Investigation of the Global Changes in Photosynthetic Electron Transport in Hosta Plants Grown Under Different Light Levels
Abstract
1. Introduction
2. Results
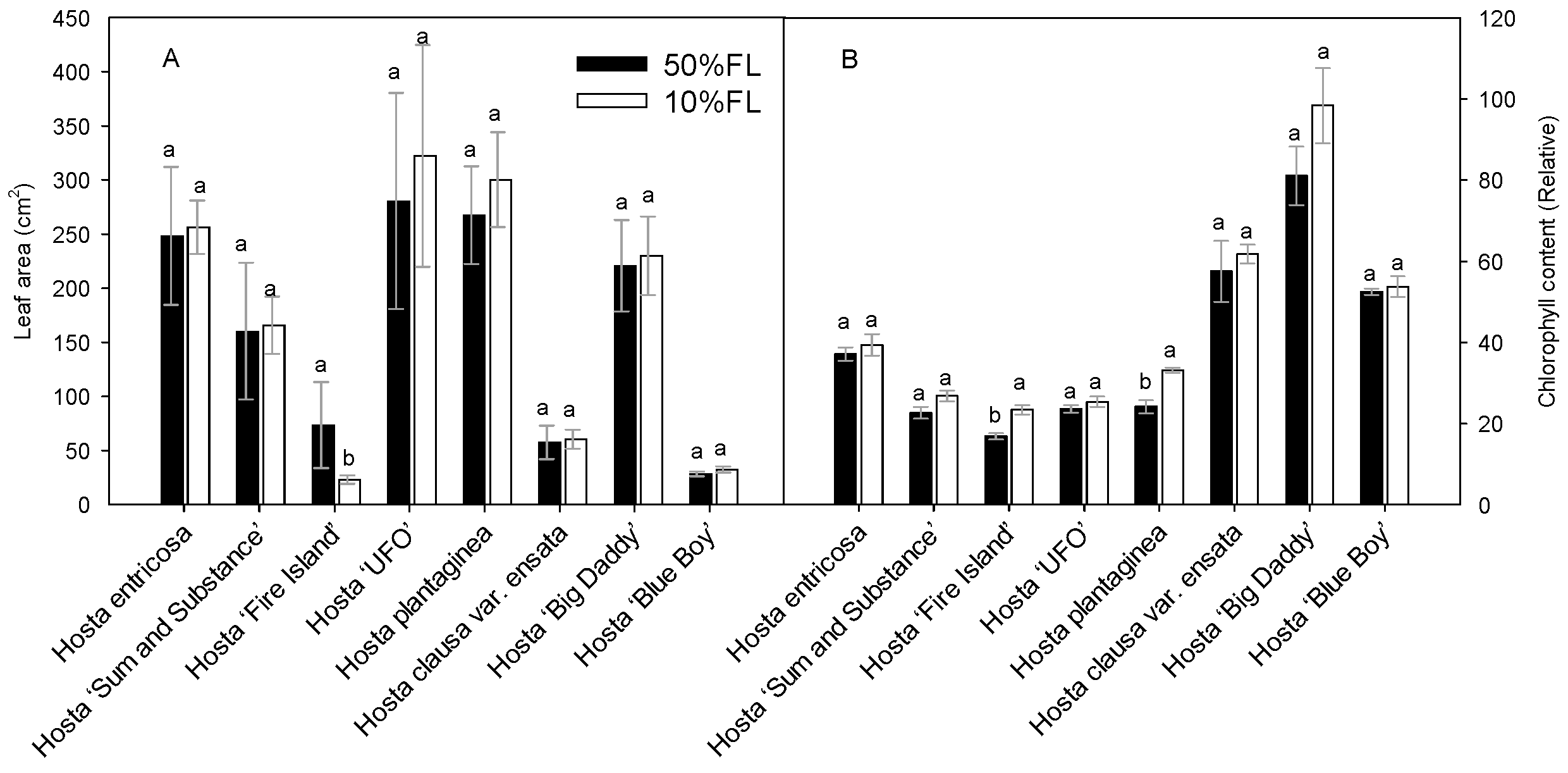
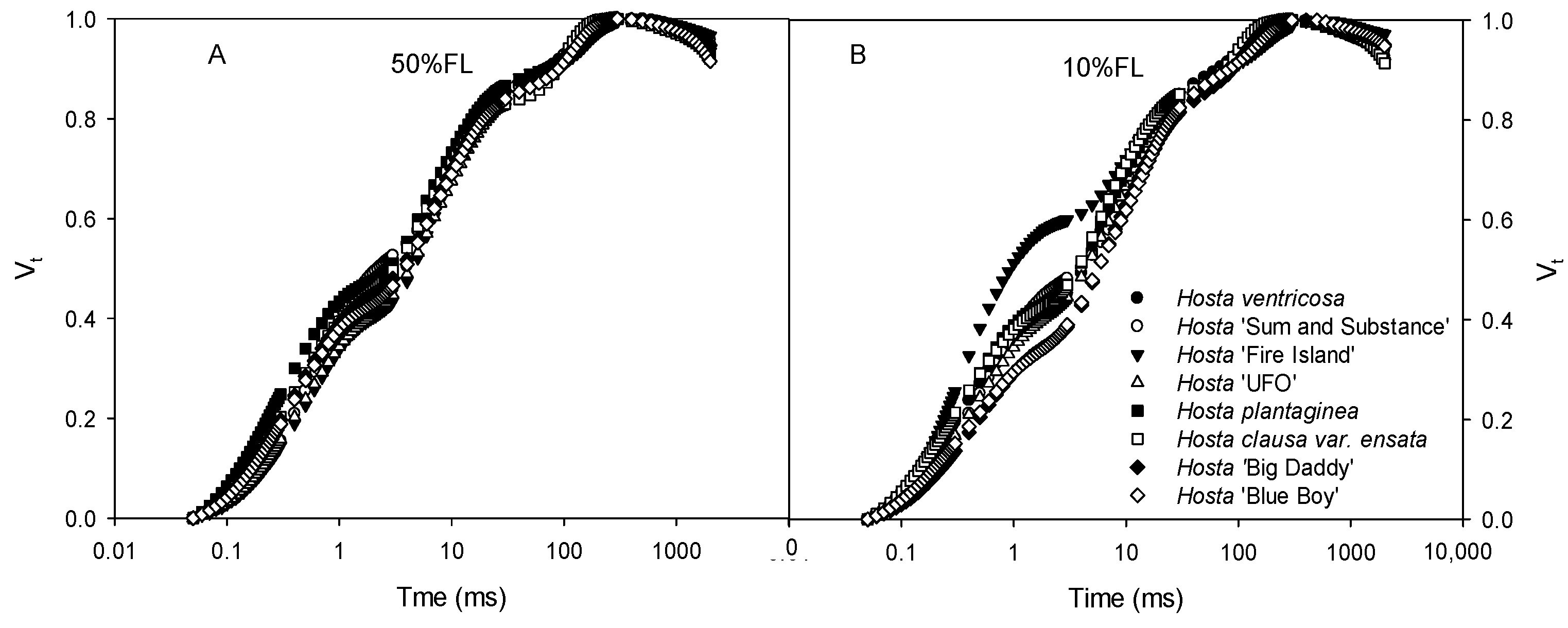
2.1. Effects of Growth Irradiance on Morphology and CFI Kinetics Among Hosta Varieties
2.2. Effects of Growth Irradiance on the Proteomes of Hosta ‘Fire Island’ and ‘Sum and Substance’
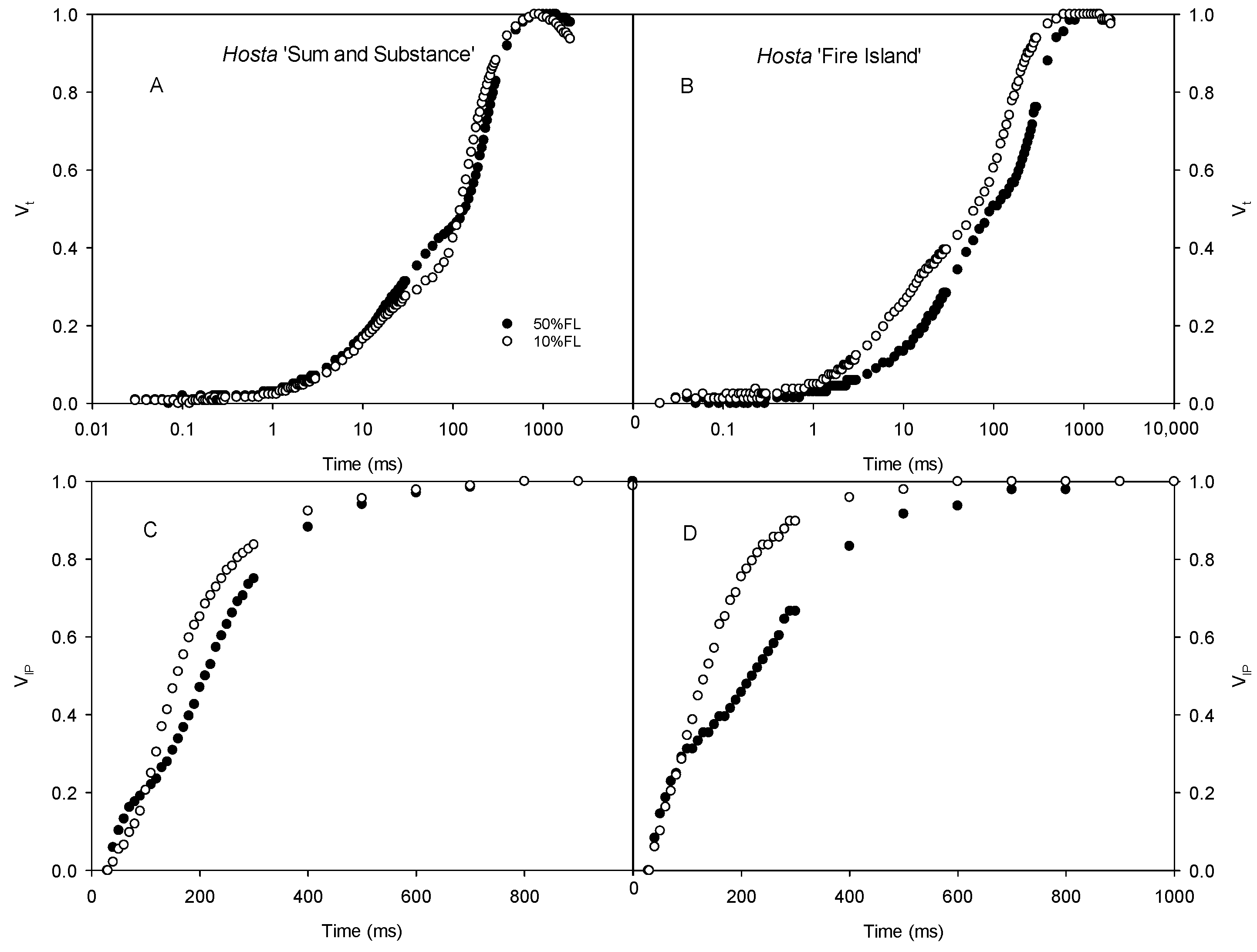
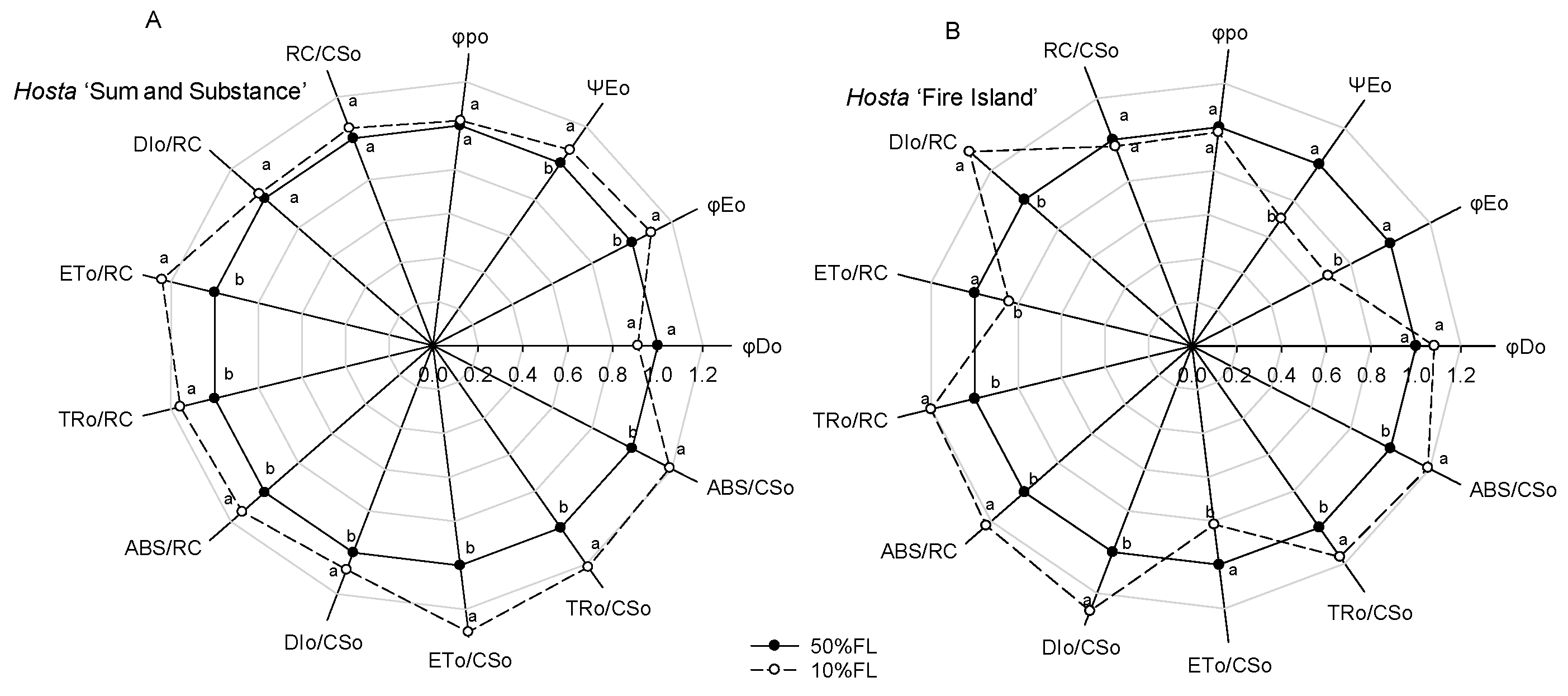
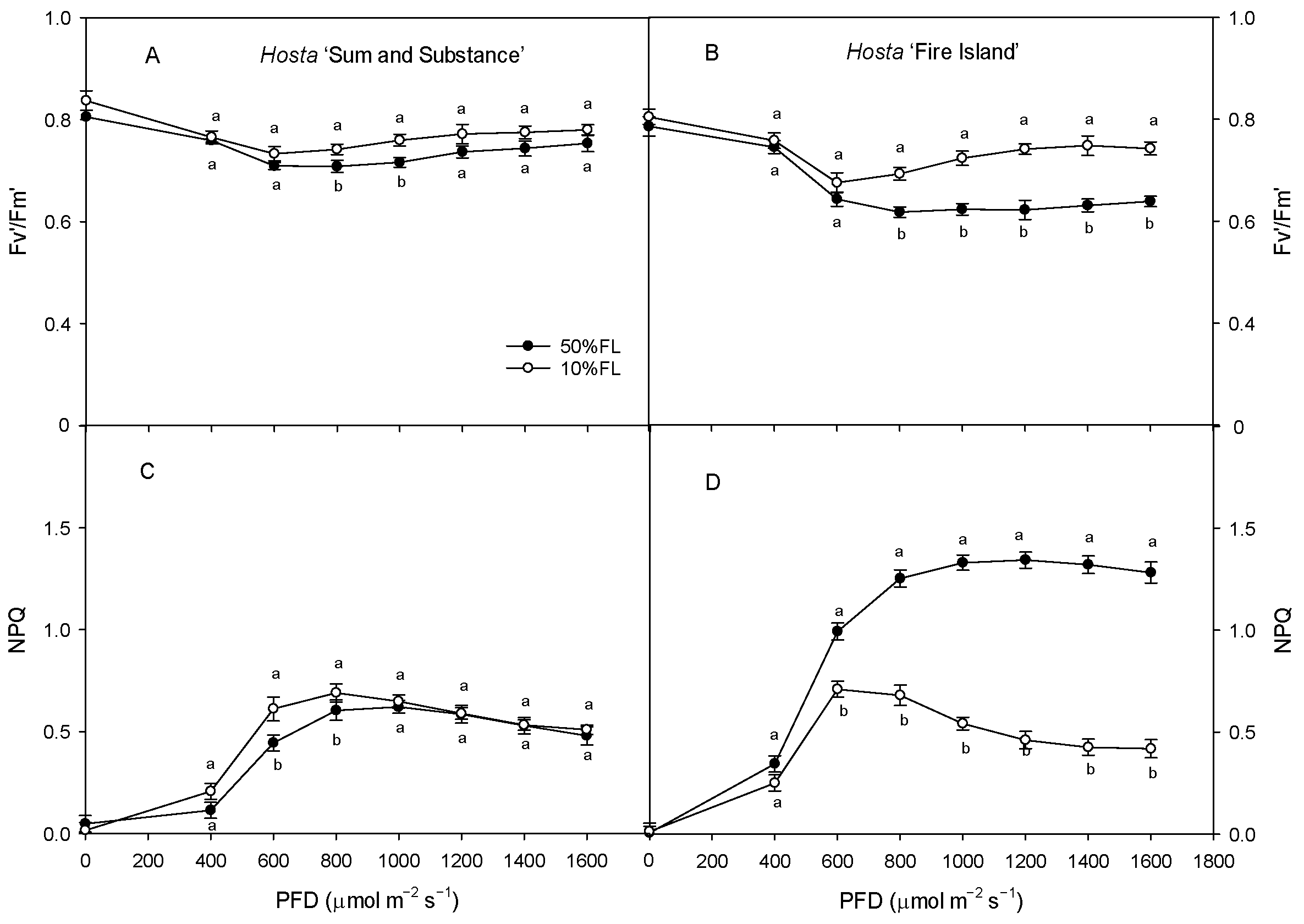
2.3. Changes in CFI Curves Probed by Various Pulse Lights in Hosta ‘Fire Island’ and ‘Sum and Substance’
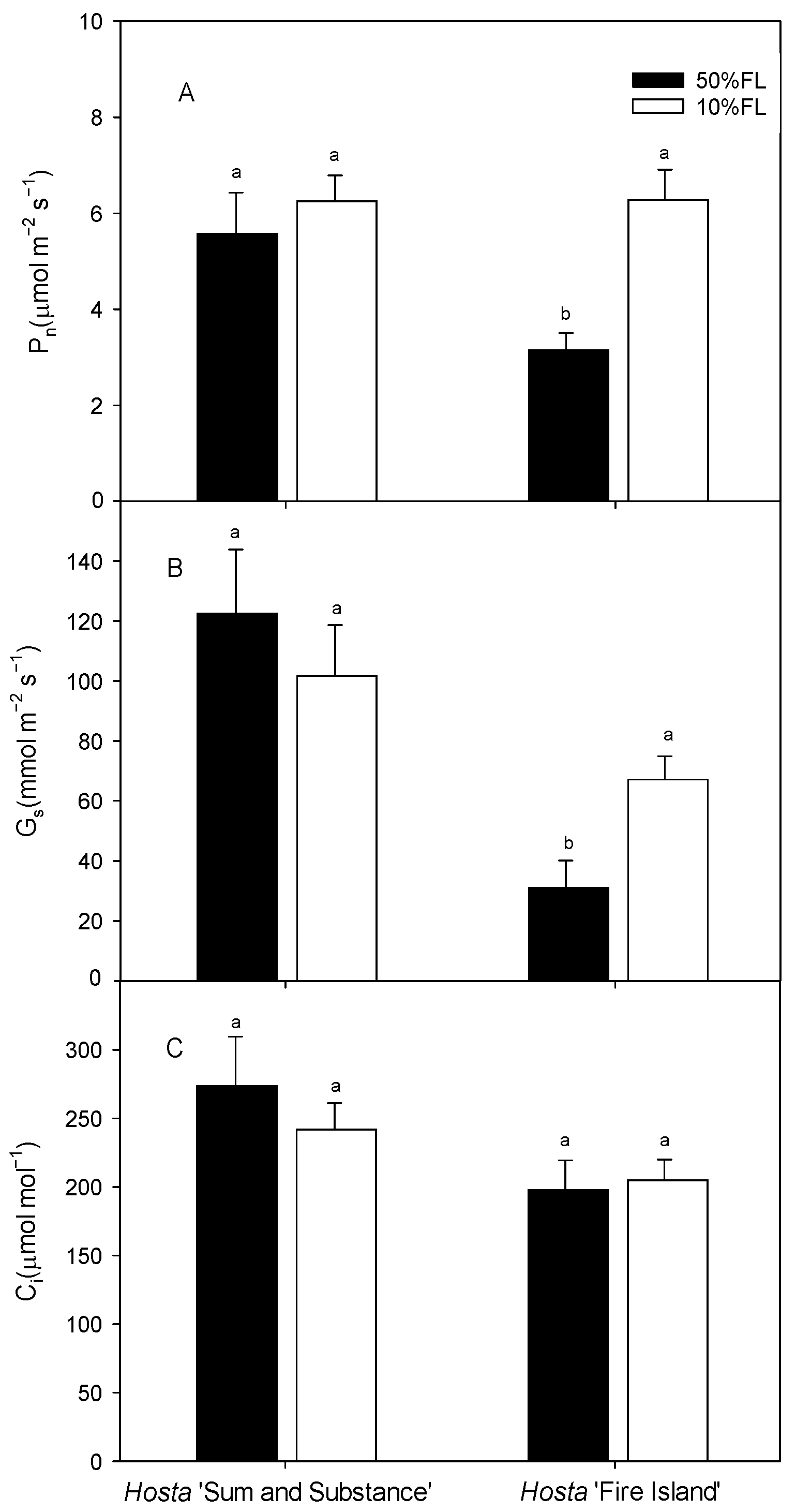
2.4. Effects of Growth Irradiance on Photosynthetic Rate and Non-Photochemical Quenching in Hosta ‘Fire Island’ and ‘Sum and Substance’
3. Discussion
3.1. Generalities in the Regulation of PET and Photoacclimation of Hosta Varieties
3.2. Differences in the Regulation of PET and Photoacclimation of Hosta Varieties
4. Materials and Methods
4.1. Plant Materials and Experimental Design
4.2. Determination of Leaf Area and Chlorophyll Content
4.3. Determination of Gas Exchange
4.4. Determinations of CFI Kinetics
4.5. Determination of Rapid Chlorophyll a Fluorescence Light Response Curve
