Combining Cold Atmospheric Plasma and Environmental Nanoparticle Removal Device Reduces Neurodegenerative Markers
Abstract
1. Introduction
2. Results
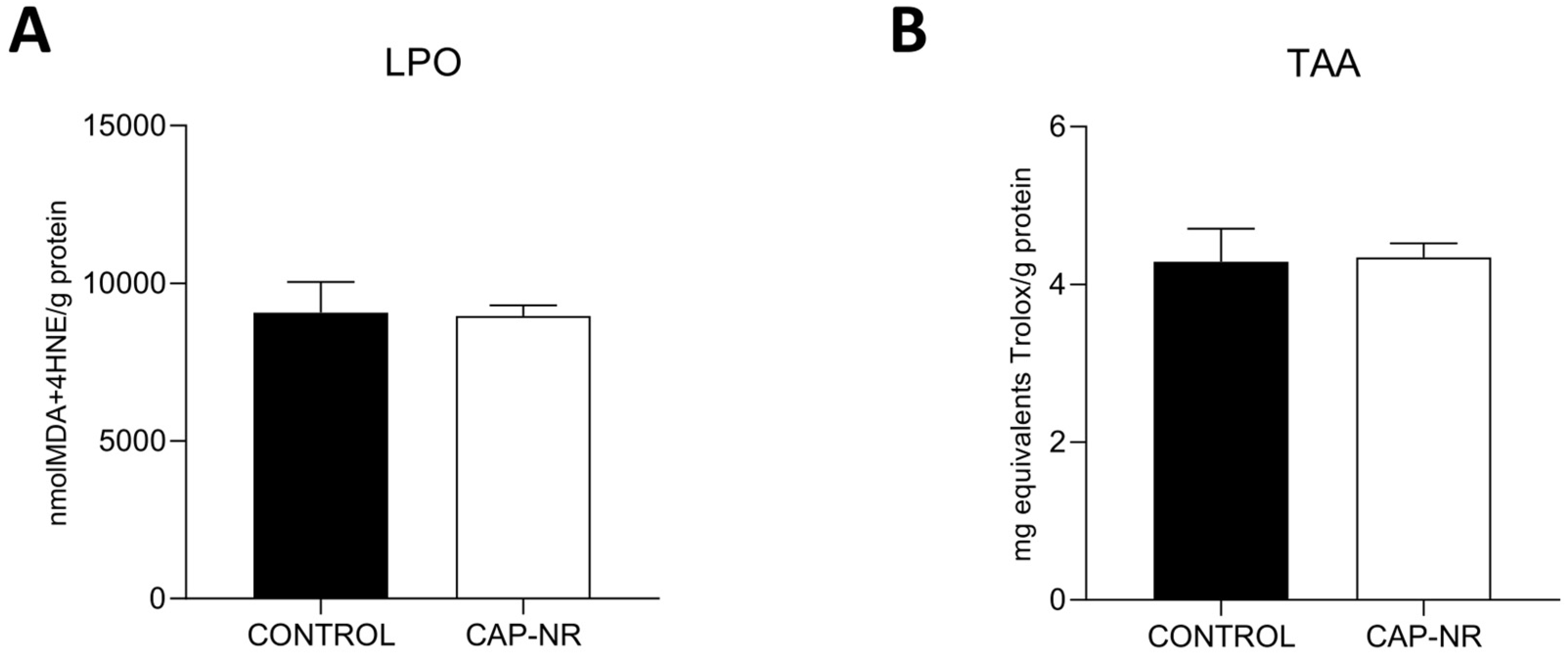
2.1. Oxidative Stress Damage
2.2. Brain Cytokine Levels
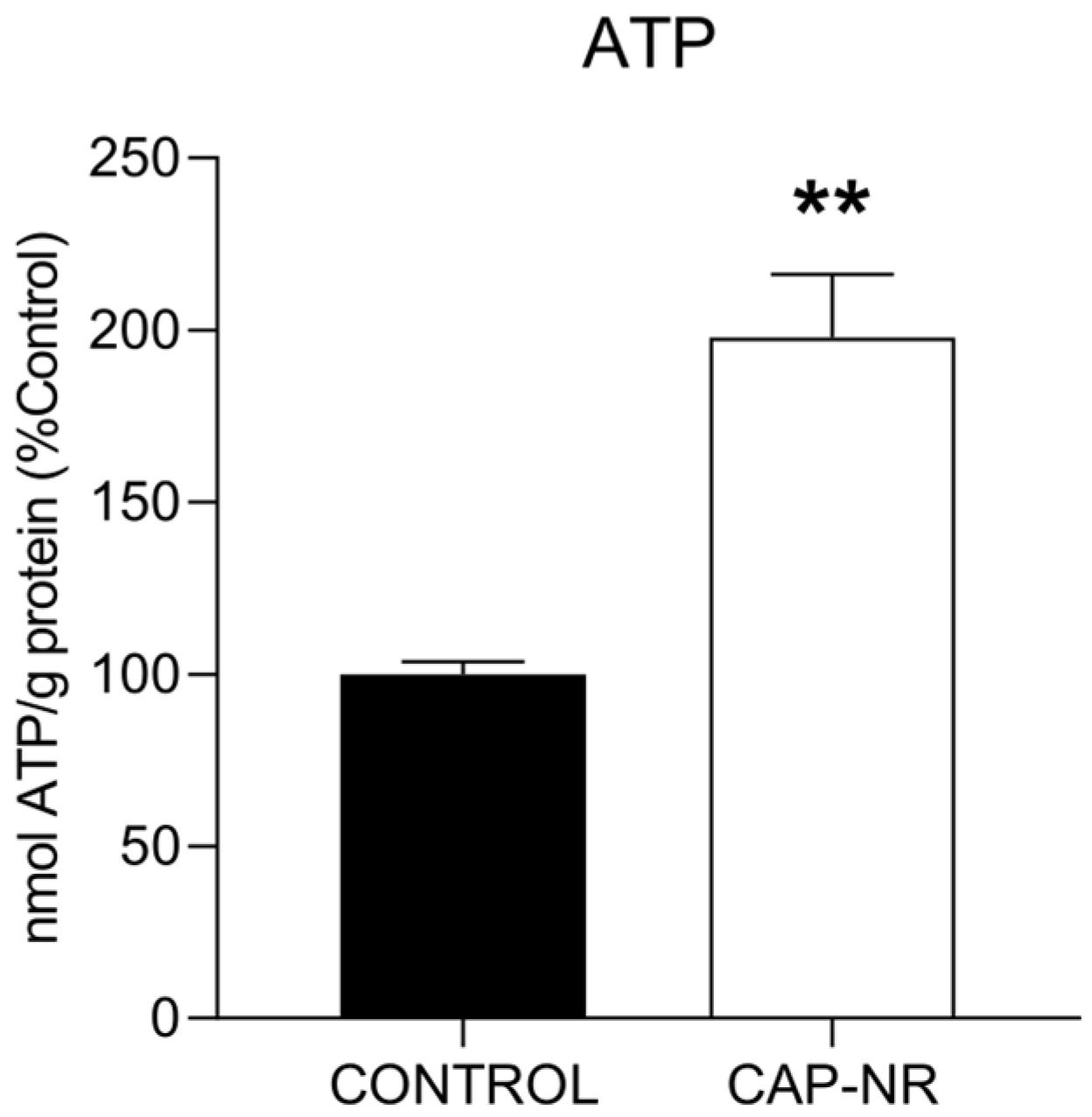
2.3. ATP Levels
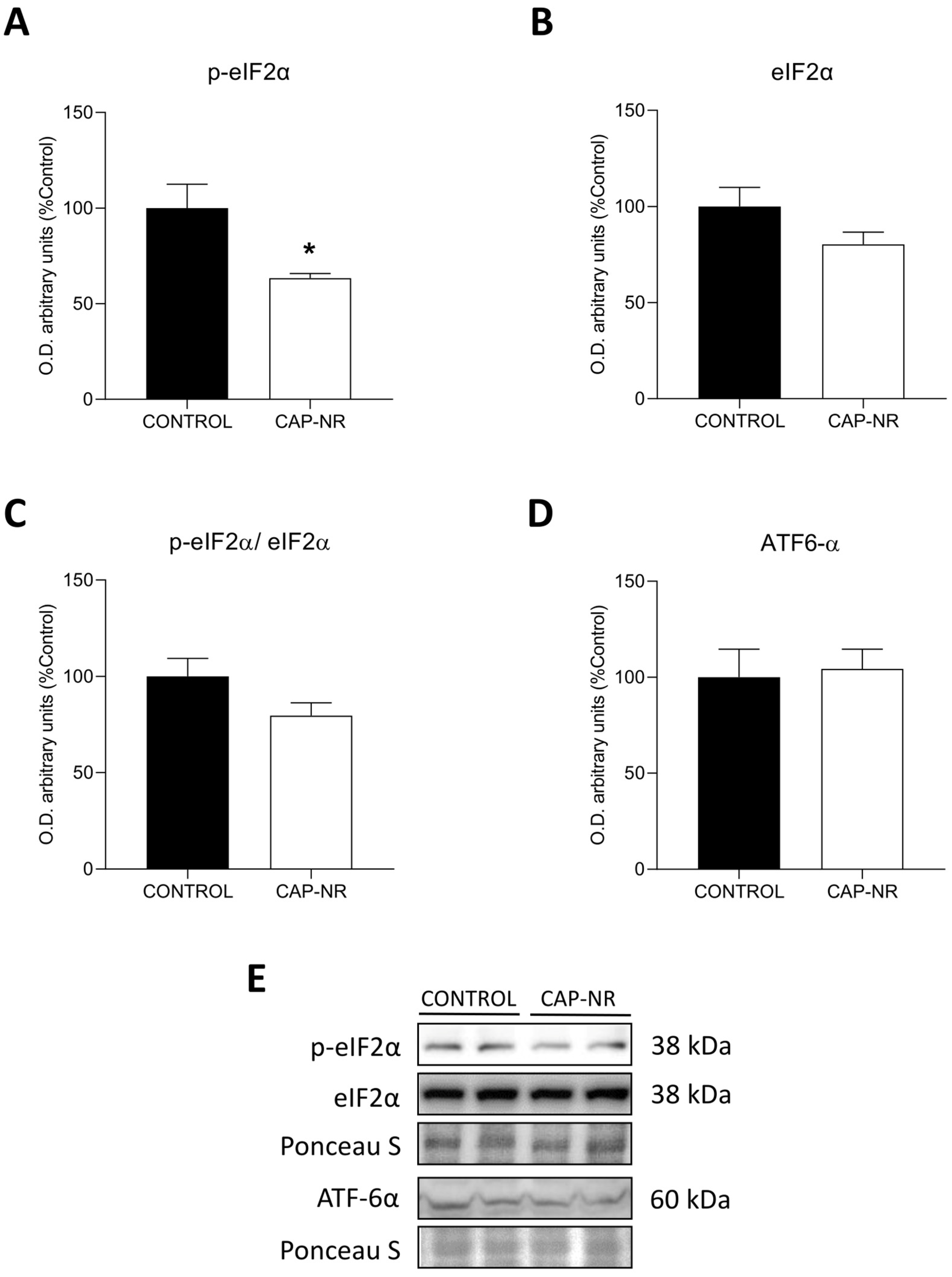
2.4. Exposure to CAP-NR Decreases ER Stress and Proteasome Activity in the Brain
2.5. UPR Pathway in CAP-NR-Treated Mouse Brains
2.6. Autophagy Activation
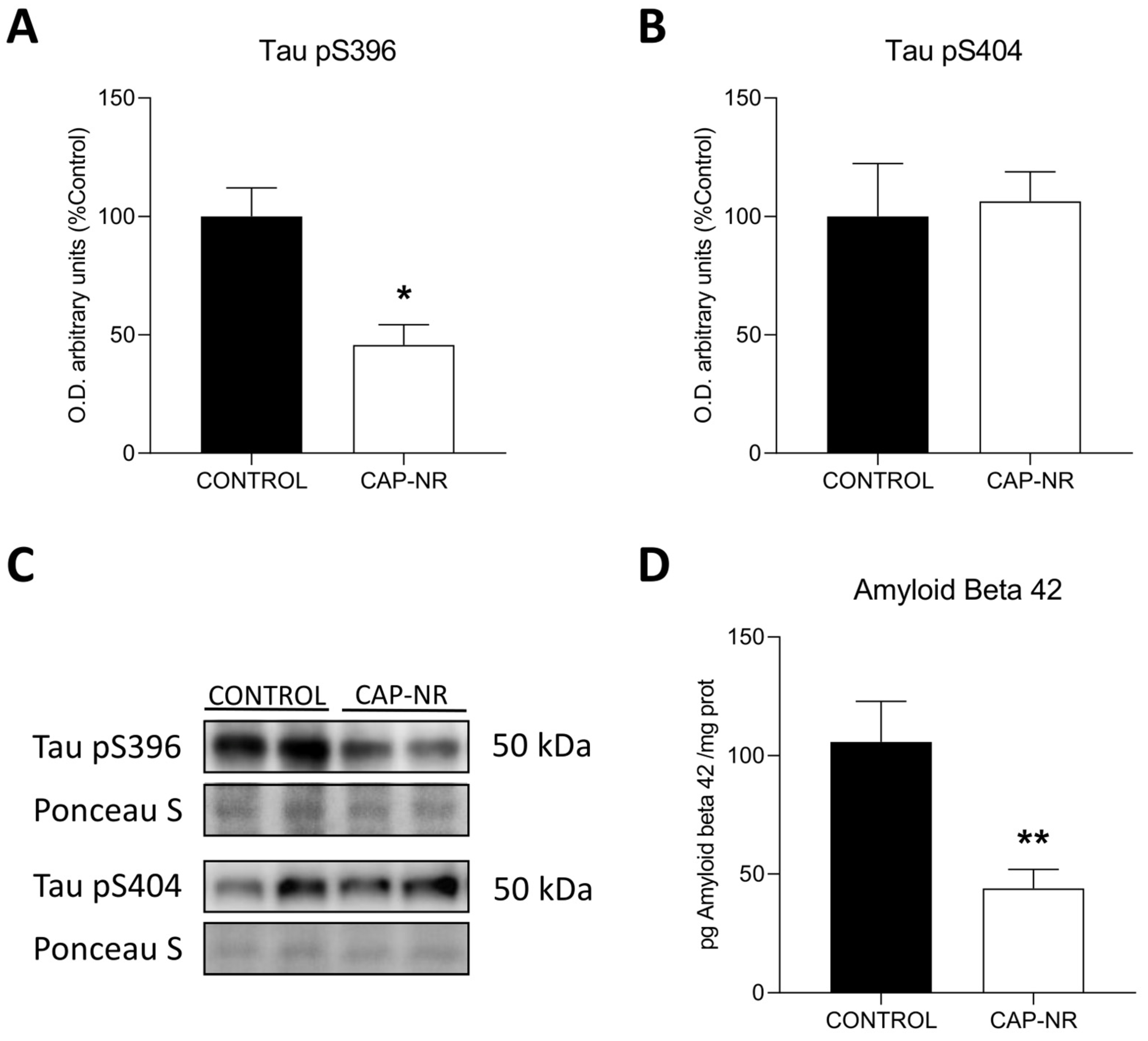
2.7. CAP-NR Treatment Decreases Neurodegeneration Markers
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Tissue Collection and Homogenisation
4.3. CAP-NR Device
- Supply voltage: 230 V;
- Frequency: 50 Hz;
- Maximum power: 525 W;
- Protection type (EN 606011-1): class 1;
- IP protection degree: IP 21;
- Functioning mode: continuous;
- Functioning temperature: 10–40 °C;
- Relative humidity: 35–75% without condensation;
- Maximum capacity for ion generation: 200,000/cm3
4.4. Oxidative Stress
4.4.1. Lipid Oxidative Damage
4.4.2. Total Antioxidant Activity
4.5. Inflammation Studies
4.5.1. Tumour Necrosis Factor α (TNF-α)
4.5.2. Interleukin 6 (IL-6)
4.5.3. Proteasome Activity
4.5.4. ATP Measurement
4.5.5. Amyloid Beta 42 Measurement
4.5.6. Western Blot Immunoassay
4.5.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mucke, L. Alzheimer’s Disease. Nature 2009, 461, 895–897. [Google Scholar] [CrossRef] [PubMed]
