In Vitro and In Vivo Translational Insights into the Intraoperative Use of Antiseptics and Lavage Solutions Against Microorganisms Causing Orthopedic Infections
Abstract
1. Introduction
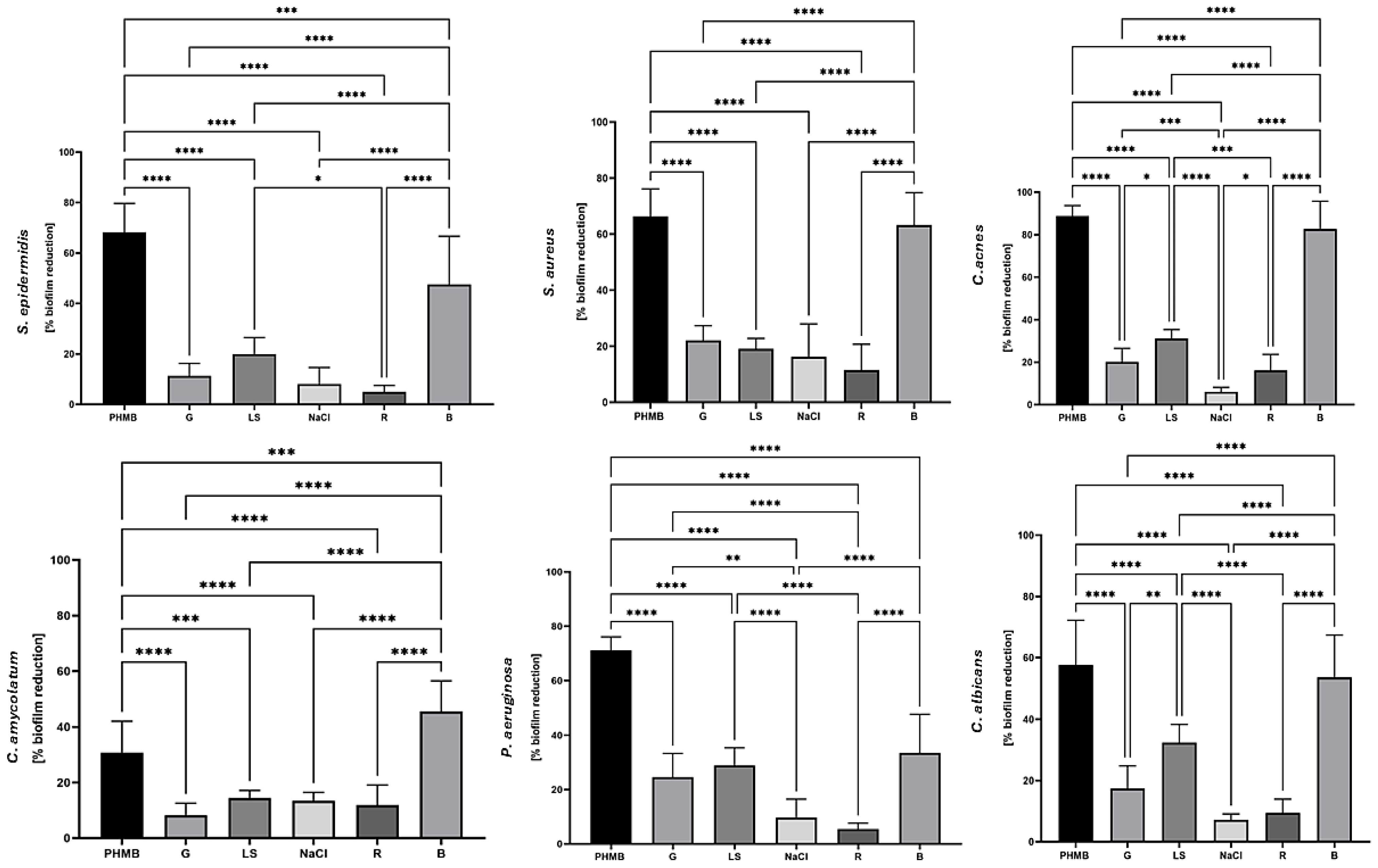
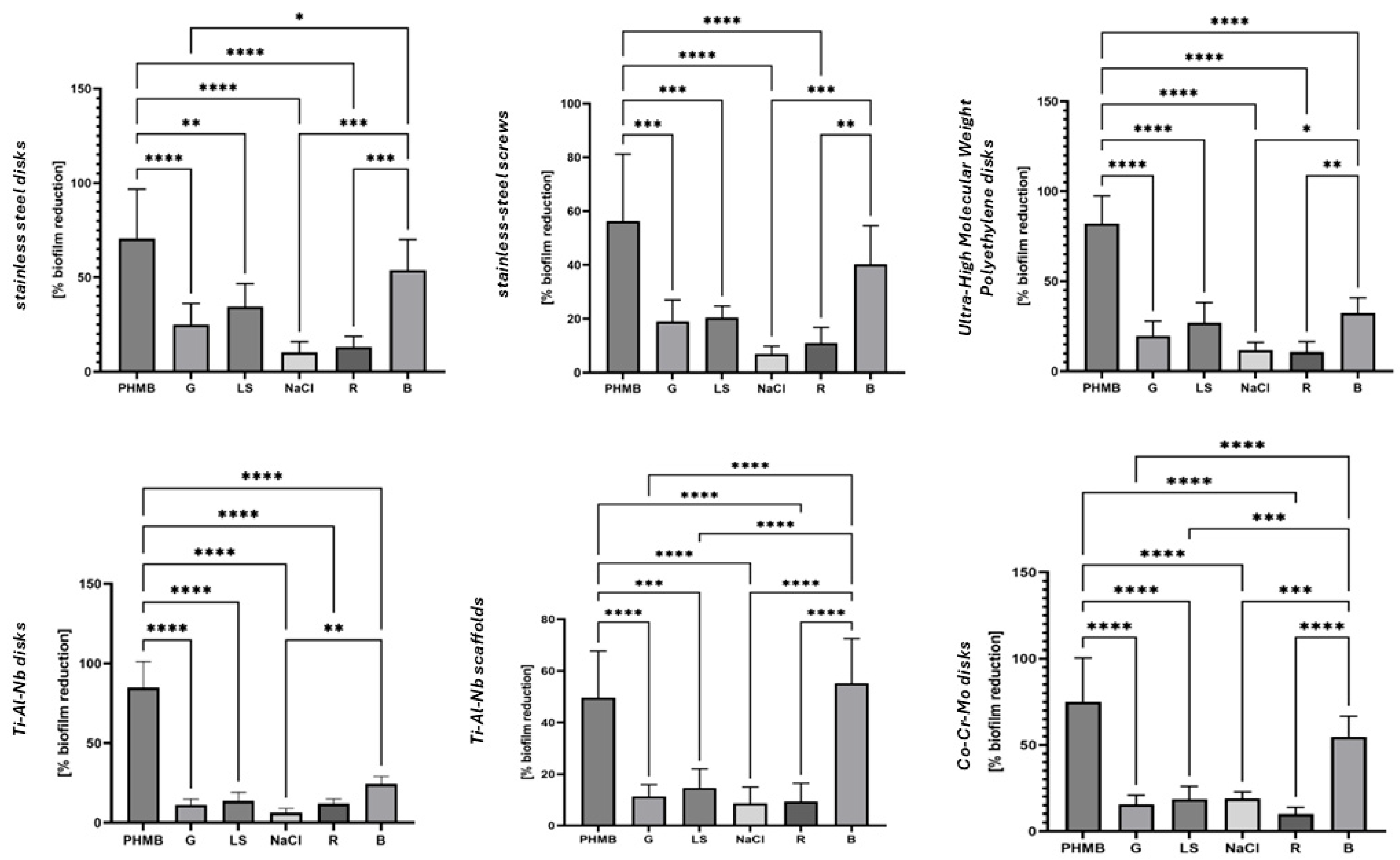
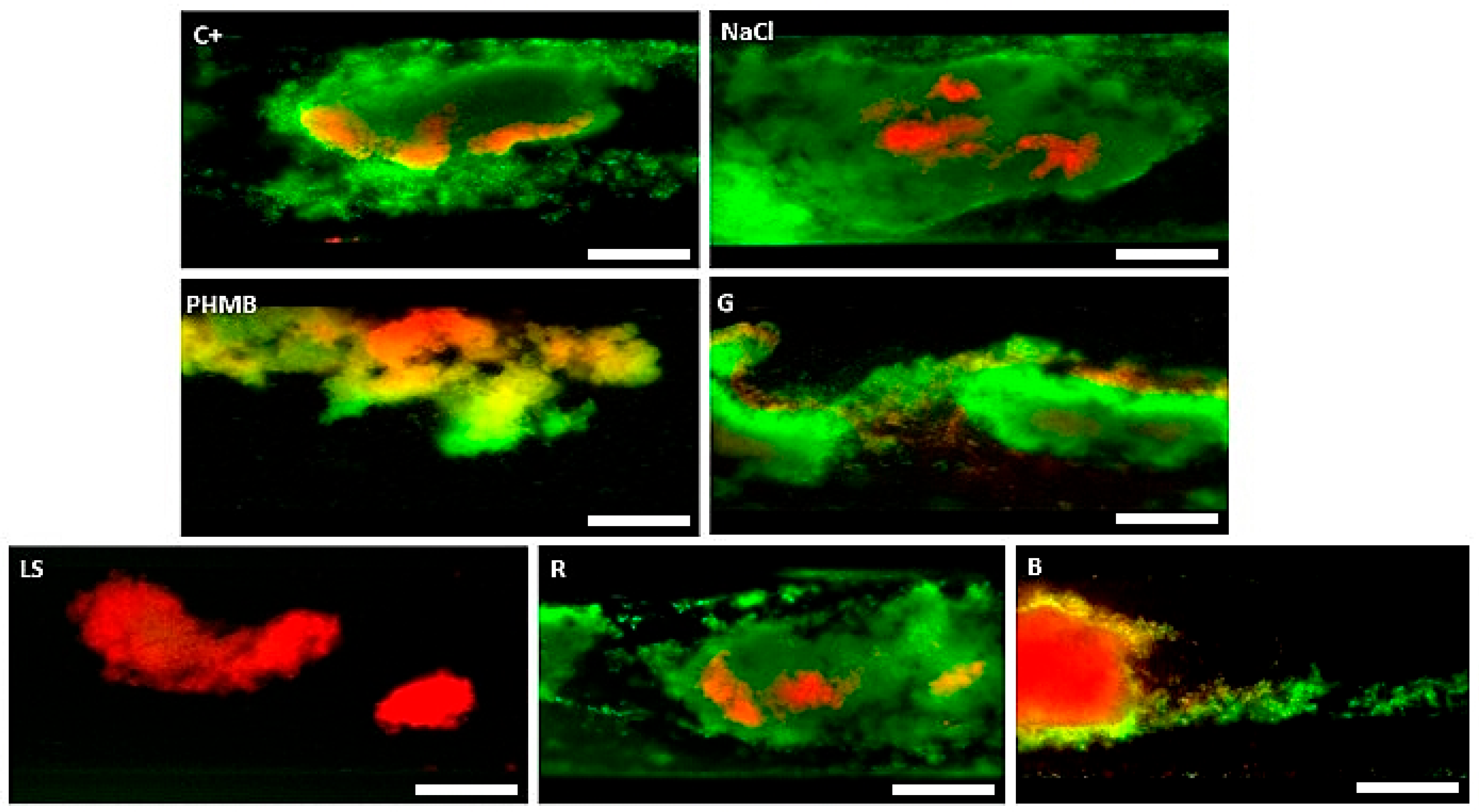
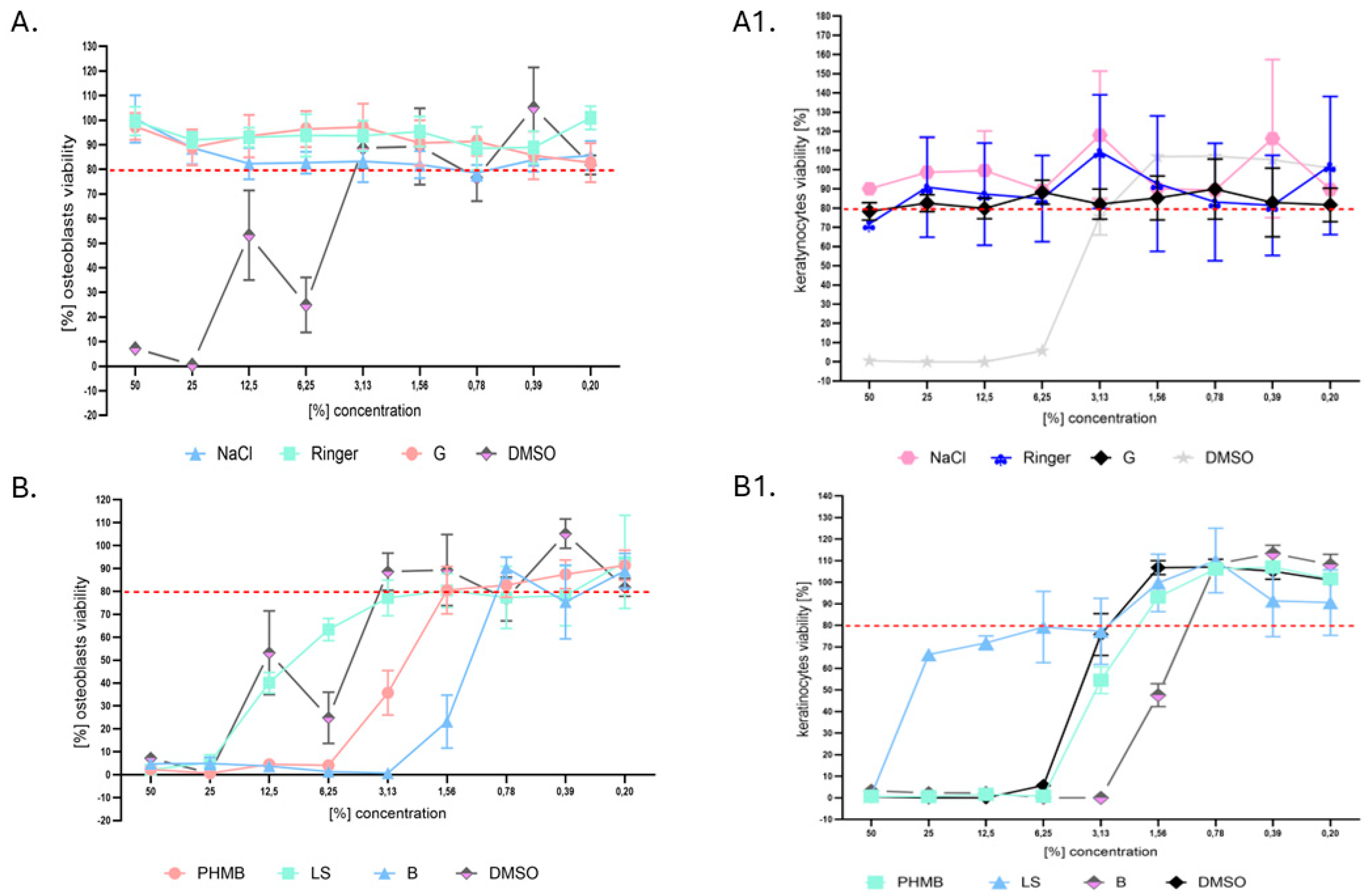
2. Results
3. Discussion
4. Materials and Methods
4.1. Antiseptic and Lavaseptic Products Analyzed
- (a)
- Polyhexanide-containing product under brand name Preventia Surgical Irrigation Solution (Paul Hartmann AG, Heidenheim an der Brenz, Germany) containing, according to the manufacturer, polyhexanide (1.0 g), and poloxamer, later referred to as “PHMB”.
- (b)
- Hypochlorous acid/sodium hypochlorite-based product under brand name Granudacyn Wound Irrigation Solution (Molnycke, Goteburg, Sweden) containing, according to the manufacturer, water, sodium chloride, hypochlorous acid (0.05 g), and sodium hypochlorite (0.05 g), later referred to as “G”.
- (c)
- Polyhexanide-based product under brand name LavaSorb (B. Braun Medical AG, Melsungen, Germany) containing, according to the manufacturer, polyhexanide (0.40 g), macrogol 4000 (0.02 g), sodium chloride (8.60 g), calcium chloride dihydrate (0.33 g), potassium chloride (0.30 g), and purified water up to 1 L, later referred to as “LS”.