4.6. Proteomic Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bailey, S.; Horton, P.; Walters, R.G. Acclimation of Arabidopsis thaliana to the light environment: The relationship between photosynthetic function and chloroplast composition. Planta 2004, 218, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Zivcak, M.; Brestic, M.; Kalaji, H.M.; Govindjee. Photosynthetic responses of sun- and shade-grown barley leaves to high light: Is the lower PS II connectivity in shade leaves associated with protection against excess of light? Photosynth. Res. 2014, 119, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Walters, R.G. Towards an understanding of photosynthetic acclimation. J. Exp. Bot. 2005, 56, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Jia, T.; Ito, H.; Tanaka, A. Simultaneous regulation of antenna size and photosystem I/II stoichiometry in Arabidopsis thaliana. Planta 2016, 244, 1041–1053. [Google Scholar] [CrossRef]
- Flannery, S.E.; Hepworth, C.; Wood, W.H.J.; Pastorelli, F.; Hunter, C.N.; Dickman, M.J.; Jackson, P.J.; Johnson, M.P. Developmental acclimation of the thylakoid proteome to light intensity in Arabidopsis. Plant J. 2021, 105, 223–244. [Google Scholar] [CrossRef]
- Kitao, M.; Kitaoka, S.; Komatsu, M.; Utsugi, H.; Tobita, H.; Koike, T.; Maruyama, Y. Leaves of Japanese oak (Quercus mongolica var. crispula) mitigate photoinhibition by adjusting electron transport capacities and thermal energy dissipation along the intra-canopy light gradient. Physiol. Plant 2012, 146, 192–204. [Google Scholar] [CrossRef]
