The Phytochemical Profile of the Petroleum Ether Extract of Purslane Leaves and Its Anticancer Effect on 4-(Methylnitrosamino)-1-(3-pyridyl)-1-buta-4 None (NNK)-Induced Lung Cancer in Rats
Abstract
1. Introduction
2. Results and Discussion
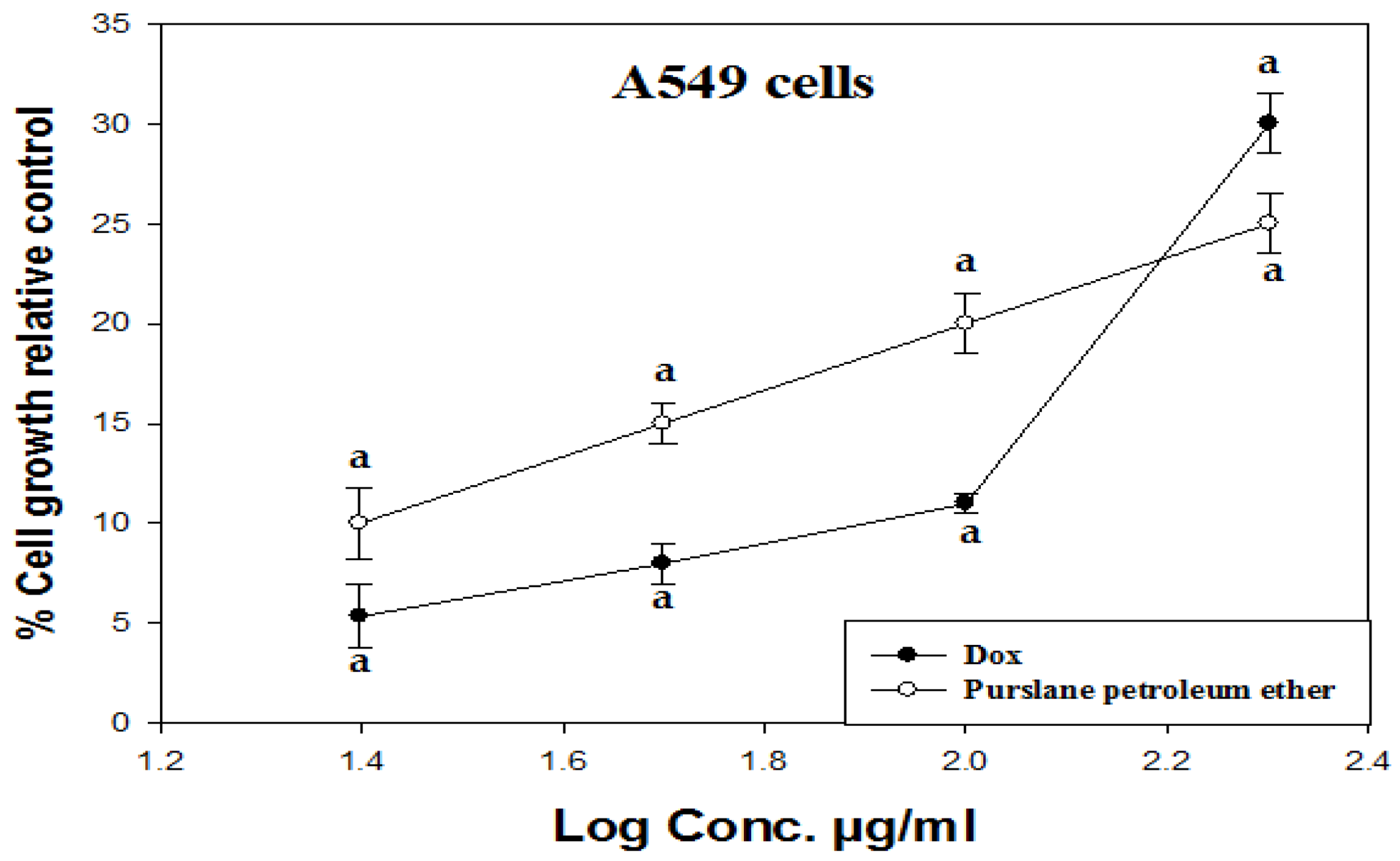
2.1. The Study on the A549 Lung Cancer Cell Line (In Vitro Investigation)
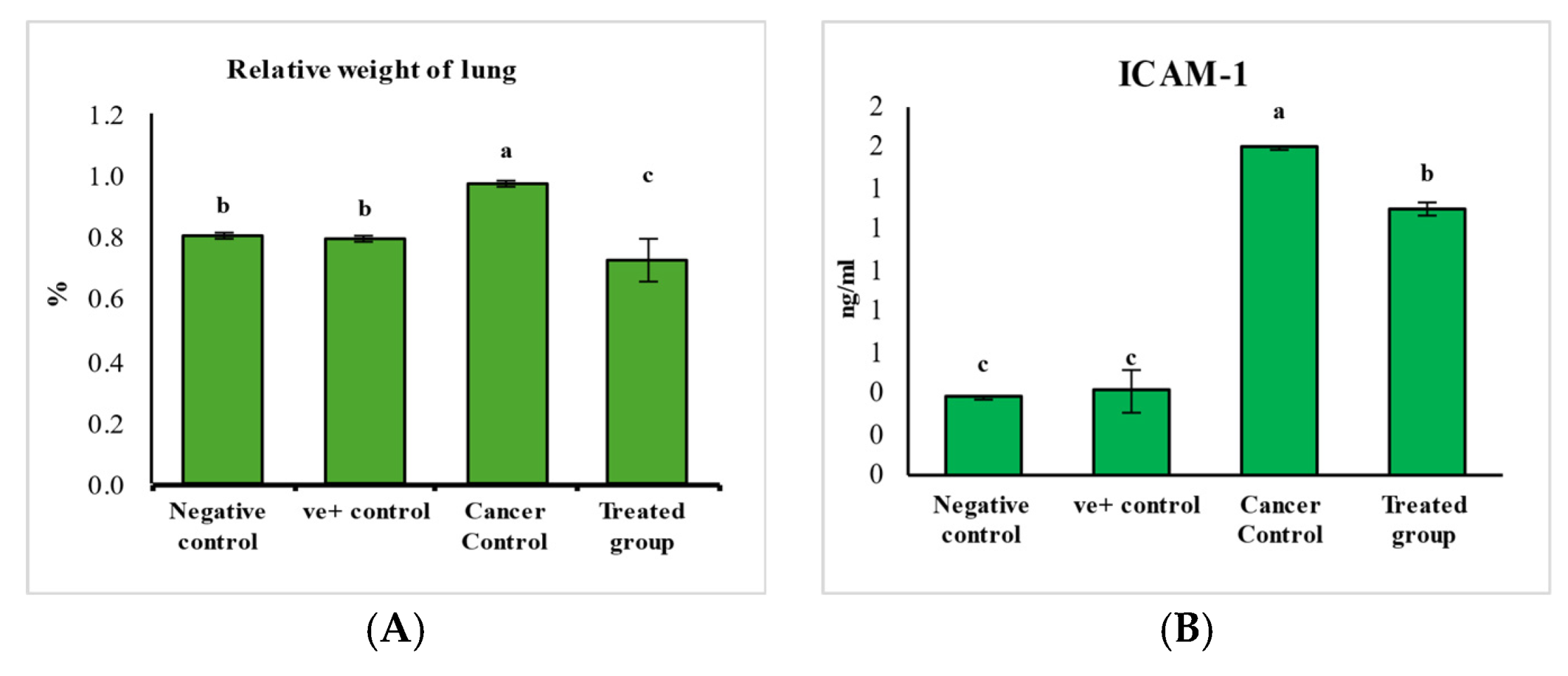
2.2. The Study on the Experimental Animals (In Vivo Investigation)
2.2.1. The Ability of Purslane Leaves Pet. Ether Extracts to Treat Lung Cancer
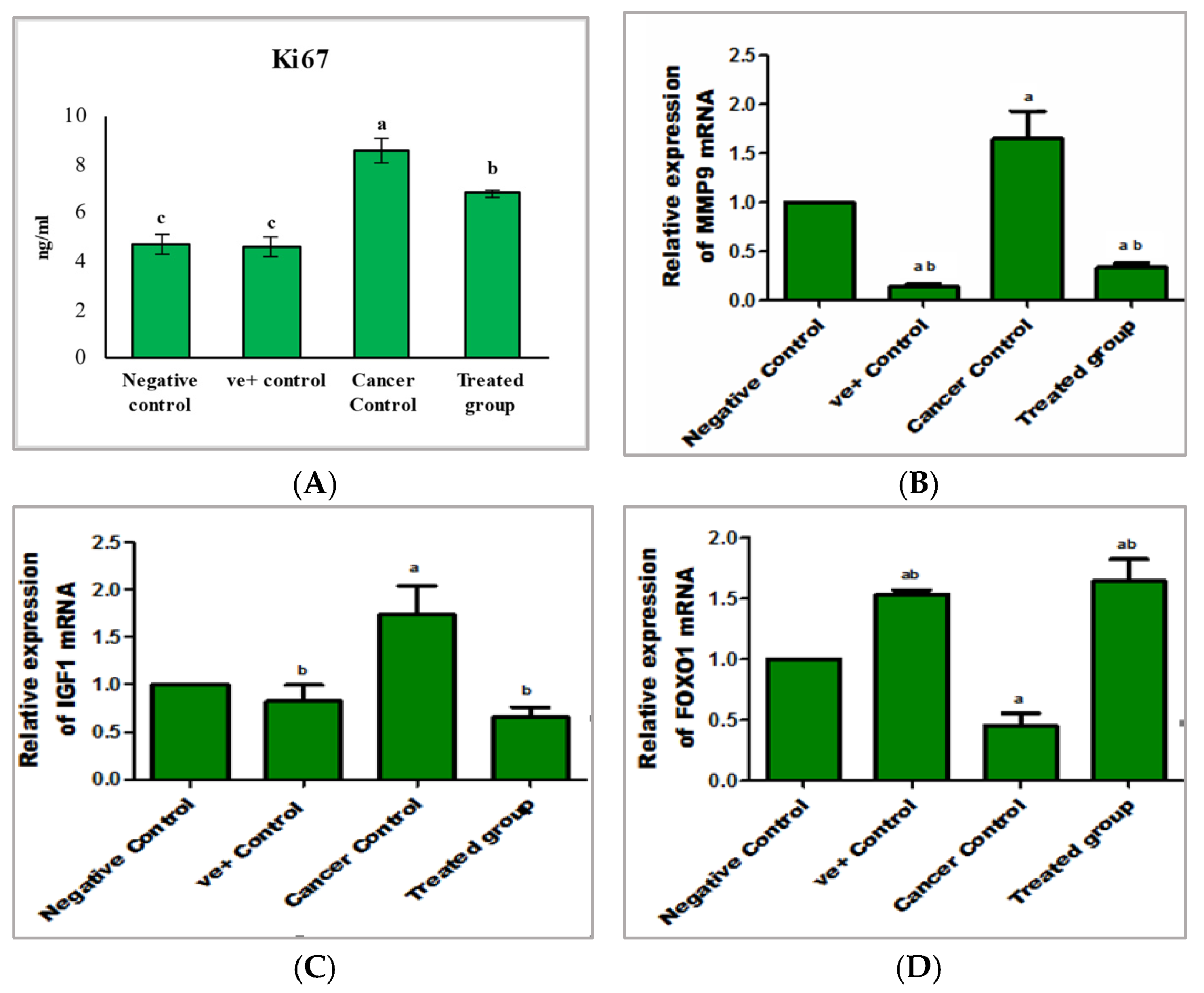
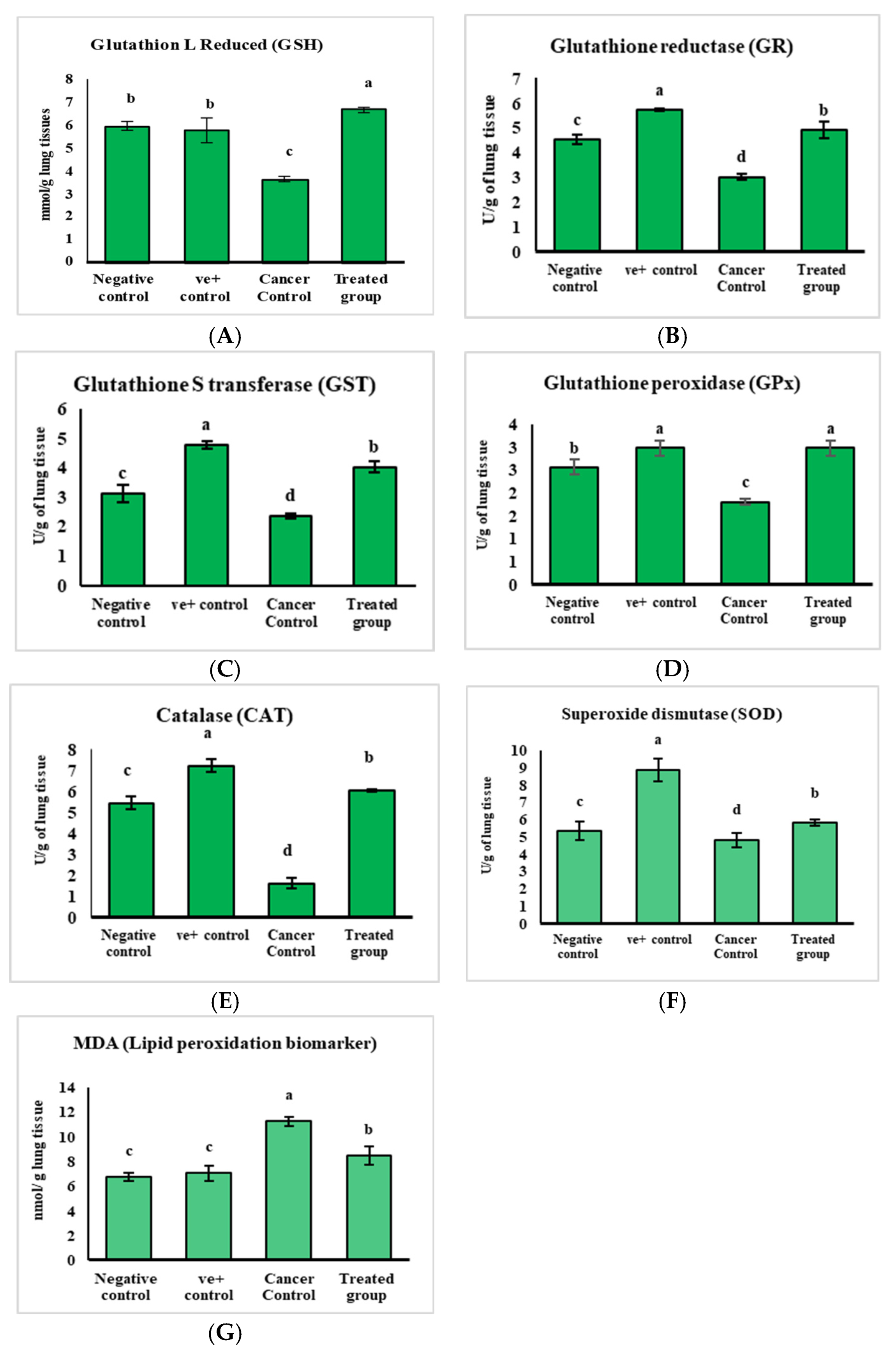
2.2.2. The Mode of Action of Purslane Leaf Pet. Ether Extract in the Treatment of Lung Cancer
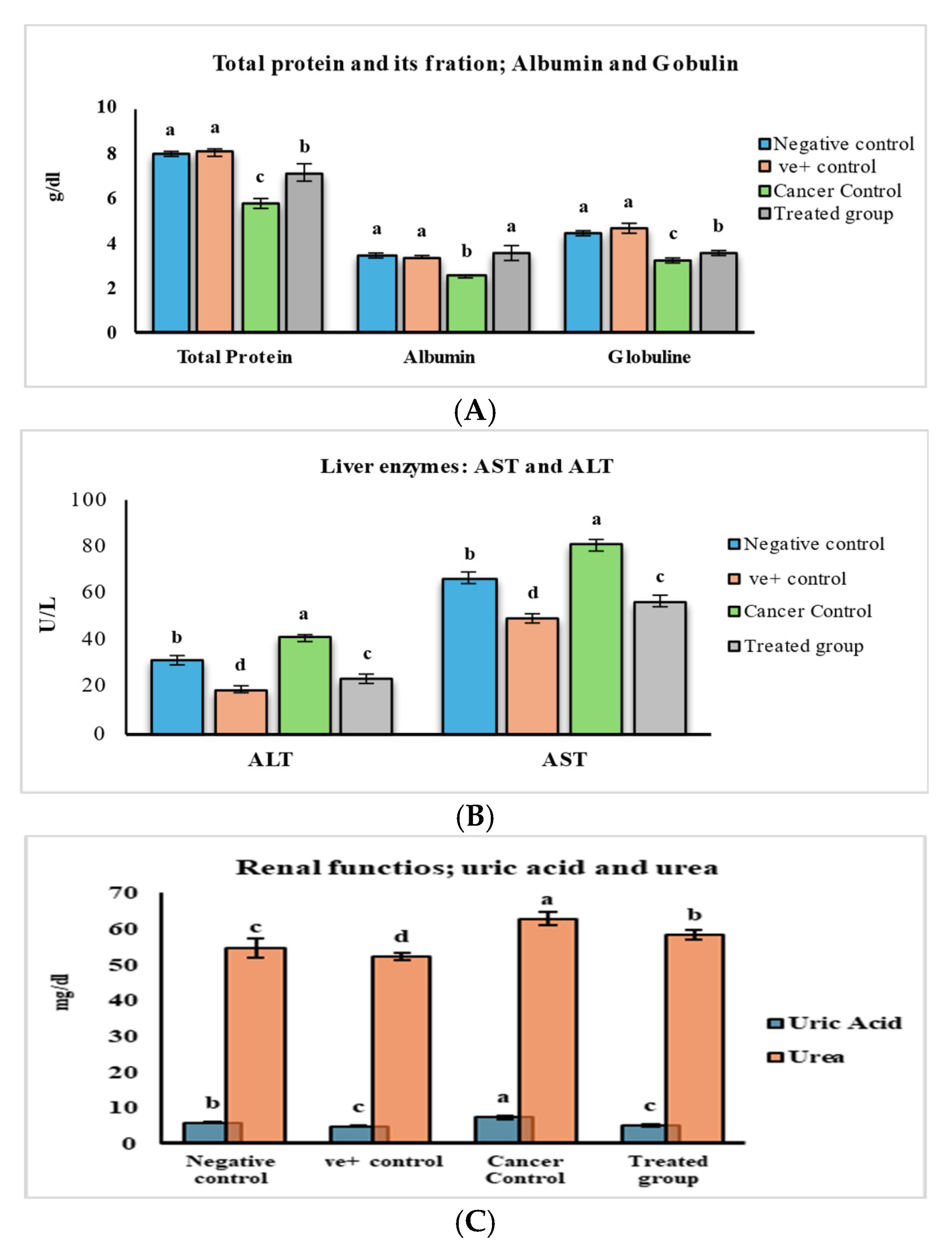
2.2.3. The Safety Profile Study of Purslane Leaf Pet. Ether Extract on the Experimental Animals for 20 Weeks
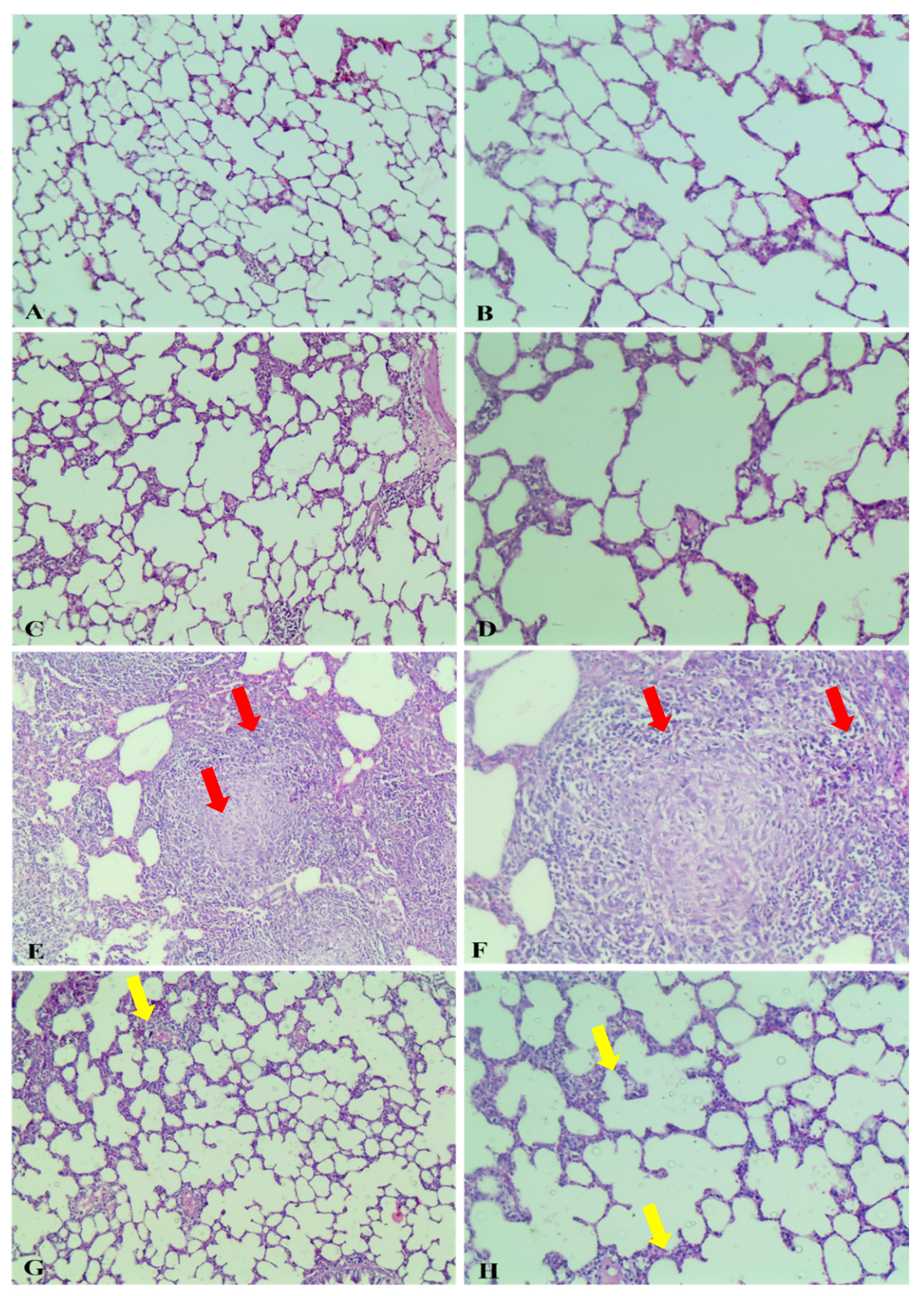