- (d)
- Povidone–iodine-containing product under brand name Betasoidona (Hermes Artzneimittel, Pullach im Isartal, Germany), containing, according to the manufacturer, povidone–iodine (100 mg), equivalent to iodine (11 mg), and excipients including glycerol, nonoxynol-9, disodium phosphate, citric acid, sodium hydroxide for pH adjustment, and potassium iodate in 1 mL of solution, later referred to as “B”.
- (e)
- Saline, 0.9% (B. Braun Medical AG, Melsungen, Germany), later referred to as “NaCl”.
- (f)
- Ringer’s solution (B. Braun Medical AG, Melsungen, Germany), containing, according to the manufacturer, sodium chloride (8.6 g), potassium chloride (0.3 g), and calcium chloride dihydrate (0.33 g/L) in 1 L of water for injections, later referred to as “R”.
4.2. The Organisms
- (a)
- Staphylococcus epidermidis, (n = 25), including ATCC strain number 12228;
- (b)
- Staphylococcus aureus MRSA (n = 25), including ATCC strain number 33591;
- (c)
- Cutibacterium acnes (n = 25), including ATCC strain number 11828;
- (d)
- Corynebacterium amycolatum (n = 25), including ATCC strain number 700207
- (e)
- Pseudomonas aeruginosa (n = 25), including ATCC strain number 27853;
- (f)
- Candida albicans (n = 25), including ATCC strain number 10231;Clinical strains isolated from chronic leg ulcers and long-bone infections under approval of the Bioethics Committee of Wroclaw Medical University, number 949/2022;
- (g)
- Cellulose-forming Komagataeibacter xylinus number 53524;
- (h)
- Galleria mellonella larvae, bred and cultivated in “P.U.M.A.” Platform for Unique Model Application, applied for the in vivo tests. The larvae applied in experiments were of 200 ± 10 mg weight.
4.3. Surfaces Applied for Biofilm Development and Subsequent Removal Using the Provided Antiseptic/Lavaseptic Products
- (a)
- Disks with 16 mm diameter/2 mm height made of medical-grade polystyrene (Compamed, Dusseldorf, Germany), later referred to as “PS”. This surface, not applied in orthopedic settings but commonly used as a reference surface for biofilm growth in vitro, was applied in the character of control setting.
- (b)
- Stainless-steel disks with 16 mm diameter/2 mm height (Kamb, Warsaw, Poland), later referred to as SSDs.
- (c)
- Co-Cr-Mo disks with 16 mm diameter/2 mm height (Schutz Dental, Rosbach vor der Hoche, Germany), later referred to as Co-Cr-Mo.
- (d)
- Ti-Al-Nb disks with 16 mm diameter/2 mm height (Kamb, Warsaw, Poland), later referred to as Ti-Al-Nb-D.
- (e)
- Ti-Al-Nb scaffold implants with 8 mm diameter/8 mm height (Kamb, Warsaw, Poland) produced by Additive Manufacturing SLM processing, as described in an earlier work of ours [44], later referred to as Ti-Al-Nb-S.
- (f)
- Ultra-high-molecular-weight polyethylene disks with 16 mm diameter/2 mm height (Enimat, Bydgoszcz, Poland), later referred to as UHMPWEs.
- (g)
- Orthopedic stainless-steel screws with a size of 6 mm × 1.8 mm (Biomedent, Houston, TX, USA), later referred to as SSCs.
- (h)
- Bacterial cellulose disks with 16 mm diameter/2 mm height, later referred to as BC, obtained as described earlier [45]. K. xylinus strain ATCC 53524 was incubated in Hestrin–Schramm medium (2% glucose (w/v; Chempur, Piekary Slaskie, Poland), 0.5% yeast extract (w/v; VWR Chemicals, Radnor, PA, USA), 0.5% bactopeptone (w/v; VWR Chemicals, Radnor, PA, USA), 0.115% citric acid (w/v; POCH, Gliwice, Poland), 0.27% Na2HPO4 (w/v; POCH, Gliwice, Poland), 0.05% MgSO4 × 7H2O (w/v; POCH, Gliwice, Poland), and 1% ethanol (v/v; Stanlab, Lublin, Poland)) for 7 days at 28 °C in 24-well microtiter plates (F type, Nest Scientific Biotechnology, Wuxi, China). Next, the formed BC disks were removed from the medium and cleansed using 0.1 M NaOH (Chempur, Piekary Slaskie, Poland) solution at 80 °C until the BC became white. Then, the BC disks were purified using water until they reached a pH of 7 (measured by pH strips, Macherey–Nagel, Düren, Germany) and sterilized in a steam autoclave. The BC disks were placed into 24-well plates (F type, Nest Scientific Biotechnology, Wuxi, China) in sterile water and incubated at 4 °C until the time of further experiments.