- Li, T.; Liu, L.N.; Jiang, C.D.; Liu, Y.J.; Shi, L. Effects of mutual shading on the regulation of photosynthesis in field-grown sorghum. J. Photochem. Photobiol. B 2014, 137, 31–38. [Google Scholar] [CrossRef]
- Sun, J.; Sui, X.L.; Huang, H.Y.; Wang, S.H.; Wei, Y.X.; Zhang, Z.X. Low light stress down-regulated rubisco gene expression and photosynthetic capacity during cucumber (Cucumis sativus L.) leaf development. J. Integr. Agric. 2014, 13, 997–1007. [Google Scholar] [CrossRef]
- Malone, L.A.; Proctor, M.S.; Hitchcock, A.; Hunter, C.N.; Johnson, M.P. Cytochrome b6f–orchestrator of photosynthetic electron transfer. Biochim. Biophys. Acta Bioenerg. 2021, 1862, 148380. [Google Scholar] [CrossRef]
- Pearcy, R.W. Responses of plants to heterogeneous light environments [M]. In Functional Plant Ecology; Pugnaire, F.I., Valladares, F., Eds.; CRC Press: Boca Raton, FL, USA, 1999; pp. 269–314. [Google Scholar]
- Wu, H.Y.; Tang, H.K.; Liu, L.A.; Shi, L.; Zhang, W.F.; Jiang, C.D. Local weak light induces the improvement of photosynthesis in adjacent illuminated leaves in maize seedlings. Physiol. Plant. 2021, 171, 125–136. [Google Scholar] [CrossRef]
