. Immunol. 2021, 39, 481–509. [Google Scholar] [CrossRef] [PubMed]
- Beiter, T.; Hoene, M.; Prenzler, F.; Mooren, F.C.; Steinacker, J.M.; Weigert, C.; Nieß, A.M.; Munz, B. Exercise, skeletal muscle and inflammation: ARE-binding proteins as key regulators in inflammatory and adaptive networks. Exerc. Immunol. Rev. 2015, 21, 42–57. [Google Scholar]
- Zubiaga, A.M.; Belasco, J.G.; Greenberg, M.E. The nonamer UUAUUUAUU is the key AU-rich sequence motif that mediates mRNA degradation. Mol. Cell. Biol. 1995, 15, 2219–2230. [Google Scholar] [CrossRef]
- Khabar, K.S.A. Post-transcriptional control during chronic inflammation and cancer: A focus on AU-rich elements. Cell. Mol. Life Sci. CMLS 2010, 67, 2937–2955. [Google Scholar] [CrossRef] [PubMed]
- Palzer, K.-A.; Bolduan, V.; Käfer, R.; Kleinert, H.; Bros, M.; Pautz, A. The Role of KH-Type Splicing Regulatory Protein (KSRP) for Immune Functions and Tumorigenesis. Cells 2022, 11, 1482. [Google Scholar] [CrossRef] [PubMed]
- Käfer, R.; Schrick, K.; Schmidtke, L.; Montermann, E.; Hobernik, D.; Bros, M.; Chen, C.-Y.; Kleinert, H.; Pautz, A. Inactivation of the KSRP gene modifies collagen antibody induced arthritis. Mol. Immunol. 2017, 87, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Schmidtke, L.; Meineck, M.; Saurin, S.; Otten, S.; Gather, F.; Schrick, K.; Käfer, R.; Roth, W.; Kleinert, H.; Weinmann-Menke, J.; et al. Knockout of the KH-Type Splicing Regulatory Protein Drives Glomerulonephritis in MRL-Faslpr Mice. Cells 2021, 10, 3167. [Google Scholar] [CrossRef] [PubMed]
- Briata, P.; Chen, C.-Y.; Ramos, A.; Gherzi, R. Functional and molecular insights into KSRP function in mRNA decay. Acta Biochim. Biophys. 2013, 1829, 689–694. [Google Scholar] [CrossRef]
- Lin, W.J.; Zheng, X.; Lin, C.C.; Tsao, J.; Zhu, X.; Cody, J.J.; Coleman, J.M.; Gherzi, R.; Luo, M.; Townes, T.M.; et al. Posttranscriptional control of type I interferon genes by KSRP in the innate immune response against viral infection. Mol. Cell Biol. 2011, 31, 3196–3207. [Google Scholar] [CrossRef] [PubMed]
- Linker, K.; Pautz, A.; Fechir, M.; Hubrich, T.; Greeve, J.; Kleinert, H. Involvement of KSRP in the post-transcriptional regulation of human iNOS expression-complex interplay of KSRP with TTP and HuR. Nucleic Acids Res. 2005, 33, 4813–4827. [Google Scholar] [CrossRef]
- Bollmann, F.; Art, J.; Henke, J.; Schrick, K.; Besche, V.; Bros, M.; Li, H.; Siuda, D.; Handler, N.; Bauer, F.; et al. Resveratrol post-transcriptionally regulates pro-inflammatory gene expression via regulation of KSRP RNA binding activity. Nucleic Acids Res. 2014, 42, 12555–12569. [Google Scholar] [CrossRef]
- Schmidtke, L.; Schrick, K.; Saurin, S.; Käfer, R.; Gather, F.; Weinmann-Menke, J.; Kleinert, H.; Pautz, A. The KH-type splicing regulatory protein (KSRP) regulates type III interferon expression post-transcriptionally. Biochem. J. 2019, 476, 333–352. [Google Scholar] [CrossRef]
- Kafer, R.; Schmidtke, L.; Schrick, K.; Montermann, E.; Bros, M.; Kleinert, H.; Pautz, A. The RNA-Binding Protein KSRP Modulates Cytokine Expression of CD4(+) T Cells. J. Immunol. Res. 2019, 2019, 4726532. [Google Scholar] [CrossRef]
- Yang, S.; Adaway, M.; Du, J.; Huang, S.; Sun, J.; Bidwell, J.P.; Zhou, B. NMP4 regulates the innate immune response to influenza A virus infection. Mucosal Immunol. 2021, 14, 209–218. [Google Scholar] [CrossRef]
