Malvidin-3-O-Glucoside Mitigates α-Syn and MPTP Co-Induced Oxidative Stress and Apoptosis in Human Microglial HMC3 Cells
Abstract
:1. Introduction
2. Results
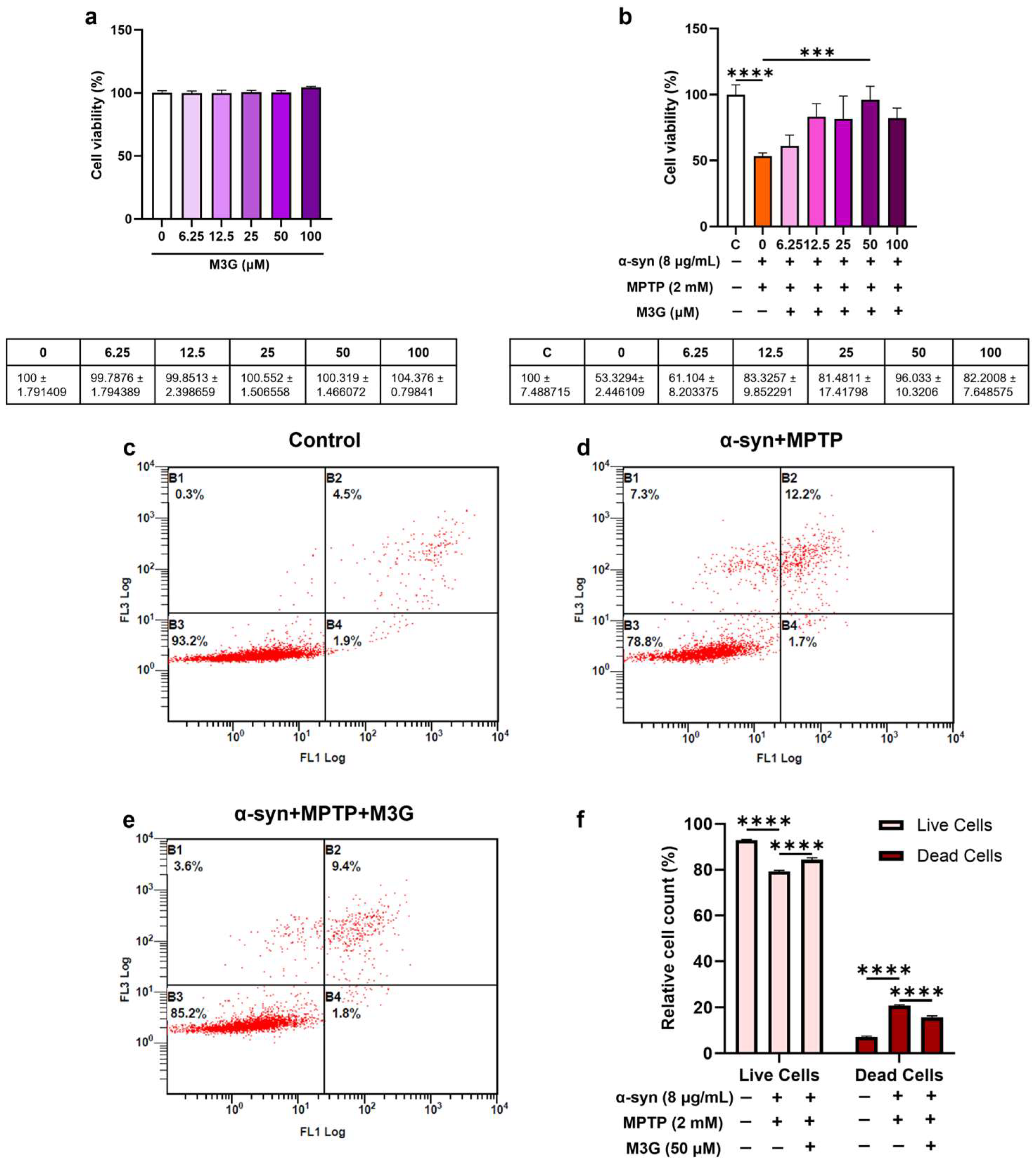
2.1. α-Syn- and MPTP-Induced Cytotoxicity in HMC3 Cells
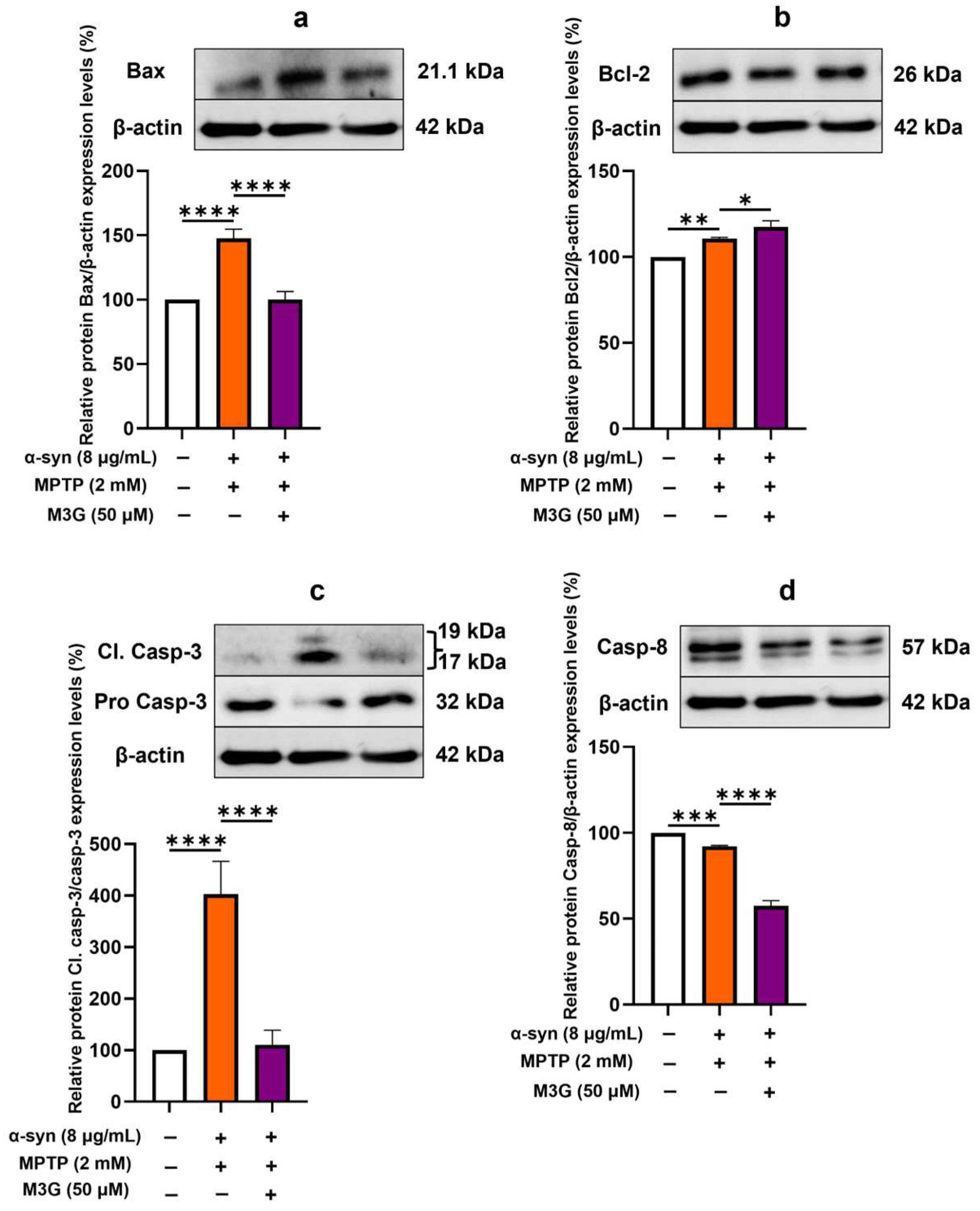
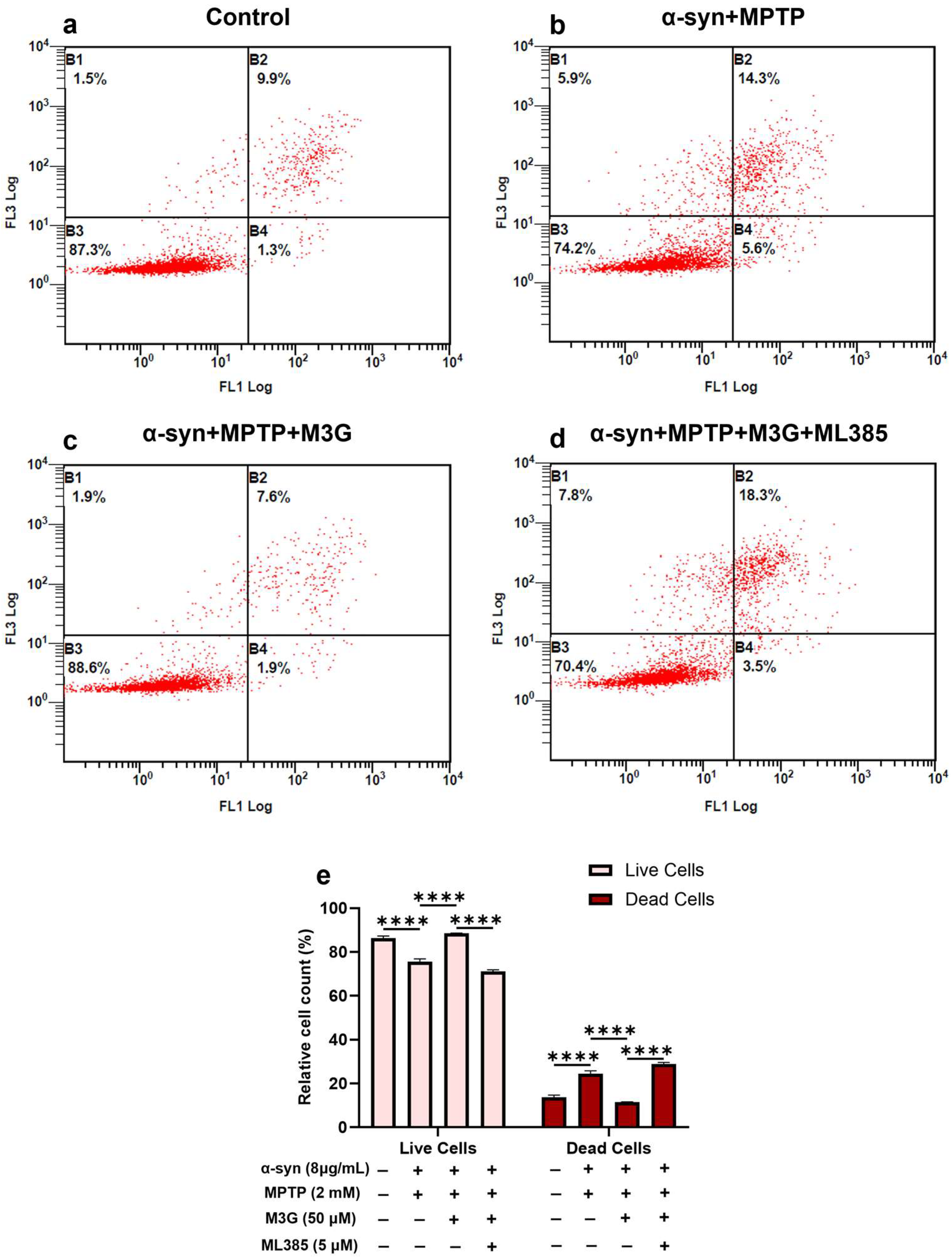
2.2. M3G Ameliorates α-Syn- and MPTP-Induced Cell Death (Apoptosis) in HMC3 Cells
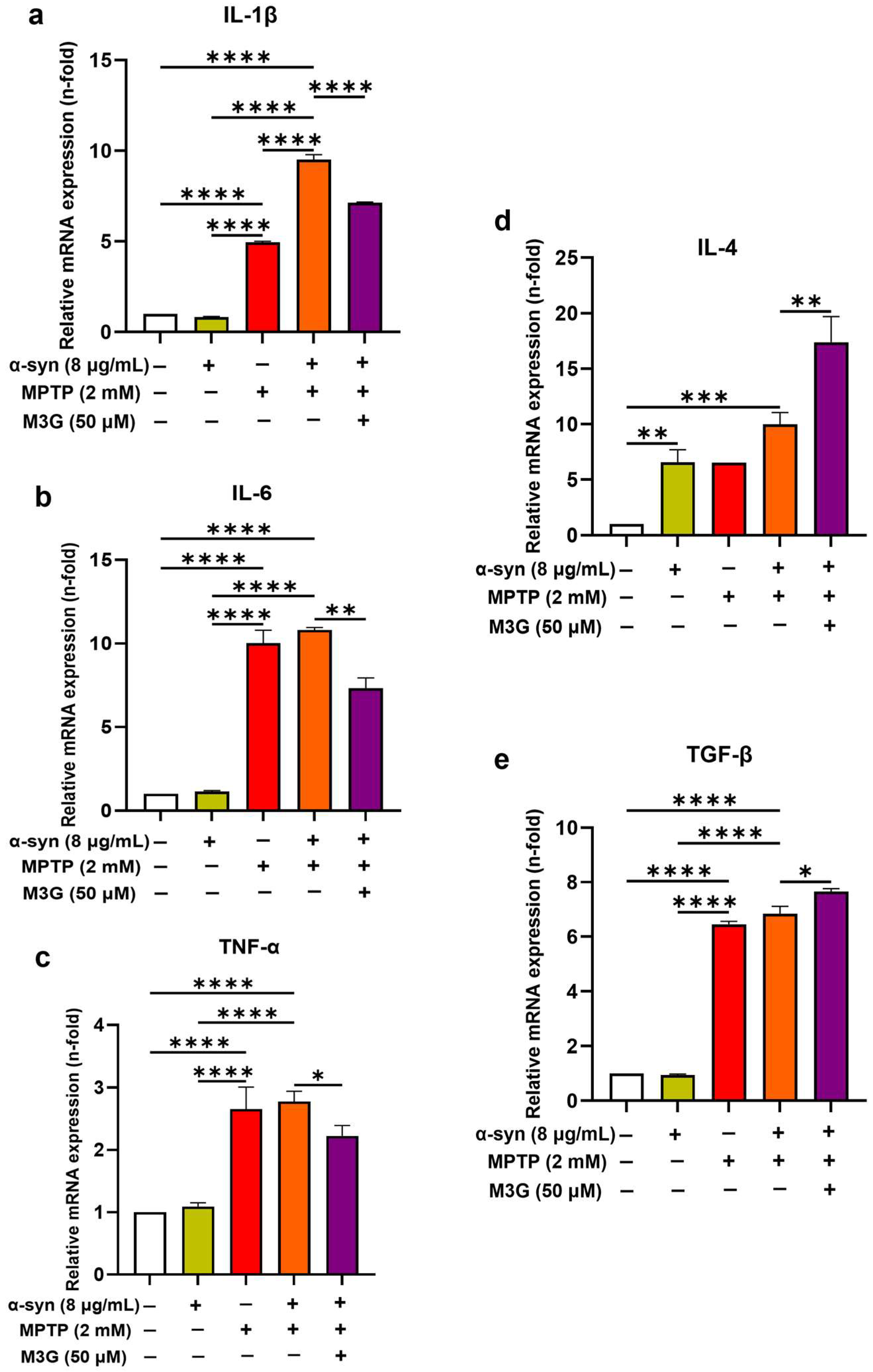
2.3. M3G Downregulates α-Syn- and MPTP-Induced Pro-Inflammatory Cytokines and Upregulates Anti-Inflammatory Cytokine Expression
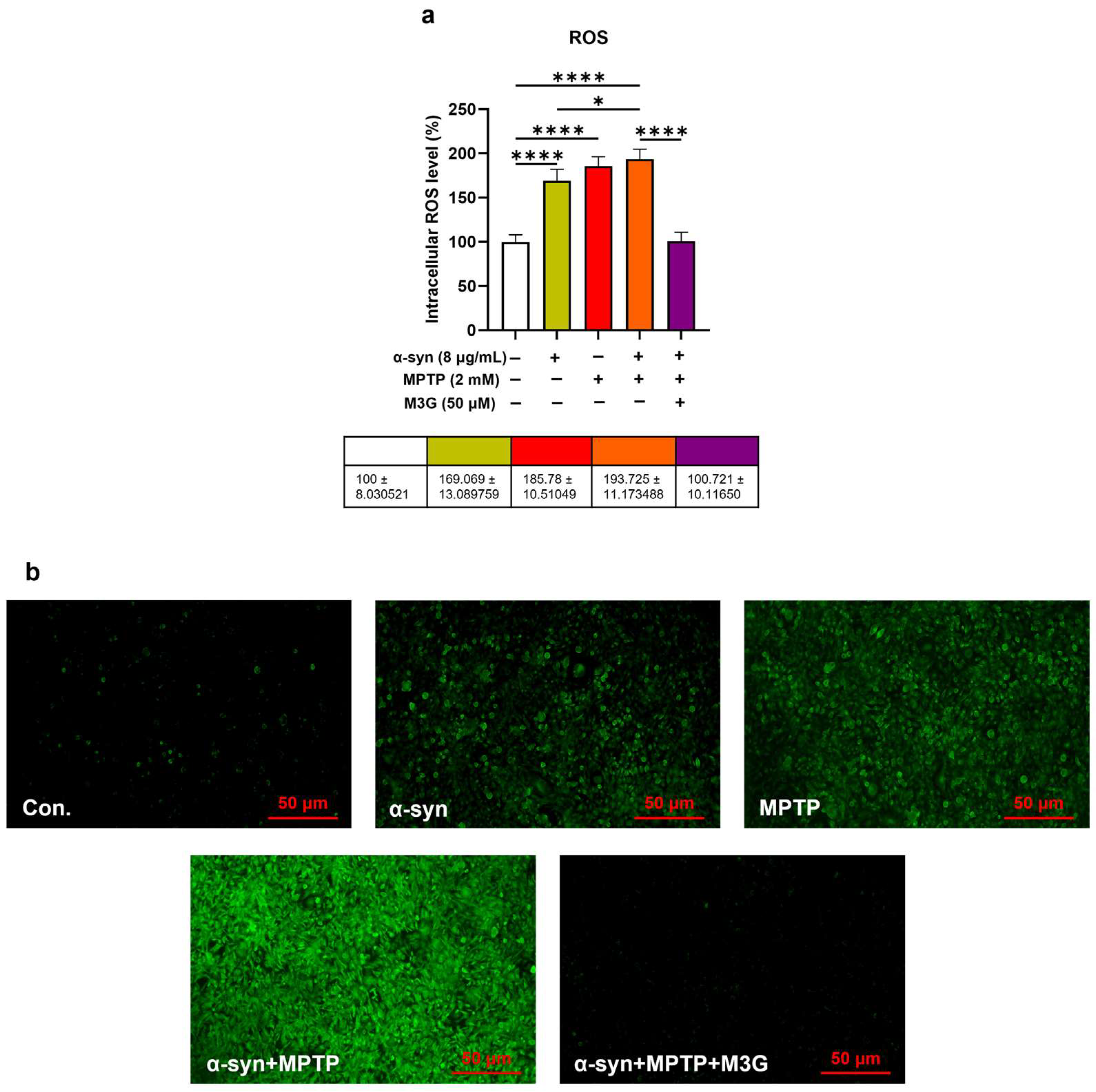
2.4. M3G Attenuates α-Syn- and MPTP-Induced ROS Production
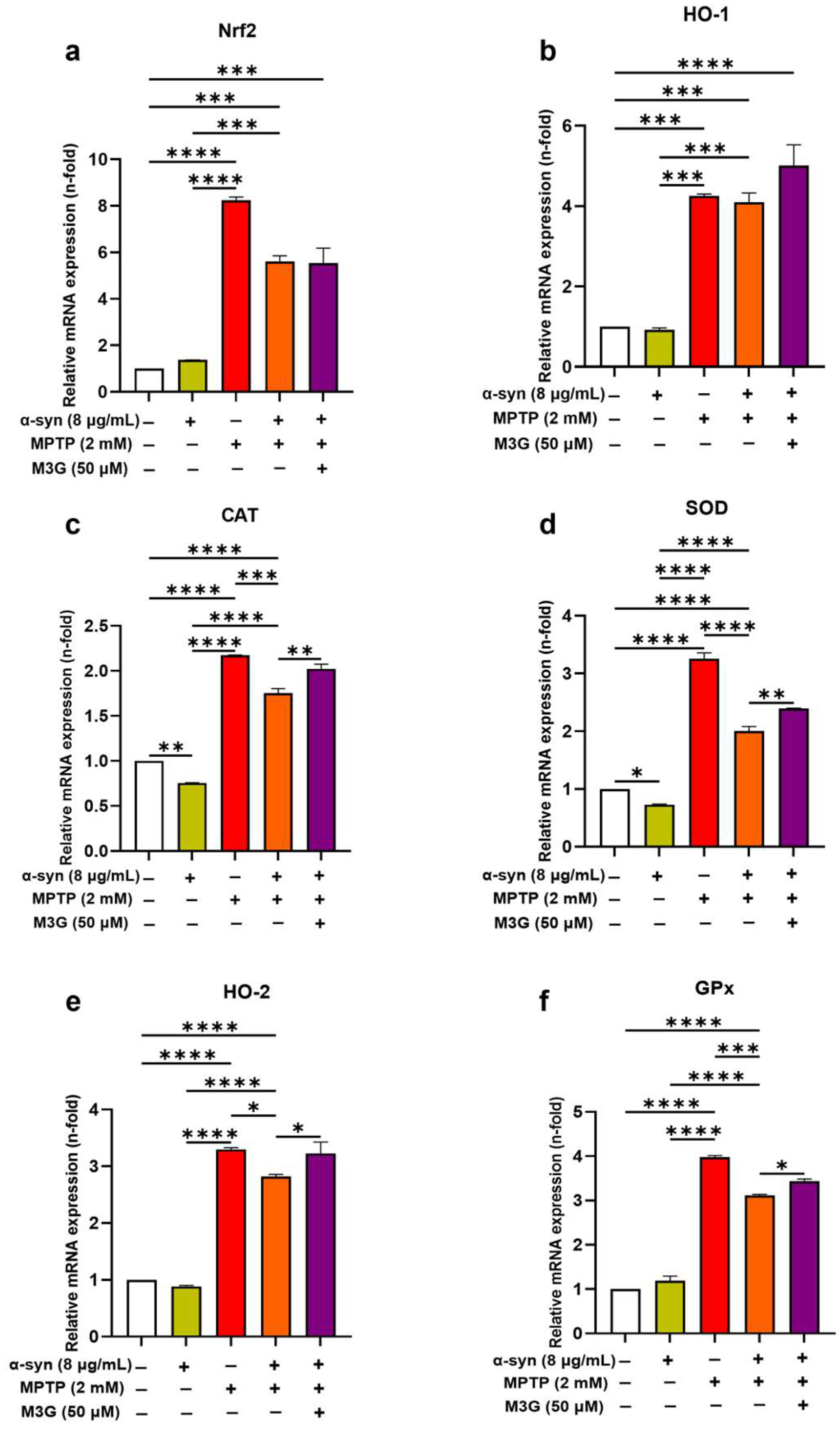
2.5. M3G Ameliorates Oxidative Stress by Upregulating the Transcriptional Expression Levels of Antioxidants in α-Syn- and MPTP-Induced HMC3 Cells
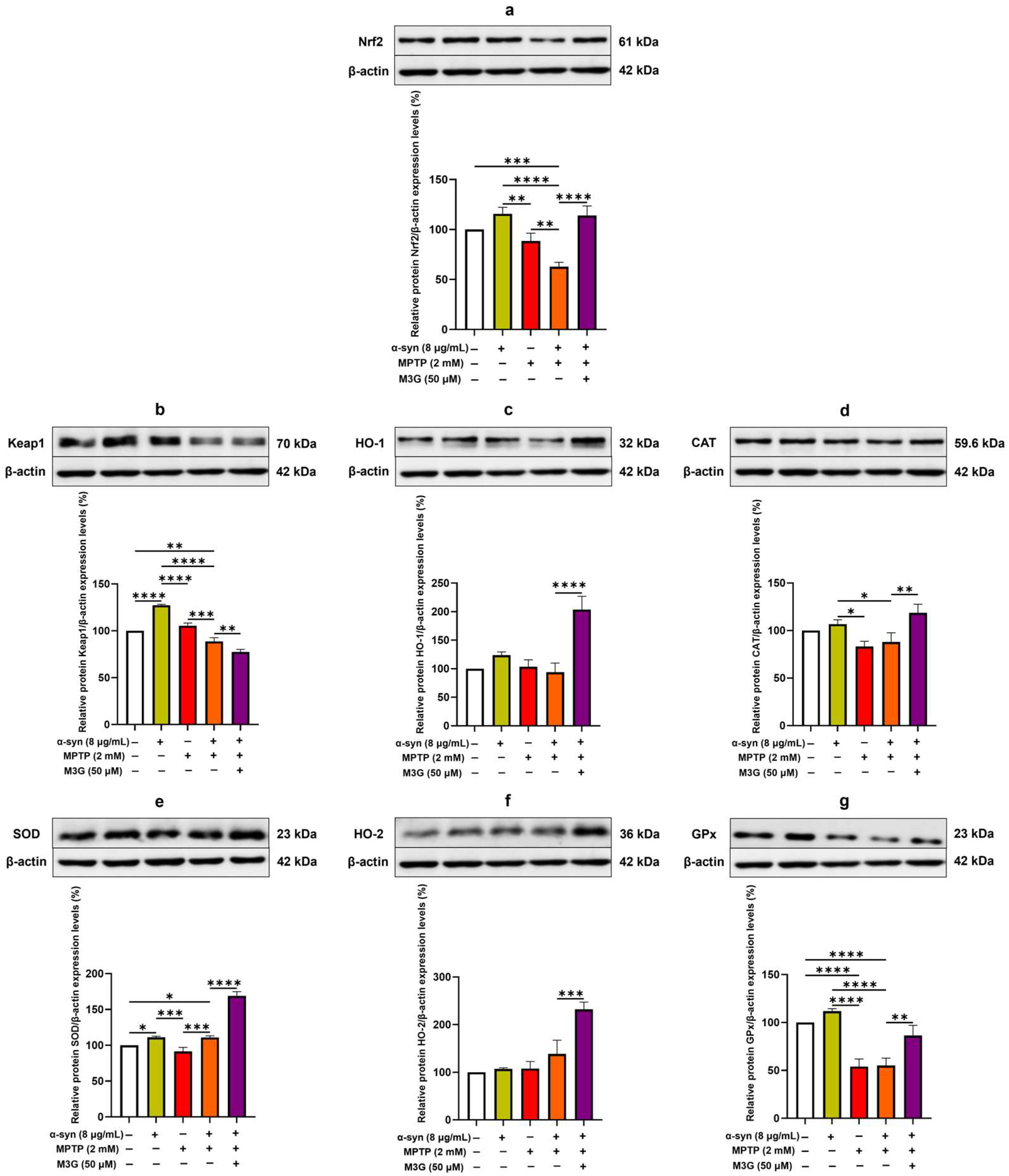
2.6. M3G Ameliorates Oxidative Stress by Upregulating the Translational Expression Levels of Antioxidants in α-Syn- and MPTP-Induced HMC3 Cells
2.7. M3G Mediates Antioxidative Effects Through Nrf2/HO-1 Signaling
2.8. M3G Mediates Anti-Apoptotic Effects Through Nrf2/HO-1 Signaling
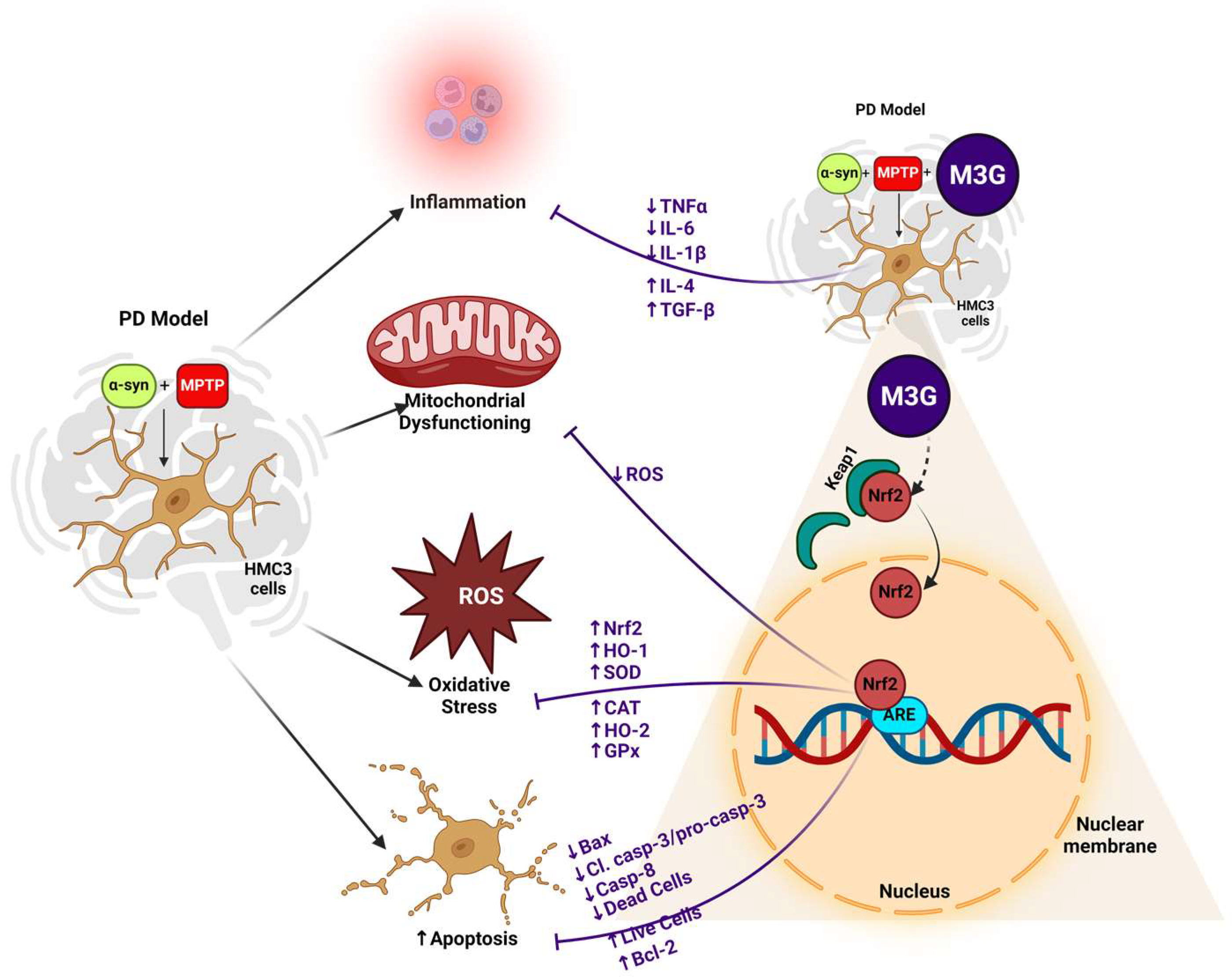
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. HMC3 Cell Culture
4.3. Cell Viability and Cytotoxicity Assay Protocol
4.4. RNA Preparation and Real-Time PCR
4.5. 2′,7′-Dichlorodihydrofluorescein Diacetate (DCFH-DA/H2DCFDA) Assay
4.6. Annexin Valinomycin–Fluorescein Isothiocyanate (V-FITC) Apoptosis Detection Assay
4.7. Western Blotting
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stykel, M.G.; Ryan, S.D. Nitrosative stress in Parkinson’s disease. NPJ Park. Dis. 2022, 8, 104. [Google Scholar] [CrossRef] [PubMed]
- Michael-Titus, A.; Revest, P.; Shortland, P. 14—DEMENTIA. In The Nervous System, 2nd ed.; Michael-Titus, A., Revest, P., Shortland, P., Eds.; Churchill Livingstone: Londaon, UK, 2010; pp. 251–266. [Google Scholar]
- Beitz, J.M. Parkinson’s disease: A review. FBS 2014, 6, 65–74. [Google Scholar] [CrossRef]
- Bernal-Conde, L.D.; Ramos-Acevedo, R.; Reyes-Hernández, M.A.; Balbuena-Olvera, A.J.; Morales-Moreno, I.D.; Argüero-Sánchez, R.; Schüle, B.; Guerra-Crespo, M. Alpha-synuclein physiology and pathology: A perspective on cellular structures and organelles. Front. Neurosci. 2020, 13, 1399. [Google Scholar] [CrossRef] [PubMed]
- Kam, T.-I.; Mao, X.; Park, H.; Chou, S.-C.; Karuppagounder, S.S.; Umanah, G.E.; Yun, S.P.; Brahmachari, S.; Panicker, N.; Chen, R. Poly (ADP-ribose) drives pathologic α-synuclein neurodegeneration in Parkinson’s disease. Science 2018, 362, eaat8407. [Google Scholar] [CrossRef]
- Lücking, C.; Brice, A. Alpha-synuclein and Parkinson’s disease. Cell. Mol. Life Sci. CMLS 2000, 57, 1894–1908. [Google Scholar] [CrossRef]
- Bengoa-Vergniory, N.; Roberts, R.F.; Wade-Martins, R.; Alegre-Abarrategui, J. Alpha-synuclein oligomers: A new hope. Acta Neuropathol. 2017, 134, 819–838. [Google Scholar] [CrossRef]
