IL-37 Isoform A Prevents Collagen-Induced Arthritis in Mice by Modulating the Th17/Treg Balance via IL1R8 Receptors
Abstract
1. Introduction
2. Results
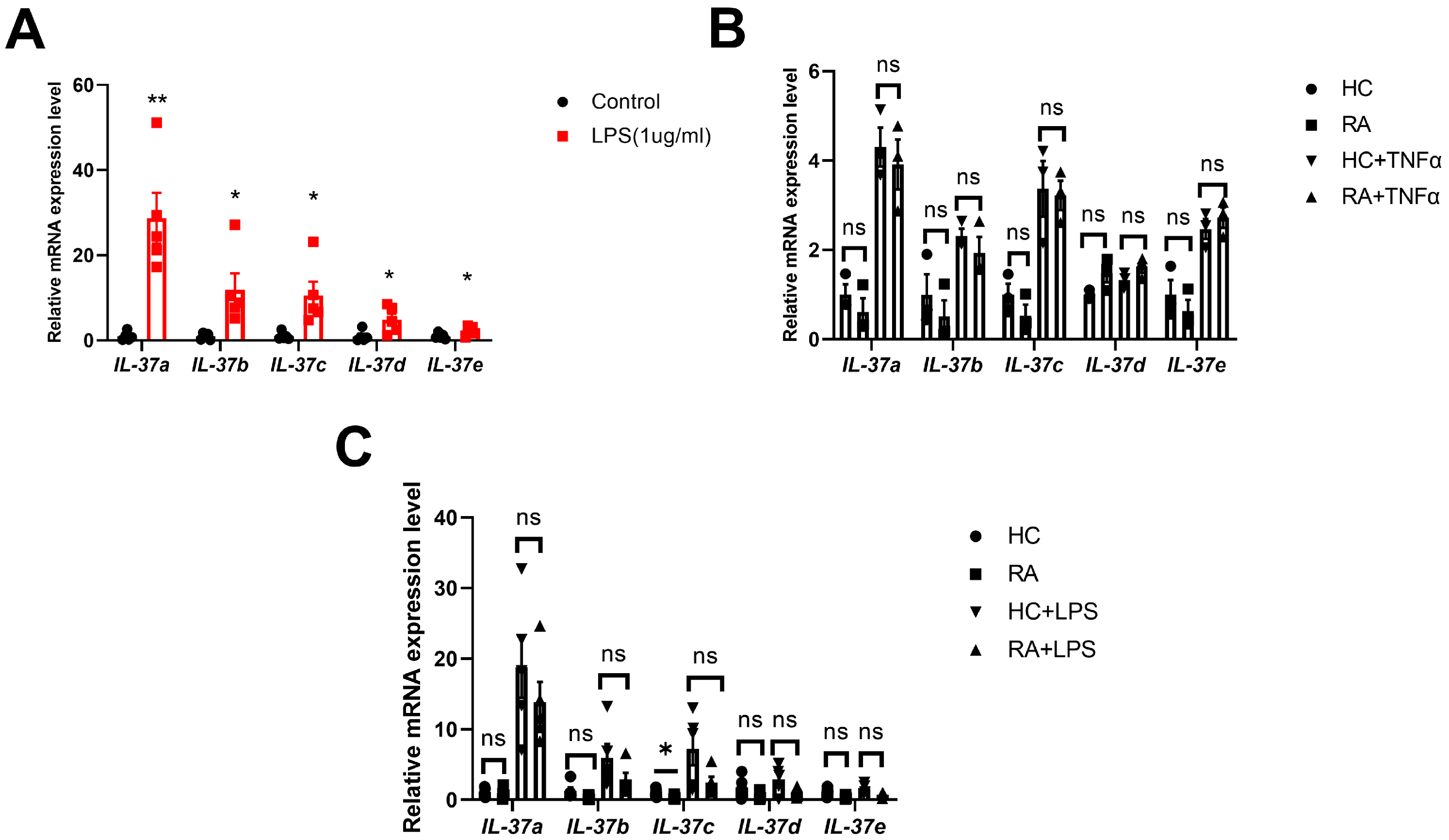
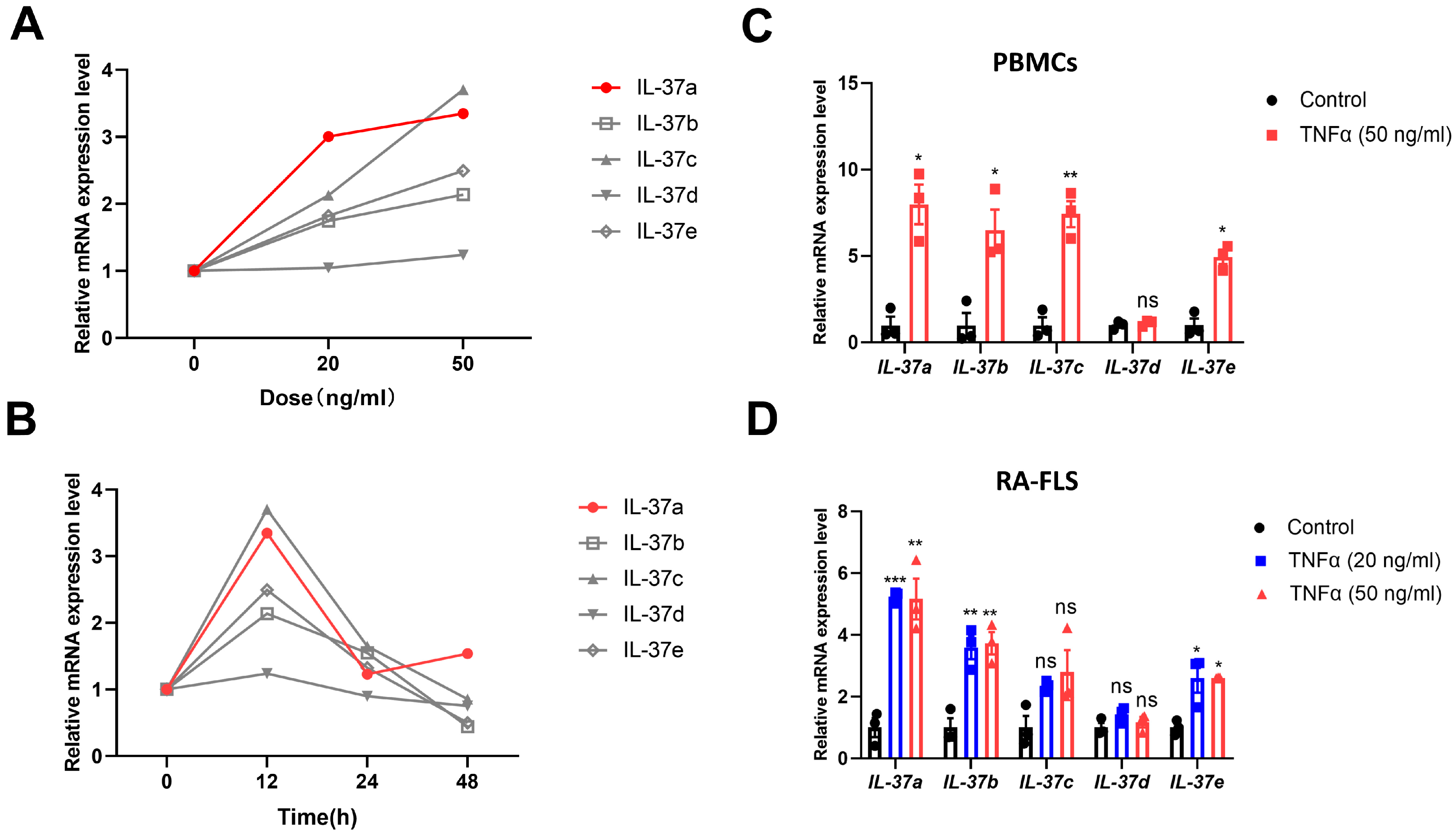
2.1. IL-37a Is Expressed in Peripheral Blood Mononuclear Cells (Pbmcs) from RA Patients and RA-Fibroblast-like Synoviocytes (FLSs) and Is Markedly Upregulated by Tnfα
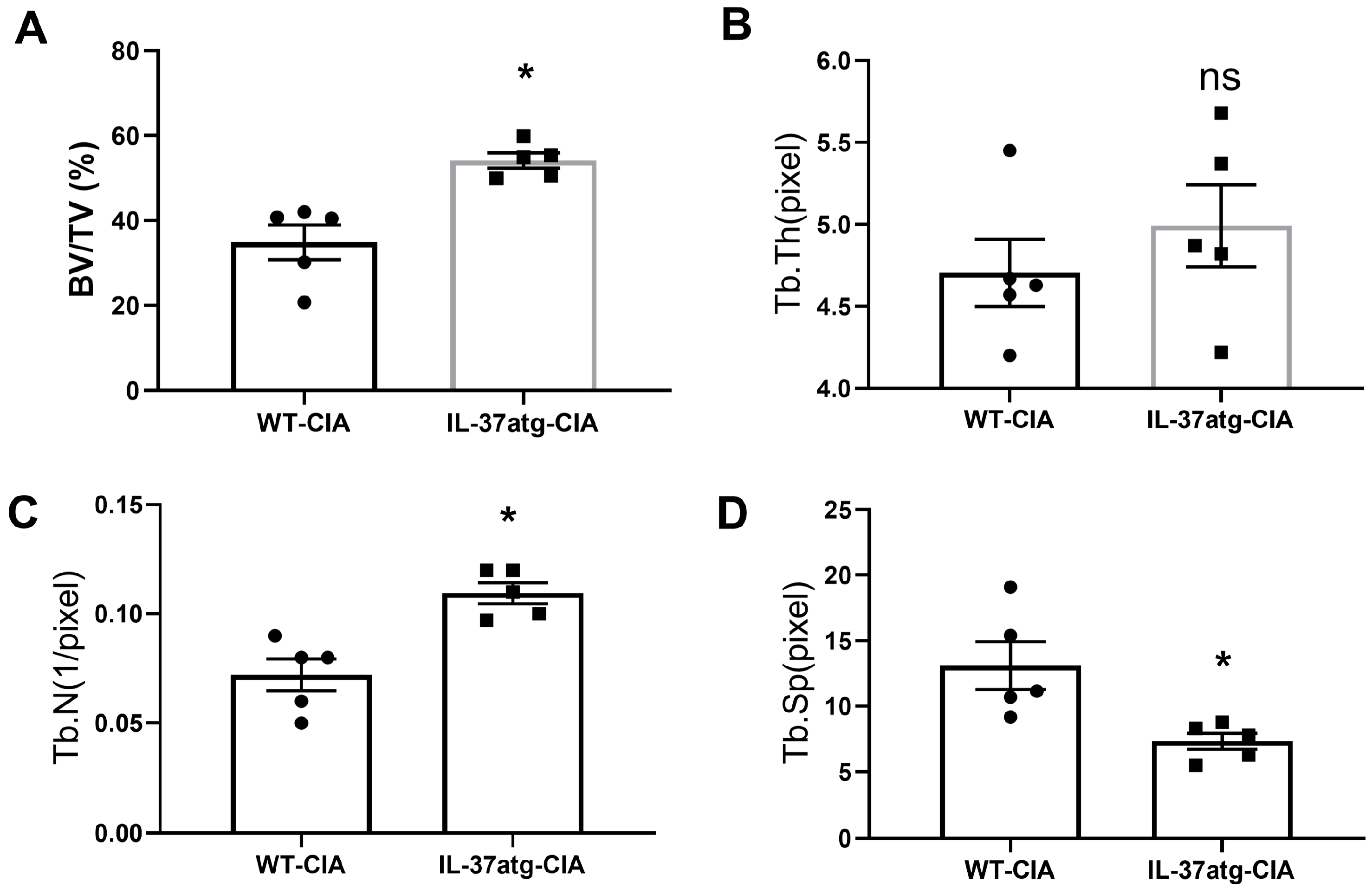
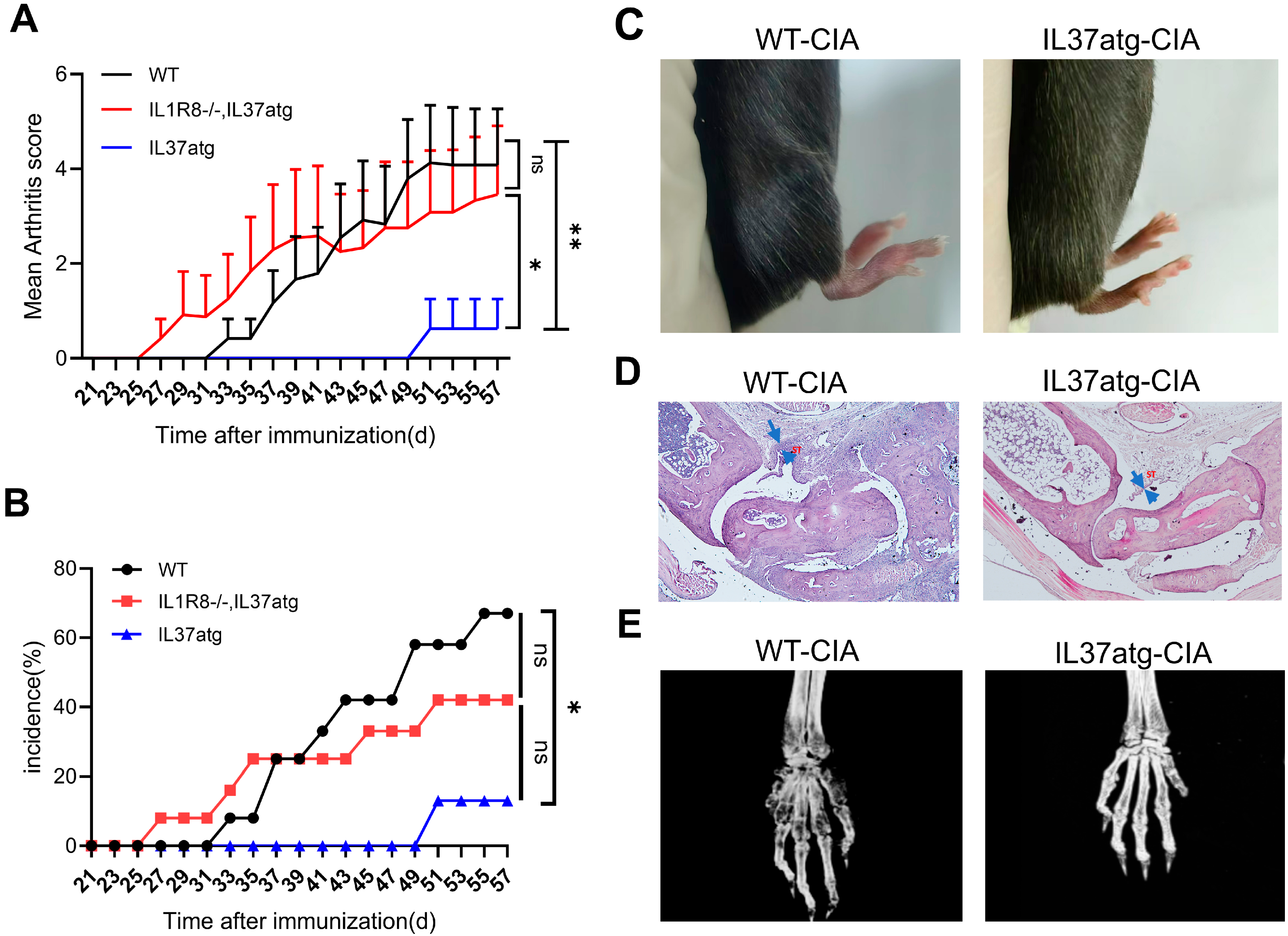
2.2. IL-37 Isoform A Is Effective in Preventing Collagen-Induced Arthritis and IL1R8 Deficiency Can Diminish the Effect of IL-37a
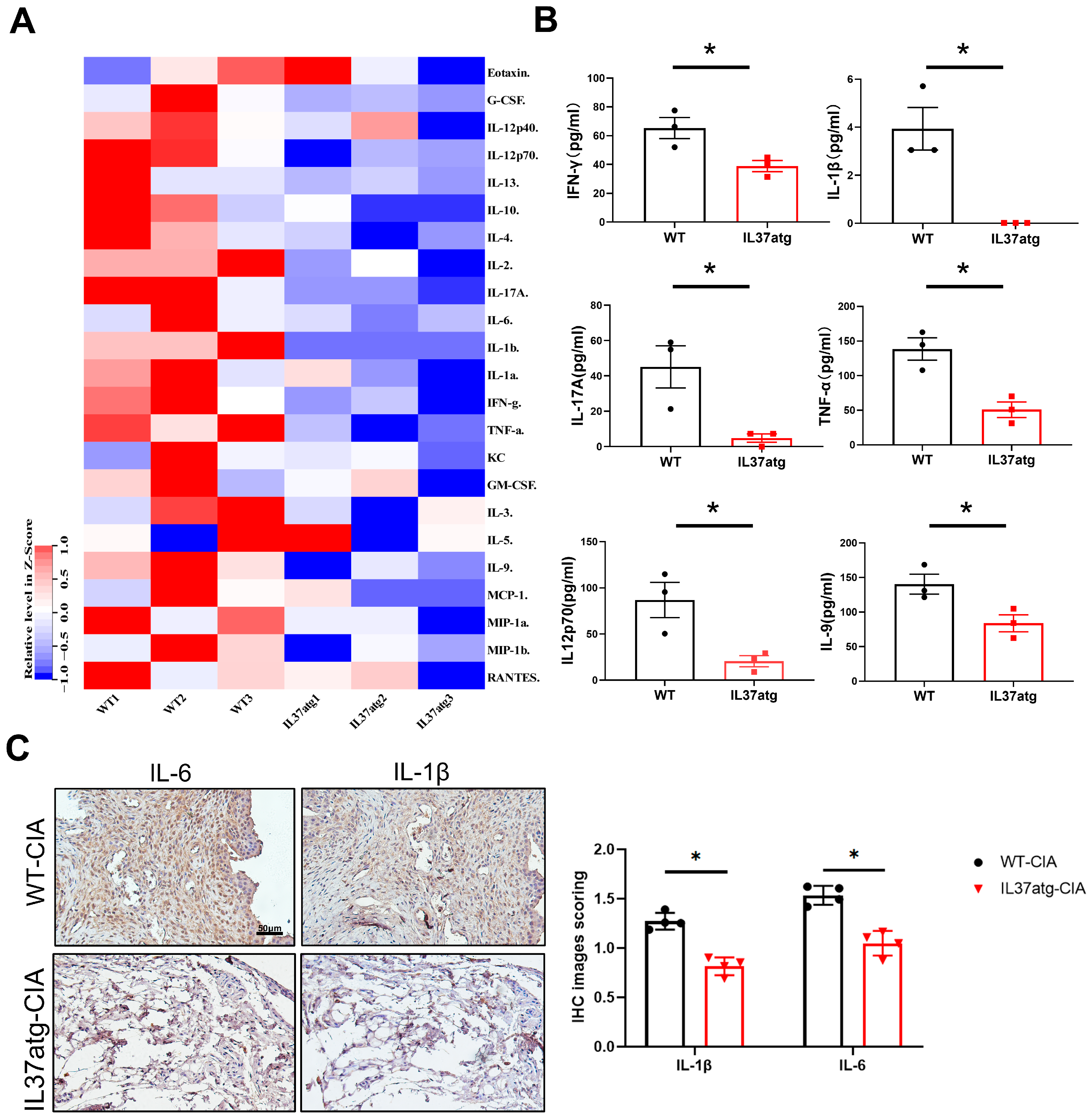
2.3. IL-37atg Mice Have Lower Levels of Proinflammatory Cytokines in the Plasma and Synovial Tissues
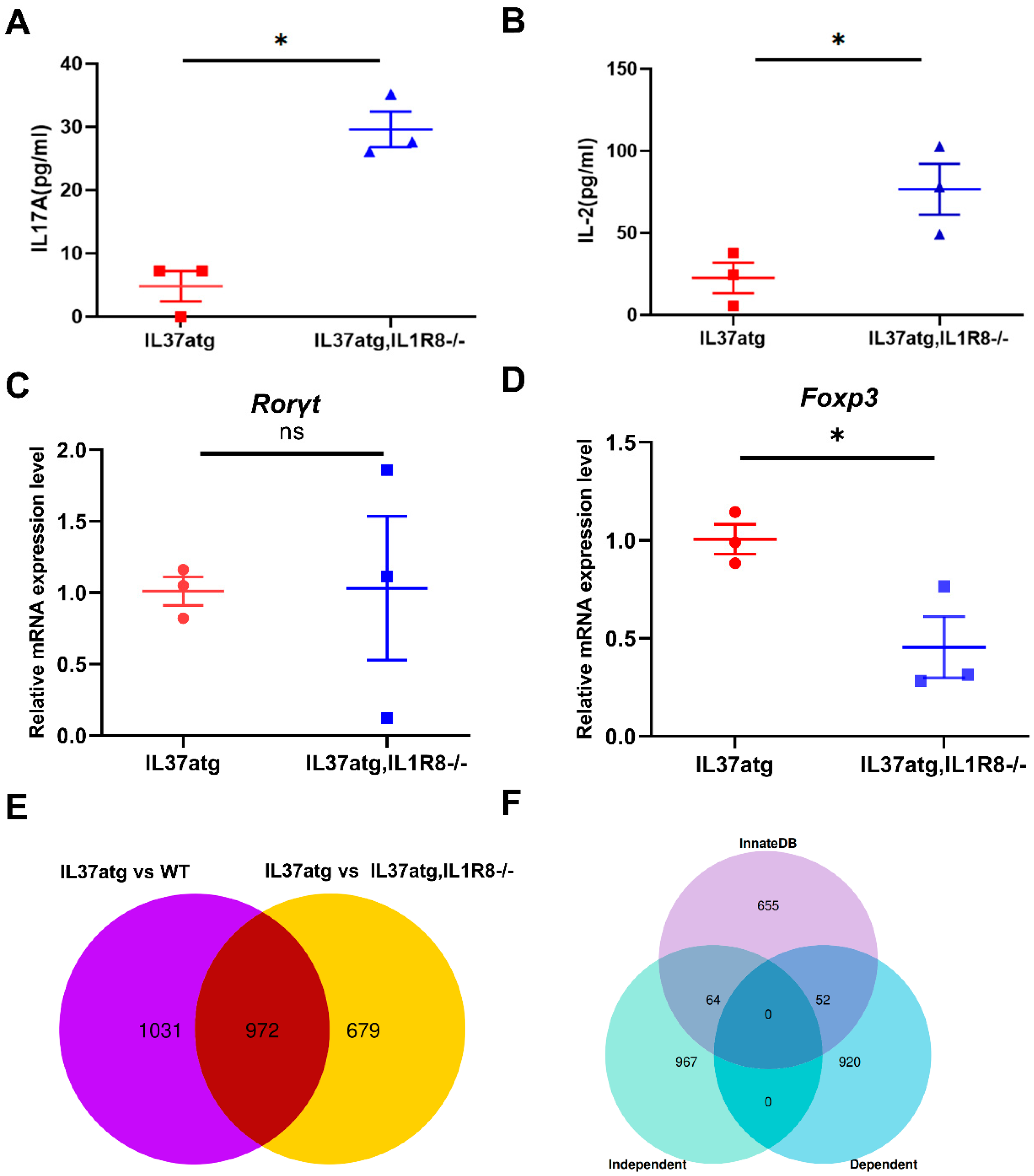
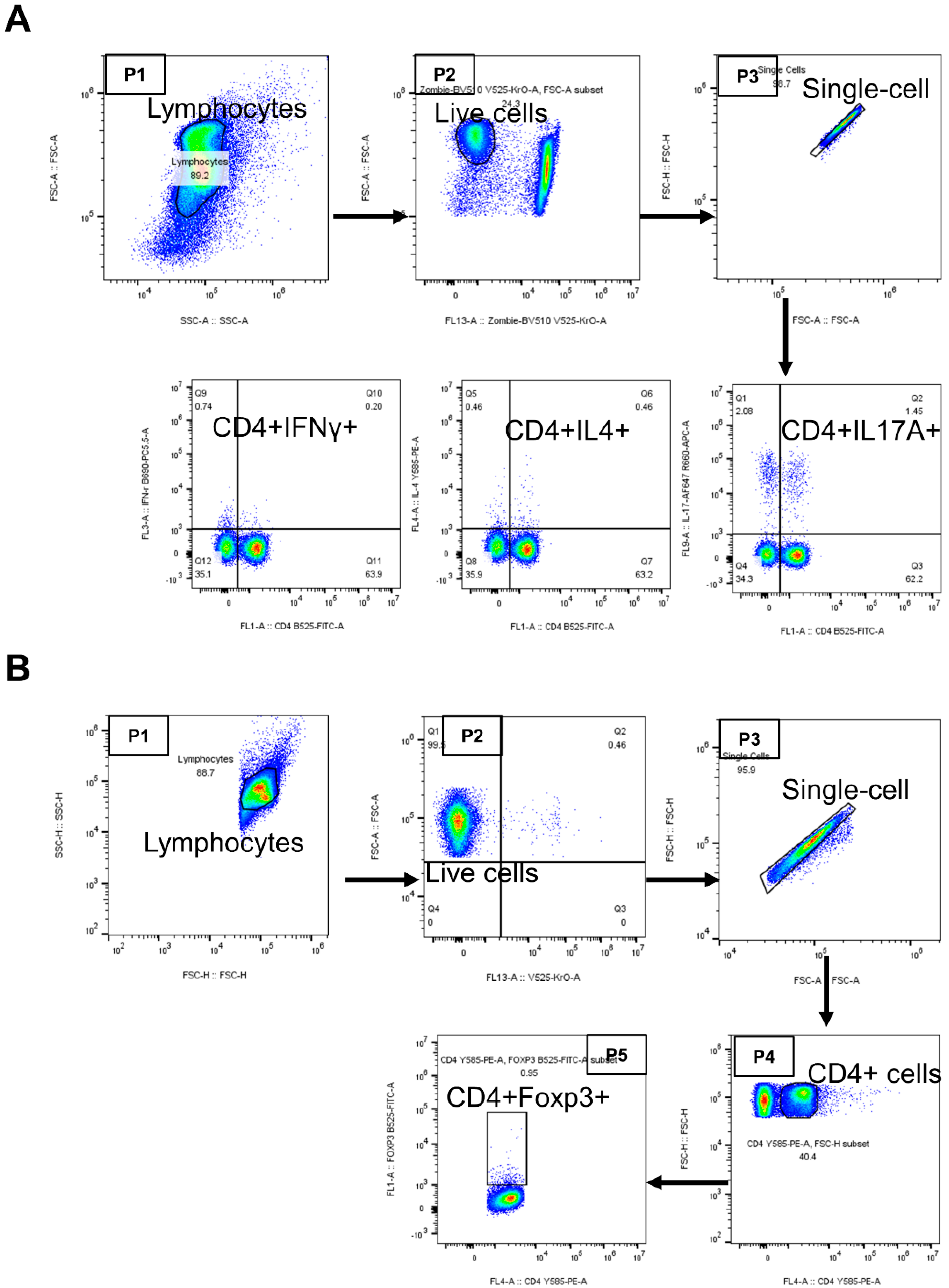
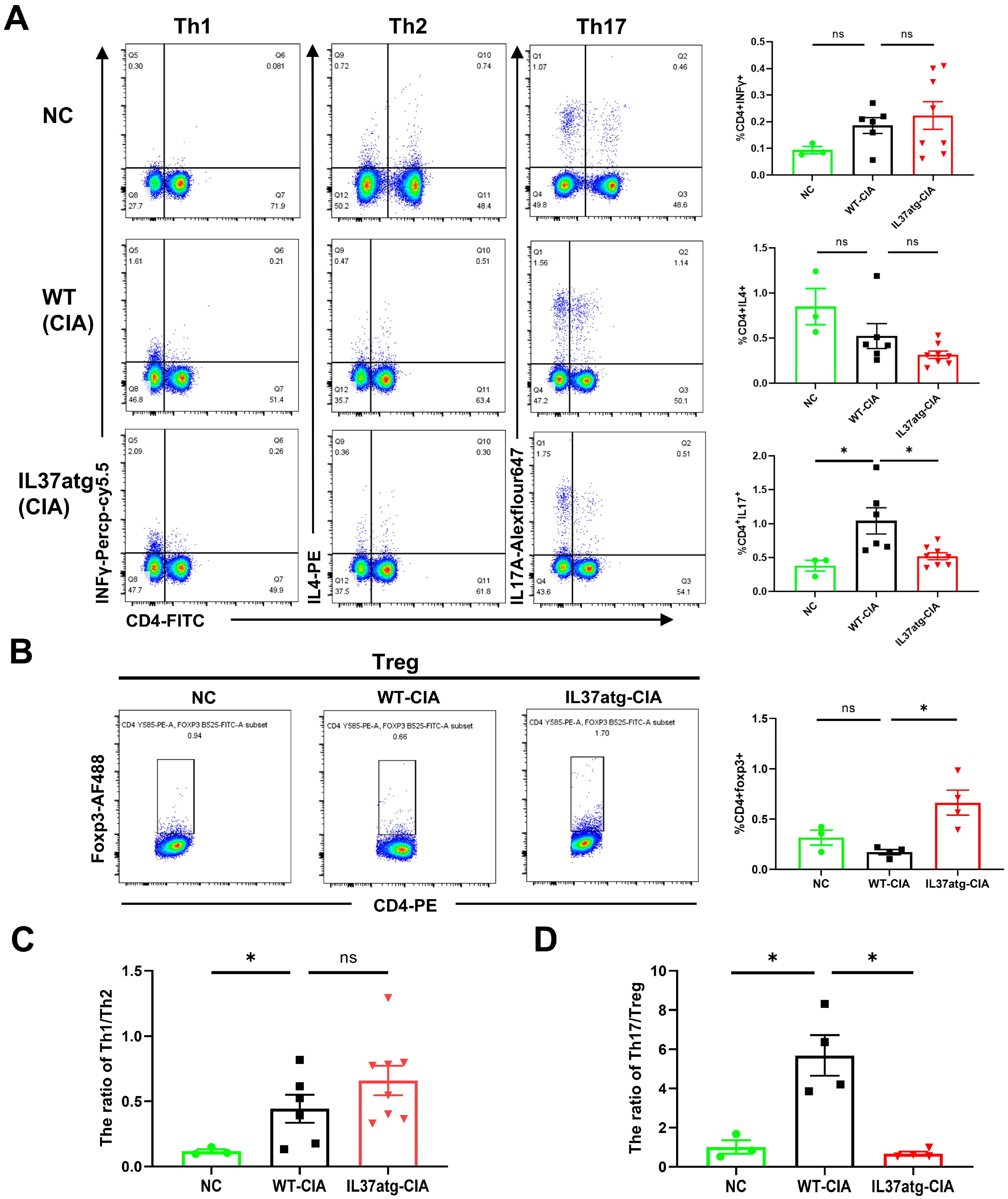
2.4. Th17 Cell Skewing Occurs in the Lymph Nodes of Wild-Type Mice with Collagen-Induced Arthritis (CIA), While the Balance Is Maintained in IL-37a Transgenic Mice with CIA
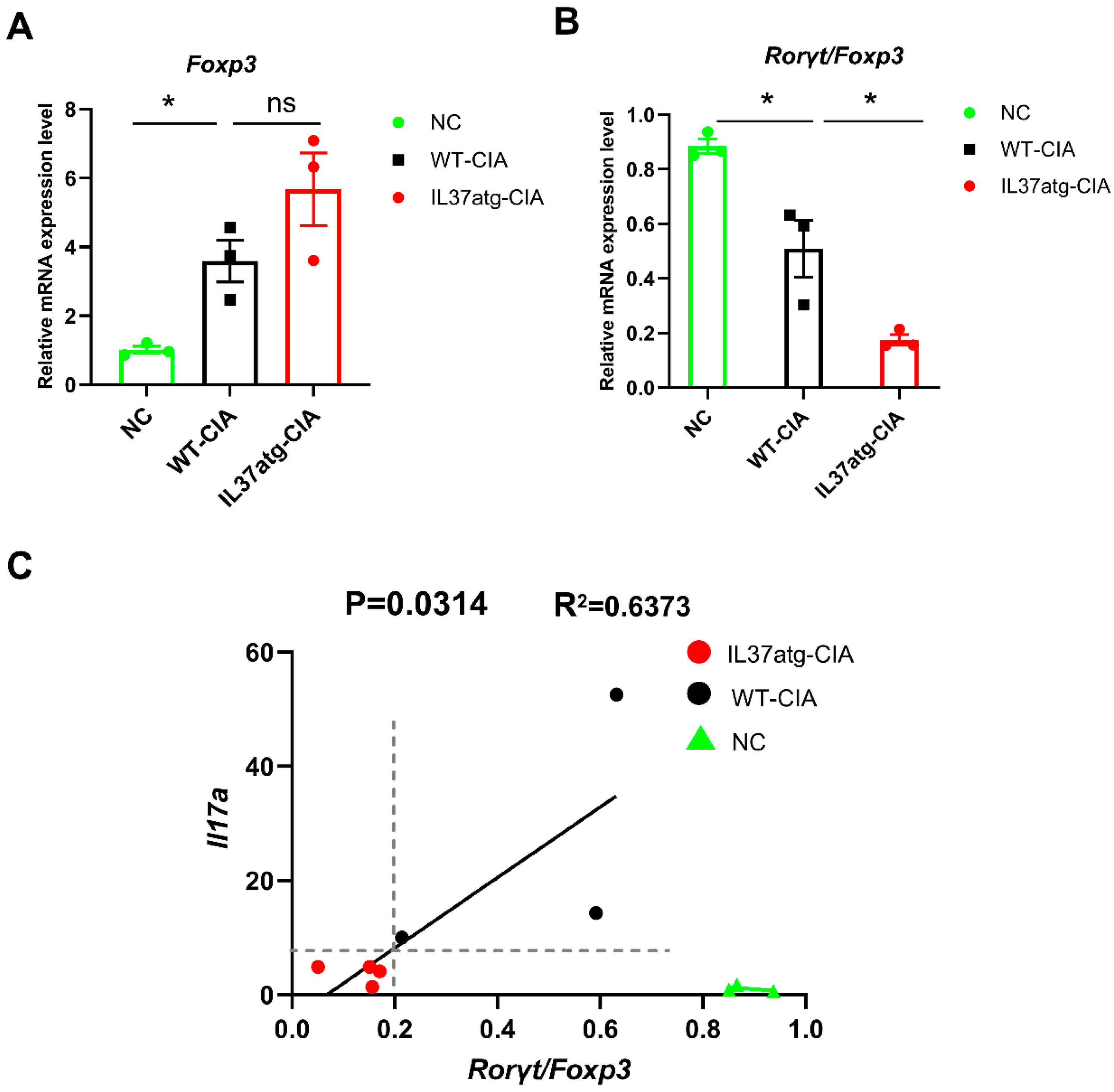
2.5. IL-37atg Mice Exhibit Reduced Rorc (RAR-Related Orphan Receptor Gamma) mRNA Expression in Lymph Nodes Compared with WT Mice, and IL-37a Suppresses the Naïve CD4+ T Cells Differentiation into Th17 Cells In Vitro
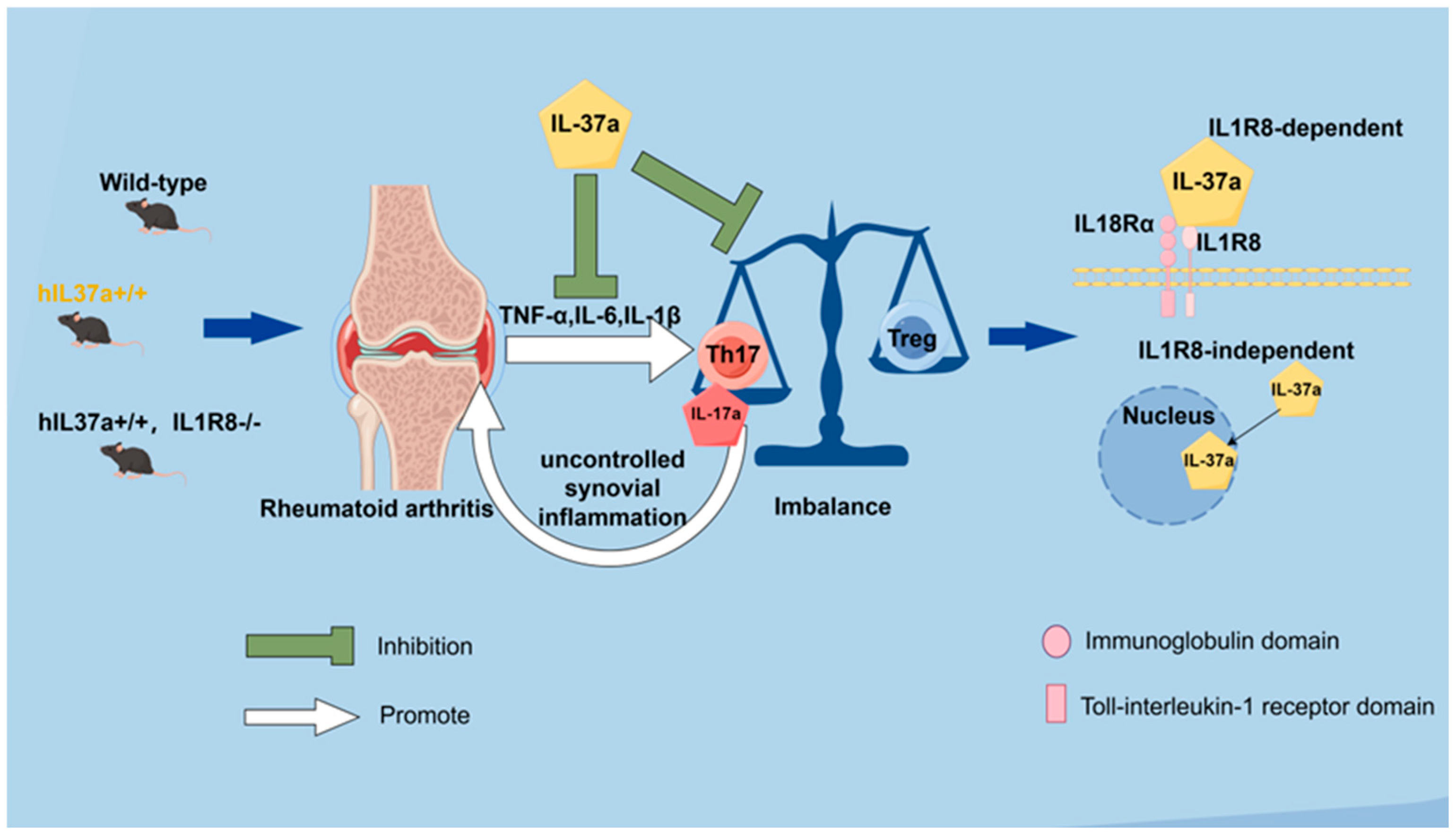
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Obtaining IL-37atg Mice and IL-37atg, IL1R8-/- Mice
4.3. Collagen-Induced Arthritis (CIA) Induction and Arthritis Assessment
4.4. Real-Time PCR After Extracting RNA
4.5. Histological Analysis
4.6. Micro-CT Detection and Image Analysis
4.7. Immunohistochemistry
4.8. Flow Cytometry
4.9. Th17 Cell Differentiation
4.10. Cytokine Assays
4.11. Transcriptomics and Differentially Expressed Gene (DEG) Analysis
4.12. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Gene | Primers | Sequence (5′>3′) |
|---|---|---|
| IL37a (Human) | Forward | GGGAAACAGAAACCAAAGGA |
| Reverse | CCCAGAGTCCAGGACCAGTA | |
| IL37b (Human) | Forward | AGCCTCCCCACCATGAATTT |