4.4. Measurement of Minimum Biocidal Concentration (MBC) and Minimum Biofilm Eradication Concentration (MBEC) of Antiseptics and Lavaseptics Using Microtitrate Plate Model
- (a)
- MBC: Microbial suspensions at a density of 0.5 McF (McFarland turbidity scale) (Densitomat II, BioMerieux, Warsaw, Poland) in 0.9% NaCl (Stanlab, Lublin, Poland) were prepared from fresh, 24 h cultures in appropriate culture broths (M-H for S. aureus, S. epidermidis, P. aeruginosa, C. albicans; BHI for C. acnes). All media were purchased from Graso, Jablowo, Poland. The suspensions were diluted 1000 times in appropriate media to reach ~105 cfu/mL and 100 μL of each suspension was introduced into 10 wells of a 96-well plate (VWR, Radnor, PA, USA). Next, serial dilutions of antiseptics/lavaseptics were added to each of well containing the microbial suspension. Therefore, the highest v/v concentration of the antimicrobial was 50%, while the lowest was 0.1%. The control setting of bacterial/fungal growth (microbial suspension without antiseptic/lavaseptic) and sterility control (broth without suspended microbes) were provided in wells number 12 and 11, respectively. The plates containing S. epidermidis, S. aureus, C. amycolatum, P. aeruginosa, and C. albicans were incubated for 24 h at 37 °C under stationary conditions in aerobic conditions, while C. acnes was incubated for 24 h/37 °C in anaerobic conditions provided by an anaerobic atmosphere generation container (Sigma-Aldritch, Taufkirchen, Germany). Analyses on C. acnes were performed with the use of an anaerobic chamber (Jacomex, Dagneux, France). The next day, a standard resazurin sodium salt solution (Acros Organics, Geel, Belgium) was applied to indicate the concentration of product, which caused the stop of metabolic activity. Wells from parallel prepared plates with no resazurin solution were introduced into 9800 µL of the appropriate medium and incubated for 72 h. If no turbidity change occurred, the specific concentration of the antiseptic/lavaseptic used was considered MBC.
- (b)
- MBEC: Microbial suspensions at a density of 0.5 McF (McFarland turbidity scale) (Densitomat II, BioMerieux, Warsaw, Poland) in 0.9% NaCl (Stanlab, Lublin, Poland) were prepared from fresh, 24 h cultures in appropriate culture broths (M-H for S. aureus, S. epidermidis, P. aeruginosa, C. albicans; BHI for C. acnes). All media were purchased from Graso, Jablowo, Poland. The suspensions were diluted 1000 times in appropriate media to reach ~105 cfu/mL and 100 μL of each suspension was introduced into 10 wells of a 96-well plate (VWR, Radnor, PA, USA). Next, the plates were incubated for 24 h (S. aureus, S. epidermidis, P. aeruginosa, C. albicans) or 48 h (C. acnes) in the conditions specified in the MBC assessment. After incubation and medium removal, serial dilutions of the antiseptics/lavaseptics were added and plates were subjected to another 24 h of incubation. Next, the medium was removed, neutralizing agents were applied for 5 min, and after that, the procedures related to resazurin staining and transfer to fresh media were performed as described in the protocol for MBC assessment.
4.5. Measurement of Activity of Antiseptics/Lavaseptics Against Biofilms Using B.O.A.T. (Biofilm-Oriented Antiseptic Test)
4.6. The Activity of Antiseptics/Lavaseptics Tested in Bacterial Cellulose Model
4.7. The Activity of Antiseptics/Lavaseptics Against Biofilms Preformed on Biomaterials in Flow Conditions
4.8. Ability of Antiseptic/Lavaseptic Products to Flush Biofilm out Measured by Means of Microfluidic System
4.9. Wettability Measurement
4.10. Normative In Vitro Neutral Red (NR) Cytotoxicity Assay Towards Keratinocytes and Osteoblasts
4.11. Analysis of Antiseptic/Lavaseptic Cytotoxicity In Vivo Towards G. mellonella Larvae Model
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2021 Antimicrobial Resistance Collaborators. Global burden of bacterial antimicrobial resistance 1990–2021: A systematic analysis with forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef] [PubMed]
- Zabaglo, M.; Leslie, S.W.; Sharman, T. Postoperative Wound Infections. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Popa, M.; Cursaru, A.; Popa, V.; Munteanu, A.; Șerban, B.; Crețu, B.; Iordache, S.; Smarandache, C.G.; Orban, C.; Cîrstoiu, C. Understanding orthopedic infections through a different perspective: Microcalorimetry growth curves. Exp. Ther. Med. 2022, 23, 263. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Motififard, M.; Teimouri, M.; Shirani, K.; Hatami, S.; Yadegari, M. Prevalence of Bacterial surgical site infection in traumatic patients undergoing orthopedic surgeries: A cross-sectional study. Int. J. Burns Trauma. 2021, 11, 191–196. [Google Scholar] [PubMed]
- Enz, A.; Mueller, S.C.; Warnke, P.; Ellenrieder, M.; Mittelmeier, W.; Klinder, A. Periprosthetic Fungal Infections in Severe Endoprosthetic Infections of the Hip and Knee Joint-A Retrospective Analysis of a Certified Arthroplasty Centre of Excellence. J. Fungi 2021, 7, 404. [Google Scholar] [CrossRef]
- Bucataru, A.; Balasoiu, M.; Ghenea, A.E.; Zlatian, O.M.; Vulcanescu, D.D.; Horhat, F.G.; Bagiu, I.C.; Sorop, V.B.; Sorop, M.I.; Oprisoni, A. Factors Contributing to Surgical Site Infections: A Comprehensive Systematic Review of Etiology and Risk Factors. Clin. Pract. 2024, 14, 52–68. [Google Scholar] [CrossRef]