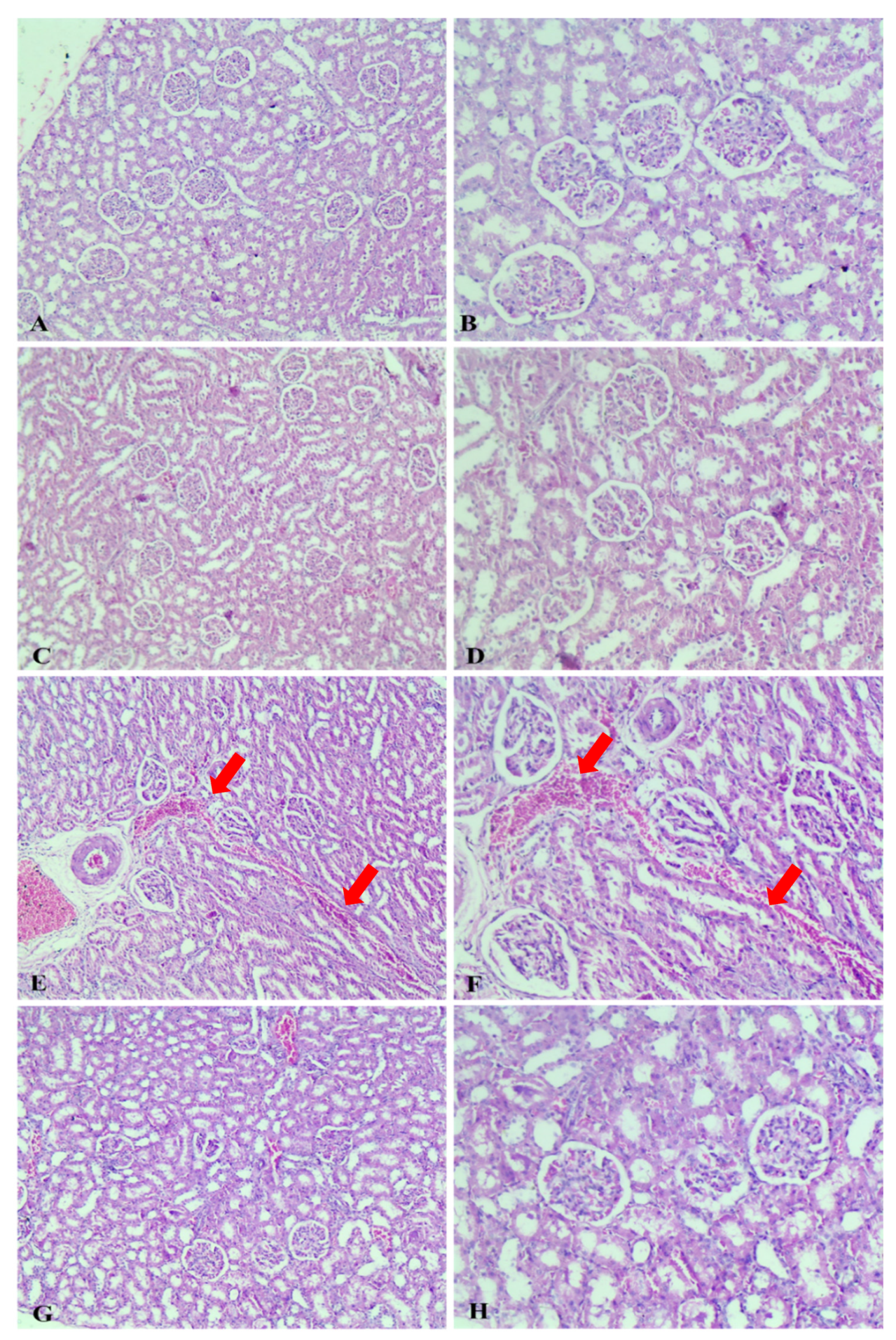
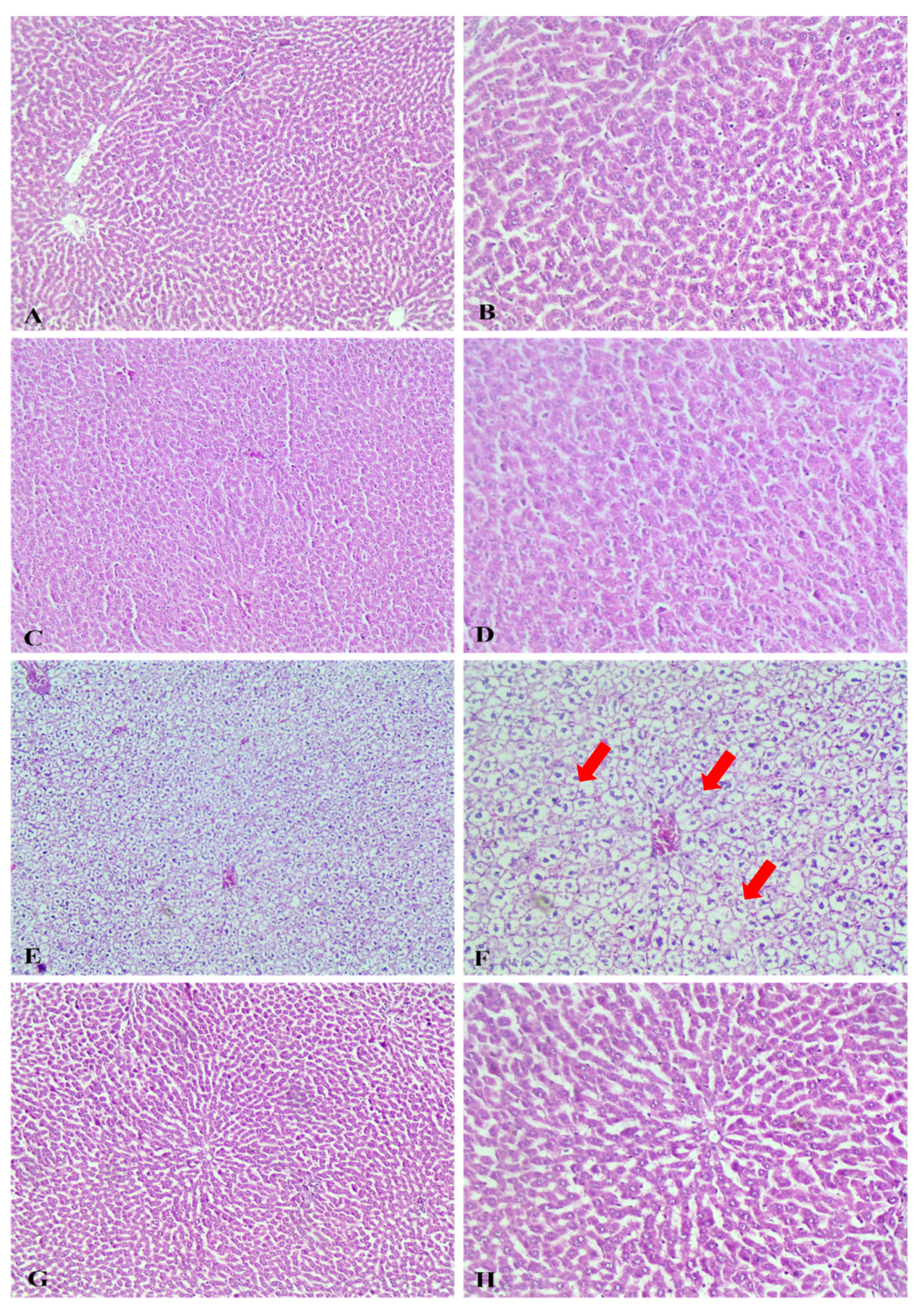
2.2.4. Histopathological Study
Gross Pathological Observations
Histopathological Examination
2.3. The First Chemical Profile of a Pet. Ether Extract from Purslane Leaves by HPLC QTOF/HR-MS/MS
2.3.1. HPLC-QTOF/HR-MS/MS
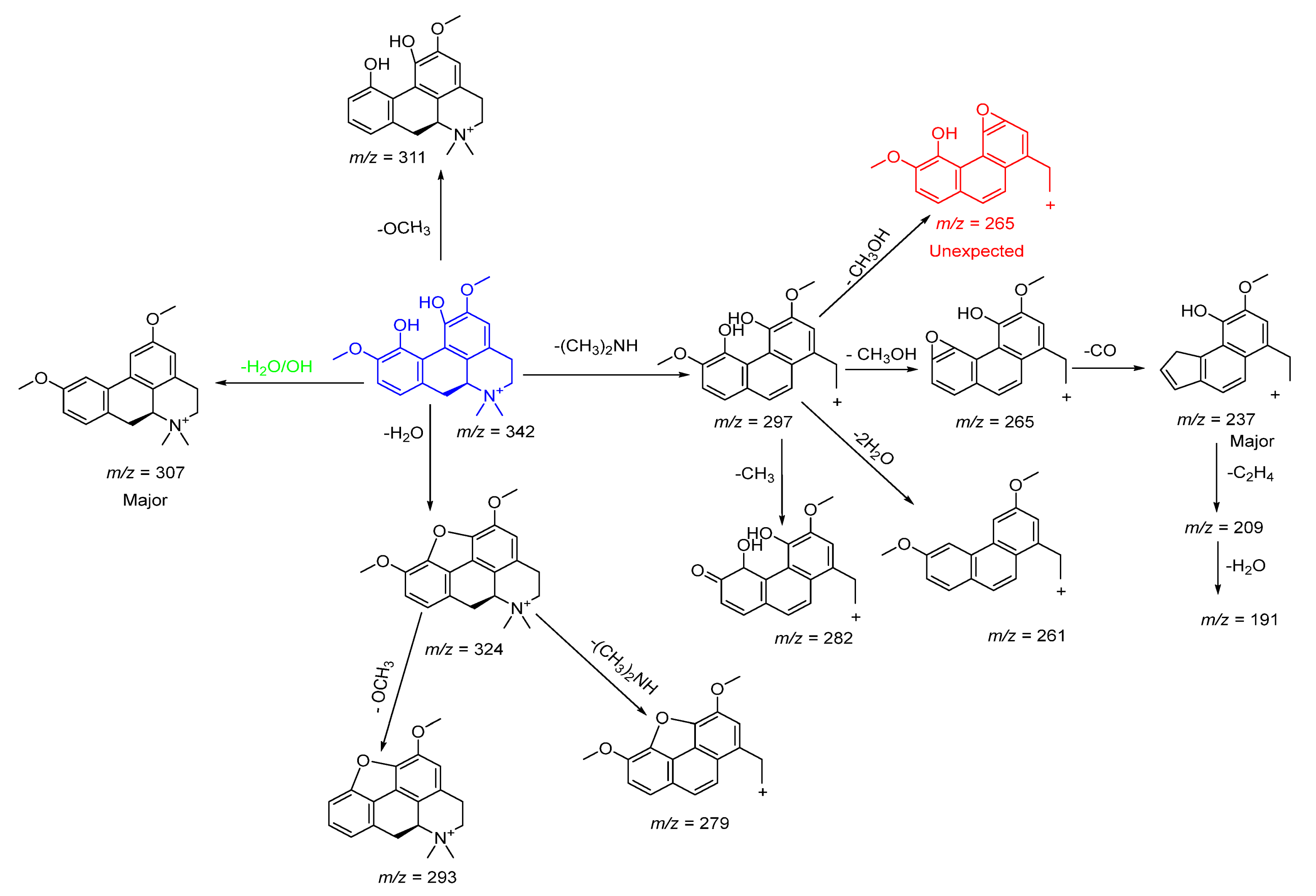
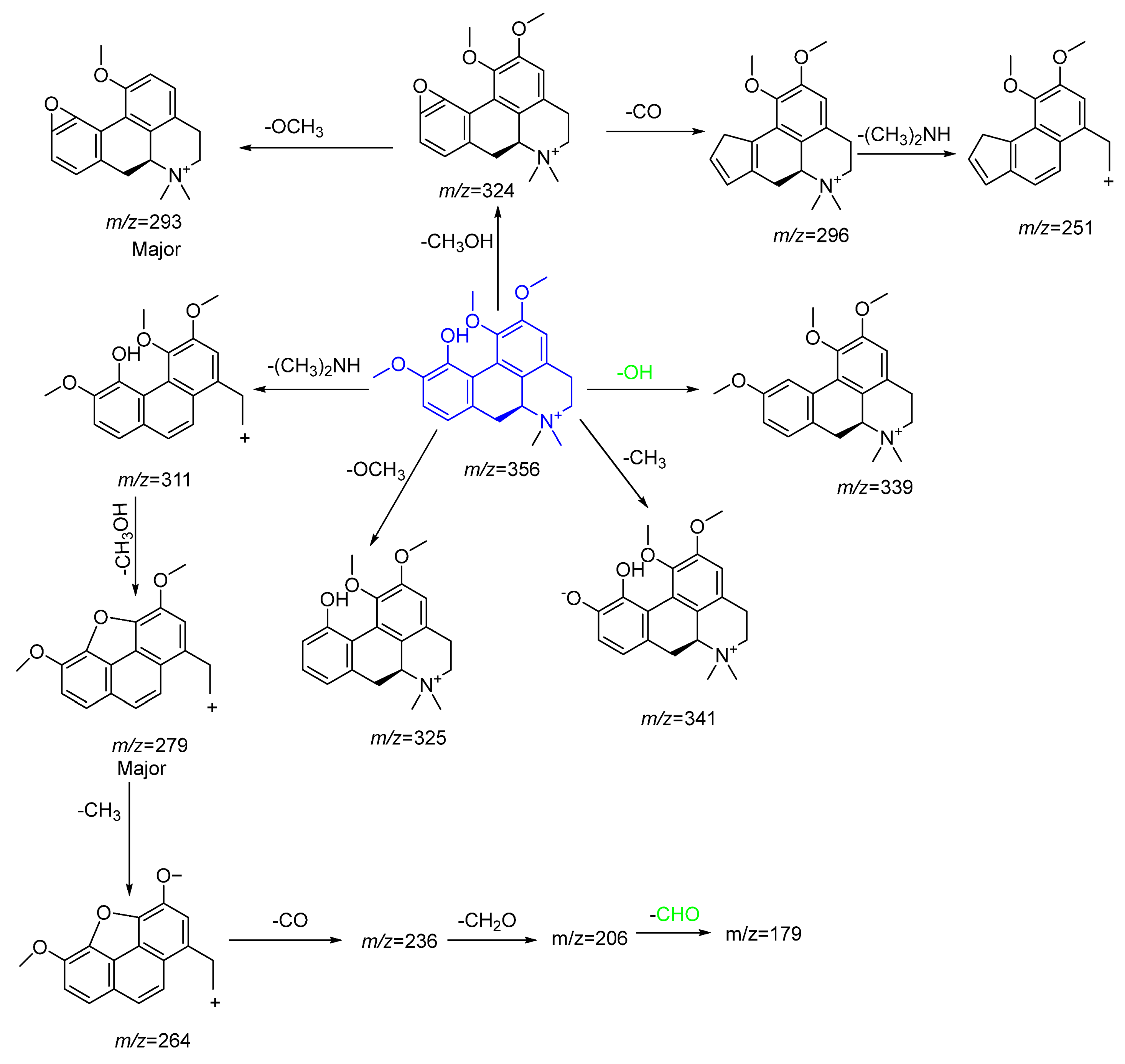
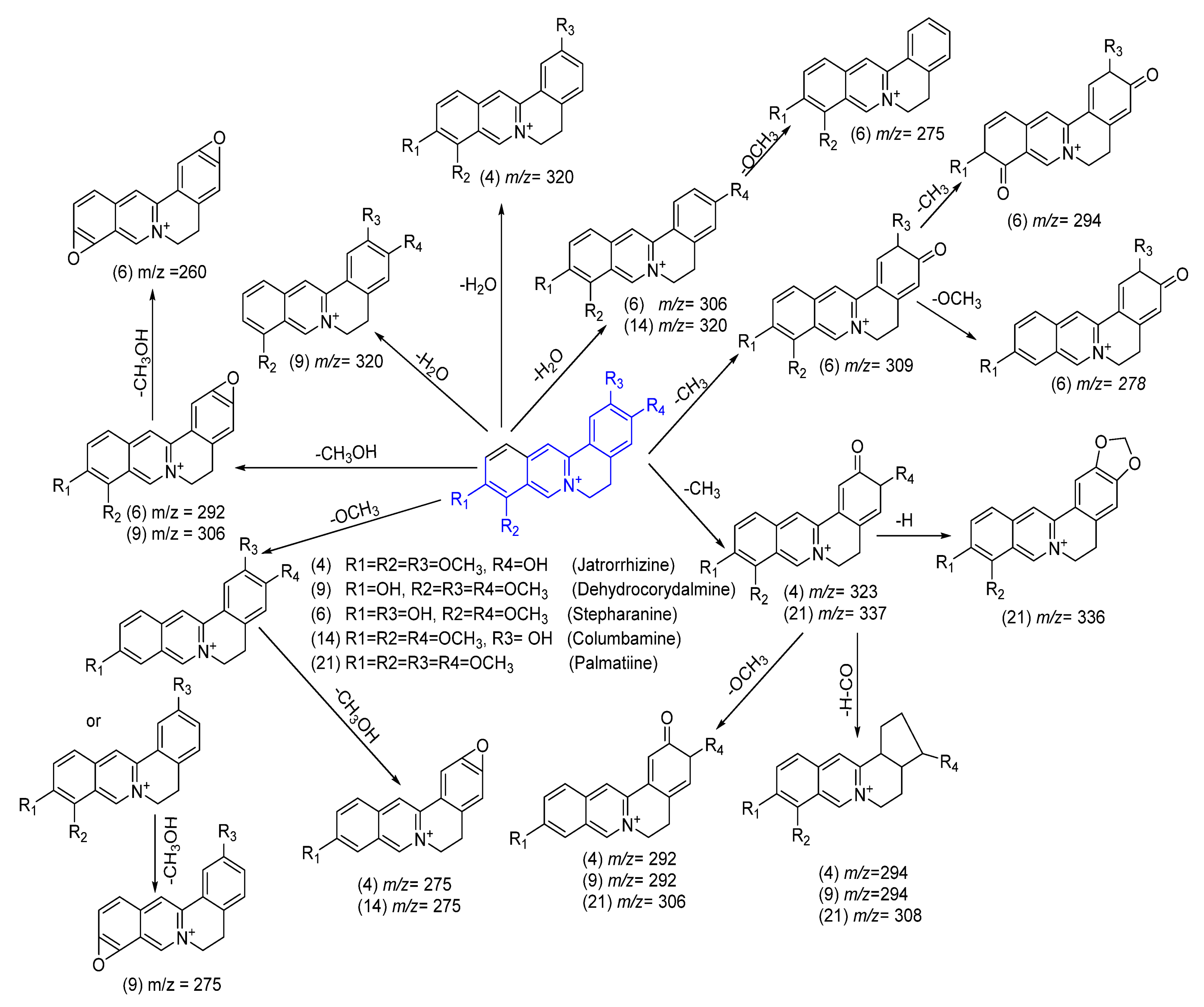
2.3.2. Characterization of Highly Abundant Alkaloids
Isoquinoline Alkaloids
Aporphine-Type Alkaloids
Protoberberine-Type Alkaloids
Alkaloids of the Benzylisoquinoline Class
Amino Alkaloids
Cyclodopa Amide Alkaloids
2.3.3. Tentative Identification of Highly Abundant Fatty Acids in Pet. Ether Extract from Purslane
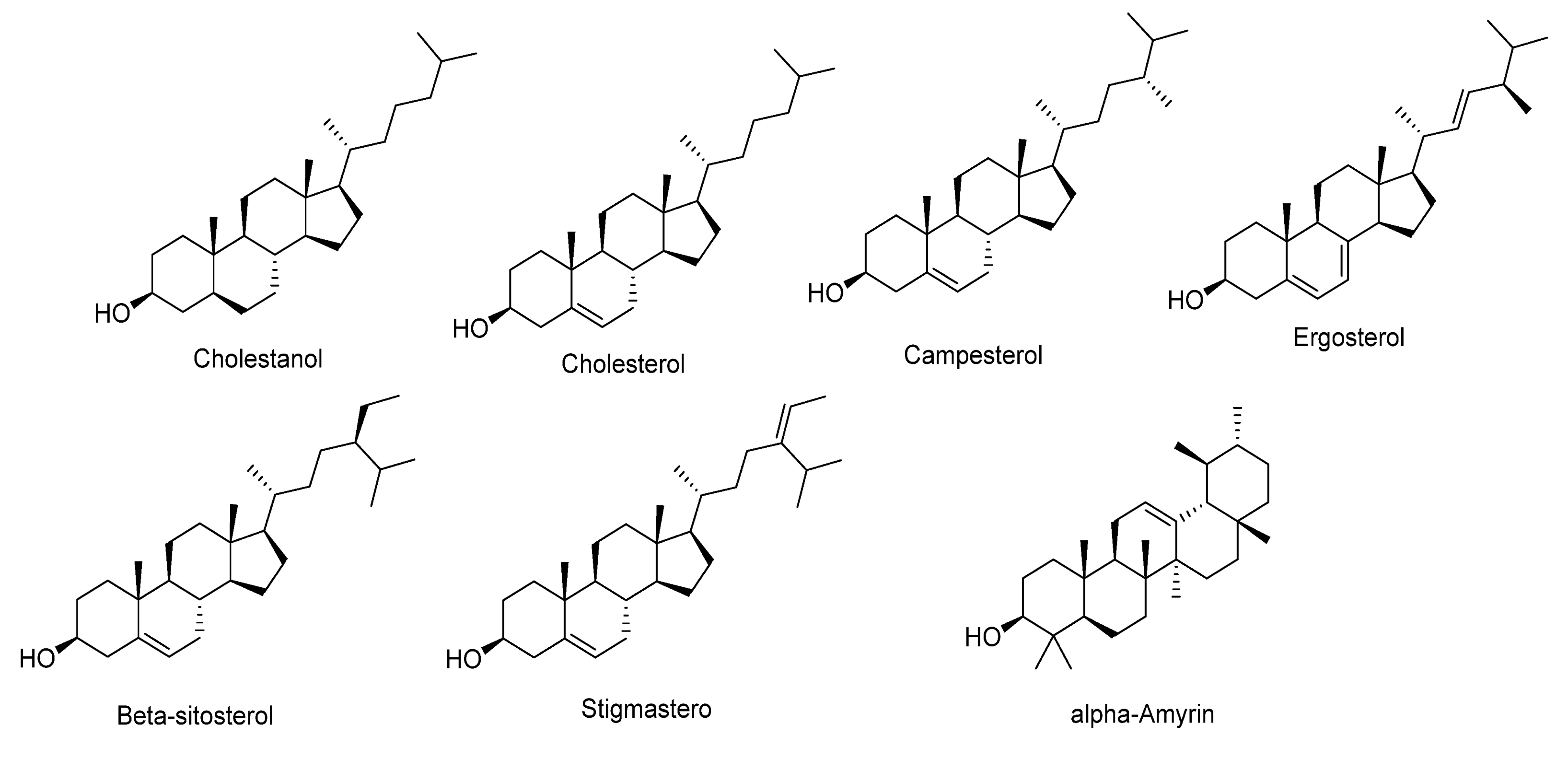
2.3.4. Tentative Identification of Sterols in Pet. Ether Extract from Purslane
3. Materials and Methods
3.1. Chemicals
3.2. Authentication of the Plant and Extraction
3.3. The Study on the A549 Lung Cancer Cell Line (In Vitro Investigation)
3.4. The Study on the Experimental Animals (In Vivo Investigation)
3.4.1. Determination of LD50
3.4.2. Animals and Accommodations
3.4.3. Lung Cancer Induction
3.4.4. Experimental Layout
3.5. Biochemical Assessments
3.6. Quantitative Real-Time PCR
3.7. Histology Assay
3.8. Qualitative Analysis of the Chemical Composition in Leaves from Pet. Ether Extract of Purslane by HPLC/HR-QTOF-MS/MS
3.8.1. Sample Preparation
3.8.2. Instruments and Acquisition Method
3.8.3. LC–MS/MS Data Processing
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Learn About Lung Cancer. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/lung-cancer?gad_source=1&gclid=CjwKCAjw1920BhA3EiwAJT3lSRuEUfsL0rxZKcq12dXH0yIcUAmSTdO8s2sN7TXq26Gle45tQwtHqxoCzxAQAvD_BwE (accessed on 26 June 2023).