- Caballero, B.; Coto-Montes, A. An Insight into the Role of Autophagy in Cell Responses in the Aging and Neurodegenerative Brain. Histol. Histopathol. 2012, 27, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Imran, S.; Ahmad, W.; Saltanat, S. Therapeutic Evaluation of Unani Medicine, Including Single Drugs and Polyherbal Formulations with Special Reference to Neurodegenerative Disorders. Altern. Ther. Health Med. 2024, 30, 54–64. [Google Scholar] [PubMed]
- Kulkarni, A.; Preeti, K.; Tryphena, K.P.; Srivastava, S.; Singh, S.B.; Khatri, D.K. Proteostasis in Parkinson’s Disease: Recent Development and Possible Implication in Diagnosis and Therapeutics. Ageing. Res. Rev. 2023, 84, 101816. [Google Scholar] [CrossRef] [PubMed]
- Amini, J.; Sanchooli, N.; Milajerdi, M.; Baeeri, M.; Haddadi, N.; Sanadgol, N. The Interplay between Tauopathy and Aging through Interruption of UPR/Nrf2/Autophagy Crosstalk in the Alzheimer’s Disease Transgenic Experimental Models. Int. J. Neurosci. 2024, 134, 1049–1067. [Google Scholar] [CrossRef]
- Rubio-González, A.; Bermejo-Millo, J.C.; de Luxán-Delgado, B.; Potes, Y.; Pérez-Martínez, Z.; Boga, J.A.; Vega-Naredo, I.; Caballero, B.; Solano, J.J.; Coto-Montes, A.; et al. Melatonin Prevents the Harmful Effects of Obesity on the Brain, Including at the Behavioral Level. Mol. Neurobiol. 2018, 55, 5830–5846. [Google Scholar] [CrossRef]