- Jiang, Y.; Ding, X.T.; Wang, J.Y.; Zou, J.; Nie, W.F. Decreased low-light regulates plant morphogenesis through the manipulation of hormone biosynthesis in Solanum lycopersicum. Environ. Exp. Bot. 2012, 185, 104409. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient [M]. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G., Govindjee, X., Eds.; Springer: Dordrecht, Netherlands, 2004; pp. 321–362. [Google Scholar]
- Stirbet, A.D.; Govindjee. Chlorophyll a fluorescence induction: A personal perspective of the thermal phase, the J-I-P rise. Photosynth. Res. 2012, 113, 15–61. [Google Scholar] [CrossRef] [PubMed]
- Tsimilli-michael, M. Revisiting JIP-test: An educative review on concepts, assumptions, approximations, definitions and terminology. Photosynthetica 2020, 58, 275–292. [Google Scholar] [CrossRef]
- Wu, H.Y.; Dong, F.Q.; Liu, A.; Shi, L.; Zhang, W.F.; Jiang, C.D. Dorsoventral variation in photosynthesis during leaf senescence probed by chlorophyll a fluorescence induction kinetics in cucumber and maize plants. Photosynthetica 2020, 58, 479–487. [Google Scholar] [CrossRef]
- Krause, G.H.; Weis, E. Chlorophyll fluorescence and photosynthesis—The basics. Annu. Rev. Plant Biol. 1991, 42, 313–349. [Google Scholar] [CrossRef]
- Tóth, S.Z.; Schansker, G.; Strasser, R.J. Intact leaves, the maximum fluorescence level (FM) is independent of the redox state of the plastoquinone pool: A DCMU inhibition study. Biochim. Biophys. Acta Bioenerg. 2005, 1708, 275–282. [Google Scholar] [CrossRef]