| Reverse | ATTCCCAGAGTCCAGGACCA | |
| IL37c (Human) | Forward | AGTGCTGCTTAGAAGACCCG |
| Reverse | CCCTTTAGAGACCCCCAGGA | |
| IL37d (Human) | Forward | GGGGGAGAACTCAGGAGTGA |
| Reverse | ACCTTTGGACCTTCTAAGCAGC | |
| IL37e (Human) | Forward | CCCAGTGCTGCTTAGAAGAGA |
| Reverse | CCTTTAGAGACCCCCAGGAGA | |
| Rorc (Mouse) | Forward | CCGCTGAGAGGGCTTCAC |
| Reverse | TGCAGGAGTAGGCCACATTACA | |
| Foxp3 (Mouse) | Forward | CCCATCCCCAGGAGTCTTG |
| Reverse | ACCATGACTAGGGGCACTGTA | |
| Il-17a (Mouse) | Forward | TTTAACTCCCTTGGCGCAAAA |
| Reverse | CTTTCCCTCCGCATTGACAC | |
| GAPDH (Human) | Forward | CTGACTTCAACAGCGACACC |
| Reverse | TAGCCAAATTCGTTGTCATAC | |
| Gapdh (Mouse) | Forward | GTGTTCCTACCCCCAATGTGT |
| Reverse | ATTGTCATACCAGGAAATGAGCTT |

References
- Finckh, A.; Gilbert, B.; Hodkinson, B.; Bae, S.-C.; Thomas, R.; Deane, K.D.; Alpizar-Rodriguez, D.; Lauper, K. Global epidemiology of rheumatoid arthritis. Nat. Rev. Rheumatol. 2022, 18, 591–602. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Schett, G. Cytokines in the pathogenesis of rheumatoid arthritis. Nat. Rev. Immunol. 2007, 7, 429–442. [Google Scholar] [CrossRef] [PubMed]
- Niu, Q.; Cai, B.; Huang, Z.-c.; Shi, Y.-y.; Wang, L.-l. Disturbed Th17/Treg balance in patients with rheumatoid arthritis. Rheumatol. Int. 2012, 32, 2731–2736. [Google Scholar] [CrossRef]
- Knochelmann, H.M.; Dwyer, C.J.; Bailey, S.R.; Amaya, S.M.; Elston, D.M.; Mazza-McCrann, J.M.; Paulos, C.M. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell Mol. Immunol. 2018, 15, 458–469. [Google Scholar] [CrossRef]
- Singh, R.P.; Hasan, S.; Sharma, S.; Nagra, S.; Yamaguchi, D.T.; Wong, D.T.W.; Hahn, B.H.; Hossain, A. Th17 cells in inflammation and autoimmunity. Autoimmun. Rev. 2014, 13, 1174–1181. [Google Scholar] [CrossRef] [PubMed]