- Fernagut, P.-O.; Chesselet, M.-F. Alpha-synuclein and transgenic mouse models. Neurobiol. Dis. 2004, 17, 123–130. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ma, H.; Yv, Q.; Ye, F.; He, Z.; Chen, S.; Keram, A.; Li, W.; Zhu, M. Alpha-synuclein/MPP+ mediated activation of NLRP3 inflammasome through microtubule-driven mitochondrial perinuclear transport. Biochem. Biophys. Res. Commun. 2022, 594, 161–167. [Google Scholar] [CrossRef] [PubMed]
- Fornai, F.; Schlüter, O.M.; Lenzi, P.; Gesi, M.; Ruffoli, R.; Ferrucci, M.; Lazzeri, G.; Busceti, C.L.; Pontarelli, F.; Battaglia, G. Parkinson-like syndrome induced by continuous MPTP infusion: Convergent roles of the ubiquitin-proteasome system and α-synuclein. Proc. Natl. Acad. Sci. USA 2005, 102, 3413–3418. [Google Scholar] [CrossRef]
- Merghani, M.M. Effect of Mptp on α-Synuclein Spreading, Accumulation and Toxicity in Mice with Intrastriatal Innoculation of Human α-Synuclein Preformed Fibril. Master’s Thesis, United Arab Emirates University, Al Ain, United Arab Emirates, 2020. [Google Scholar]
- Song, L.-K.; Ma, K.-L.; Yuan, Y.-H.; Mu, Z.; Song, X.-Y.; Niu, F.; Han, N.; Chen, N.-H. Targeted overexpression of α-synuclein by rAAV2/1 vectors induces progressive nigrostriatal degeneration and increases vulnerability to MPTP in mouse. PLoS ONE 2015, 10, e0131281. [Google Scholar] [CrossRef]
- Richardson, J.R.; Caudle, W.M.; Guillot, T.S.; Watson, J.L.; Nakamaru-Ogiso, E.; Seo, B.B.; Sherer, T.B.; Greenamyre, J.T.; Yagi, T.; Matsuno-Yagi, A. Obligatory role for complex I inhibition in the dopaminergic neurotoxicity of 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP). Toxicol. Sci. 2007, 95, 196–204. [Google Scholar] [CrossRef] [PubMed]
- Arduíno, D.M.; Esteves, A.R.; Cardoso, S.M. Mitochondria drive autophagy pathology via microtubule disassembly: A new hypothesis for Parkinson disease. Autophagy 2013, 9, 112–114. [Google Scholar] [CrossRef]
- Jenner, P.; Olanow, C.W. Understanding cell death in Parkinson’s disease. Ann. Neurol. 1998, 44 (Suppl. S1), S72–S84. [Google Scholar] [CrossRef]
- Dauer, W.; Przedborski, S. Parkinson’s disease: Mechanisms and models. Neuron 2003, 39, 889–909. [Google Scholar] [CrossRef]
- Hirsch, E.C.; Hunot, S. Neuroinflammation in Parkinson’s disease: A target for neuroprotection? Lancet Neurol. 2009, 8, 382–397. [Google Scholar] [CrossRef]
- Fu, S.-P.; Wang, J.-F.; Xue, W.-J.; Liu, H.-M.; Liu, B.-R.; Zeng, Y.-L.; Li, S.-N.; Huang, B.-X.; Lv, Q.-K.; Wang, W. Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson’s disease models are mediated by GPR109A-dependent mechanisms. J. Neuroinflamm. 2015, 12, 9. [Google Scholar] [CrossRef]
- Barcia, C.; Ros, C.; Annese, V.; Gomez, A.; Ros-Bernal, F.; Aguado-Llera, D.; Martinez-Pagan, M.; De Pablos, V.; Fernandez-Villalba, E.; Herrero, M. IFN-γ signaling, with the synergistic contribution of TNF-α, mediates cell specific microglial and astroglial activation in experimental models of Parkinson’s disease. Cell Death Dis. 2011, 2, e142. [Google Scholar] [CrossRef] [PubMed]