- Leiter, A.; Veluswamy, R.R.; Wisnivesky, J.P. The global burden of lung cancer: Current status and future trends. Nat. Rev. Clin. Oncol. 2023, 20, 624–639. [Google Scholar] [CrossRef] [PubMed]
- Bracken-Clarke, D.; Kapoor, D.; Baird, A.M.; Buchanan, P.J.; Gately, K.; Cuffe, S.; Finn, S.P. Vaping and lung cancer—A review of current data and recommendations. Lung Cancer 2021, 153, 11–20. [Google Scholar] [CrossRef]
- Aini, N.S.; Ansori, A.N.M.; Kharisma, V.D.; Syadzha, M.F.; Widyananda, M.H.; Murtadlo, A.A.A.; Probojati, R.T.; Ullah, E.; Naw, S.W.; Jakhmola, V.; et al. Potential roles of Purslane (Portulaca oleracea L.) as antimetabolic syndrome: A review. Pharmacogn. J. 2022, 14, 710–714. [Google Scholar] [CrossRef]
- Damavandi, R.D.; Shidfar, F.; Najafi, M.; Janani, L.; Masoodi, M.; Heshmati, J.; Ziaei, S. Effect of portulaca oleracea (purslane) extract on inflammatory factors in nonalcoholic fatty liver disease: A randomized, double-blind clinical trial. J. Funct. Foods 2023, 102, 105465. [Google Scholar] [CrossRef]
- Liu, J.; Jiu, J.; Zhang, X.; Sun, J.; Ying, X. Four alkaloids from Portulaca oleracea L. and their anti-inflammatory. Nat. Prod. Res. 2024, 13, 1–7. [Google Scholar] [CrossRef]
- Dkhil, M.A.; Moniem AE, A.; Al-Quraishy, S.; Saleh, R.A. Antioxidant effect of purslane (Portulaca oleracea) and its mechanism of action. J. Med. Plants Res. 2011, 5, 1589–1593. [Google Scholar]
- Hozayen, W.; Bastawy, M.; Elshafeey, H. Effects of aqueous purslane (Portulaca Oleracea) extract and fish oil on gentamicin nephrotoxicity in albino rats. Nat. Sci. 2011, 9, 47–62. [Google Scholar]
- Obukohwo, O.M. Nutraceutical health benefit and safety utility of Portulaca oleracea: A review focus on neuroendocrine function. Clin. Tradit. Med. Pharmacol. 2024, 5, 200168. [Google Scholar] [CrossRef]
- Qiao, J.-Y.; Li, H.-W.; Liu, F.-G.; Li, Y.-C.; Tian, S.; Cao, L.-H.; Hu, K.; Wu, X.-X.; Miao, M.-S. Effects of Portulaca oleracea extract on acute alcoholic liver injury of rats. Molecules 2019, 24, 2887. [Google Scholar] [CrossRef]
- El-Newary, S.A. The hypolipidemic effect of Portulaca oleracea L. stem on hyperlipidemic Wister albino rats. Ann. Agric. Sci. 2016, 61, 111–124. [Google Scholar] [CrossRef]
- Salman, K.H.; Mahmoud, E.A.; Abd-Alla, A.A. Preparing Untraditional Kishk formula with Purslane as natural source of bioactive compounds. J. Food Dairy Sci. Mansoura Univ. 2020, 11, 299–305. [Google Scholar] [CrossRef]
- Yehia, R.; Lone, I.M.; Yehia, I.; Iraqi, F.A. Studying the Pharmagenomic effect of Portulaca oleracea extract on anti-diabetic therapy using the Collaborative Cross mice. Phytomed. Plus 2023, 3, 100394. [Google Scholar] [CrossRef]
- Van Anh, N.T.; Luyen, L.H. Potential inhibitory effects on human platelet aggregation and blood coagulation of the aerial part of Portulaca oleracea L. Trop. J. Nat. Prod. Res. 2024, 8, 6001. [Google Scholar]
- Mostafa, M.E.; Geneedy, M.R.M.; El-Lessy, F.M. Potential therapeutic and prophylactic effects of purslane (Portulaca oleracea) oil extract in murine Schistosomiasis mansoni. J. Egypt. Soc. Parasitol. 2024, 54, 11–18. [Google Scholar] [CrossRef]
- Khazdair, M.R.; Anaeigoudari, A.; Kianmehr, M. Anti-Asthmatic effects of Portulaca oleracea and its constituents, a review. J. Pharmacopunct. 2019, 22, 122–130. [Google Scholar] [CrossRef]
- Alfwuaires, M.A.; Algefare, A.I.; Afkar, E.; Abdel Salam, S.; Abd El-Moaty, H.I.; Badr, G.M. Immunomodulatory assessment of Portulaca oleracea L. extract in a mouse model of colitis. Biomed. Pharmacother. 2021, 143, 112148. [Google Scholar] [CrossRef]
- Aboulthana, W.M.; Omar, N.I.; Hasan, E.A.; Ahmed, K.A.; Youssef, A.M. Assessment of the biological activities of Egyptian Purslane (Portulaca oleracea) extract after incorporating metal nanoparticles, in vitro and in vivo study. Asian Pac. J. Cancer Prev. 2022, 23, 287–310. [Google Scholar] [CrossRef]
- Al-Sheddi, E.S.; Farshori, N.N.; Al-Oqail, M.M.; Musarrat, J.; Al-Khedhairy, A.A.; Siddiqui, M.A. Portulaca oleracea seed oil exerts cytotoxic effects on human liver cancer (HepG2) and human lung cancer (A-549) cell lines. Asian Pac. J. Cancer Prev. 2015, 16, 3383–3387. [Google Scholar] [CrossRef]
- Asnani, G.P.; Kokare, C.R. In vitro and in vivo evaluation of colon cancer targeted epichlorohydrin crosslinked Portulaca-alginate beads. Biomol. Concepts 2018, 9, 190–199. [Google Scholar] [CrossRef]
- Rahimi, V.B.; Mousavi, S.H.; Haghghi, S.; Soheili-Far, S.; Askari, V.R. Cytotoxicity and apoptogenic properties of the standardized extract of Portulaca oleracea on glioblastoma multiforme cancer cell line (U-87): A mechanistic study. EXCLI J. 2019, 18, 165–186. [Google Scholar]
- Ghorani, V.; Saadat, S.; Khazdair, M.R.; Gholamnezhad, Z.; El-Seedi, H.; Boskabady, M.H. Phytochemical Characteristics and Anti-Inflammatory, Immunoregulatory, and Antioxidant Effects of Portulaca oleracea L.: A Comprehensive Review. Evid.-Based Complement. Altern. Med. 2023, 2023, 2075444. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Yang, M.; Liu, Y. Portulaca oleracea L. polysaccharide inhibits ovarian cancer via inducing ACSL4-dependent ferroptosis. Aging 2024, 16, 5108–5122. [Google Scholar] [CrossRef]
- Zhao, R.; Shao, X.; Jia, G.; Huang, Y.; Liu, Z.; Song, B.; Hou, J. Anti-cervical carcinoma effect of Portulaca oleracea L. polysaccharides by oral administration on intestinal dendritic cells. BMC Complement. Altern. Med. 2019, 19, 161. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hu, Y.; Shi, S.; Jiang, L. Evaluation of antioxidant and immuno-enhancing activities of Purslane polysaccharides in gastric cancer rats. Int. J. Biolog. Macromol. 2014, 68, 113–116. [Google Scholar] [CrossRef]
- Alipour, S.; Pishkar, L.; Chaleshi, V. Cytotoxic effect of Portulaca oleracea extract on the regulation of CDK1 and P53 gene expression in pancreatic cancer cell line. Nutr. Cancer 2022, 74, 1792–1801. [Google Scholar] [CrossRef] [PubMed]
- de Souza, P.G.; Rosenthal, A.; Ayres, E.M.M.; Teodoro, A.J. Potential functional food products and molecular mechanisms of Portulaca oleracea L. on anticancer activity: A review. Oxi. Med. Cell. Long. 2022, 2022, 7235412. [Google Scholar] [CrossRef]
- Kumar, A.; Sreedharan, S.; Kashyap, A.K.; Singh, P.; Ramchiary, N. A review on bioactive phytochemicals and ethnopharmacological potential of purslane (Portulaca oleracea L.). Heliyon 2022, 8, e08669. [Google Scholar] [CrossRef]
- El-Newary, S.A.; Youness, E.R.; Ibrahim, A.Y. Vitex berries attenuates chemically-induced mammary carcinomas in rats through modulation of the cancer growth rate-limiting enzymes activities: Aromatase and Na+/K+ ATPase. Egypt. J. Chem. 2024, 67, 235–255. [Google Scholar] [CrossRef]
- Ali, N.A.; Elsayed, G.H.; Mohamed, S.H.; Abd Elkarim, A.S.; Aly, M.S.; Elgamal, A.M.; Elsayed, W.M.; El-Newary, S.A. Chia seed (Salvia hispanica) attenuates chemically induced lung carcinomas in rats through suppression of proliferation and angiogenesis. Pharmaceuticals 2024, 17, 1129. [Google Scholar] [CrossRef]
- Farag, M.-A.; Shakour, Z.-T. Metabolomics driven analysis of 11 Portulaca leaf taxa as analysed via UPLC-ESI-MS/MS and chemometrics. Phytochemistry 2019, 161, 117–129. [Google Scholar] [CrossRef]
- Chiocchio, I.; Mandrone, M.; Tomasi, P.; Marincich, L.; Poli, F. Plant secondary metabolites: An opportunity for circular economy. Molecules 2021, 26, 495. [Google Scholar] [CrossRef] [PubMed]
- Qing, Z.; Xu, Y.; Yu, L.; Liu, J.; Huang, X.; Tang, Z.; Cheng, P.; Zeng, J. Investigation of fragmentation behaviours of isoquinoline alkaloids by mass spectrometry combined with computational chemistry. Sci. Rep. 2020, 10, 733. [Google Scholar] [CrossRef] [PubMed]
- Deng, A.P.; Zhang, Y.; Zhou, L.; Kang, C.Z.; Lv, C.G.; Kang, L.P.; Nan, T.G.; Zhan, Z.L.; Guo, L.P.; Huang, L.Q. Systematic review of the alkaloid constituents in several important medicinal plants of the Genus Corydalis. Phytochem 2021, 183, 112644. [Google Scholar] [CrossRef]
- Luo, H.; Wu, H.; Yu, X.; Zhang, X.; Lu, Y.; Fan, J.; Tang, L.; Wang, Z. A review of the phytochemistry and pharmacological activities of Magnoliae officinalis cortex. J. Ethnopharm. 2019, 236, 412–442. [Google Scholar] [CrossRef]
- Singh, A.; Bajpai, V.; Kumar, S.; Rawat, A.K.; Kumar, B. Analysis of isoquinoline alkaloids from Mahonia leschenaultia and Mahonia napaulensis roots using UHPLC-Orbitrap-MSn and UHPLC-QqQLIT-MS/MS. J. Pharm. Anal. 2017, 7, 77–86. [Google Scholar] [CrossRef]
- Stévigny, C.; Jiwan, J.L.H.; Rozenberg, R.; de Hoffmann, E.; Quetin-Leclercq, J. Key fragmentation patterns of aporphine alkaloids by electrospray ionization with multistage mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 523–528. [Google Scholar] [CrossRef]
- Qing, Z.X.; Cheng, P.; Zeng, J.G. Research progress on mass spectral fragmentation behavior of alkaloids in Macleaya cordata. Chin. Tradit. Herb. Drugs 2013, 44, 2929. [Google Scholar]
- Jiao, Q.S.; Xu, L.L.; Zhang, J.Y.; Wang, Z.J.; Jiang, Y.Y.; Liu, B. Rapid characterization and identification of non-diterpenoid constituents in Tinospora sinensis by HPLC-LTQ-orbitrap MSn. Molecules 2018, 23, 274. [Google Scholar] [CrossRef]
- Maurya, R.; Gupta, P.; Chand, K.; Kumar, M.; Dixit, P.; Singh, N.; Dube, A. Constituents of Tinospora sinensis and their antileishmanial activity against Leishmania donovani. Nat. Prod. Res. 2009, 23, 1134–1143. [Google Scholar] [CrossRef]
- Wu, W.; Song, F.; Yan, C.; Liu, Z.; Liu, S. Structural analyses of protoberberine alkaloids in medicine herbs by using ESI–FT-ICR-MS and HPLC–ESI–MSn. J. Pharm. Biomed. Analy. 2005, 37, 437–446. [Google Scholar] [CrossRef]
- Xiang, Q.; Hashi, Y.; Chen, Z. Simultaneous detection of eight active components in Radix Tinosporae by ultra high performance liquid chromatography coupled with electrospray tandem mass spectrometry. J. Sep. Sci. 2016, 39, 2036–2042. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, W.; Zhong, Y.; Zhang, L.; Su, T.; Liang, G.; Zhang, D.; Zhang, Y.; Chen, J.; Gong, M. Identification of α-glucosidase inhibitors from Cortex Lycii based on a bioactivity-labeling high-resolution mass spectrometry–metabolomics investigation. J. Chromatogr. A 2021, 1642, 462041. [Google Scholar] [CrossRef] [PubMed]
- El-Newary, S.A.; Abd Elkarim, A.S.; Abdelwahed, N.A.; Omer, E.A.; Elgamal, A.M.; Elsayed, W.M. Chenopodium murale Juice shows anti-Fungal efficacy in experimental oral candidiasis in immunosuppressed rats in relation to its chemical profile. Molecules 2023, 28, 4304. [Google Scholar] [CrossRef] [PubMed]
- Abd Elkarim, A.S.; Ahmed, A.H.; Taie, H.A.A.; Elgamal, A.M.; Abu-elghait, M.; Shabana, S. Synadenium grantii hook f.: HPLC/QTOF-MS/MS tentative identification of the phytoconstituents, antioxidant, antimicrobial and antibiofilm evaluation of the aerial parts. Rasayan J. Chem. 2021, 14, 811–828. [Google Scholar] [CrossRef]
- Nagy, A.M.; Abdelhameed, M.F.; Abd Elkarim, A.S.; Sarker, T.C.; Abd-ElGawad, A.M.; Elshamy, A.I.; Hammam, A.M. Enhancement of female rat fertility via ethanolic extract from Nigella sativa L. (Black Cumin) seeds assessed via HPLC-ESI-MS/MS and molecular docking. Molecules 2024, 29, 735. [Google Scholar] [CrossRef]
- van Agthoven, M.A.; Barrow, M.P.; Chiron, L.; Coutouly, M.A.; Kilgour, D.; Wootton, C.A.; Wei, J.; Soulby, A.; Delsuc, M.A.; Rolando, C.; et al. Differentiating fragmentation pathways of cholesterol by two-dimensional Fourier transform ion cyclotron resonance mass spectrometry. J. Am. Soc. Mass Spectrom. 2015, 26, 2105–2114. [Google Scholar] [CrossRef]
- Ory, L.; Gentil, E.; Kumla, D.; Kijjoa, A.; Nazih, E.H.; Roullier, C. Detection of ergosterol using liquid chromatography/electrospray ionization mass spectrometry: Investigation of unusual in-source reactions. Rapid Commun. Mass Spectrom. 2020, 34, e8780. [Google Scholar] [CrossRef]
- Yan, H.; Zhang, S.; Yang, L.; Jiang, M.; Xin, Y.; Liao, X.; Li, Y.; Lu, J. The antitumor effects of α-linolenic acid. J. Pers. Med. 2024, 14, 260. [Google Scholar] [CrossRef]
- Yang, L.; Yuan, J.; Liu, L.; Shi, C.; Wang, L.; Tian, F.; Liu, F.; Wang, H.; Shao, C.; Zhang, Q. α-linolenic acid inhibits human renal cell carcinoma cell proliferation through PPAR-γ activation and COX-2 inhibition. Oncol. Lett. 2013, 6, 197–202. [Google Scholar] [CrossRef]