- Nakae, S.; Komiyama, Y.; Nambu, A.; Sudo, K.; Iwase, M.; Homma, I.; Sekikawa, K.; Asano, M.; Iwakura, Y. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity 2002, 17, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Samson, M.; Audia, S.; Janikashvili, N.; Ciudad, M.; Trad, M.; Fraszczak, J.; Ornetti, P.; Maillefert, J.-F.; Miossec, P.; Bonnotte, B. Brief report: Inhibition of interleukin-6 function corrects Th17/Treg cell imbalance in patients with rheumatoid arthritis. Arthritis Rheum. 2012, 64, 2499–2503. [Google Scholar] [CrossRef]
- Cavalli, G.; Dinarello, C.A. Suppression of inflammation and acquired immunity by IL-37. Immunol. Rev. 2018, 281, 179–190. [Google Scholar] [CrossRef]
- Taylor, S.L.; Renshaw, B.R.; Garka, K.E.; Smith, D.E.; Sims, J.E. Genomic organization of the interleukin-1 locus. Genomics 2002, 79, 726–733. [Google Scholar] [CrossRef]
- Nold, M.F.; Nold-Petry, C.A.; Zepp, J.A.; Palmer, B.E.; Bufler, P.; Dinarello, C.A. IL-37 is a fundamental inhibitor of innate immunity. Nat. Immunol. 2010, 11, 1014–1022. [Google Scholar] [CrossRef]
- Nold-Petry, C.A.; Lo, C.Y.; Rudloff, I.; Elgass, K.D.; Li, S.; Gantier, M.P.; Lotz-Havla, A.S.; Gersting, S.W.; Cho, S.X.; Lao, J.C.; et al. IL-37 requires the receptors IL-18Rα and IL-1R8 (SIGIRR) to carry out its multifaceted anti-inflammatory program upon innate signal transduction. Nat. Immunol. 2015, 16, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Neff, C.P.; Barber, K.; Hong, J.; Luo, Y.; Azam, T.; Palmer, B.E.; Fujita, M.; Garlanda, C.; Mantovani, A.; et al. Extracellular forms of IL-37 inhibit innate inflammation in vitro and in vivo but require the IL-1 family decoy receptor IL-1R8. Proc. Natl. Acad. Sci. USA 2015, 112, 2497–2502. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Shu, Y.; Luo, J.; Liu, D.; Huang, D.S.; Han, Y.; Chen, C.; Li, Y.C.; Zou, J.M.; Qin, J.; et al. Intracellular IL-37b interacts with Smad3 to suppress multiple signaling pathways and the metastatic phenotype of tumor cells. Oncogene 2017, 36, 2889–2899. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Kulk, N.; Nold, M.F.; Gräf, R.; Kim, S.-H.; Reinhardt, D.; Dinarello, C.A.; Bufler, P. The IL-1 family member 7b translocates to the nucleus and down-regulates proinflammatory cytokines. J. Immunol. 2008, 180, 5477–5482. [Google Scholar] [CrossRef]