- Cuanalo-Contreras, K.; Schulz, J.; Mukherjee, A.; Park, K.-W.; Armijo, E.; Soto, C. Extensive Accumulation of Misfolded Protein Aggregates during Natural Aging and Senescence. Front. Aging Neurosci. 2022, 14, 1090109. [Google Scholar] [CrossRef]
- Kelly, F.J.; Fussell, J.C. Air Pollution and Public Health: Emerging Hazards and Improved Understanding of Risk. Environ. Geochem. Health 2015, 37, 631–649. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.L.; HEI Health Review Committee. Assessment of the Health Impacts of Particulate Matter Characteristics. Res. Rep. Health Eff. Inst. 2012, 161, 5–38. [Google Scholar]
- Calderón-Garcidueñas, L.; Torres-Jardón, R.; Franco-Lira, M.; Kulesza, R.; González-Maciel, A.; Reynoso-Robles, R.; Brito-Aguilar, R.; García-Arreola, B.; Revueltas-Ficachi, P.; Barrera-Velázquez, J.A.; et al. Environmental Nanoparticles, SARS-CoV-2 Brain Involvement, and Potential Acceleration of Alzheimer’s and Parkinson’s Diseases in Young Urbanites Exposed to Air Pollution. J. Alzheimer’s Dis. 2020, 78, 479–503. [Google Scholar] [CrossRef]
- Bernhardt, T.; Semmler, M.L.; Schäfer, M.; Bekeschus, S.; Emmert, S.; Boeckmann, L. Plasma Medicine: Applications of Cold Atmospheric Pressure Plasma in Dermatology. Oxid. Med. Cell. Longev. 2019, 2019, 3873928. [Google Scholar] [CrossRef] [PubMed]
- Bolgeo, T.; Maconi, A.; Gardalini, M.; Gatti, D.; Di Matteo, R.; Lapidari, M.; Longhitano, Y.; Savioli, G.; Piccioni, A.; Zanza, C. The Role of Cold Atmospheric Plasma in Wound Healing Processes in Critically Ill Patients. J. Pers. Med. 2023, 13, 736. [Google Scholar] [CrossRef] [PubMed]