- Schansker, G.; Tóth, S.Z.; Strasser, R.J. Methylviologen and dibromothymoquinone treatments of pea leaves reveal the role of photosystem I in the Chl a fluorescence rise OJIP. Biochim. Biophys. Acta Bioenerg. 2005, 1706, 250–261. [Google Scholar] [CrossRef]
- Wisniewski, J.R.; Zougman, A.; Nagaraj, N.; Mann, M. Universal sample preparation method for proteome analysis. Nat. Methods 2009, 6, 359–362. [Google Scholar] [CrossRef]
- Wu, H.Y.; Qiao, M.Y.; Zhang, Y.J.; Kang, W.J.; Ma, Q.H.; Gao, H.Y.; Zhang, W.F.; Jiang, C.D. Photosynthetic mechanism of maize yield under fluctuating light environments in the field. Plant Physiol. 2023, 191, 957–973. [Google Scholar] [CrossRef]
- Li, Z.Z.; Liu, D.H.; Zhao, S.W.; Jiang, C.D.; Shi, L. Mechanisms of photoinhibition induced by high light in Hosta grown outdoors. Chin. J. Plant Ecol. 2014, 38, 720–728. [Google Scholar]
- Liu, D.H.; Zhao, S.W. The impacts of light levels on growth and ornamental characteristics of Hosta. Acta Hortic. 2012, 977, 183–188. [Google Scholar] [CrossRef]
- Lazar, D.; Ilik, P. High-temperature induced chlorophyll fluorescence changes in barley leaves—Comparison of the critical temperatures determined from fluorescence induction and from fluorescence temperature curve. Plant Sci. 1997, 124, 159–164. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Qiang, S.; Goltsev, V. Simultaneous in vivo recording of prompt and delayed fluorescence and 820-nm reflection changes during drying and after rehydration of the resurrection plant Haberlea rhodopensis. Biochim. Biophys. Acta Bioenerg. 