- Jayaram, S.; Krishnamurthy, P.T. Role of microgliosis, oxidative stress and associated neuroinflammation in the pathogenesis of Parkinson’s disease: The therapeutic role of Nrf2 activators. Neurochem. Int. 2021, 145, 105014. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Shi, H.; Zhang, C.; Ren, M.; Han, M.; Wei, X.; Zhang, X.; Lou, H. Dimethyl fumarate attenuates 6-OHDA-induced neurotoxicity in SH-SY5Y cells and in animal model of Parkinson’s disease by enhancing Nrf2 activity. Neuroscience 2015, 286, 131–140. [Google Scholar] [CrossRef]
- Carvalho, A.N.; Marques, C.; Guedes, R.C.; Castro-Caldas, M.; Rodrigues, E.; Van Horssen, J.; Gama, M.J. S-Glutathionylation of Keap1: A new role for glutathione S-transferase pi in neuronal protection. FEBS Lett. 2016, 590, 1455–1466. [Google Scholar] [CrossRef]
- Trachootham, D.; Lu, W.; Ogasawara, M.A.; Valle, N.R.-D.; Huang, P. Redox regulation of cell survival. Antioxid. Redox Signal. 2008, 10, 1343–1374. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Cheng, E.; Brooke, S.; Chang, P.; Sapolsky, R. Over-expression of antioxidant enzymes protects cultured hippocampal and cortical neurons from necrotic insults. J. Neurochem. 2003, 87, 1527–1534. [Google Scholar] [CrossRef]
- Watzl, B.; Bub, A.; Briviba, K.; Rechkemmer, G. Acute intake of moderate amounts of red wine or alcohol has no effect on the immune system of healthy men. Eur. J. Nutr. 2002, 41, 264–270. [Google Scholar] [CrossRef]
- Sood, R.; Choi, H.-K.; Lee, H.-J. Potential anti-cancer properties of malvidin and its glycosides: Evidence from in vitro and in vivo studies. J. Funct. Foods 2024, 116, 106191. [Google Scholar] [CrossRef]
- Herrera-Balandrano, D.D.; Chai, Z.; Hutabarat, R.P.; Beta, T.; Feng, J.; Ma, K.; Li, D.; Huang, W. Hypoglycemic and hypolipidemic effects of blueberry anthocyanins by AMPK activation: In vitro and in vivo studies. Redox Biol. 2021, 46, 102100. [Google Scholar] [CrossRef]
- Bognar, E.; Sarszegi, Z.; Szabo, A.; Debreceni, B.; Kalman, N.; Tucsek, Z.; Sumegi, B.; Gallyas, F., Jr. Antioxidant and anti-inflammatory effects in RAW264. 7 macrophages of malvidin, a major red wine polyphenol. PLoS ONE 2013, 8, e65355. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.R.; Choi, M.J.; Choi, J.M.; Ko, J.C.; Ko, J.Y.; Cho, E.J. Malvidin protects WI-38 human fibroblast cells against stress-induced premature senescence. J. Cancer Prev. 2016, 21, 32. [Google Scholar] [CrossRef] [PubMed]
- Gutierres, J.M.; Carvalho, F.B.; Schetinger, M.R.C.; Marisco, P.; Agostinho, P.; Rodrigues, M.; Rubin, M.A.; Schmatz, R.; da Silva, C.R.; Cognato, G.D.P. Anthocyanins restore behavioral and biochemical changes caused by streptozotocin-induced sporadic dementia of Alzheimer’s type. Life Sci. 2014, 96, 7–17. [Google Scholar] [CrossRef]
- Tansey, M.G.; McCoy, M.K.; Frank-Cannon, T.C. Neuroinflammatory mechanisms in Parkinson’s disease: Potential environmental triggers, pathways, and targets for early therapeutic intervention. Exp. Neurol. 2007, 208, 1–25. [Google Scholar] [CrossRef]