- Marches, A.; Clement, E.; Albérola, G.; Rols, M.-P.; Cousty, S.; Simon, M.; Merbahi, N. Cold Atmospheric Plasma Jet Treatment Improves Human Keratinocyte Migration and Wound Closure Capacity without Causing Cellular Oxidative Stress. Int. J. Mol. Sci. 2022, 23, 10650. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, C.; Berganza, C.; Zhang, J. Cold Atmospheric Plasma: Methods of Production and Application in Dentistry and Oncology. Med. Gas. Res. 2013, 3, 21. [Google Scholar] [CrossRef] [PubMed]
- Izadjoo, M.; Zack, S.; Kim, H.; Skiba, J. Medical Applications of Cold Atmospheric Plasma: State of the Science. J. Wound Care 2018, 27, S4–S10. [Google Scholar] [CrossRef]
- Kazemi, A.; Nicol, M.J.; Bilén, S.G.; Kirimanjeswara, G.S.; Knecht, S.D. Cold Atmospheric Plasma Medicine: Applications, Challenges, and Opportunities for Predictive Control. Plasma 2024, 7, 233–257. [Google Scholar] [CrossRef]
- Adhikari, B.R.; Khanal, R. Introduction to the Plasma State of Matter. Himal. Phys. 2013, 4, 60–64. [Google Scholar] [CrossRef][Green Version]
- Fundamentals of Plasma Physics|SpringerLink. Available online: https://link.springer.com/book/10.1007/978-1-4757-4030-1 (accessed on 8 October 2024).
- Plasma Kinetic Theory|IntechOpen. Available online: https://www.intechopen.com/chapters/57376 (accessed on 8 October 2024).