- Crader, M.F.; Varacallo, M. Preoperative Antibiotic Prophylaxis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar] [PubMed]
- Kadirvelu, L.; Sivaramalingam, S.S.; Jothivel, D.; Chithiraiselvan, D.D.; Karaiyagowder Govindarajan, D.; Kandaswamy, K. A review on antimicrobial strategies in mitigating biofilm-associated infections on medical implants. Curr. Res. Microb. Sci. 2024, 6, 100231. [Google Scholar] [CrossRef]
- Rozis, M.; Evangelopoulos, D.S.; Pneumaticos, S.G. Orthopedic Implant-Related Biofilm Pathophysiology: A Review of the Literature. Cureus 2021, 13, e15634. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bąchor, U.; Junka, A.; Brożyna, M.; Mączyński, M. The In Vitro Impact of Isoxazole Derivatives on Pathogenic Biofilm and Cytotoxicity of Fibroblast Cell Line. Int. J. Mol. Sci. 2023, 24, 2997. [Google Scholar] [CrossRef]
- Tyski, S.; Bocian, E.; Laudy, A.E. Application of normative documents for determination of biocidal activity of disinfectants and antiseptics dedicated to the medical area: A narrative review. J. Hosp. Infect. 2022, 125, 75–91. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Dissemond, J.; Kim, S.; Willy, C.; Mayer, D.; Papke, R.; Tuchmann, F.; Assadian, O. Consensus on Wound Antisepsis: Update 2018. Skin Pharmacol. Physiol. 2018, 31, 28–58. [Google Scholar] [CrossRef]
- Böhle, S.; Röhner, E.; Zippelius, T.; Jacob, B.; Matziolis, G.; Rohe, S. Cytotoxic effect of sodium hypochlorite (Lavanox 0.08%) and chlorhexidine gluconate (Irrisept 0.05%) on human osteoblasts. Eur. J. Orthop. Surg. Traumatol. 2022, 32, 81–89. [Google Scholar] [CrossRef] [PubMed]
- Hackett, D.J.; Rothenberg, A.C.; Chen, A.F.; Gutowski, C.; Jaekel, D.; Tomek, I.M.; Parsley, B.S.; Ducheyne, P.; Manner, P.A. The economic significance of orthopaedic infections. J. Am. Acad. Orthop. Surg. 2015, 23, S1–S7. [Google Scholar] [CrossRef] [PubMed]
- Pirisi, L.; Pennestrì, F.; Viganò, M.; Banfi, G. Prevalence and burden of orthopaedic implantable-device infections in Italy: A hospital-based national study. BMC Infect. Dis. 2020, 20, 337. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Prabhoo, R.; Chaddha, R.; Iyer, R.; Mehra, A.; Ahdal, J.; Jain, R. Overview of methicillin resistant Staphylococcus aureus mediated bone and joint infections in India. Orthop. Rev. 2019, 11, 8070. [Google Scholar] [CrossRef] [PubMed]
- Büttner, H.; Mack, D.; Rohde, H. Structural basis of Staphylococcus epidermidis biofilm formation: Mechanisms and molecular interactions. Front. Cell Infect. Microbiol. 2015, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Sambri, A.; Zunarelli, R.; Fiore, M.; Bortoli, M.; Paolucci, A.; Filippini, M.; Zamparini, E.; Tedeschi, S.; Viale, P.; De Paolis, M. Epidemiology of Fungal Periprosthetic Joint Infection: A Systematic Review of the Literature. Microorganisms 2022, 11, 84. [Google Scholar] [CrossRef]
- Corbisiero, M.F.; Batta, N.; Kyllo, H.; Smyth, A.; Allen, L.; Franco-Paredes, C. Clinical spectrum of Cutibacterium acnes infections: The SAPHO syndrome. IDCases 2023, 32, e01784. [Google Scholar] [CrossRef]
- Prié, H.; Meyssonnier, V.; Kerroumi, Y.; Heym, B.; Lidove, O.; Marmor, S.; Zeller, V. Pseudomonas aeruginosa prosthetic joint-infection outcomes: Prospective, observational study on 43 patients. Front. Med. 2022, 9, 1039596. [Google Scholar] [CrossRef]
- Roux, V.; Drancourt, M.; Stein, A.; Riegel, P.; Raoult, D.; La Scola, B. Corynebacterium species isolated from bone and joint infections identified by 16S rRNA gene sequence analysis. J. Clin. Microbiol. 2004, 42, 2231–2233. [Google Scholar] [CrossRef]
- Krasowski, G.; Junka, A.; Paleczny, J.; Czajkowska, J.; Makomaska-Szaroszyk, E.; Chodaczek, G.; Majkowski, M.; Migdał, P.; Fijałkowski, K.; Kowalska-Krochmal, B.; et al. In Vitro Evaluation of Polihexanide, Octenidine and NaClO/HClO-Based Antiseptics against Biofilm Formed by Wound Pathogens. Membranes 2021, 11, 62. [Google Scholar] [CrossRef]
- Fazli, M.M.; Kirketerp-Møller, K.; Sonne, D.P.; Balchen, T.; Gundersen, G.; Jørgensen, E.; Bjarnsholt, T. A First-in-Human Randomized Clinical Study Investigating the Safety and Tolerability of Stabilized Hypochlorous Acid in Patients with Chronic Leg Ulcers. Adv. Wound Care 2024, 13, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Giordano, V.; Giannoudis, P.V. Biofilm Formation, Antibiotic Resistance, and Infection (BARI): The Triangle of Death. J. Clin. Med. 2024, 13, 5779. [Google Scholar] [CrossRef] [PubMed]
- Paleczny, J.; Junka, A.F.; Krzyżek, P.; Czajkowska, J.; Kramer, A.; Benkhai, H.; Żyfka-Zagrodzińska, E.; Bartoszewicz, M. Comparison of antibiofilm activity of low-concentrated hypochlorites vs polyhexanide-containing antiseptic. Front. Cell Infect. Microbiol. 2023, 13, 1119188. [Google Scholar] [CrossRef] [PubMed]
- Junka, A.; Bartoszewicz, M.; Smutnicka, D.; Secewicz, A.; Szymczyk, P. Efficacy of antiseptics containing povidone-iodine, octenidine dihydrochloride and ethacridine lactate against biofilm formed by Pseudomonas aeruginosa and Staphylococcus aureus measured with the novel biofilm-oriented antiseptics test. Int. Wound J. 2014, 11, 730–734. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Junka, A.F.; Żywicka, A.; Szymczyk, P.; Dziadas, M.; Bartoszewicz, M.; Fijałkowski, K. A.D.A.M. test (Antibiofilm Dressing’s Activity Measurement)—Simple method for evaluating anti-biofilm activity of drug-saturated dressings against wound pathogens. J. Microbiol. Methods 2017, 143, 6–12. [Google Scholar] [CrossRef]