- Block, M.L.; Zecca, L.; Hong, J.-S. Microglia-mediated neurotoxicity: Uncovering the molecular mechanisms. Nat. Rev. Neurosci. 2007, 8, 57–69. [Google Scholar] [CrossRef]
- Choi, I.; Zhang, Y.; Seegobin, S.P.; Pruvost, M.; Wang, Q.; Purtell, K.; Zhang, B.; Yue, Z. Microglia clear neuron-released α-synuclein via selective autophagy and prevent neurodegeneration. Nat. Commun. 2020, 11, 1386. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in neurodegenerative diseases: Mechanism and potential therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef]
- Block, M.; Hong, J.-S. Chronic microglial activation and progressive dopaminergic neurotoxicity. Biochem. Soc. Trans. 2007, 35, 1127–1132. [Google Scholar] [CrossRef]
- Li, N.; Alam, J.; Venkatesan, M.I.; Eiguren-Fernandez, A.; Schmitz, D.; Di Stefano, E.; Slaughter, N.; Killeen, E.; Wang, X.; Huang, A.; et al. Nrf2 is a key transcription factor that regulates antioxidant defense in macrophages and epithelial cells: Protecting against the proinflammatory and oxidizing effects of diesel exhaust chemicals. J. Immunol. 2004, 173, 3467–3481. [Google Scholar] [CrossRef] [PubMed]
- Rathore, A.S.; Singh, S.S.; Birla, H.; Zahra, W.; Keshri, P.K.; Dilnashin, H.; Singh, R.; Singh, S.; Singh, S.P. Curcumin Modulates p62–Keap1–Nrf2-Mediated Autophagy in Rotenone-Induced Parkinson’s Disease Mouse Models. ACS Chem. Neurosci. 2023, 14, 1412–1423. [Google Scholar]
- Upadhayay, S.; Mehan, S. Targeting Nrf2/HO-1 anti-oxidant signaling pathway in the progression of multiple sclerosis and influences on neurological dysfunctions. Brain Disord. 2021, 3, 100019. [Google Scholar] [CrossRef]
- Song, S.; Nie, Q.; Li, Z.; Du, G. Curcumin improves neurofunctions of 6-OHDA-induced parkinsonian rats. Pathol.-Res. Pract. 2016, 212, 247–251. [Google Scholar] [CrossRef]
- Dorsey, E.; Sherer, T.; Okun, M.S.; Bloem, B.R. The emerging evidence of the Parkinson pandemic. J. Park. Dis. 2018, 8 (Suppl. S1), S3–S8. [Google Scholar] [CrossRef]
- Ingelsson, M. Alpha-synuclein oligomers—Neurotoxic molecules in Parkinson’s disease and other Lewy body disorders. Front. Neurosci. 2016, 10, 408. [Google Scholar] [CrossRef]
- Van Den Eeden, S.K.; Tanner, C.M.; Bernstein, A.L.; Fross, R.D.; Leimpeter, A.; Bloch, D.A.; Nelson, L.M. Incidence of Parkinson’s disease: Variation by age, gender, and race/ethnicity. Am. J. Epidemiol. 2003, 157, 1015–1022. [Google Scholar] [CrossRef]
- Scheperjans, F.; Pekkonen, E.; Kaakkola, S.; Auvinen, P. Linking smoking, coffee, urate, and Parkinson’s disease–a role for gut microbiota? J. Park. Dis. 2015, 5, 255–262. [Google Scholar]
- Wanneveich, M.; Moisan, F.; Jacqmin-Gadda, H.; Elbaz, A.; Joly, P. Projections of prevalence, lifetime risk, and life expectancy of Parkinson’s disease (2010–2030) in France. Mov. Disord. 2018, 33, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Goldman, S.M. Environmental toxins and Parkinson’s disease. Annu. Rev. Pharmacol. Toxicol. 2014, 54, 141–164. [Google Scholar] [CrossRef] [PubMed]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T.D. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. 2014, 29, 1583–1590. [Google Scholar] [CrossRef]