- Roy, S.; Rawat, A.K.; Sammi, S.R.; Devi, U.; Singh, M.; Gautam, S.; Yadav, R.K.; Rawat, J.K.; Singh, L.; Ansari, M.N. Alpha-linolenic acid stabilizes HIF-1 α and downregulates FASN to promote mitochondrial apoptosis for mammary gland chemoprevention. Oncotarget 2017, 8, 70049–70071. [Google Scholar] [CrossRef]
- Fan, N.; Fusco, J.L.; Rosenberg, D.W. Antioxidant and anti-inflammatory properties of walnut constituents: Focus on personalized cancer prevention and the microbiome. Antioxidants 2023, 12, 982. [Google Scholar] [CrossRef] [PubMed]
- Sala-Vila, A.; Fleming, J.; Kris-Etherton, P.; Ros, E. Impact of α-linolenic acid, the vegetable ω-3 fatty acid, on cardiovascular disease and cognition. Adv. Nutr. 2022, 13, 1584–1602. [Google Scholar] [CrossRef]
- Polavarapu, S.; Mani, A.M.; Gundala, N.K.V.; Hari, A.D.; Bathina, S.; Das, U.N. Effect of Polyunsaturated Fatty Acids and Their Metabolites on Bleomycin-Induced Cytotoxic Action on Human Neuroblastoma Cells In Vitro. PLoS ONE 2014, 9, e114766. [Google Scholar] [CrossRef] [PubMed]
- Słowikowski, B.K.; Drzewiecka, H.; Malesza, M.; Mądry, I.; Sterzyńska, K.; Jagodziński, P.P. The influence of conjugated linoleic acid on the expression of peroxisome proliferator-activated receptor-γ and selected apoptotic genes in non-small cell lung cancer. Mol. Cell. Biochem. 2020, 466, 65–82. [Google Scholar] [CrossRef] [PubMed]
- Cabral, M.; Martín-Venegas, R.; Moreno, J.J. Differential cell growth/apoptosis behavior of 13-hydroxyoctadecadienoic acid enantiomers in a colorectal cancer cell line. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G664–G671. [Google Scholar] [CrossRef]
- Vaezi, M.A.; Safizadeh, B.; Eghtedari, A.R.; Ghorbanhosseini, S.S.; Rastegar, M.; Salimi, V.; Tavakoli-Yaraki, M. 15-Lipoxygenase and its metabolites in the pathogenesis of breast cancer: A doubleedged sword. Lipids Health Dis. 2021, 20, 169. [Google Scholar] [CrossRef]
- Li, M.-Y.; Yuan, H.-L.; Ko, F.W.S.; Wu, B.; Long, X.; Du, J.; Wu, J.; Ng, C.S.H.; Wan, I.Y.P.; Mok, T.S.K.; et al. Antineoplastic Effects of 15(S)-Hydroxyeicosatetraenoic Acid and 13-S-Hydroxyoctadecadienoic Acid in Non–Small Cell Lung Cancer. Cancer 2015, 121 (Suppl. S17), 3130–3145. [Google Scholar] [CrossRef]
- Li, Z.; Chen, B.; Wang, P.; Li, X.; Cai, G.; Wei, W.; Dong, W. A proteomic analysis of acute leukemia cells treated with 9-hydroxyoctadecadienoic acid. Lip. Health Dis. 2016, 15, 192. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, C.; Bao, N. Molecular mechanism of palmitic acid and its derivatives in tumor progression. Front. Oncol. 2023, 13, 1224125. [Google Scholar] [CrossRef]
- Deyab, M.A.; Habbak, L.Z.; Ward, F.M. Antitumor activity of water extract and some fatty acids of Turbinaria ornata (Turner) J. Agardh. Egypt. J. Exp. Biol. (Bot.) 2012, 8, 199–204. [Google Scholar]
- Yu, X.; Peng, W.; Wang, Y.; Xu, W.; Chen, W.; Huang, L.; Xu, H.; He, X.; Wang, S.; Sun, Q.; et al. Palmitic acid inhibits the growth and metastasis of gastric cancer by blocking the STAT3 signaling pathway. Cancers 2023, 15, 388. [Google Scholar] [CrossRef]
- Zhao, Z.; Wang, J.; Kong, W.; Newton, M.A.; Burkett, W.C.; Sun, W.; Buckingham, L.; O’Donnell, J.; Suo, H.; Deng, B. Palmitic acid exerts anti-tumorigenic activities by modulating cellular stress and lipid droplet formation in endometrial cancer. Biomolecules 2024, 14, 601. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Yang, X.; Huang, X.; Yao, Y.; Wei, X.; Yang, S.; Zhou, D.; Zhang, W.; Long, Z.; Xu, X.; et al. Hydroxylation of fatty acids represses colorectal tumorigenesis and metastasis via the YAP transcriptional axis. Cancer Res. 2021, 81, 289–302. [Google Scholar] [CrossRef] [PubMed]
- Rajavel, T.; Packiyaraj, P.; Suryanarayanan, V.; Singh, S.K.; Ruckmani, K.; Devi, K.P. β-Sitosterol targets Trx/Trx1 reductase to induce apoptosis in A549 cells via ROS mediated mitochondrial dysregulation and p53 activation. Sci. Rep. 2018, 8, 2071. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Zhang, Y.; Zhang, H.; Xia, L. Molecular mechanism of b-sitosterol and its derivatives in tumor progression. Front. Oncol. 2022, 12, 926975. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Zhang, Z.; Liu, J.; Hong, L. β-Sitosterol as a promising anticancer agent for chemoprevention and chemotherapy: Mechanisms of action and future prospects. Adv. Nutr. 2023, 14, 1085–1110. [Google Scholar] [CrossRef]
- Yun, D.; Yoon, S.Y.; Park, S.J.; Park, Y.J. The anticancer effect of natural plant alkaloid isoquinolines. Int. J. Mol. Sci. 2021, 22, 1653. [Google Scholar] [CrossRef]
- Tang, Z.-H.; Cao, W.-X.; Wang, Z.-Y.; Lu, J.-H.; Liu, B.; Chen, X.; Lu, J.-J. Induction of reactive oxygen species-stimulated distinctive autophagy by chelerythrine in non-small cell lung cancer cells. Redox Biol. 2017, 12, 367–376. [Google Scholar] [CrossRef]
- Xie, Y.-J.; Gao, W.-N.; Wu, Q.-B.; Yao, X.-J.; Jiang, Z.-B.; Wang, Y.-W.; Wang, W.-J.; Li, W.; Hussain, S.; Liu, L. Chelidonine selectively inhibits the growth of gefitinib-resistant non-small cell lung cancer cells through the EGFR-AMPK pathway. Pharmacol. Res. 2020, 159, 104934. [Google Scholar] [CrossRef]
- Zhao, Y.; Jing, Z.; Lv, J.; Zhang, Z.; Lin, J.; Cao, X.; Zhao, Z.; Liu, P.; Mao, W. Berberine activates caspase-9/cytochrome c-mediated apoptosis to suppress triple-negative breast cancer cells in vitro and in vivo. Biomed. Pharmacother. 2017, 95, 18–24. [Google Scholar] [CrossRef]
- Kalaiarasi, A.; Anusha, C.; Sankar, R.; Rajasekaran, S.; Marshal, J.J.; Muthusamy, K.; Ravikumar, V. Plant isoquinoline alkaloid berberine exhibits chromatin remodeling by modulation of histone deacetylase to induce growth arrest and apoptosis in the A549 cell line. J. Agric. Food Chem. 2016, 64, 9542–9550. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Jiang, K.; Wu, H.; Yang, C.; Yang, Y.; Yang, J.; Zhao, G.; Deng, G. Magnoflorine ameliorates lipopolysaccharide-induced acute lung injury via suppressing NF-κB and MAPK activation. Front. Pharmacol. 2018, 30, 982. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.W.; Jeong, J.S.; Kim, J.H.; Aggarwal, B.B. Cancer prevention and therapy: Integrating traditional Korean medicine into modern cancer care. Integr. Cancer Ther. 2014, 13, 310–331. [Google Scholar] [CrossRef]
- Folescu, R.; Levai, C.M.; Grigoraş, M.L.; Arghirescu, T.S.; Talpoş, I.C.; Gîndac, C.M.; Zamfir, C.L.; Poroch, V.; Anghel, M.D. Expression and significance of Ki-67 in lung cancer. Rom. J. Morphol. Embryol. 2018, 59, 227–233. [Google Scholar]
- Saman, H.; Raza, S.S.; Uddinm, S.; Rasulm, K. Inducing angiogenesis, a key step in cancer vascularization, and treatment approaches. Cancers 2023, 15, 1172. [Google Scholar] [CrossRef]
- Taghvaei, S.; Saremi, L.; Motovali-Bashi, M. A single nucleotide polymorphism in the MMP9 promoter affects lung cancer and clinicopathological properties in Iranian population. J. Biomed. Res. Environ. Sci. 2021, 2, 1274–1282. [Google Scholar] [CrossRef]
- Li, S.; Pritchard, D.M.; Yu, L.-G. Regulation and Function of Matrix Metalloproteinase-13 in Cancer Progression and Metastasis. Cancers 2022, 14, 3263. [Google Scholar] [CrossRef]
- Guirado, E.; George, A. Dentine matrix metalloproteinases as potential mediators of dentine regeneration. Eur. Cell Mater. 2022, 42, 392–400. [Google Scholar] [CrossRef]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of matrix metalloproteinases in angiogenesis and cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Macvanin, M.; Gluvic, Z.; Radovanovic, J.; Essack, M.; Gao, X.; Isenovic, E.R. New insights on the cardiovascular effects of IGF-1. Front. Endocrinol. 2023, 14, 1142644. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, S.; Li, J.; Wang, D.; Li, Q. Effect of microRNA-135a on cell proliferation, migration, invasion, apoptosis and tumor angiogenesis through the IGF-1/PI3K/Akt signaling pathway in non-small cell lung cancer. Cell Physiol. Biochem. 2017, 42, 1431–1446. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.; Teng, P.; Hu, P. Effects of ANCR lncRNA on the biological behaviors of lung cancer cells A549 and the mechanism. Transl. Cancer Res. 2020, 9, 4693–4702. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.-H.; Chen, G.; Deng, C.; Tang, J.-M.; Xie, L.; Zhou, H.-Y.; Ye, X.; Zhang, D.-K.; Shi, R.-Q.; Tian, D.; et al. TCF19 contributes to cell proliferation of non-small cell lung cancer through inhibiting FOXO1. Cell Biol. Int. 2019, 43, 1416–1424. [Google Scholar] [CrossRef]
- Mayer, I.A.; Arteaga, C.L. The PI3K/AKT Pathway as a Target for Cancer Treatment. Annu. Rev. Med. 2016, 67, 11–28. [Google Scholar] [CrossRef]
- Lei, Z.; Ali, I.; Yang, M.; Yang, C.; Li, Y.; Li, L. Non-esterified fatty acid-induced apoptosis in bovine granulosa cells via ROS-activated PI3K/AKT/FoxO1 pathway. Antioxidants 2023, 12, 434. [Google Scholar] [CrossRef] [PubMed]
- Jian, H.; Liu, B.; Zhang, J. Hypoxia and hypoxia-inducible factor 1 repress SEMA4B expression to promote non-small cell lung cancer invasion. Tumor Biol. 2014, 35, 4949–4955. [Google Scholar] [CrossRef]
- Jian, H.; Zhao, Y.; Liu, B.; Lu, S. SEMA4B inhibits growth of non-small cell lung cancer in vitro and in vivo. Cell Signal. 2015, 27, 1208–1213. [Google Scholar] [CrossRef]
- Gheghiani, L.; Shang, S.; Fu, Z. Targeting the PLK1-FOXO1 pathway as a novel therapeutic approach for treating advanced prostate cancer. Sci. Rep. 2020, 10, 12327. [Google Scholar] [CrossRef]
- Jiang, S.; Xu, Z.; Shi, Y.; Liang, S.; Jiang, X.; Xiao, M.; Wang, K.; Ding, L. Circulating insulin-like growth factor-1 and risk of lung diseases: A Mendelian randomization analysis. Front. Endocrinol. 2023, 14, 1126397. [Google Scholar] [CrossRef]
- Li, S.; Pinard, M.; Wang, Y.; Yang, L.; Lin, R.; Hiscott, J.; Su, B.; Brodt, P. Crosstalk between the TNF and IGF pathways enhances NF-kappa B activation and signaling in cancer cells. Growth Horm. IGF Res. 2015, 25, 253–261. [Google Scholar] [CrossRef]
- Spadaro, O.; Goldberg, E.L.; Camell, C.D.; Youm, Y.-H.; Kopchick, J.J.; Nguyen, K.Y.; Bartke, A.; Sun, L.Y.; Dixit, V.D. Growth hormone receptor deficiency protects against age-related NLRP3 inflammasome activation and immune senescence. Cell Rep. 2016, 14, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Reales-Calderon, J.A.; Aguilera-Montilla, N.; Corbi, A.L.; Molero, G.; Gil, C. Proteomic characterization of human proinflammatory M1 and anti-inflammatory M2 macrophages and their response to Candida albicans. Proteomics 2014, 14, 1503–1518. [Google Scholar] [CrossRef] [PubMed]
- Piñeiro-Hermida, S.; López, I.P.; Alfaro-Arnedo, E.; Torrens, R.; Iñiguez, M.; Alvarez-Erviti, L.; Ruíz-Martínez, C.; Pichel, J.G. IGF1R deficiency attenuates acute inflammatory response in a bleomycin-induced lung injury mouse model. Sci. Rep. 2017, 7, 4290. [Google Scholar] [CrossRef] [PubMed]
- Alfaro-Arnedo, E.; Lopez, I.P.; Pineiro-Hermida, S.; Canalejo, M.; Gotera, C.; Sola, J.J.; Roncero, A.; Peces-Barba, G.; Ruiz-Martinez, C.; Pichel, J.G. IGF1R acts as a cancer-promoting factor in the tumor microenvironment facilitating lung metastasis implantation and progression. Oncogene 2022, 41, 3625–3639. [Google Scholar] [CrossRef] [PubMed]
- Zalewska-Ziob, M.; Adamek, B.; Kasperczyk, J.; Romuk, E.; Hudziec, E.; Chwalińska, E.; Dobija-Kubica, K.; Rogoziński, P.; Bruliński, K. Activity of antioxidant enzymes in the tumor and adjacent noncancerous tissues of non-small-cell lung cancer. Oxi. Med. Cell. Long. 2019, 2019, 2901840. [Google Scholar] [CrossRef]
- Udomsak, W.; Kucinska, M.; Pospieszna, J.; Dams-Kozlowska, H.; Chatuphonprasert, W.; Murias, M. Antioxidant enzymes in cancer cells: Their role in photodynamic therapy resistance and potential as targets for improved treatment outcomes. Int. J. Mol. Sci. 2024, 25, 3164. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- OECD. OECD Guidelines for the Testing of Chemicals No. 423: Acute Oral Toxicity—Acute Toxic Class Method; OECD: Paris, France, 1996. [Google Scholar]
- Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academic Press: Washington, DC, USA, 2011.