- Ghemrawi, R.; Khair, M. Endoplasmic Reticulum Stress and Unfolded Protein Response in Neurodegenerative Diseases. Int. J. Mol. Sci. 2020, 21, 6127. [Google Scholar] [CrossRef]
- Schneider, B.; Baudry, A.; Pietri, M.; Alleaume-Butaux, A.; Bizingre, C.; Nioche, P.; Kellermann, O.; Launay, J.-M. The Cellular Prion Protein-ROCK Connection: Contribution to Neuronal Homeostasis and Neurodegenerative Diseases. Front. Cell. Neurosci. 2021, 15, 660683. [Google Scholar] [CrossRef]
- Takamatsu, T.; Uehara, K.; Sasaki, Y.; Miyahara, H.; Matsumura, Y.; Iwasawa, A.; Ito, N.; Azuma, T.; Kohno, M.; Okino, A. Investigation of Reactive Species Using Various Gas Plasmas. RSC Adv. 2014, 4, 39901–39905. [Google Scholar] [CrossRef]
- Stańczyk, B.; Wiśniewski, M. The Promising Potential of Cold Atmospheric Plasma Therapies. Plasma 2024, 7, 465–497. [Google Scholar] [CrossRef]
- Monetto, I. The Effects of an Interlayer Debond on the Flexural Behavior of Three-Layer Beams. Coatings 2019, 9, 258. [Google Scholar] [CrossRef]
- Gross, T.; Ledernez, L.A.; Birrer, L.; Bergmann, M.E.; Altenburger, M.J. Guided Plasma Application in Dentistry—An Alternative to Antibiotic Therapy. Antibiotics 2024, 13, 735. [Google Scholar] [CrossRef] [PubMed]
- Alqutaibi, A.Y.; Aljohani, A.; Alduri, A.; Masoudi, A.; Alsaedi, A.M.; Al-Sharani, H.M.; Farghal, A.E.; Alnazzawi, A.A.; Aboalrejal, A.N.; Mohamed, A.-A.H.; et al. The Effectiveness of Cold Atmospheric Plasma (CAP) on Bacterial Reduction in Dental Implants: A Systematic Review. Biomolecules 2023, 13, 1528. [Google Scholar] [CrossRef] [PubMed]
- Borges, A.C.; Kostov, K.G.; Pessoa, R.S.; de Abreu, G.M.A.; Lima, G.d.M.G.; Figueira, L.W.; Koga-Ito, C.Y. Applications of Cold Atmospheric Pressure Plasma in Dentistry. Appl. Sci. 2021, 11, 1975. [Google Scholar] [CrossRef]
- Park, J.; Lee, H.; Lee, H.J.; Kim, G.C.; Kim, D.Y.; Han, S.; Song, K. Non-Thermal Atmospheric Pressure Plasma Efficiently Promotes the Proliferation of Adipose Tissue-Derived Stem Cells by Activating NO-Response Pathways. Sci. Rep. 2016, 6, 39298. [Google Scholar] [CrossRef]
- Ermakov, A.; Ermakova, O.; Skavulyak, A.; Kreshchenko, N.; Gudkov, S.; Maevsky, E. The Effects of the Low Temperature Argon Plasma on Stem Cells Proliferation and Regeneration in Planarians. Plasma Process. Polym. 2016, 13, 788–801. [Google Scholar] [CrossRef]
- Eggers, B.; Marciniak, J.; Deschner, J.; Stope, M.B.; Mustea, A.; Kramer, F.-J.; Nokhbehsaim, M. Cold Atmospheric Plasma Promotes Regeneration-Associated Cell Functions of Murine Cementoblasts In Vitro. Int. J. Mol. Sci. 2021, 22, 5280. [Google Scholar] [CrossRef]
- Tan, F.; Fang, Y.; Zhu, L.; Al-Rubeai, M. Controlling Stem Cell Fate Using Cold Atmospheric Plasma. Stem Cell Res. Ther. 2020, 11, 368. [Google Scholar] [CrossRef]
- Braný, D.; Dvorská, D.; Halašová, E.; Škovierová, H. Cold Atmospheric Plasma: A Powerful Tool for Modern Medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef]
- Elsaadany, M.; Subramanian, G.; Ayan, H.; Yildirim-Ayan, E. Exogenous Nitric Oxide (NO) Generated by NO-Plasma Treatment Modulates Osteoprogenitor Cells Early Differentiation. J. Phys. D Appl. Phys. 2015, 48, 345401. [Google Scholar] [CrossRef]
- Miletić, M.; Mojsilović, S.; Đorđević, I.O.; Maletić, D.; Puač, N.; Lazović, S.; Malović, G.; Milenković, P.; Petrović, Z.L.; Bugarski, D. Effects of Non-Thermal Atmospheric Plasma on Human Periodontal Ligament Mesenchymal Stem Cells. J. Phys. D Appl. Phys. 2013, 46, 345401. [Google Scholar] [CrossRef]
- Katiyar, K.S.; Lin, A.; Fridman, A.; Keating, C.E.; Cullen, D.K.; Miller, V. Non-Thermal Plasma Accelerates Astrocyte Regrowth and Neurite Regeneration Following Physical Trauma In Vitro. Appl. Sci. 2019, 9, 3747. [Google Scholar] [CrossRef]
- Duchesne, C.; Banzet, S.; Lataillade, J.-J.; Rousseau, A.; Frescaline, N. Cold Atmospheric Plasma Modulates Endothelial Nitric Oxide Synthase Signalling and Enhances Burn Wound Neovascularisation. J. Pathol. 2019, 249, 368–380. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Om, J.-Y.; Kim, Y.-H.; Kim, K.-M.; Choi, E.-H.; Kim, K.-N. Selective Killing Effects of Cold Atmospheric Pressure Plasma with NO Induced Dysfunction of Epidermal Growth Factor Receptor in Oral Squamous Cell Carcinoma. PLoS ONE 2016, 11, e0150279. [Google Scholar] [CrossRef]
- Topala, I.; Nastuta, A. Helium Atmospheric Pressure Plasma Jet: Diagnostics and Application for Burned Wounds Healing. In Plasma for Bio-Decontamination, Medicine and Food Security; Machala, Z., Hensel, K., Akishev, Y., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 335–345. [Google Scholar]
- Antuña, E.; Carlos Bermejo-Millo, J.; Caso-Onzain, E.; Caso-Peláez, E.; Potes, Y.; Coto-Montes, A. Removal of Environmental Nanoparticles Increases Protein Synthesis and Energy Production in Healthy Humans. Front. Bioeng. Biotechnol. 2022, 10, 800011. [Google Scholar] [CrossRef]