- Driver, J.A.; Logroscino, G.; Gaziano, J.M.; Kurth, T. Incidence and remaining lifetime risk of Parkinson disease in advanced age. Neurology 2009, 72, 432–438. [Google Scholar] [CrossRef]
- Zafra-Stone, S.; Yasmin, T.; Bagchi, M.; Chatterjee, A.; Vinson, J.A.; Bagchi, D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol. Nutr. Food Res. 2007, 51, 675–683. [Google Scholar] [CrossRef]
- Rashid, K.; Wachira, F.N.; Nyabuga, J.N.; Wanyonyi, B.; Murilla, G.; Isaac, A.O. Kenyan purple tea anthocyanins ability to cross the blood brain barrier and reinforce brain antioxidant capacity in mice. Nutr. Neurosci. 2014, 17, 178–185. [Google Scholar] [CrossRef]
- Strathearn, K.E.; Yousef, G.G.; Grace, M.H.; Roy, S.L.; Tambe, M.A.; Ferruzzi, M.G.; Wu, Q.-L.; Simon, J.E.; Lila, M.A.; Rochet, J.-C. Neuroprotective effects of anthocyanin-and proanthocyanidin-rich extracts in cellular models of Parkinson’s disease. Brain Res. 2014, 1555, 60–77. [Google Scholar] [CrossRef]
- Matsunaga, N.; Imai, S.; Inokuchi, Y.; Shimazawa, M.; Yokota, S.; Araki, Y.; Hara, H. Bilberry and its main constituents have neuroprotective effects against retinal neuronal damage in vitro and in vivo. Mol. Nutr. Food Res. 2009, 53, 869–877. [Google Scholar] [CrossRef]
- Matsunaga, N.; Chikaraishi, Y.; Shimazawa, M.; Yokota, S.; Hara, H. Vaccinium myrtillus (bilberry) extracts reduce angiogenesis in vitro and in vivo. Evid.-Based Complement. Altern. Med. 2010, 7, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Marko, D.; Puppel, N.; Tjaden, Z.; Jakobs, S.; Pahlke, G. The substitution pattern of anthocyanidins affects different cellular signaling cascades regulating cell proliferation. Mol. Nutr. Food Res. 2004, 48, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.; Han, D.; Kim, B.; Baek, N.; Baik, B.-K. Antioxidant and anti-hypertensive activity of anthocyanin-rich extracts from hulless pigmented barley cultivars. Int. J. Food Sci. Technol. 2013, 48, 984–991. [Google Scholar] [CrossRef]
- Hyun, J.W.; Chung, H.S. Cyanidin and malvidin from Oryza sativa cv. Heugjinjubyeo mediate cytotoxicity against human monocytic leukemia cells by arrest of G2/M phase and induction of apoptosis. J. Agric. Food Chem. 2004, 52, 2213–2217. [Google Scholar] [CrossRef] [PubMed]
- Schober, A. Classic toxin-induced animal models of Parkinson’s disease: 6-OHDA and MPTP. Cell Tissue Res. 2004, 318, 215–224. [Google Scholar] [CrossRef]
- Qian, F.; Wang, M.; Wang, J.; Lu, C. Anthocyanin-rich blueberry extract ameliorates the behavioral deficits of MPTP-induced mouse model of Parkinson’s disease via anti-oxidative mechanisms. Yangtze Med. 2018, 3, 72–78. [Google Scholar] [CrossRef]
- Dauer, W.; Kholodilov, N.; Vila, M.; Trillat, A.-C.; Goodchild, R.; Larsen, K.E.; Staal, R.; Tieu, K.; Schmitz, Y.; Yuan, C.A. Resistance of α-synuclein null mice to the parkinsonian neurotoxin MPTP. Proc. Natl. Acad. Sci. USA 2002, 99, 14524–14529. [Google Scholar] [CrossRef]