- Chung, F.L.; Wang, M.; Rivenson, A.; Iatropoulos, M.J.; Reinhardt, J.C.; Pittman, B.; Ho, C.T.; Amin, S.G. Inhibition of lung carcinogenesis by black tea in Fischer rats treated with a tobacco-specific carcinogen: Caffeine as an important constituent. Cancer Res. 1998, 58, 4096–4101. [Google Scholar]
- Van Pelt, L. Ketamine and xylazine for surgical anesthesia in rats. J. Am. Vet. Med. Assoc. 1977, 171, 842–844. [Google Scholar]
- Henry, R. Clinical Chemistry. Principles and Techniques; Harper & Row, Publishers: New York, NY, USA, 1964. [Google Scholar]
- Doumas, B.T.; Watson, W.A.; Biggs, H.G. Albumin standards and the measurement of serum albumin with bromcresol green. Clin. Chim. Acta 1971, 31, 87–96. [Google Scholar] [CrossRef]
- Rettman, S.; Frankel, L.S. A colorimetric method for the determination of serum glutamic oxalacetic and glutamic pyruvic transaminases. Am. J. Clin. Pathol. 1957, 28, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Tabacco, A.; Meiattini, F.; Moda, E.; Tarli, P. Simplified enzymic/colorimetric serum urea nitrogen determination. Clin. Chem. 1979, 25, 336–337. [Google Scholar] [CrossRef] [PubMed]
- Gochman, N.; Schmitz, J.M. Automated determination of uric acid, with use of auricase—Peroxidase system. Clin. Chem. 1971, 17, 1154–1159. [Google Scholar] [CrossRef] [PubMed]
- Reinhold, J.G. Standard Methods in Clinical Chemistry; Academic Press: New York, NY, USA, 1953; p. 95. [Google Scholar]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Goldberg, D.M.; Spooner, R.J. Glutathione reductase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Bergmeyer, J., Gra, B.I.M., Eds.; Verlag Chemie: Basel, Switzerland, 1983; Volume 3, pp. 258–265. [Google Scholar]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Habig, W.H.; Pabst, M.J.; Jakoby, W.B. Glutathione S-transferases the first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974, 249, 7130–7139. [Google Scholar] [CrossRef]
- Beers, R.F.; Sizer, I.W. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J. Biol. Chem. 1952, 195, 133–140. [Google Scholar] [CrossRef]
- Fridovich, I. Superoxide dismutases. Adv. Enzymol. Relat. Areas J. Mol. Biol. 1974, 41, 35–97. [Google Scholar]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Suvarna, S.K.; Layton, C.; Bancroft, J.D. Bancroft’s Theory and Practice of Histological Techniques, 8th ed.; Elsevier: Oxford, UK, 2019. [Google Scholar]

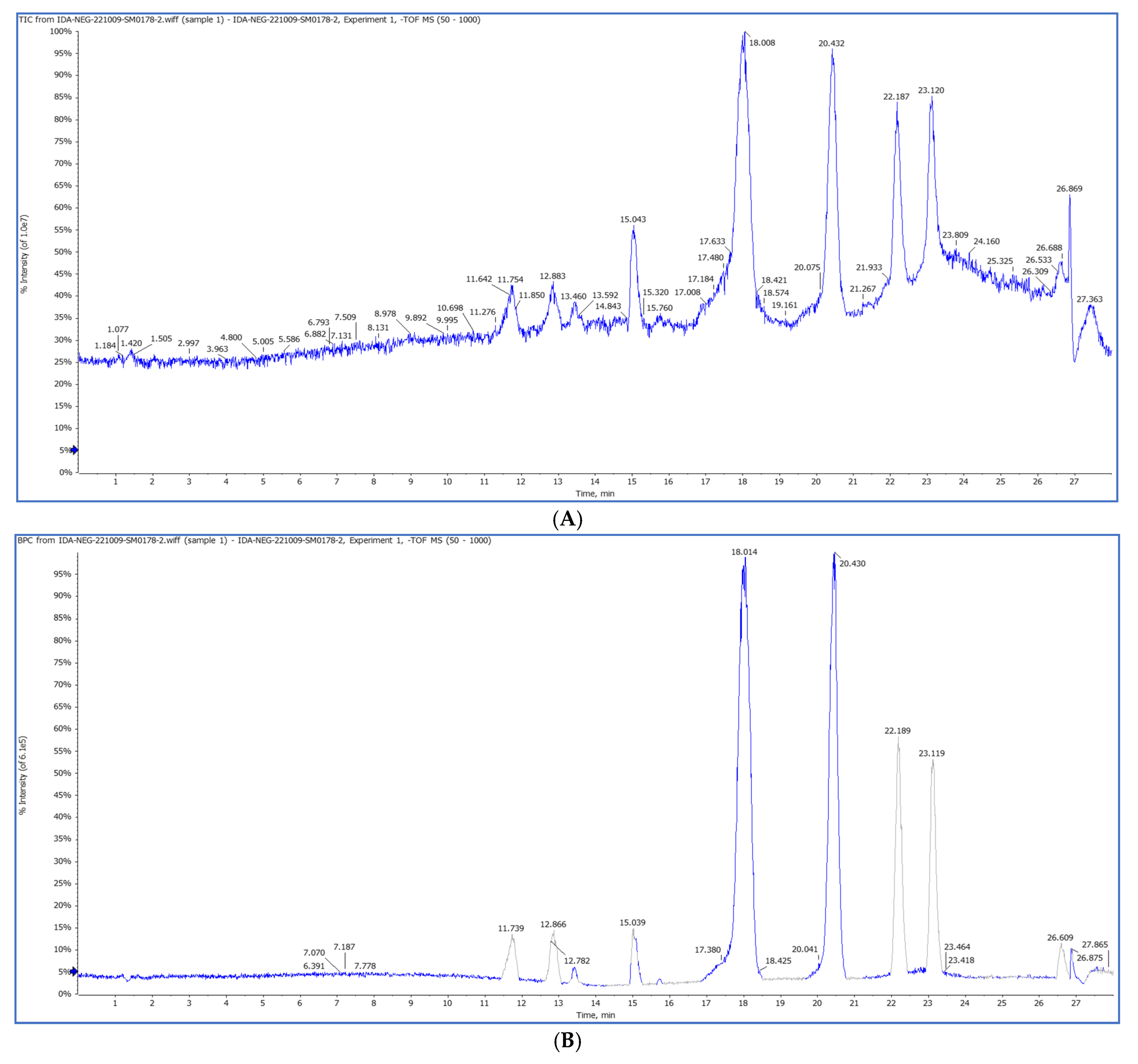
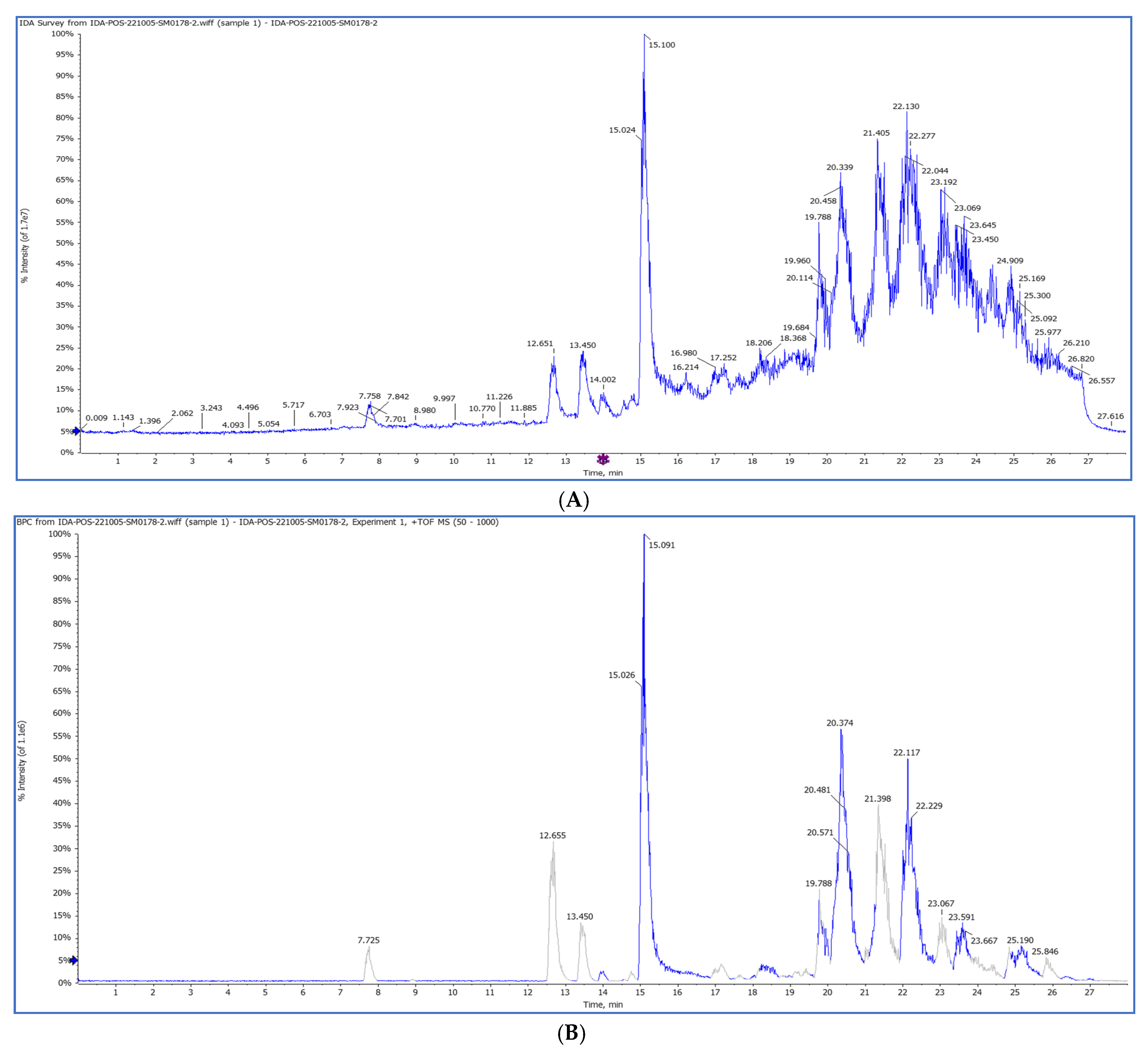
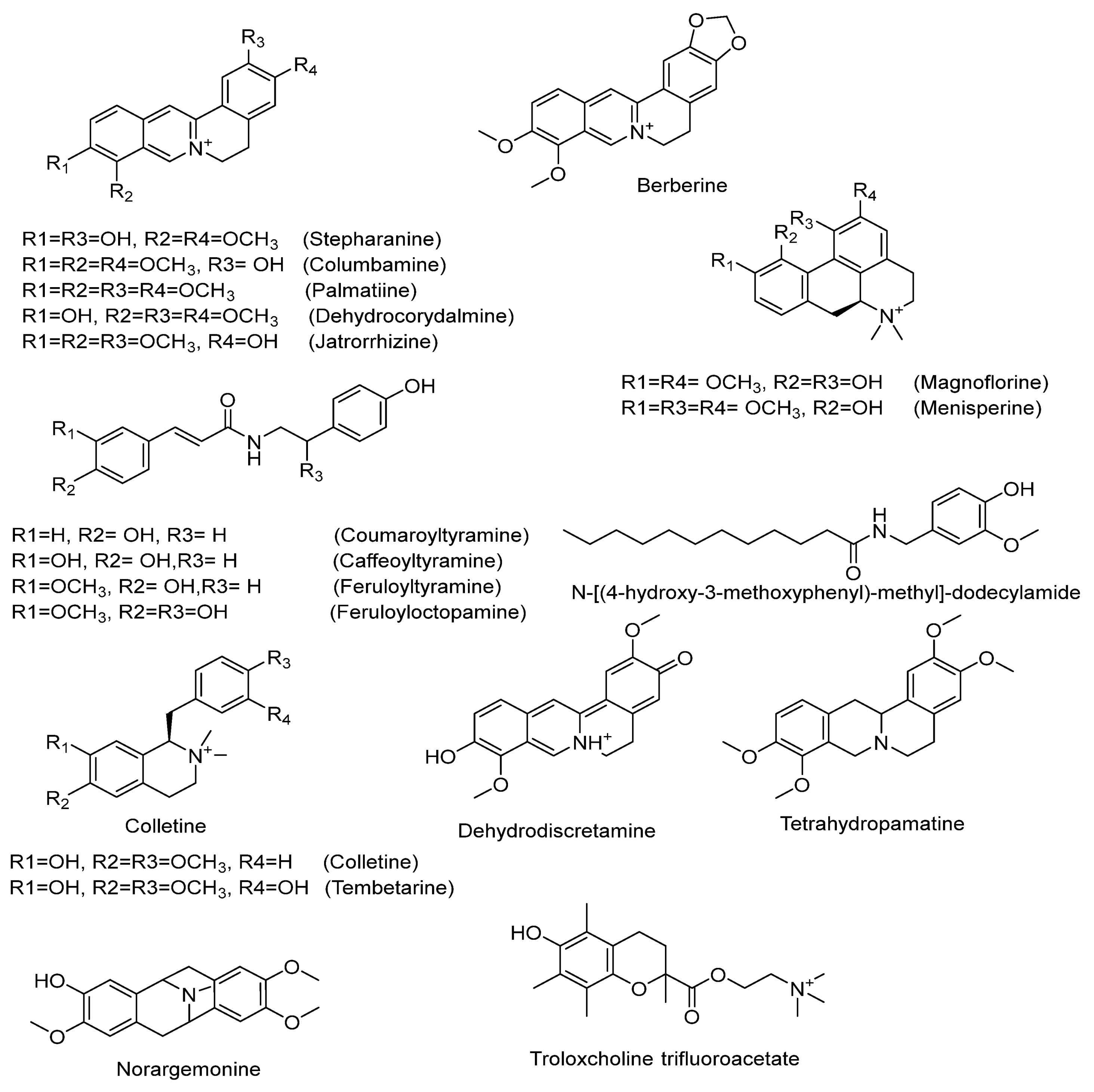
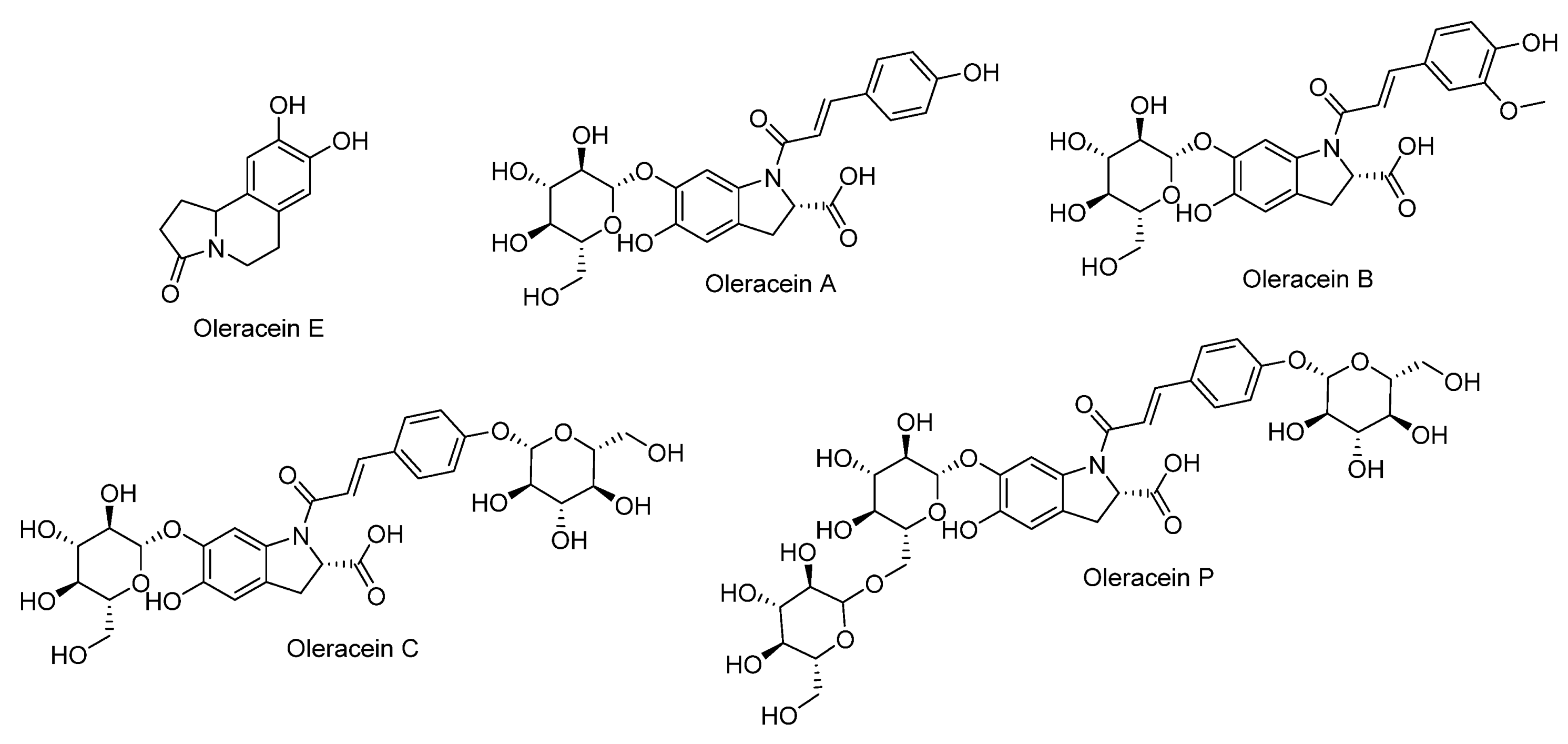
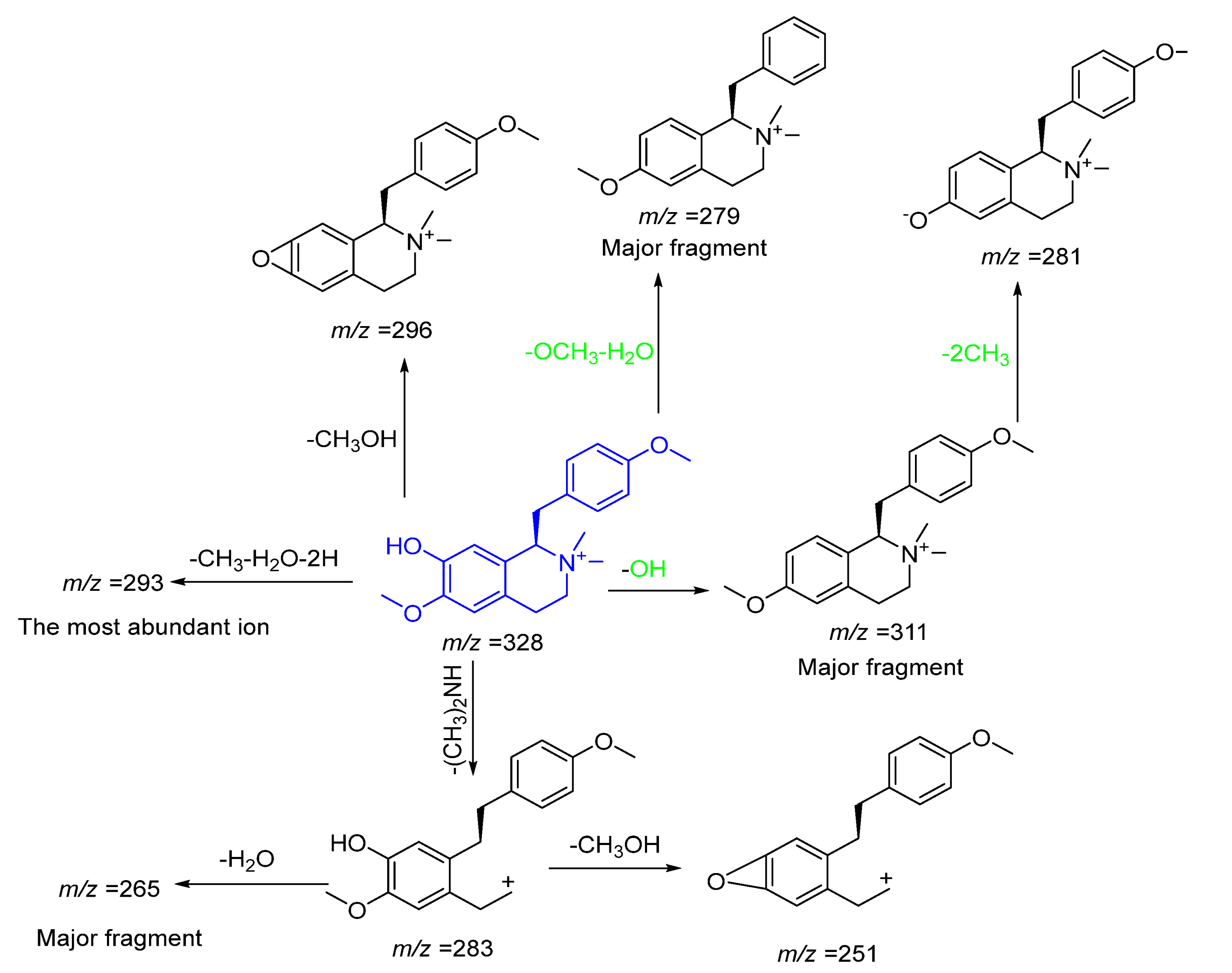
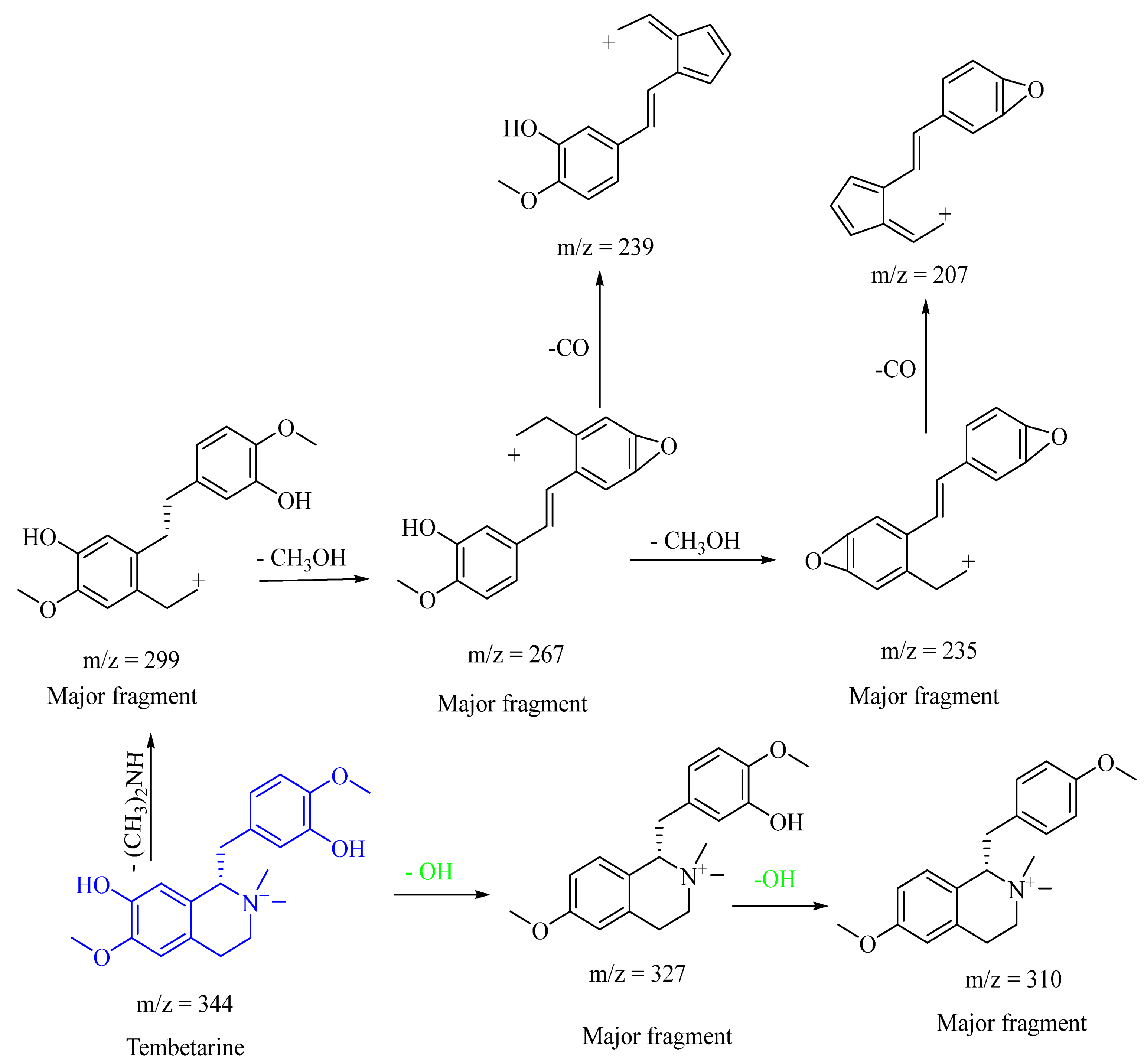
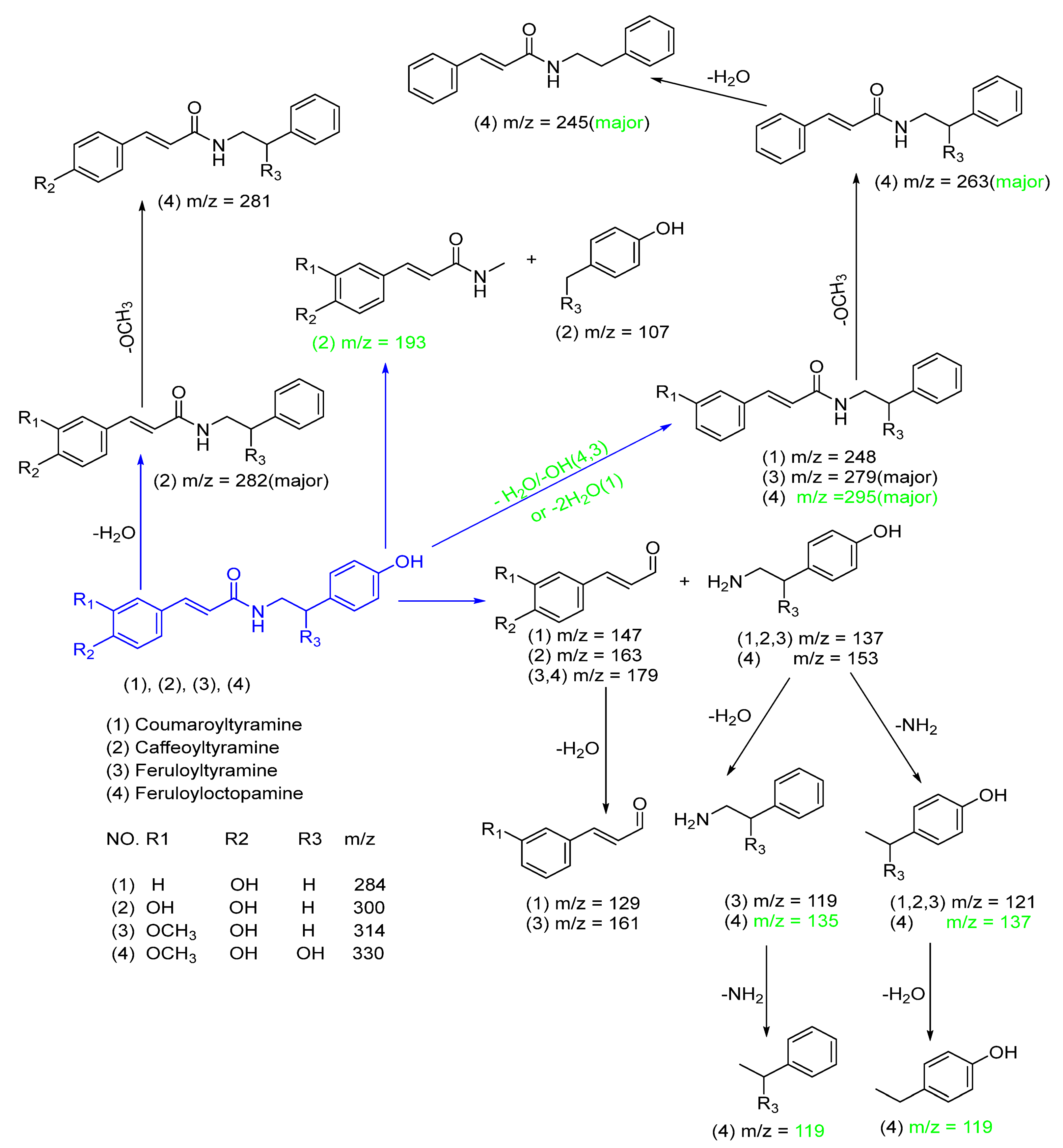
| No. | Rt min | Tentatively Identified Compounds | Class | Mol. Ion m/z ppm (±) | Molecular Formula | Error (ppm) | MS/MS |
|---|---|---|---|---|---|---|---|
| 1 | 12.662 | * Nigellidine | Alkaloid | 295.1922 | C18H19N2O2+ | 0.1 | 277.2204[M+H-H2O]+, 280.1874[M+H-CH3]+, 282.900, 203.0165[M+H-C6H6OH]+, 267.0979[M+H-CO]+, 249.2220[M+H-H2O-CO]+ 163.0501 |
| 2 | 13.062 | Oleracein E | Alkaloid | 220.1441 | C21H14NO3+ | 0.17 | 202.12510[M+H-H2O]+, 192.1367, 202.12510[M+H-CO]+, 162.0900, 177.1242, 171.1007, 122.0620 |
| 3 | 13.163 | Tembetarine | Alkaloid | 344.3013 | C20H26NO4+ | 0.6 | 327.0811[M+-OH]+, 299.1520[M+-(CH3)2NH]+, 267.2640[M+-(CH3)2NH-CH3OH]+, 235.2196[M+-(CH3)2NH-2CH3OH]+, 177.1141 |
| 4 | 15.075 | *** Jatrorrhizine | Alkaloid | 338.3384 | C20H20NO4+ | 0.8 | 320.2390[M+-H2O]+, 323.3271[M+-CH3]+, 294.1001[M+-CH3-H-CO]+, 292.2130[M+-OCH3-CH3]+, 275.2001[M+-OCH3-CH3OH]+, 274.2024[M+-OCH3-CH3-H2O]+, 256.2677 |
| 5 | 20.369 | ** Magnoflorine | Alkaloid | 342.0910 | C20H24NO4+ | 0.33 | 324.0550[M+-H2O]+, 307.2593[M+-H2O-OH]+, 296.8945 [M+-(CH3)2NH]+, 293.2233[M+-H2O-OCH3]+, 282.2715[M+-(CH3)2NH-CH3]+, 279.186 [M+-H2O-(CH3)2NH]+, 265.2275[M+-CH3OH]+, 261.2180[M+-(CH3)2NH-H2O]+, 243.2086, 237.1840[M+-(CH3)2NH-CH3OH-CO]+, 209.1366[M+-(CH3)2NH-CH3OH-CO-C2H4]+, 191.1449[M+-(CH3)2NH-CH3OH-CO-C2H4-H2O]+ |
| 6 | 20.418 | ** Stepharanine | Alkaloid | 324.2862 | C19H18NO4+ | 1.7 | 309.2202[M+-CH3]+, 306.2829[M+-H2O]+, 294.2925[M+-2CH3]+, 292.2032[M+-CH3OH]+, 260.2106[M+-2CH3OH]+, 263.2321[M+-2OCH3]+, 278.2199[M+-OCH3-CH3]+, 275.1972[M+-H2O-OCH3]+, 256.2619[M+-H2O-OCH3]+, 179.1082 |
| 7 | 20.446 | ** Oleracein A | Alkaloid | 504.3752 | C24H26NO11+ | 0.4 | 487.3500, 486.3576[M+H-H2O]+, 469.2950[M+H-2H2O]+, 423.3385[M+H-2H2O-COO]+, 451.3341[M+H-3H2O]+, 342.0766[M+H-gl(162)]+, 254.1595, 271.1985, 197.1095 [M +H-C9H6O2(P-coumaroyl)]+ |
| 8 | 21.18 | * Colletine | Alkaloid | 328.1046 | C20H26NO3+ | 0.7 | 311.2313 [M+-OH]+, 310.2640, 296.3214[M+-CH3OH]+, 281.1769 [M+-OH-2CH3]+, 283.1830[M+-(CH3)2NH]+, 279.2029 [M+-OCH3-H2O]+, 265.1799[M+-(CH3)2NH-H2O]+, 269.2070, 251.2050[M+-(CH3)2NH-CH3OH]+, 243.2094, 265.1799, 261.2265, 179.1420, 173.1339 |
| 9 | 21.252 | * Dehydrocorydalmine | Alkaloid | 338.3391 | C20H20NO4+ | −3.7 | 321.2424, 320.2627[M-H2O]+, 306.2180[M+-CH3OH]+, 294.1305[M+-OCH3-H-CO]+, 292.2049[M+-OCH3-CH3]+, 275.2012[M+-OCH3-CH3OH]+, 256.2639[M+-2OCH3-H2O]+, 243.1604[M+-2OCH3-H2O-CH3]+, 233.1604 |
| 10 | 21.364 | * Dehydrodiscretamine | Alkaloid | 324.2878 | C19H18NO4+ | 1.0 | 307.2221, 306.2587[M+-H2O]+, 292.1013[M+-CH3OH]+, 278.1936[M+-OCH3-CH3]+, 277.1705, 275.2005, 257.1810, 250.1868, 239.1841, 232.1763 |