- Li, C.; Zhao, M.; Zhao, M.; Chen, N.; Guo, Y.; Du, Y.; Zhang, Y.; Cao, B.; Zhan, B.; Guo, C.; et al. IL-37 isoform D acts as an inhibitor of soluble ST2 to boost type 2 immune homeostasis in white adipose tissue. Cell Death Discov. 2022, 8, 163. [Google Scholar] [CrossRef]
- Zhao, M.; Li, Y.; Guo, C.; Wang, L.; Chu, H.; Zhu, F.; Li, Y.; Wang, X.; Wang, Q.; Zhao, W.; et al. IL-37 isoform D downregulates pro-inflammatory cytokines expression in a Smad3-dependent manner. Cell Death Dis. 2018, 9, 582. [Google Scholar] [CrossRef]
- Li, Y.; Chu, H.; Zhao, M.; Li, C.; Guan, Y.; Guo, C.; Li, Y.; Wang, Q.; Shi, Y.; Zhu, F.; et al. IL-37d Negatively Regulates NLRP3 Transcription via Receptor-mediated Pathway and Alleviates DSS-induced Colitis. Inflamm. Bowel Dis. 2021, 27, 84–93. [Google Scholar] [CrossRef]
- Jia, H.; Liu, J.; Han, B. Reviews of Interleukin-37: Functions, Receptors, and Roles in Diseases. Biomed. Res. Int. 2018, 2018, 3058640. [Google Scholar] [CrossRef]
- Mesjasz, A.; Trzeciak, M.; Gleń, J.; Jaskulak, M. Potential Role of IL-37 in Atopic Dermatitis. Cells 2023, 12, 2766. [Google Scholar] [CrossRef]
- Papasavva, M.; Amvrosiou, S.; Pilala, K.-M.; Soureas, K.; Christodoulou, P.; Ji, Y.; Stravodimos, K.; Xu, D.; Scorilas, A.; Avgeris, M.; et al. Deregulated Expression of IL-37 in Patients with Bladder Urothelial Cancer: The Diagnostic Potential of the IL-37e Isoform. Int. J. Mol. Sci. 2023, 24, 9258. [Google Scholar] [CrossRef]
- Wei, R.; Han, X.; Li, M.; Ji, Y.; Zhang, L.; Christodoulou, M.-I.; Hameed Aga, N.J.; Zhang, C.; Gao, R.; Liu, J.; et al. The nuclear cytokine IL-37a controls lethal cytokine storms primarily via IL-1R8-independent transcriptional upregulation of PPARγ. Cell Mol. Immunol. 2023, 20, 1428–1444. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.X.; Rudloff, I.; Ellisdon, A.M.; Nold-Petry, C.A.; Nold, M.F. Thirteen years to get from b to a: One of the neglected isoforms of IL-37 enters the stage. Cell. Mol. Immunol. 2024, 21, 201–202. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and therapeutic potential of interleukin-10. J. Exp. Med. 2020, 217, e20190418. [Google Scholar] [CrossRef] [PubMed]