2010, 1797, 1313–1326. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.Q.; Liu, D.H.; Sang, M.; Jiang, C.D. Sunflower leaf structure affects chlorophyll a fluorescence induction kinetics in vivo. Int. J. Mol. Sci. 2022, 23, 14996. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Adams, W.W. Xanthophyll cycle and light stress in nature uniform response to excess direct sunlight among higher plant species. Planta 1996, 198, 460–470. [Google Scholar] [CrossRef]

| Protein Identification | Protein Name | Fold Change | ||
|---|---|---|---|---|
| Protein Types | Protein IDs | ‘Sum and Substance’ | ‘Fire Island’ | |
| Rubisco | CL2106.Contig5_All | Rubisco large subunit | 2.16 ↑ | |
| CL4327_Contig1_All | Rubisco large subunit | 2.03 ↑ | ||
| Unigene52506_All | Rubisco small subunit | 3.44 ↑ | ||
| PS II | CL24649_Contig5_All | PS II protein D1 | 4.8 ↑ | |
| CL12408_Contig5_All | PS II 47KDa protein | 2.04 ↑ | ||
| Unigene121480_All | PS II protein D1 | 4.06 ↑ | ||
| CL6272_Contig6_All | PS II | 3.49 ↑ | ||
| OEC | CL11213_Contig1_All | PS II oxygen-evolving complex | −2.56 ↓ | |
| CL9703_Contig3_All | PS II oxygen-evolving complex | −2.61 ↓ | ||
| CL11958_Contig10_All | PS II oxygen-evolving complex | −2.81 ↓ | ||
| CL30452_Contig2_All | PS II oxygen-evolving complex | −3.32 ↓ | ||