- Kim, M.-Y.; Koh, D.-I.; Choi, W.-I.; Jeon, B.-N.; Jeong, D.-y.; Kim, K.-S.; Kim, K.; Kim, S.-H.; Hur, M.-W. ZBTB2 increases PDK4 expression by transcriptional repression of RelA/p65. Nucleic Acids Res. 2015, 43, 1609–1625. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Lee, J.-O. Recognition of lipopolysaccharide pattern by TLR4 complexes. Exp. Mol. Med. 2013, 45, e66. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.-Y.; Sadri, N.; Schneider, R.J. Endotoxic shock in AUF1 knockout mice mediated by failure to degrade proinflammatory cytokine mRNAs. Genes Dev. 2006, 20, 3174–3184. [Google Scholar] [CrossRef]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Kharraz, Y.; Lefort, A.; Libert, F.; Mann, C.J.; Gueydan, C.; Kruys, V. Genome-wide analysis of TIAR RNA ligands in mouse macrophages before and after LPS stimulation. Genom. Data 2016, 7, 297–300. [Google Scholar] [CrossRef] [PubMed]
- Kratochvill, F.; Machacek, C.; Vogl, C.; Ebner, F.; Sedlyarov, V.; Gruber, A.R.; Hartweger, H.; Vielnascher, R.; Karaghiosoff, M.; Rülicke, T.; et al. Tristetraprolin-driven regulatory circuit controls quality and timing of mRNA decay in inflammation. Mol. Syst. Biol. 2011, 7, 560. [Google Scholar] [CrossRef] [PubMed]
- Sedlyarov, V.; Fallmann, J.; Ebner, F.; Huemer, J.; Sneezum, L.; Ivin, M.; Kreiner, K.; Tanzer, A.; Vogl, C.; Hofacker, I.; et al. Tristetraprolin binding site atlas in the macrophage transcriptome reveals a switch for inflammation resolution. Mol. Syst. Biol. 2016, 12, 868. [Google Scholar] [CrossRef] [PubMed]
- Stoecklin, G.; Tenenbaum, S.A.; Mayo, T.; Chittur, S.V.; George, A.D.; Baroni, T.E.; Blackshear, P.J.; Anderson, P. Genome-wide analysis identifies interleukin-10 mRNA as target of tristetraprolin. J. Biol. Chem. 2008, 283, 11689–11699. [Google Scholar] [CrossRef]
- Gherzi, R.; Chen, C.-Y.; Ramos, A.; Briata, P. KSRP controls pleiotropic cellular functions. Semin. Cell Dev. Biol. 2014, 34, 2–8. [Google Scholar] [CrossRef]
- Dhamija, S.; Kuehne, N.; Winzen, R.; Doerrie, A.; Dittrich-Breiholz, O.; Thakur, B.K.; Kracht, M.; Holtmann, H. Interleukin-1 activates synthesis of interleukin-6 by interfering with a KH-type splicing regulatory protein (KSRP)-dependent translational silencing mechanism. J. Biol. Chem. 2011, 286, 33279–33288. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Kumar, P.; Tsuchiya, M.; Bhattacharyya, A.; Biswas, R. Regulation of miR-155 biogenesis in cystic fibrosis lung epithelial cells: Antagonistic role of two mRNA-destabilizing proteins, KSRP and TTP. Biochem. Biophys. Res. Commun. 2013, 433, 484–488. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, T.; Trabucchi, M.; De Santa, F.; Zupo, S.; Harfe, B.D.; McManus, M.T.; Rosenfeld, M.G.; Briata, P.; Gherzi, R. LPS induces KH-type splicing regulatory protein-dependent processing of microRNA-155 precursors in macrophages. FASEB J. 2009, 23, 2898–2908. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lin, W.-J.; Chen, C.-Y.; Si, Y.; Zhang, X.; Lu, L.; Suswam, E.; Zheng, L.; King, P.H. KSRP: A checkpoint for inflammatory cytokine production in astrocytes. Glia 2012, 60, 1773–1784. [Google Scholar] [CrossRef] [PubMed]