- Rauber, S.; Luber, M.; Weber, S.; Maul, L.; Soare, A.; Wohlfahrt, T.; Lin, N.-Y.; Dietel, K.; Bozec, A.; Herrmann, M.; et al. Resolution of inflammation by interleukin-9-producing type 2 innate lymphoid cells. Nat. Med. 2017, 23, 938–944. [Google Scholar] [CrossRef]
- Varghese, F.; Bukhari, A.B.; Malhotra, R.; De, A. IHC Profiler: An open source plugin for the quantitative evaluation and automated scoring of immunohistochemistry images of human tissue samples. PLoS ONE 2014, 9, e96801. [Google Scholar] [CrossRef]
- Wehrens, E.J.; Prakken, B.J.; van Wijk, F. T cells out of control--impaired immune regulation in the inflamed joint. Nat. Rev. Rheumatol. 2013, 9, 34–42. [Google Scholar] [CrossRef]
- Inglis, J.J.; Criado, G.; Medghalchi, M.; Andrews, M.; Sandison, A.; Feldmann, M.; Williams, R.O. Collagen-induced arthritis in C57BL/6 mice is associated with a robust and sustained T-cell response to type II collagen. Arthritis Res. Ther. 2007, 9, R113. [Google Scholar] [CrossRef]
- Chi, X.; Jin, W.; Zhao, X.; Xie, T.; Shao, J.; Bai, X.; Jiang, Y.; Wang, X.; Dong, C. RORγt expression in mature TH17 cells safeguards their lineage specification by inhibiting conversion to TH2 cells. Sci. Adv. 2022, 8, eabn7774. [Google Scholar] [CrossRef]
- Kaneko, S.; Kondo, Y.; Yokosawa, M.; Furuyama, K.; Segawa, S.; Tsuboi, H.; Kanamori, A.; Matsumoto, I.; Yamazaki, M.; Sumida, T. The RORγt-CCR6-CCL20 axis augments Th17 cells invasion into the synovia of rheumatoid arthritis patients. Mod. Rheumatol. 2018, 28, 814–825. [Google Scholar] [CrossRef]
- Williams, L.M.; Rudensky, A.Y. Maintenance of the Foxp3-dependent developmental program in mature regulatory T cells requires continued expression of Foxp3. Nat. Immunol. 2007, 8, 277–284. [Google Scholar] [CrossRef]