| Unigene45629_All | PS II oxygen-evolving complex | −3.54 ↓ | ||
| ETC | CL26278_Contig7_All | Cytochrome b6-f complex | −1.37 ↓ | |
| CL26278_Contig3_All | Cytochrome b6-f complex | −1.31 ↓ | −2.4 ↓ | |
| Unigene12003_All | Cytochrome b6-f complex | −2.91 ↓ | ||
| CL7316_Contig4_All | Plastocyanin 1 | −11.81 ↓ | ||
| PS I | CL1094_Contig1_All | PS I reaction center subunit VI | −2.19 ↓ | |
| CL11407_Contig2_All | PS I reaction center subunit N | −3.05 ↓ | ||
| Unigene725_All | PS I reaction center subunit III | −4.94 ↓ | ||
| CL2739_Contig2_All | Photosynthetic electron transport in PS I | −2.83 ↓ | ||
| CL26881_Contig1_All | Photosynthetic electron transport in PS I | −2.14 ↓ | ||
| PSI antenna proteins | Unigene124018_All | PS I chlorophyll a/b-binding protein 5 | −3.17 ↓ | −2.13 ↓ |
| Unigene9117_All | PS I chlorophyll a/b-binding protein 4 | −2.53 ↓ | −3.86 ↓ | |
| CL29551_Contig2_All | PS I chlorophyll a/b-binding protein | −2.41 ↓ | ||
| CL31036_Contig7_All | PS I chlorophyll a/b-binding protein 1 | −61.06 ↓ | ||
| CL31202_Contig6_All | PS I chlorophyll a/b-binding protein 3 | −5.33 ↓ | ||
| CL32131_Contig2_All | PS I chlorophyll a/b-binding protein 4 | −5.07 ↓ | ||
| Unigene12354_All | PS I chlorophyll a/b-binding protein 2 | −5.11 ↓ | ||
| NPQ | Unigene435_All | Chlorophyll a/b-binding protein CP24 10B | −2.48 ↓ | |