| 11 | 21.437 | * Caffeoyltyramine | Alkaloid | 300.2835 | C17H18NO4+ | 0.27 | 283.2577, 282.2772[M+H-H2O]+, 265.2273, 191.1745[M+H-C7H8O−]+, 107.038651[M+H-C10H11NO3−]+, 163.10785[M+H-C8H10NO−2]+, 137.1215[M+H-C9H7O3−]+, 123.1193, 121.0939[M+H-C9H7O3−-NH2]+, 165.1594, 129.0651[M+H-C8H10NO−2-2H2O]+ |
| 12 | 21.453 | ** Berberine | Alkaloid | 336.2517 | C20H18NO4+ | 0.12 | 334.10100[M+-2H]+, 319.2826, 320.2473[M+-CH3-H]+, 321.1630[M+-CH3]+, 306.1610[M+-2CH3]+, 292.0728[M+-CH3-H-CO]+, 274.2216[M+-2OCH3]+, 262.1481[M+-OCH3-CH3-CO]+, 246.48002[M+-2OCH3-CO]+, 145.1005, 135.1135 |
| 13 | 21.514 | * Coumaroyltyramine (prapazine) | Alkaloid | 284.2812 | C17H18NO3+ | 3.3 | 267.0846[M+H-OH]+, 248.2024[M+H-2H2O]+, 147.0994[M+H-C8H10NO−2]+, 137.1385[M+H-C9H8O2−]+, 133.1021, 121.10351[M+H-C9H8O2−-NH2]+, 122.1078, 129.05255[M+H-C8H10NO−2-H2O]+ |
| 14 | 21.780 | C olumbamine | Alkaloid | 338.3382 | C20H20NO4+ | −1.9 | 320.2450[M+-H2O]+, 303.2401, 307.2104[M+-H-OCH3]+ 275.2061[M+-OCH3-CH3OH]+, 257.1927[M+-2OCH3-H2O-H]+, 256.2644[M+-2OCH3-H2O-2H]+, 243.0915[M+-2OCH3-H2O-CH3]+, 233.4401 |
| 15 | 21.854 | * Feruloyltyramine | Alkaloid | 314.2883 | C18H20NO4+ | 4.7 | 297.2309, 298.7040[M+H-CH3]+, 279.2309, 278.3351[M+H-2H2O]+, 265.1851[M+H-H2O-OCH3]+, 261.2226, 243.2086, 223.1661, 179.1451[M+H-C8H11NO−]+, 161.1316[M+H-C8H11NO−-H2O]+,137.1287[M+H-C10H11O3−]+, 121.0997 [M+H-C10H11O3-NH2]+, 119.0847[M+H-C10H11O3-H2O]+ |
| 16 | 22.025 | * Norargemonine | Alkaloid | 340.2202 | C20H22NO4− | 0 | 322.2092[M+-H-H2O]−, 294.0803[M+-OCH3-CH3-H]−, 278.2505[M+-2OCH3-H]−, 265.0680, 256.2393, 241.0059 |
| 17 | 22.129 | ** Menisperine | Alkaloid | 356.3600 | C21H26NO4+ | 0.31 | 341.1823[M+-CH3]+, 310.9110[M+-(CH3)2NH]+, 339.2567[M+-H2O]+, 324.2220 [M+-CH3OH]+, 324.9888[M-OCH3]+, 293.2103[M+-CH3OH+-OCH3]+, 279.1961[M+-(CH3)2NH-CH3OH]+, 296.3794[M+-CH3OH-CO]+, 251.2101[M+-CH3OH-CO-(CH3)2NH]+, 179.1054 |
| 18 | 22.298 | * Feruloylglycine | Alkaloid | 250.1734 | C12H12NO5− | 1.2 | 234.1130, 235.1130[M-H-OCH3]−, 218.85265[M-H-OCH3]−, 205.11970[M-H-COOH]−, 177.1267 [M-H-C2H4NO2]−, 113.9887[M-H-C7H7O2]− |
| 19 | 22.315 | Troloxcholine | Alkaloid | 336.2572 | C19H30NO4+ | 2.28 | 319.2743[M+-H2O]+, 277.2139[M+-C3H9N•+]+, 293[M+-CH3-CO]+ |
| 20 | 22.468 | Feruloyloctopamine | Alkaloid | 330.0861 | C18H20NO5+ | 299.6067, 295.2597[M+H-OH-H2O]+, 294.3407[M+H-2H2O]+, 312.2778[M+H-H2O]+, 294.3407[M+H-2H2O]+, 280.9635[M+H-H2O-OCH3]+, 263.2357[M+H-2H2O-OCH3]+, 245.2264[M+H-3H2O-OCH3]+, 179.1799[M+H-C8H12NO2−]+, 153.0845[M+H-C10H11O3−]+, 137.1320[M+H-C10H11O3-H2O]+, 135.1139[M+H-C10H11O3-NH2]+, 122.1030[M+H-C10H11O3-NH2-H2O]+, 121.0997[M+H-C10H11O3-H2O-NH2]+, 133.0999 | |
| 21 | 22.481 | Palmatine | Alkaloid | 352.2412 | C21H22NO4+ | 0.6 | 337.8639[M+-CH3]+, 335.2989 [M+-CH3-2H]+, 323.9123, [M+-CO]+, 307.9925 [M+-CH3-H-CO]+, 306.2431[M+-CH3-OCH3]+, 295.8660, 275.1961[M+-2OCH3-H]+, 291.2285[M+-OCH3-2CH3]+, 232.9020 |
| 22 | 24.457 | Oleracein B | Alkaloid | 534.3407 | C25H28NO12+ | 0.24 | 517.4145[M+H-H2O]+, 516.4204, 471.3734[M+H-H2O-COO]+, 372.3595[M+H-gl(162)]+, 329.2121[M+H-gl(162)-COO]+, 331.2160, 145.0132 |
| 23 | 24.858 | Tetrahydropamatine | Alkaloid | 356.3455 | C21H26NO4+ | 0.2 | 341.080[M+H-CH3]+, 339.2951, 324.9885[ M+H-OCH3]+, 321.2728, 293.8981[ M+H-2OCH3]+, 293.2381, 279.2328, 261.2191 |
| 24 | 25.692 | Oleracein C | Alkaloid | 666.4575 | C30 H35NO16+ | 504.3685[M+H-hex(162)]+, 342.3380[M+H-dihex(324)]+, 324.2831[M+H-dihex(324)-H2O]+, 177.9566[M+H-dihex(324)-H2O-146(coumaroyl]+, 297.2211[M+H-dihex(324)-COOH]+, 151.1112[M+H-dihex(324)-COOH-146(coumaroyl]+ | |
| 25 | 26.530 | N-[(4-hydroxy-3-methoxyphenyl)-methyl]-dodecylamide | Alkaloid | 336.3225 | C20H34NO3+ | 4.2 | 319.2974, 318.2473[M+H-H2O]+, 198.1920[M+H-C12H24NO (dodecanamide)]+, 301.0460[M+H-H2O-2H-CH3]+ |
| 26 | 26.686 | Oleracein P | Alkaloid | 826.2395 | C36H45NO21− | 1.5 | 664.4680[M+H-hex(162)]+, 502.4850[M+H-dihex(324)]+, 340.4720[M+H-trihex(486)]+, 295.2294[M+H-trihex(486)-COOH]+ |
| Terpenes | |||||||
| 27 | 18.283 | Nigellic acid | Monoterpenes | 279.2222 | C15H19O5− | 7.0 | 261.1911[M-H-H2O]−, 235.2300[M-H-COO]−, 170.9436, 104.9581 |
| 28 | 15.443 | Triptophenolide | Terpenoids | 311.2586 | C20H23O3− | 0.26 | 293.1921[M-H-H2O]−, 296.0617[M-H-CH3]−, 295.0300, 281.0381[M-H-2CH3]−, 283.2449[M-CO]−, 267.2654[M-H-CO2]−, 237.0612 |
| Fatty acids | |||||||
| 29 | 12.770 | Hydroxy octadecadienoic acid | Fatty acid | 295.2259 | C18H31O3− | 1.8 | 277.2164[M-H-H2O]−, 233.2243[M-H-H2O-CO2]−, 259.2061[M-H-2H2O]−, 195.1389, 183.1025, 188.9388 |
| 30 | 13.273 | Hydroxy octadecenoic acid | Fatty acid | 297.2425 | C18H33O3− | 0.2 | 279.2344[M-H-H2O]−, 251.2292[M-H-H2O-CO]−, 228.9338, 183.0116, 225.0638[M-H-C2H2O3]−, 237.0507[M-H-CH3-COOH]− |
| 31 | 14.091 | Ricinoleic | Fatty acid | 297.2434 | C18H33O3− | 3.3 | 279.2327[M-H-H2O]−, 261.2215[M-H-2H2O]−, 228.9309, 188.9388 |
| 32 | 15.040 | ** D-Glucitol monomyristate (D-Glucitol,tetradecanoate) | Fatty acid | 393.2857 | C20H41O7+ | 1.9 | 376.2586[M+H-H2O]+, 302.1852[M+H-C3H7O3∙]+, 293.1858[M+H-C7H15]+, 209.1282[M+H-C7H13O7]+ |