- Nygaard, G.; Firestein, G.S. Restoring synovial homeostasis in rheumatoid arthritis by targeting fibroblast-like synoviocytes. Nat. Rev. Rheumatol. 2020, 16, 316–333. [Google Scholar] [CrossRef] [PubMed]
- Asquith, D.L.; Miller, A.M.; McInnes, I.B.; Liew, F.Y. Animal models of rheumatoid arthritis. Eur. J. Immunol. 2009, 39, 2040–2044. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Moudgil, K.D. Immunomodulation of autoimmune arthritis by pro-inflammatory cytokines. Cytokine 2017, 98, 87–96. [Google Scholar] [CrossRef]
- Burger, D.; Dayer, J.-M.; Palmer, G.; Gabay, C. Is IL-1 a good therapeutic target in the treatment of arthritis? Best Pract. Res. Clin. Rheumatol. 2006, 20, 879–896. [Google Scholar] [CrossRef]
- Elyaman, W.; Bradshaw, E.M.; Uyttenhove, C.; Dardalhon, V.; Awasthi, A.; Imitola, J.; Bettelli, E.; Oukka, M.; van Snick, J.; Renauld, J.-C.; et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc. Natl. Acad. Sci. USA 2009, 106, 12885–12890. [Google Scholar] [CrossRef]
- Nowak, E.C.; Weaver, C.T.; Turner, H.; Begum-Haque, S.; Becher, B.; Schreiner, B.; Coyle, A.J.; Kasper, L.H.; Noelle, R.J. IL-9 as a mediator of Th17-driven inflammatory disease. J. Exp. Med. 2009, 206, 1653–1660. [Google Scholar] [CrossRef]
- Teng, M.W.L.; Bowman, E.P.; McElwee, J.J.; Smyth, M.J.; Casanova, J.-L.; Cooper, A.M.; Cua, D.J. IL-12 and IL-23 cytokines: From discovery to targeted therapies for immune-mediated inflammatory diseases. Nat. Med. 2015, 21, 719–729. [Google Scholar] [CrossRef]
- Wing, K.; Fehérvári, Z.; Sakaguchi, S. Emerging possibilities in the development and function of regulatory T cells. Int. Immunol. 2006, 18, 991–1000. [Google Scholar] [CrossRef]
- Yang, X.O.; Nurieva, R.; Martinez, G.J.; Kang, H.S.; Chung, Y.; Pappu, B.P.; Shah, B.; Chang, S.H.; Schluns, K.S.; Watowich, S.S.; et al. Molecular antagonism and plasticity of regulatory and inflammatory T cell programs. Immunity 2008, 29, 44–56. [Google Scholar] [CrossRef]
- Mailer, R.K.W.; Joly, A.-L.; Liu, S.; Elias, S.; Tegner, J.; Andersson, J. IL-1β promotes Th17 differentiation by inducing alternative splicing of FOXP3. Sci. Rep. 2015, 5, 14674. [Google Scholar] [CrossRef]