- Hartmann, A.; Hunot, S.; Michel, P.P.; Muriel, M.-P.; Vyas, S.; Faucheux, B.A.; Mouatt-Prigent, A.; Turmel, H.; Srinivasan, A.; Ruberg, M. Caspase-3: A vulnerability factor and final effector in apoptotic death of dopaminergic neurons in Parkinson’s disease. Proc. Natl. Acad. Sci. USA 2000, 97, 2875–2880. [Google Scholar] [CrossRef]
- Tatton, N.A. Increased caspase 3 and Bax immunoreactivity accompany nuclear GAPDH translocation and neuronal apoptosis in Parkinson’s disease. Exp. Neurol. 2000, 166, 29–43. [Google Scholar] [CrossRef]
- Junn, E.; Mouradian, M.M. Apoptotic signaling in dopamine-induced cell death: The role of oxidative stress, p38 mitogen-activated protein kinase, cytochrome c and caspases. J. Neurochem. 2001, 78, 374–383. [Google Scholar] [CrossRef]
- Fan, H.; Cui, J.; Liu, F.; Zhang, W.; Yang, H.; He, N.; Dong, Z.; Dong, J. Malvidin protects against lipopolysaccharide-induced acute liver injury in mice via regulating Nrf2 and NLRP3 pathways and suppressing apoptosis and autophagy. Eur. J. Pharmacol. 2022, 933, 175252. [Google Scholar] [CrossRef] [PubMed]
- Tansey, M.G.; Goldberg, M.S. Neuroinflammation in Parkinson’s disease: Its role in neuronal death and implications for therapeutic intervention. Neurobiol. Dis. 2010, 37, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Dai, F.-R.; Du, X.-P.; Yang, Q.-D.; Chen, Y. Neuroprotection by silencing iNOS expression in a 6-OHDA model of Parkinson’s disease. J. Mol. Neurosci. 2012, 48, 225–233. [Google Scholar] [CrossRef]
- Huang, W.-Y.; Liu, Y.-M.; Wang, J.; Wang, X.-N.; Li, C.-Y. Anti-inflammatory effect of the blueberry anthocyanins malvidin-3-glucoside and malvidin-3-galactoside in endothelial cells. Molecules 2014, 19, 12827–12841. [Google Scholar] [CrossRef]
- Lull, M.E.; Block, M.L. Microglial activation and chronic neurodegeneration. Neurotherapeutics 2010, 7, 354–365. [Google Scholar] [CrossRef]
- Sonne, J.; Reddy, V.; Beato, M.R. Neuroanatomy, Substantia Nigra; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Lotharius, J.; O’Malley, K.L. The parkinsonism-inducing drug 1-methyl-4-phenylpyridinium triggers intracellular dopamine oxidation. A novel mechanism of toxicity. J. Biol. Chem. 2000, 275, 38581–38588. [Google Scholar] [CrossRef] [PubMed]
- Floor, E.; Wetzel, M.G. Increased Protein Oxidation in Human Substantia Nigra Pars Compacta in Comparison with Basal Ganglia and Prefrontal Cortex Measured with an Improved Dinitrophenylhydrazine Assay. J. Neurochem. 1998, 70, 268–275. [Google Scholar] [CrossRef]
- Votyakova, T.V.; Reynolds, I.J. DeltaPsi(m)-Dependent and -independent production of reactive oxygen species by rat brain mitochondria. J. Neurochem. 2001, 79, 266–277. [Google Scholar] [CrossRef]
- Ramsay, R.R.; Krueger, M.J.; Youngster, S.K.; Gluck, M.R.; Casida, J.E.; Singer, T.P. Interaction of 1-methyl-4-phenylpyridinium ion (MPP+) and its analogs with the rotenone/piericidin binding site of NADH dehydrogenase. J. Neurochem. 1991, 56, 1184–1190. [Google Scholar] [CrossRef]