- Latka, A.; Drulis-Kawa, Z. Advantages and limitations of microtiter biofilm assays in the model of antibiofilm activity of Klebsiella phage KP34 and its depolymerase. Sci. Rep. 2020, 10, 20338. [Google Scholar] [CrossRef]
- Yang, S.; Li, X.; Cang, W.; Mu, D.; Ji, S.; An, Y.; Wu, R.; Wu, J. Biofilm tolerance, resistance and infections increasing threat of public health. Microb. Cell. 2023, 10, 233–247. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lepelletier, D.; Maillard, J.Y.; Pozzetto, B.; Simon, A. Povidone Iodine: Properties, Mechanisms of Action, and Role in Infection Control and Staphylococcus aureus Decolonization. Antimicrob. Agents Chemother. 2020, 64, e00682-20. [Google Scholar] [CrossRef] [PubMed]
- Smock, E.; Demertzi, E.; Abdolrasouli, A.; Azadian, B.; Williams, G. Antiseptic Efficacy of Povidone Iodine and Chlorhexidine Gluconate Skin Preparation Solutions Used in Burns Surgery. J. Burn. Care Res. 2018, 39, 440–444. [Google Scholar] [CrossRef] [PubMed]
- Linder, N.; Davidovitch, N.; Reichman, B.; Kuint, J.; Lubin, D.; Meyerovitch, J.; Sela, B.A.; Dolfin, Z.; Sack, J. Topical iodine-containing antiseptics and subclinical hypothyroidism in preterm infants. J. Pediatr. 1997, 131, 434–439. [Google Scholar] [CrossRef] [PubMed]
- Gryson, L.; Meaume, S.; Feldkaemper, I.; Favalli, F. Anti-biofilm Activity of Povidone-Iodine and Polyhexamethylene Biguanide: Evidence from In Vitro Tests. Curr. Microbiol. 2023, 80, 161. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bas, S.; Kramer, M.; Stopar, D. Biofilm Surface Density Determines Biocide Effectiveness. Front. Microbiol. 2017, 8, 2443. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Postek, W.; Staśkiewicz, K.; Lilja, E.; Wacław, B. Substrate geometry affects population dynamics in a bacterial biofilm. bioRxiv 2023, Preprint. [Google Scholar] [CrossRef] [PubMed]
- Müller, G.; Kramer, A. Biocompatibility index of antiseptic agents by parallel assessment of antimicrobial activity and cellular cytotoxicity. J. Antimicrob. Chemother. 2008, 61, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Severing, A.L.; Rembe, J.D.; Koester, V.; Stuermer, E.K. Safety and efficacy profiles of different commercial sodium hypochlorite/hypochlorous acid solutions (NaClO/HClO): Antimicrobial efficacy, cytotoxic impact and physicochemical parameters in vitro. J. Antimicrob. Chemother. 2019, 74, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Serrano, I.; Verdial, C.; Tavares, L.; Oliveira, M. The Virtuous Galleria mellonella Model for Scientific Experimentation. Antibiotics 2023, 12, 505. [Google Scholar] [CrossRef]
- Wojda, I.; Staniec, B.; Sułek, M.; Kordaczuk, J. The greater wax moth Galleria mellonella: Biology and use in immune studies. Pathog. Dis. 2020, 78, ftaa057. [Google Scholar] [CrossRef]
- Pompili, S.; Latella, G.; Gaudio, E.; Sferra, R.; Vetuschi, A. The Charming World of the Extracellular Matrix: A Dynamic and Protective Network of the Intestinal Wall. Front. Med. 2021, 8, 610189. [Google Scholar] [CrossRef]
- Sowlati-Hashjin, S.; Carbone, P.; Karttunen, M. Insights into the Polyhexamethylene Biguanide (PHMB) Mechanism of Action on Bacterial Membrane and DNA: A Molecular Dynamics Study. J. Phys. Chem. B 2020, 124, 4487–4497. [Google Scholar] [CrossRef]
- Dörfel, D.; Maiwald, M.; Daeschlein, G.; Müller, G.; Hudek, R.; Assadian, O.; Kampf, G.; Kohlmann, T.; Harnoss, J.C.; Kramer, A. Comparison of the antimicrobial efficacy of povidone-iodine-alcohol versus chlorhexidine-alcohol for surgical skin preparation on the aerobic and anaerobic skin flora of the shoulder region. Antimicrob. Resist. Infect. Control. 2021, 10, 17. [Google Scholar] [CrossRef]
- Zhang, M.; Jin, J.; Liu, Y.; Ben, C.; Li, H.; Cheng, D.; Sun, Y.; Guang-Yi, W.; Zhu, S. Analysis of povidone iodine, chlorhexidine acetate and polyhexamethylene biguanide as wound disinfectants: In vitro cytotoxicity and antibacterial activity. BMJ Nutr. Prev. Health. 2023, 6, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Szymczyk, P.; Junka, A.; Ziółkowski, G.; Smutnicka, D.; Bartoszewicz, M.; Chlebus, E. The Ability of, S. Aureus to Form Biofilm on the Ti-6Al-7Nb Scaffolds Produced by Selective Laser Melting and Subjected to the Different Types of Surface Modifications. Acta Bioeng. Biomech. 2013, 15, 69–76. [Google Scholar] [PubMed]
- Brożyna, M.; Dudek, B.; Kozłowska, W.; Malec, K.; Paleczny, J.; Detyna, J.; Fabianowska-Majewska, K.; Junka, A. The chronic wound milieu changes essential oils’ antibiofilm activity-an in vitro and larval model study. Sci. Rep. 2024, 14, 2218. [Google Scholar] [CrossRef]
- Kos, M.; Junka, A.; Smutnicka, D.; Szymczyk, P.; Gluza, K.; Bartoszewicz, M. Bisphosphonates enhance bacterial adhesion and biofilm formation on bone hydroxyapatite. J. Craniomaxillofac Surg. 2015, 43, 863–869. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. ISO: Geneva, Switzerland, 2009.