- Ye, L.; Jiang, B.; Deng, J.; Du, J.; Xiong, W.; Guan, Y.; Wen, Z.; Huang, K.; Huang, Z. IL-37 Alleviates Rheumatoid Arthritis by Suppressing IL-17 and IL-17-Triggering Cytokine Production and Limiting Th17 Cell Proliferation. J. Immunol. 2015, 194, 5110–5119. [Google Scholar] [CrossRef]
- Lunding, L.; Webering, S.; Vock, C.; Schröder, A.; Raedler, D.; Schaub, B.; Fehrenbach, H.; Wegmann, M. IL-37 requires IL-18Rα and SIGIRR/IL-1R8 to diminish allergic airway inflammation in mice. Allergy 2015, 70, 366–373. [Google Scholar] [CrossRef]
- Lin, X.; Lin, T.; Wang, X.; He, J.; Gao, X.; Lyu, S.; Wang, Q.; Chen, J. Sesamol serves as a p53 stabilizer to relieve rheumatoid arthritis progression and inhibits the growth of synovial organoids. Phytomedicine 2023, 121, 155109. [Google Scholar] [CrossRef]
- Fang, Z.; Lv, J.; Wang, J.; Qin, Q.; He, J.; Wang, M.; Zhou, G.; Liu, G.; Zhong, F.; Zheng, Y.; et al. C-Reactive Protein Promotes the Activation of Fibroblast-Like Synoviocytes From Patients With Rheumatoid Arthritis. Front. Immunol. 2020, 11, 958. [Google Scholar] [CrossRef]
- Inglis, J.J.; Simelyte, E.; McCann, F.E.; Criado, G.; Williams, R.O. Protocol for the induction of arthritis in C57BL/6 mice. Nat. Protoc. 2008, 3, 612–618. [Google Scholar] [CrossRef]
- Brenner, M.; Meng, H.-C.; Yarlett, N.C.; Griffiths, M.M.; Remmers, E.F.; Wilder, R.L.; Gulko, P.S. The non-major histocompatibility complex quantitative trait locus Cia10 contains a major arthritis gene and regulates disease severity, pannus formation, and joint damage. Arthritis Rheum. 2005, 52, 322–332. [Google Scholar] [CrossRef]
- Lin, Z.-M.; Yang, X.-Q.; Zhu, F.-H.; He, S.-J.; Tang, W.; Zuo, J.-P. Artemisinin analogue SM934 attenuate collagen-induced arthritis by suppressing T follicular helper cells and T helper 17 cells. Sci. Rep. 2016, 6, 38115. [Google Scholar] [CrossRef]