- Nakano, M.; Imamura, H.; Sasaoka, N.; Yamamoto, M.; Uemura, N.; Shudo, T.; Fuchigami, T.; Takahashi, R.; Kakizuka, A. ATP Maintenance via Two Types of ATP Regulators Mitigates Pathological Phenotypes in Mouse Models of Parkinson’s Disease. EBioMedicine 2017, 22, 225–241. [Google Scholar] [CrossRef]
- Hetz, C.; Mollereau, B. Disturbance of Endoplasmic Reticulum Proteostasis in Neurodegenerative Diseases. Nat. Rev. Neurosci. 2014, 15, 233–249. [Google Scholar] [CrossRef]
- Mondragón-Rodríguez, S.; Perry, G.; Luna-Muñoz, J.; Acevedo-Aquino, M.C.; Williams, S. Phosphorylation of Tau Protein at Sites Ser(396-404) Is One of the Earliest Events in Alzheimer’s Disease and Down Syndrome. Neuropathol. Appl. Neurobiol. 2014, 40, 121–135. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia Prevention, Intervention, and Care: 2020 Report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- Turrini, E.; Laurita, R.; Stancampiano, A.; Catanzaro, E.; Calcabrini, C.; Maffei, F.; Gherardi, M.; Colombo, V.; Fimognari, C. Cold Atmospheric Plasma Induces Apoptosis and Oxidative Stress Pathway Regulation in T-Lymphoblastoid Leukemia Cells. Oxid. Med. Cell. Longev. 2017, 2017, 4271065. [Google Scholar] [CrossRef]
- Sreedevi, P.R.; Suresh, K.; Yugeswaran, S. Cold Atmospheric Plasma-Induced Oxidative Stress and Ensuing Immunological Response—A Neo-Vista in Immunotherapy. Free Radic. Res. 2022, 56, 498–510. [Google Scholar] [CrossRef]
- Lee, K.H.; Cha, M.; Lee, B.H. Neuroprotective Effect of Antioxidants in the Brain. Int. J. Mol. Sci. 2020, 21, 7152. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.H.; Seo, Y.; Jo, Y.S.; Lee, S.; Cho, E.; Cazenave-Gassiot, A.; Shin, Y.-S.; Moon, M.H.; An, H.J.; Wenk, M.R.; et al. Brain Lipidomics: From Functional Landscape to Clinical Significance. Sci. Adv. 2022, 8, eadc9317. [Google Scholar] [CrossRef]
- Chen, B.; Dong, X.; Zhang, J.; Wang, W.; Song, Y.; Sun, X.; Zhao, K.; Sun, Z. Effects of Oxidative Stress Regulation in Inflammation-Associated Gastric Cancer Progression Treated Using Traditional Chinese Medicines: A Review. Medicine 2023, 102, e36157. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory Responses and Inflammation-Associated Diseases in Organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Andronie-Cioara, F.L.; Ardelean, A.I.; Nistor-Cseppento, C.D.; Jurcau, A.; Jurcau, M.C.; Pascalau, N.; Marcu, F. Molecular Mechanisms of Neuroinflammation in Aging and Alzheimer’s Disease Progression. Int. J. Mol. Sci. 2023, 24, 1869. [Google Scholar] [CrossRef]
- Hirasawa, I.; Odagiri, H.; Park, G.; Sanghavi, R.; Oshita, T.; Togi, A.; Yoshikawa, K.; Mizutani, K.; Takeuchi, Y.; Kobayashi, H.; et al. Anti-Inflammatory Effects of Cold Atmospheric Plasma Irradiation on the THP-1 Human Acute Monocytic Leukemia Cell Line. PLoS ONE 2023, 18, e0292267. [Google Scholar] [CrossRef]
- Anisimova, A.S.; Alexandrov, A.I.; Makarova, N.E.; Gladyshev, V.N.; Dmitriev, S.E. Protein Synthesis and Quality Control in Aging. Aging 2018, 10, 4269–4288. [Google Scholar] [CrossRef]
- Huang, T.; Qin, L.; Zhang, D.; Tong, Q.; Zhu, Q.; Ding, G.; Liu, J. The Mitochondrial Function of Peripheral Blood Mononuclear Cells in Frail Older Patients. Exp. Gerontol. 2024, 197, 112594. [Google Scholar] [CrossRef] [PubMed]
- Błaszczyk, J.W. Energy Metabolism Decline in the Aging Brain-Pathogenesis of Neurodegenerative Disorders. Metabolites 2020, 10, 450. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Dai, C.; Chen, N.; Zhou, X.; Li, H.; Xu, Q.; Xu, Y. Plasma-Activated Medium Exerts Tumor-Specific Inhibitory Effect on Hepatocellular Carcinoma via Disruption of the Salvage Pathway. J. Clin. Biochem. Nutr. 2024, 75, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Maybin, J.-A.; Thompson, T.P.; Flynn, P.B.; Skvortsov, T.; Hickok, N.J.; Freeman, T.A.; Gilmore, B.F. Cold Atmospheric Pressure Plasma-Antibiotic Synergy in Pseudomonas Aeruginosa Biofilms Is Mediated via Oxidative Stress Response. Biofilm 2023, 5, 100122. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, N.; Anathy, V. Pathological Consequences of the Unfolded Protein Response and Downstream Protein Disulphide Isomerases in Pulmonary Viral Infection and Disease. J. Biochem. 2020, 167, 173–184. [Google Scholar] [CrossRef]
- Alshareef, M.H.; Hartland, E.L.; McCaffrey, K. Effectors Targeting the Unfolded Protein Response during Intracellular Bacterial Infection. Microorganisms 2021, 9, 705. [Google Scholar] [CrossRef]
- Cade, S.; Zhou, X.-F.; Bobrovskaya, L. The Role of Brain-Derived Neurotrophic Factor and the Neurotrophin Receptor p75NTR in Age-Related Brain Atrophy and the Transition to Alzheimer’s Disease. Rev. Neurosci. 2022, 33, 515–529. [Google Scholar] [CrossRef]
- Valenzuela, V.; Martínez, G.; Duran-Aniotz, C.; Hetz, C. Gene Therapy to Target ER Stress in Brain Diseases. Brain Res. 2016, 1648, 561–570. [Google Scholar] [CrossRef]
- Okamura, K.; Kimata, Y.; Higashio, H.; Tsuru, A.; Kohno, K. Dissociation of Kar2p/BiP from an ER Sensory Molecule, Ire1p, Triggers the Unfolded Protein Response in Yeast. Biochem. Biophys. Res. Commun. 2000, 279, 445–450. [Google Scholar] [CrossRef]