| CL25573_Contig1_All | Chlorophyll a/b-binding protein CP24 10B | −7.87 ↓ | ||
| CL28869_Contig9_All | photosystem II 22 kDa protein | −2.02 ↓ | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.-H.; Ji, W.-T.; Zou, Q.-Q.; Wu, H.-Y.; Li, T.; Shi, W.-B.; Jiang, C.-D. Investigation of the Global Changes in Photosynthetic Electron Transport in Hosta Plants Grown Under Different Light Levels. Int. J. Mol. Sci. 2024, 25, 12876. https://doi.org/10.3390/ijms252312876
Liu D-H, Ji W-T, Zou Q-Q, Wu H-Y, Li T, Shi W-B, Jiang C-D. Investigation of the Global Changes in Photosynthetic Electron Transport in Hosta Plants Grown Under Different Light Levels. International Journal of Molecular Sciences. 2024; 25(23):12876. https://doi.org/10.3390/ijms252312876
Chicago/Turabian StyleLiu, Dong-Huan, Wen-Tao Ji, Qing-Qing Zou, Han-Yu Wu, Tao Li, Wen-Bin Shi, and Chuang-Dao Jiang. 2024. "Investigation of the Global Changes in Photosynthetic Electron Transport in Hosta Plants Grown Under Different Light Levels" International Journal of Molecular Sciences 25, no. 23: 12876. https://doi.org/10.3390/ijms252312876
APA StyleLiu, D.-H., Ji, W.-T., Zou, Q.-Q., Wu, H.-Y., Li, T., Shi, W.-B., & Jiang, C.-D. (2024). Investigation of the Global Changes in Photosynthetic Electron Transport in Hosta Plants Grown Under Different Light Levels. International Journal of Molecular Sciences, 25(23), 12876. https://doi.org/10.3390/ijms252312876