- Li, G.; Liu, S.; Chen, Y.; Zhao, J.; Xu, H.; Weng, J.; Yu, F.; Xiong, A.; Udduttula, A.; Wang, D.; et al. An injectable liposome-anchored teriparatide incorporated gallic acid-grafted gelatin hydrogel for osteoarthritis treatment. Nat. Commun. 2023, 14, 3159. [Google Scholar] [CrossRef]
- He, J.; Li, X.; Zhuang, J.; Han, J.; Luo, G.; Yang, F.; Sun, Y.; Liao, P.; Han, Y.; He, Y.; et al. Blocking Matrix Metalloproteinase-9 Abrogates Collagen-Induced Arthritis via Inhibiting Dendritic Cell Migration. J. Immunol. 2018, 201, 3514–3523. [Google Scholar] [CrossRef]
- Shen, W.; Song, Z.; Zhong, X.; Huang, M.; Shen, D.; Gao, P.; Qian, X.; Wang, M.; He, X.; Wang, T.; et al. Sangerbox: A comprehensive, interaction-friendly clinical bioinformatics analysis platform. iMeta 2022, 1, e36. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lyu, S.; Fang, Z.; Hu, Y.; Zhang, M.; He, J.; Wang, X.; He, J.; Gao, X.; Wang, H.; Xu, D.; et al. IL-37 Isoform A Prevents Collagen-Induced Arthritis in Mice by Modulating the Th17/Treg Balance via IL1R8 Receptors. Int. J. Mol. Sci. 2024, 25, 12878. https://doi.org/10.3390/ijms252312878
Lyu S, Fang Z, Hu Y, Zhang M, He J, Wang X, He J, Gao X, Wang H, Xu D, et al. IL-37 Isoform A Prevents Collagen-Induced Arthritis in Mice by Modulating the Th17/Treg Balance via IL1R8 Receptors. International Journal of Molecular Sciences. 2024; 25(23):12878. https://doi.org/10.3390/ijms252312878
Chicago/Turabian StyleLyu, Shuyan, Zhengyu Fang, Yiping Hu, Miaomiao Zhang, Jiaxin He, Xiaocheng Wang, Juan He, Xu Gao, Hongli Wang, Damo Xu, and et al. 2024. "IL-37 Isoform A Prevents Collagen-Induced Arthritis in Mice by Modulating the Th17/Treg Balance via IL1R8 Receptors" International Journal of Molecular Sciences 25, no. 23: 12878. https://doi.org/10.3390/ijms252312878
APA StyleLyu, S., Fang, Z., Hu, Y., Zhang, M., He, J., Wang, X., He, J., Gao, X., Wang, H., Xu, D., & Wang, Q. (2024). IL-37 Isoform A Prevents Collagen-Induced Arthritis in Mice by Modulating the Th17/Treg Balance via IL1R8 Receptors. International Journal of Molecular Sciences, 25(23), 12878. https://doi.org/10.3390/ijms252312878