- Winzen, R.; Thakur, B.K.; Dittrich-Breiholz, O.; Shah, M.; Redich, N.; Dhamija, S.; Kracht, M.; Holtmann, H. Functional analysis of KSRP interaction with the AU-rich element of interleukin-8 and identification of inflammatory mRNA targets. Mol. Cell Biol. 2007, 27, 8388–8400. [Google Scholar] [CrossRef] [PubMed]
- Trabucchi, M.; Briata, P.; Garcia-Mayoral, M.; Haase, A.D.; Filipowicz, W.; Ramos, A.; Gherzi, R.; Rosenfeld, M.G. The RNA-binding protein KSRP promotes the biogenesis of a subset of microRNAs. Nature 2009, 459, 1010–1014. [Google Scholar] [CrossRef] [PubMed]
- Gulei, D.; Raduly, L.; Broseghini, E.; Ferracin, M.; Berindan-Neagoe, I. The extensive role of miR-155 in malignant and non-malignant diseases. Mol. Asp. Med. 2019, 70, 33–56. [Google Scholar] [CrossRef] [PubMed]
- Yazarlou, F.; Kadkhoda, S.; Ghafouri-Fard, S. Emerging role of let-7 family in the pathogenesis of hematological malignancies. Biomed. Pharmacother. 2021, 144, 112334. [Google Scholar] [CrossRef]
- Deng, B.; Tang, X.; Wang, Y. Role of microRNA-129 in cancer and non-cancerous diseases (Review). Exp. Ther. Med. 2021, 22, 918. [Google Scholar] [CrossRef]
- Jiang, S.; Yan, W.; Wang, S.E.; Baltimore, D. Dual mechanisms of posttranscriptional regulation of Tet2 by Let-7 microRNA in macrophages. Proc. Natl. Acad. Sci. USA 2019, 116, 12416–12421. [Google Scholar] [CrossRef]
- Jarczak, D.; Kluge, S.; Nierhaus, A. Sepsis-Pathophysiology and Therapeutic Concepts. Front. Med. 2021, 8, 628302. [Google Scholar] [CrossRef]
- Anderson, P. Post-transcriptional regulons coordinate the initiation and resolution of inflammation. Nat. Rev. Immunol. 2010, 10, 24–35. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2014, 30, 923–930. [Google Scholar] [CrossRef]
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S.; Ellis, B.; Gautier, L.; Ge, Y.; Gentry, J.; et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004, 5, R80. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Mootha, V.K.; Lindgren, C.M.; Eriksson, K.F.; Subramanian, A.; Sihag, S.; Lehar, J.; Puigserver, P.; Carlsson, E.; Ridderstråle, M.; Laurila, E.; et al. PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat. Genet. 2003, 34, 267–273. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bolduan, V.; Palzer, K.-A.; Hieber, C.; Schunke, J.; Fichter, M.; Schneider, P.; Grabbe, S.; Pautz, A.; Bros, M. The mRNA-Binding Protein KSRP Limits the Inflammatory Response of Macrophages. Int. J. Mol. Sci. 2024, 25, 3884. https://doi.org/10.3390/ijms25073884
Bolduan V, Palzer K-A, Hieber C, Schunke J, Fichter M, Schneider P, Grabbe S, Pautz A, Bros M. The mRNA-Binding Protein KSRP Limits the Inflammatory Response of Macrophages. International Journal of Molecular Sciences. 2024; 25(7):3884. https://doi.org/10.3390/ijms25073884
Chicago/Turabian StyleBolduan, Vanessa, Kim-Alicia Palzer, Christoph Hieber, Jenny Schunke, Michael Fichter, Paul Schneider, Stephan Grabbe, Andrea Pautz, and Matthias Bros. 2024. "The mRNA-Binding Protein KSRP Limits the Inflammatory Response of Macrophages" International Journal of Molecular Sciences 25, no. 7: 3884. https://doi.org/10.3390/ijms25073884
APA StyleBolduan, V., Palzer, K.-A., Hieber, C., Schunke, J., Fichter, M., Schneider, P., Grabbe, S., Pautz, A., & Bros, M. (2024). The mRNA-Binding Protein KSRP Limits the Inflammatory Response of Macrophages. International Journal of Molecular Sciences, 25(7), 3884. https://doi.org/10.3390/ijms25073884