- Aouichat, S.; Navarro-Alarcon, M.; Alarcón-Guijo, P.; Salagre, D.; Ncir, M.; Zourgui, L.; Agil, A. Melatonin Improves Endoplasmic Reticulum Stress-Mediated IRE1α Pathway in Zücker Diabetic Fatty Rat. Pharmaceuticals 2021, 14, 232. [Google Scholar] [CrossRef]
- Coto-Montes, A.; González-Blanco, L.; Antuña, E.; Menéndez-Valle, I.; Bermejo-Millo, J.C.; Caballero, B.; Vega-Naredo, I.; Potes, Y. The Interactome in the Evolution From Frailty to Sarcopenic Dependence. Front. Cell. Dev. Biol. 2021, 9, 792825. [Google Scholar] [CrossRef] [PubMed]
- Rubio-González, A.; Reiter, R.J.; Luxán-Delgado, B.d.; Potes, Y.; Caballero, B.; Boga, J.A.; Solano, J.J.; Vega-Naredo, I.; Coto-Montes, A. Pleiotropic Role of Melatonin in Brain Mitochondria of Obese Mice. Melatonin Res. 2020, 3, 538–557. [Google Scholar] [CrossRef]
- Olzmann, J.A.; Li, L.; Chin, L.S. Aggresome Formation and Neurodegenerative Diseases: Therapeutic Implications. Curr. Med. Chem. 2008, 15, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Potes, Y.; de Luxán-Delgado, B.; Rodriguez-González, S.; Guimarães, M.R.M.; Solano, J.J.; Fernández-Fernández, M.; Bermúdez, M.; Boga, J.A.; Vega-Naredo, I.; Coto-Montes, A. Overweight in Elderly People Induces Impaired Autophagy in Skeletal Muscle. Free Radic. Biol. Med. 2017, 110, 31–41. [Google Scholar] [CrossRef]
- Yen, S.H.; Liu, W.K.; Hall, F.L.; Yan, S.D.; Stern, D.; Dickson, D.W. Alzheimer Neurofibrillary Lesions: Molecular Nature and Potential Roles of Different Components. Neurobiol. Aging 1995, 16, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Münch, G.; Kuhla, B.; Lüth, H.-J.; Arendt, T.; Robinson, S.R. Anti-AGEing Defences against Alzheimer’s Disease. Biochem. Soc. Trans. 2003, 31, 1397–1399. [Google Scholar] [CrossRef]
- Nagy, Z. Cell Cycle Regulatory Failure in Neurones: Causes and Consequences. Neurobiol. Aging 2000, 21, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Jellinger, K.A.; Bancher, C. Neuropathology of Alzheimer’s Disease: A Critical Update. J. Neural Transm. Suppl. 1998, 54, 77–95. [Google Scholar] [CrossRef]
- Barresi, E.; Baglini, E.; Poggetti, V.; Castagnoli, J.; Giorgini, D.; Salerno, S.; Taliani, S.; Da Settimo, F. Indole-Based Compounds in the Development of Anti-Neurodegenerative Agents. Molecules 2024, 29, 2127. [Google Scholar] [CrossRef]
- Santos, M.A.; Chand, K.; Chaves, S. Recent Progress in Repositioning Alzheimer’s Disease Drugs Based on a Multitarget Strategy. Future Med. Chem. 2016, 8, 2113–2142. [Google Scholar] [CrossRef]
- Hasse, S.; Seebauer, C.; Wende, K.; Schmidt, A.; Metelmann, H.-R.; von Woedtke, T.; Bekeschus, S. Cold Argon Plasma as Adjuvant Tumour Therapy on Progressive Head and Neck Cancer: A Preclinical Study. Appl. Sci. 2019, 9, 2061. [Google Scholar] [CrossRef]
- Utsumi, F.; Kajiyama, H.; Nakamura, K.; Tanaka, H.; Mizuno, M.; Ishikawa, K.; Kondo, H.; Kano, H.; Hori, M.; Kikkawa, F. Effect of Indirect Nonequilibrium Atmospheric Pressure Plasma on Anti-Proliferative Activity against Chronic Chemo-Resistant Ovarian Cancer Cells in Vitro and in Vivo. PLoS ONE 2013, 8, e81576. [Google Scholar] [CrossRef] [PubMed]
- Chatraie, M.; Torkaman, G.; Khani, M.; Salehi, H.; Shokri, B. In Vivo Study of Non-Invasive Effects of Non-Thermal Plasma in Pressure Ulcer Treatment. Sci. Rep. 2018, 8, 5621. [Google Scholar] [CrossRef] [PubMed]
- Koban, I.; Holtfreter, B.; Hübner, N.-O.; Matthes, R.; Sietmann, R.; Kindel, E.; Weltmann, K.-D.; Welk, A.; Kramer, A.; Kocher, T. Antimicrobial Efficacy of Non-Thermal Plasma in Comparison to Chlorhexidine against Dental Biofilms on Titanium Discs in Vitro—Proof of Principle Experiment. J. Clin. Periodontol. 2011, 38, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Laroussi, M. Low Temperature Plasma-Based Sterilization: Overview and State-of-the-Art. Plasma Process. Polym. 2005, 2, 391–400. [Google Scholar] [CrossRef]
- Harikrishna, S.; Anil, P.P.; Shams, R.; Dash, K.K. Cold plasma as an emerging nonthermal technology for food processing: A comprehensive review. J. Agric. Food Res. 2023, 14, 100747. [Google Scholar] [CrossRef]
- Nicol, M.J.; Brubaker, T.R.; Honish, B.J.; Simmons, A.N.; Kazemi, A.; Geissel, M.A.; Whalen, C.T.; Siedlecki, C.A.; Bilén, S.G.; Knecht, S.D.; et al. Antibacterial Effects of Low-Temperature Plasma Generated by Atmospheric-Pressure Plasma Jet Are Mediated by Reactive Oxygen Species. Sci. Rep. 2020, 10, 3066. [Google Scholar] [CrossRef]
- Schmidt, A.; Wende, K.; Bekeschus, S.; Bundscherer, L.; Barton, A.; Ottmüller, K.; Weltmann, K.-D.; Masur, K. Non-Thermal Plasma Treatment Is Associated with Changes in Transcriptome of Human Epithelial Skin Cells. Free Radic. Res. 2013, 47, 577–592. [Google Scholar] [CrossRef]
- Nanotoxicology: Toxicity and Biological Effects of Nanoparticles for New Evaluation Standards. Available online: https://www.researchgate.net/publication/51525344_Nanotoxicology_Toxicity_and_biological_effects_of_nanoparticles_for_new_evaluation_standards (accessed on 8 October 2024).