- Czajkowska, J.; Junka, A.; Hoppe, J.; Toporkiewicz, M.; Pawlak, A.; Migdał, P.; Oleksy-Wawrzyniak, M.; Fijałkowski, K.; Śmiglak, M.; Markowska-Szczupak, A. The Co-Culture of Staphylococcal Biofilm and Fibroblast Cell Line: The Correlation of Biological Phenomena with Metabolic NMR1 Footprint. Int. J. Mol. Sci. 2021, 22, 5826. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| PHMB | G | LS | B | NaCl | R | ||
| S. epidermidis | MBC | 0.2% | none | 1.56% | 6.25% | none | none |
| MBEC | 3.13% | none | 25% | 50% | none | none | |
| MRSA | MBC | 0.1% | none | 1.56% | 6.25% | none | none |
| MBEC | 3.13% | none | 25% | 12.5% | none | none | |
| P. aeruginosa | MBC | 3.13% | none | 6.25% | 12.5% | none | none |
| MBEC | 12.5% | none | 50% | 50% | none | none | |
| C. albicans | MBC | 1.56% | none | 6.25% | 12.5% | none | none |
| MBEC | 6.25% | none | 12.5% | 50% | none | none | |
| C. acnes | MBC | 0.4% | none | 0.8% | 6.25% | none | none |
| MBEC | 6.25% | none | 25% | 50% | none | none | |
| C. amycolatum | MBC | 0.8% | none | 3.13% | 12.5% | none | none |
| MBEC | 6.25% | none | 50% | 50% | none | none |
| PHMB | G | LS | NaCl | R | B | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Species/Time | 30’ | 1 h | 24 h | 30’ | 1 h | 24 h | 30’ | 1 h | 24 h | 30’ | 1 h | 24 h | 30’ | 1 h | 24 h | 30’ | 1 h | 24 h |
| S. epidermidis [n = 10] | + | +[60%] | - | + | + | + | + | + | + | + | + | + | + | + | + | + | +[10%] | - |
| S. aureus [n = 10] | + | +[50%] | - | + | + | + | + | + | + | + | + | + | + | + | + | + | +[20%] | - |
| C. acnes [n = 10] | + | +[20%] | - | + | + | + | + | + | + | + | + | + | + | + | + | + | +[10%] | - |
| C. amycolatum [n = 10] | + | +[70%] | +[10%] | + | + | + | + | + | + | + | + | + | + | + | + | +[40%] | +[20%] | - |
| P. aeruginosa [n = 10] | + | - | - | + | + | + | + | + | + | + | + | + | + | + | + | + | - | - |
| C. albicans [n = 10] | + | - | - | + | + | + | + | + | + | + | + | + | + | + | + | + | +[30%] | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dudek, B.; Brożyna, M.; Karoluk, M.; Frankiewicz, M.; Migdał, P.; Szustakiewicz, K.; Matys, T.; Wiater, A.; Junka, A. In Vitro and In Vivo Translational Insights into the Intraoperative Use of Antiseptics and Lavage Solutions Against Microorganisms Causing Orthopedic Infections. Int. J. Mol. Sci. 2024, 25, 12720. https://doi.org/10.3390/ijms252312720
Dudek B, Brożyna M, Karoluk M, Frankiewicz M, Migdał P, Szustakiewicz K, Matys T, Wiater A, Junka A. In Vitro and In Vivo Translational Insights into the Intraoperative Use of Antiseptics and Lavage Solutions Against Microorganisms Causing Orthopedic Infections. International Journal of Molecular Sciences. 2024; 25(23):12720. https://doi.org/10.3390/ijms252312720
Chicago/Turabian StyleDudek, Bartłomiej, Malwina Brożyna, Michał Karoluk, Mariusz Frankiewicz, Paweł Migdał, Konrad Szustakiewicz, Tomasz Matys, Adrian Wiater, and Adam Junka. 2024. "In Vitro and In Vivo Translational Insights into the Intraoperative Use of Antiseptics and Lavage Solutions Against Microorganisms Causing Orthopedic Infections" International Journal of Molecular Sciences 25, no. 23: 12720. https://doi.org/10.3390/ijms252312720
APA StyleDudek, B., Brożyna, M., Karoluk, M., Frankiewicz, M., Migdał, P., Szustakiewicz, K., Matys, T., Wiater, A., & Junka, A. (2024). In Vitro and In Vivo Translational Insights into the Intraoperative Use of Antiseptics and Lavage Solutions Against Microorganisms Causing Orthopedic Infections. International Journal of Molecular Sciences, 25(23), 12720. https://doi.org/10.3390/ijms252312720