| 33 | 15.235 | 2-hydroxyeicosanoic acid | Fatty acid | 326.9309 | C20H39O3− | 0.82 | 312.0544[M-H-CH3]−,291.2029[M-H-2H2O]− 282.9245[M-H-CO2]−, 264.8793[M-H-CO2-H2O]−, 258.9391, 190.9550 |
| 34 | 17.276 | Trihydroxy-octadecadienoic acid | Fatty acid | 327.2889 | C18H31O5− | 0.43 | 281.2420[M-H-H2O-CO]−, 258.9403, 218.9488, 190.9547, 188.9331 |
| 35 | 18.060 | ** Linolenic acid | Fatty acid | 277.2161 | C18H29O2−/C18H33O2+ | 0.14 | 259.2064[M-H-H2O]−, 233.2271[M-H-CO2]−, 205.1974, 182.1308, 127.0762, 149.0237 |
| 36 | 18.47 | Stearic acid | Fatty acid | 283.2650 | C16H35O2− | 2.3 | 265.255[M-H-H2O]−, 255.1884[M-H-CO2]−, 223.0362[M-H-COOH-CH3]−, 209.0418 |
| 37 | 19.824 | Dihydroxy octadecatrienoic acid | Fatty acid | 309.2042 | C18H29O4− | 1.81 | 291.2028[M-H-H2O]−, 265.0540[M-H-CO2]−, 281.1030[M-H-CO]−, 241.2192, 198.0627, 180.9669 |
| 38 | 19.916 | Arachidonic acid | Fatty acid | 303.2290 | C19H32O2− | 2.82 | 258.9405[M-H-CO2]−, 234.9808, 244.1144[M-H-CO2-CH3]−, 218.9479, 220.9537, 190.9508 |
| 39 | 20.369 | ** Hydroxy octadecatrienoic acid | Fatty acid | 293.2124 | C18H29O3− | 0.8 | 275.2055[M-H-H2O]−, 257.1020 [M-H-2H2O]−, 265.1780[M-H-CO]−, 249.1800[M-H-CO2]−, 236.1065[M-H-CO-C2H5]−, 221.1552, 220.1456[M-H-CO2-C2H5]−, 136.0850, 205.1241[M-H-C3H5O3]− |
| 40 | 20.380 | ** alpha-Linoleic acid | Fatty acid | 279.2303 | C18H31O2− | 1.1 | 261.2224[M-H-H2O]− |
| 41 | 22.174 | * Palmitic acid | Fatty acid | 255.2297 | C16H31O2− | 2.1 | 237.2191[M-H-H2O]− |
| 42 | 22.186 | * 2-Hydroxypalmitic acid | Fatty acid | 271.2281 | C16H31O3− | 1.3 | 253.1769[M-H-H2O]−, 235.1970 [M-H-2H2O]−, 225.2154[M-H-H2O-CO]−, 212.0614, 183.0366 |
| 43 | 22.225 | * Oleic acid | Fatty acid | 281.2461 | C18H33O2− | 1.4 | 253.0588[M-H-CO]−, 222.1093[M-H-CH3-CO2]−, 130.9880, 112.9885, 104.9558 |
| 44 | 22.299 | Eicosapentaenoic acid | Fatty acid | 301.2165 | C20H29O2− | 1.1 | 255.2320[M-H-COOH]− |
| 45 | 22.458 | Methyl octadeca-9,12-dienoate | Fatty acid | 293.2467 | C19H31O2− | 2.7 | 278.1045[M-H-CH3]−, 236.1085, 235.1245[M-H-2CH3-CO]−, 221.1532, 220.1466[M-H-COOCH3-CH3]−, 197.0990, 148.0559 |
| 46 | 24.304 | Trans-11-methyl-12-octadecenoic acid | Fatty acid | 295.2641 | C19H34O2− | 1.3 | 267.0340[M-H-CO]−, 251.1715[M-H-CO2]−, 233.0780, 230.9859 |
| Sterols | |||||||
| 47 | 21.907 | Campesterol | 401.2657 | C28H49O+ | −4.1 | 383.2570[M+H-H2O]+, 369.2416[M+H-2H-2CH3]+, 355.1109[M+H-H-3CH3]+, 351.2255, 289.1842, 321.2049, 337.2780 | |
| 48 | 23.303 | Cholestanol | 389.2447 | C27H49O+ | 0.7 | 371.2918[M+H-H2O]+, 356.0695[M+H-H2O-CH3]+, 309.2895[M+H-H2O-60(4CH3)-2H]+ | |
| 49 | 23.354 | Cholesterol | Phytosterols | 387.2398 | C27H47O+ | 2.10 | 369.2651[M+H-H2O]+, 354.4301[M+H-H2O-CH3]+, 309.2600[M+H-H2O-60(4CH3)]+, 243.1957, 228.1601, 205.0653 |
| 50 | 24.705 | β-sitosterols | Sterols | 415.2208 | C29H51O+ | 1.7 | 397.3486[M+H-H2O]+, 379.3319, 380.3282, 275.1148, 171.1013 |
| 51 | 25.533 | Stigmasterols (Fucosterol) | Sterols | 413.3771 | C29H49O+ | 3.2 | 395.3650[M+H-H2O]+, 301.1288, 245.1789, 109.0655 |
| 52 | 25.779 | α-Amyrin | Sterols | 427.3549 | C30H51O+ | −2.19 | 409.3421[M+H-H2O]+, 287.1999[M+H-C9H16O]+ |
| 53 | 26.532 | Ergosterol | Sterols | 397.3432 | C28H45O+ | −3.26 | 380.2905, 379.2682[M+H-H2O]+, 363.2307 [M+H-2CH3-4H]+, 349.5022 [M+H-3CH3-3H]+, 339.3045, 269.2025, 285. 2570, 257.254, 245.2287, 179.1057 |
| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| β-actin | CACGTGGGCCGCTCTAGGCACCAA | CTCTTTGATGTCACGCACGATTTC |
| MMP9 | CGGCACGCCTTGGTGTAGCA | AGGCAGAGTAGGAGCGGCCC |
| IGF-I | GCTATGGCTCCAGCATTCG | TCCGGAAGCAACACTCATCC |
| FOXO1 | CACCTTGCTATTCGTTTGC | CTGTCCTGAAGTGTCTGC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd Elkarim, A.S.; Mohamed, S.H.; Ali, N.A.; Elsayed, G.H.; Aly, M.S.; Elgamal, A.M.; Elsayed, W.M.; El-Newary, S.A. The Phytochemical Profile of the Petroleum Ether Extract of Purslane Leaves and Its Anticancer Effect on 4-(Methylnitrosamino)-1-(3-pyridyl)-1-buta-4 None (NNK)-Induced Lung Cancer in Rats. Int. J. Mol. Sci. 2024, 25, 13024. https://doi.org/10.3390/ijms252313024
Abd Elkarim AS, Mohamed SH, Ali NA, Elsayed GH, Aly MS, Elgamal AM, Elsayed WM, El-Newary SA. The Phytochemical Profile of the Petroleum Ether Extract of Purslane Leaves and Its Anticancer Effect on 4-(Methylnitrosamino)-1-(3-pyridyl)-1-buta-4 None (NNK)-Induced Lung Cancer in Rats. International Journal of Molecular Sciences. 2024; 25(23):13024. https://doi.org/10.3390/ijms252313024
Chicago/Turabian StyleAbd Elkarim, Asmaa S., Safaa H. Mohamed, Naglaa A. Ali, Ghada H. Elsayed, Mohamed S. Aly, Abdelbaset M. Elgamal, Wael M. Elsayed, and Samah A. El-Newary. 2024. "The Phytochemical Profile of the Petroleum Ether Extract of Purslane Leaves and Its Anticancer Effect on 4-(Methylnitrosamino)-1-(3-pyridyl)-1-buta-4 None (NNK)-Induced Lung Cancer in Rats" International Journal of Molecular Sciences 25, no. 23: 13024. https://doi.org/10.3390/ijms252313024
APA StyleAbd Elkarim, A. S., Mohamed, S. H., Ali, N. A., Elsayed, G. H., Aly, M. S., Elgamal, A. M., Elsayed, W. M., & El-Newary, S. A. (2024). The Phytochemical Profile of the Petroleum Ether Extract of Purslane Leaves and Its Anticancer Effect on 4-(Methylnitrosamino)-1-(3-pyridyl)-1-buta-4 None (NNK)-Induced Lung Cancer in Rats. International Journal of Molecular Sciences, 25(23), 13024. https://doi.org/10.3390/ijms252313024