- Souza, J.M.; Giasson, B.I.; Chen, Q.; Lee, V.M.; Ischiropoulos, H. Dityrosine cross-linking promotes formation of stable alpha -synuclein polymers. Implication of nitrative and oxidative stress in the pathogenesis of neurodegenerative synucleinopathies. J. Biol. Chem. 2000, 275, 18344–18349. [Google Scholar] [CrossRef]
- Hsu, L.J.; Sagara, Y.; Arroyo, A.; Rockenstein, E.; Sisk, A.; Mallory, M.; Wong, J.; Takenouchi, T.; Hashimoto, M.; Masliah, E. Alpha-synuclein promotes mitochondrial deficit and oxidative stress. Am. J. Pathol. 2000, 157, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Merecz-Sadowska, A.; Sitarek, P.; Kowalczyk, T.; Zajdel, K.; Jęcek, M.; Nowak, P.; Zajdel, R. Food anthocyanins: Malvidin and its glycosides as promising antioxidant and anti-inflammatory agents with potential health benefits. Nutrients 2023, 15, 3016. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Li, X.; Yang, Q.; Lu, Y.; Wang, G.; Yang, H.; Dong, J.; Zhang, H. Malvidin alleviates mitochondrial dysfunction and ROS accumulation through activating AMPK-α/UCP2 axis, thereby resisting inflammation and apoptosis in SAE mice. Front. Pharmacol. 2023, 13, 1038802. [Google Scholar] [CrossRef]
- Ji, L.; Moghal, N.; Zou, X.; Fang, Y.; Hu, S.; Wang, Y.; Tsao, M.S. The NRF2 antagonist ML385 inhibits PI3K-mTOR signaling and growth of lung squamous cell carcinoma cells. Cancer Med. 2023, 12, 5688–5702. [Google Scholar] [CrossRef]
- Najjar, R.S.; Mu, S.; Feresin, R.G. Blueberry polyphenols increase nitric oxide and attenuate angiotensin II-induced oxidative stress and inflammatory signaling in human aortic endothelial cells. Antioxidants 2022, 11, 616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, T.; Pei, Z.; Miller, D.S.; Wu, X.; Block, M.L.; Wilson, B.; Zhang, W.; Zhou, Y.; Hong, J.-S.; et al. Aggregated α-synuclein activates microglia: A process leading to disease progression in Parkinson’s disease. FASEB J. 2005, 19, 533–542. [Google Scholar] [CrossRef]
- Biasini, E.; Fioriti, L.; Ceglia, I.; Invernizzi, R.; Bertoli, A.; Chiesa, R.; Forloni, G. Proteasome inhibition and aggregation in Parkinson’s disease: A comparative study in untransfected and transfected cells. J. Neurochem. 2004, 88, 545–553. [Google Scholar] [CrossRef]
- Feng, L.R.; Federoff, H.J.; Vicini, S.; Maguire-Zeiss, K.A. Alpha-synuclein mediates alterations in membrane conductance: A potential role for alpha-synuclein oligomers in cell vulnerability. Eur. J. Neurosci. 2010, 32, 10–17. [Google Scholar] [CrossRef]
- McNaught, K.S.P.; Olanow, C.W.; Halliwell, B.; Isacson, O.; Jenner, P. Failure of the ubiquitin–proteasome system in Parkinson’s disease. Nat. Rev. Neurosci. 2001, 2, 589–594. [Google Scholar] [CrossRef]
- Bassil, F.; Brown, H.J.; Pattabhiraman, S.; Iwasyk, J.E.; Maghames, C.M.; Meymand, E.S.; Cox, T.O.; Riddle, D.M.; Zhang, B.; Trojanowski, J.Q. Amyloid-beta (Aβ) plaques promote seeding and spreading of alpha-synuclein and tau in a mouse model of Lewy body disorders with Aβ pathology. Neuron 2020, 105, 260–275.e6. [Google Scholar] [CrossRef]