- Liu, S.; Huang, Q.; Wu, Y.; Song, Y.; Dong, W.; Chu, M.; Yang, D.; Zhang, X.; Zhang, J.; Chen, C.; et al. Metabolic Linkages between Indoor Negative Air Ions, Particulate Matter and Cardiorespiratory Function: A Randomized, Double-Blind Crossover Study among Children. Environ. Int. 2020, 138, 105663. [Google Scholar] [CrossRef]
- Xiao, S.; Wei, T.; Petersen, J.D.; Zhou, J.; Lu, X. Biological Effects of Negative Air Ions on Human Health and Integrated Multiomics to Identify Biomarkers: A Literature Review. Environ. Sci. Pollut. Res. Int. 2023, 30, 69824–69836. [Google Scholar] [CrossRef]
- Jiang, S.-Y.; Ma, A.; Ramachandran, S. Negative Air Ions and Their Effects on Human Health and Air Quality Improvement. Int. J. Mol. Sci. 2018, 19, 2966. [Google Scholar] [CrossRef] [PubMed]
- Tarnowska, M.; Briançon, S.; Resende de Azevedo, J.; Chevalier, Y.; Bolzinger, M.-A. Inorganic Ions in the Skin: Allies or Enemies? Int. J. Pharm. 2020, 591, 119991. [Google Scholar] [CrossRef] [PubMed]
- Linju, M.C.; Rekha, M.R. Role of Inorganic Ions in Wound Healing: An Insight into the Various Approaches for Localized Delivery. Ther. Deliv. 2023, 14, 649–667. [Google Scholar] [CrossRef] [PubMed]
- Gérard-Monnier, D.; Erdelmeier, I.; Régnard, K.; Moze-Henry, N.; Yadan, J.C.; Chaudière, J. Reactions of 1-Methyl-2-Phenylindole with Malondialdehyde and 4-Hydroxyalkenals. Analytical Applications to a Colorimetric Assay of Lipid Peroxidation. Chem. Res. Toxicol. 1998, 11, 1176–1183. [Google Scholar] [CrossRef] [PubMed]
- de Gonzalo-Calvo, D.; Neitzert, K.; Fernández, M.; Vega-Naredo, I.; Caballero, B.; García-Macía, M.; Suárez, F.M.; Rodríguez-Colunga, M.J.; Solano, J.J.; Coto-Montes, A. Differential Inflammatory Responses in Aging and Disease: TNF-Alpha and IL-6 as Possible Biomarkers. Free Radic. Biol. Med. 2010, 49, 733–737. [Google Scholar] [CrossRef]
- González-Blanco, L.; Bermúdez, M.; Bermejo-Millo, J.C.; Gutiérrez-Rodríguez, J.; Solano, J.J.; Antuña, E.; Menéndez-Valle, I.; Caballero, B.; Vega-Naredo, I.; Potes, Y.; et al. Cell Interactome in Sarcopenia during Aging. J. Cachexia Sarcopenia Muscle 2022, 13, 919–931. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Menéndez-Coto, N.; Garcia-Gonzalez, C.; Baena-Huerta, F.J.; Zapata-Pérez, R.; Rabadán-Ros, R.; Núñez-Delicado, E.; González-Llorente, L.; Caso-Peláez, E.; Coto-Montes, A. Combining Cold Atmospheric Plasma and Environmental Nanoparticle Removal Device Reduces Neurodegenerative Markers. Int. J. Mol. Sci. 2024, 25, 12986. https://doi.org/10.3390/ijms252312986
Menéndez-Coto N, Garcia-Gonzalez C, Baena-Huerta FJ, Zapata-Pérez R, Rabadán-Ros R, Núñez-Delicado E, González-Llorente L, Caso-Peláez E, Coto-Montes A. Combining Cold Atmospheric Plasma and Environmental Nanoparticle Removal Device Reduces Neurodegenerative Markers. International Journal of Molecular Sciences. 2024; 25(23):12986. https://doi.org/10.3390/ijms252312986
Chicago/Turabian StyleMenéndez-Coto, Nerea, Claudia Garcia-Gonzalez, Francisco Javier Baena-Huerta, Rubén Zapata-Pérez, Rubén Rabadán-Ros, Estrella Núñez-Delicado, Lucía González-Llorente, Enrique Caso-Peláez, and Ana Coto-Montes. 2024. "Combining Cold Atmospheric Plasma and Environmental Nanoparticle Removal Device Reduces Neurodegenerative Markers" International Journal of Molecular Sciences 25, no. 23: 12986. https://doi.org/10.3390/ijms252312986
APA StyleMenéndez-Coto, N., Garcia-Gonzalez, C., Baena-Huerta, F. J., Zapata-Pérez, R., Rabadán-Ros, R., Núñez-Delicado, E., González-Llorente, L., Caso-Peláez, E., & Coto-Montes, A. (2024). Combining Cold Atmospheric Plasma and Environmental Nanoparticle Removal Device Reduces Neurodegenerative Markers. International Journal of Molecular Sciences, 25(23), 12986. https://doi.org/10.3390/ijms252312986