- Sengupta, U.; Kayed, R. Amyloid β, Tau, and α-Synuclein aggregates in the pathogenesis, prognosis, and therapeutics for neurodegenerative diseases. Prog. Neurobiol. 2022, 214, 102270. [Google Scholar] [CrossRef] [PubMed]
- Tosun, D.; Hausle, Z.; Iwaki, H.; Thropp, P.; Lamoureux, J.; Lee, E.B.; MacLeod, K.; McEvoy, S.; Nalls, M.; Perrin, R.J. A cross-sectional study of α-synuclein seed amplification assay in Alzheimer’s disease neuroimaging initiative: Prevalence and associations with Alzheimer’s disease biomarkers and cognitive function. In Alzheimer’s & Dementia; Wiley: Hoboken, NJ, USA, 2024. [Google Scholar]



| Target Gene | Forward Primer Sequence | Reverse Primer Sequence |
|---|---|---|
| β-actin | 5′-CTCTTCCAGCCTTCCTTCCT-3′ | 5′-AGCACTGTGTTGGCGTACAG-3′ |
| Nrf2 | 5′-TCCGGGTGTGTTTGTTCCAA-3′ | 5′-CGCCCGCGAGATAAAGAGTT-3′ |
| HO-1 | 5′-AGACTGCGTTCCTGCTCAAC-3′ | 5′-AAAGCCCTACAGCAACTGTCG-3′ |
| CAT | 5′-GTGCGGAGATTCAACACTGCCA-3′ | 5′-CGGCAATGTTCTCACACAGACG-3′ |
| SOD | 5′-CTCACTCTCAGGAGACCATTGC-3′ | 5′-CCACAAGCCAAACGACTTCCAG-3′ |
| HO-2 | 5′-CCACCACGGCACTTTACTTCA-3′ | 5′-CGTTCTGCCCTATGTAGTGGA-3′ |
| GPx | 5′-GTGCTCGGCTTCCCGTGCAAC-3′ | 5′-CTCGAAGAGCATGAAGTTGGGC-3′ |
| IL-1β | 5′-GGGATAACGAGGCTTATGTGC-3′ | 5′-AGGTGGAGAGCTTTCAGTTCA-3′ |
| IL-6 | 5′-GACCCAACCACAAATGCCAG-3′ | 5′-GAGTTGTCATGTCCTGCAGC-3′ |
| TNF-α | 5′-TGAGCACTGAAAGCATGATCC-3′ | 5′-GGAGAAGAGGCTGAGGAACA-3′ |
| IL-4 | 5′-CCGTAACAGACATCTTTGCTGCC-3′ | 5′-GAGTGTCCTTCTCATGGTGGCT-3′ |
| TGF-β | 5′-TACCTGAACCCGTGTTGCTCTC-3′ | 5′-GTTGCTGAGGTATCGCCAGGAA-3′ |
| Bax | 5′-TCAGGATGCGTCCACCAAGAAG-3′ | 5′-TGTGTCCACGGCGGCAATCATC-3′ |
| Bcl2 | 5′-ATCGCCCTGTGGATGACTGAGT-3′ | 5′-GCCAGGAGAAATCAAACAGAGGC-3′ |
| Casp-3 | 5′-GGAAGCGAATCAATGGACTCTGG-3′ | 5′-GCATCGACATCTGTACCAGACC-3′ |
| Casp-8 | 5′-AGAAGAGGGTCATCCTGGGAGA-3′ | 5′-TCAGGACTTCCTTCAAGGCTGC-3′ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sood, R.; Sanjay; Kang, S.-U.; Yoon, N.Y.; Lee, H.-J. Malvidin-3-O-Glucoside Mitigates α-Syn and MPTP Co-Induced Oxidative Stress and Apoptosis in Human Microglial HMC3 Cells. Int. J. Mol. Sci. 2024, 25, 12733. https://doi.org/10.3390/ijms252312733
Sood R, Sanjay, Kang S-U, Yoon NY, Lee H-J. Malvidin-3-O-Glucoside Mitigates α-Syn and MPTP Co-Induced Oxidative Stress and Apoptosis in Human Microglial HMC3 Cells. International Journal of Molecular Sciences. 2024; 25(23):12733. https://doi.org/10.3390/ijms252312733
Chicago/Turabian StyleSood, Rachit, Sanjay, Sung-Ung Kang, Na Young Yoon, and Hae-Jeung Lee. 2024. "Malvidin-3-O-Glucoside Mitigates α-Syn and MPTP Co-Induced Oxidative Stress and Apoptosis in Human Microglial HMC3 Cells" International Journal of Molecular Sciences 25, no. 23: 12733. https://doi.org/10.3390/ijms252312733
APA StyleSood, R., Sanjay, Kang, S.-U., Yoon, N. Y., & Lee, H.-J. (2024). Malvidin-3-O-Glucoside Mitigates α-Syn and MPTP Co-Induced Oxidative Stress and Apoptosis in Human Microglial HMC3 Cells. International Journal of Molecular Sciences, 25(23), 12733. https://doi.org/10.3390/ijms252312733










