Exogenous Calcium Enhances Castor Tolerance to Saline–Alkaline Stress by Regulating Antioxidant Enzyme Activity and Activating Ca2+ and ROS Signaling Crosstalk
Abstract
1. Introduction
2. Results
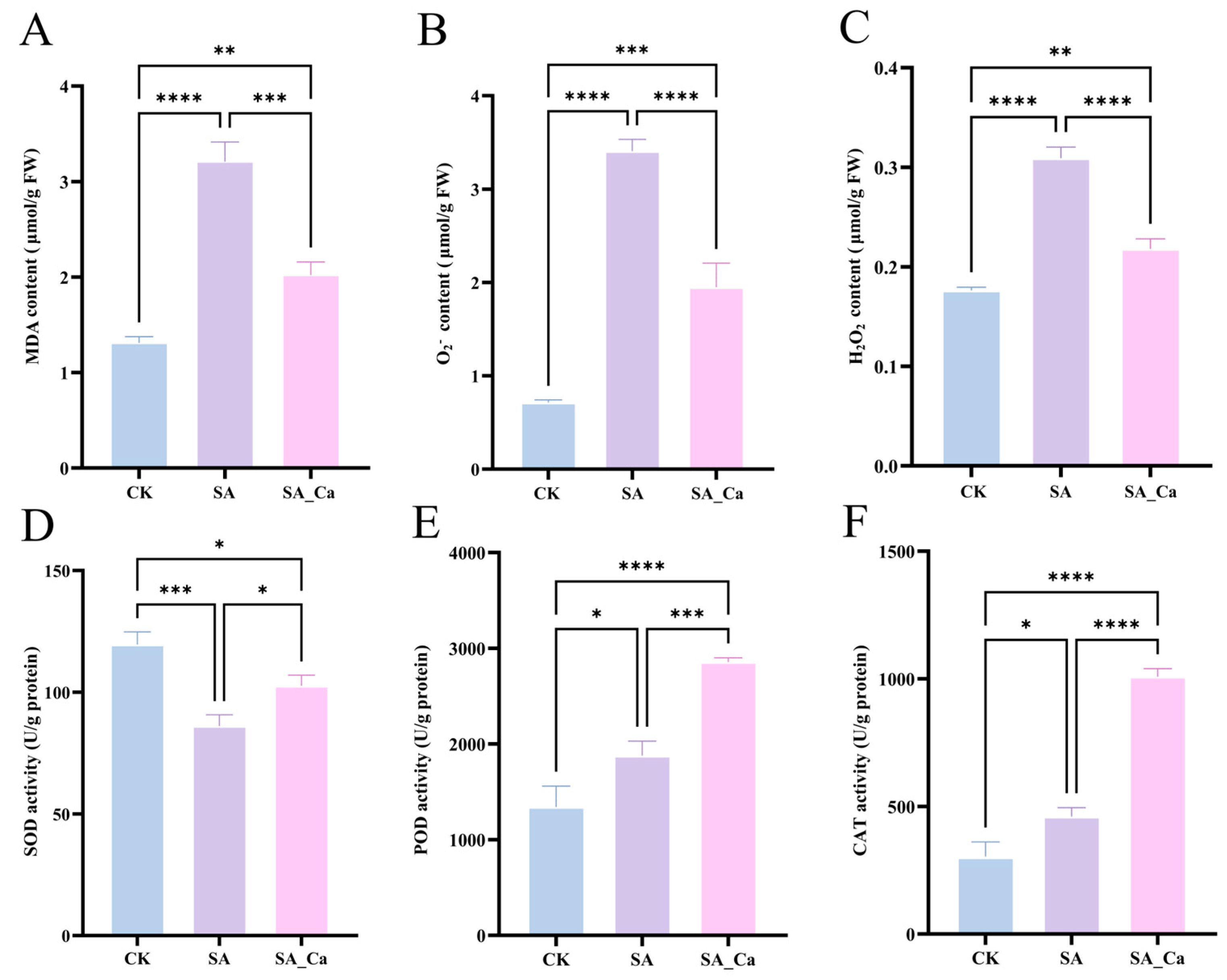
2.1. Effect of Exogenous Calcium on the Antioxidant System of Castor Root Under Saline-Alkaline Stress
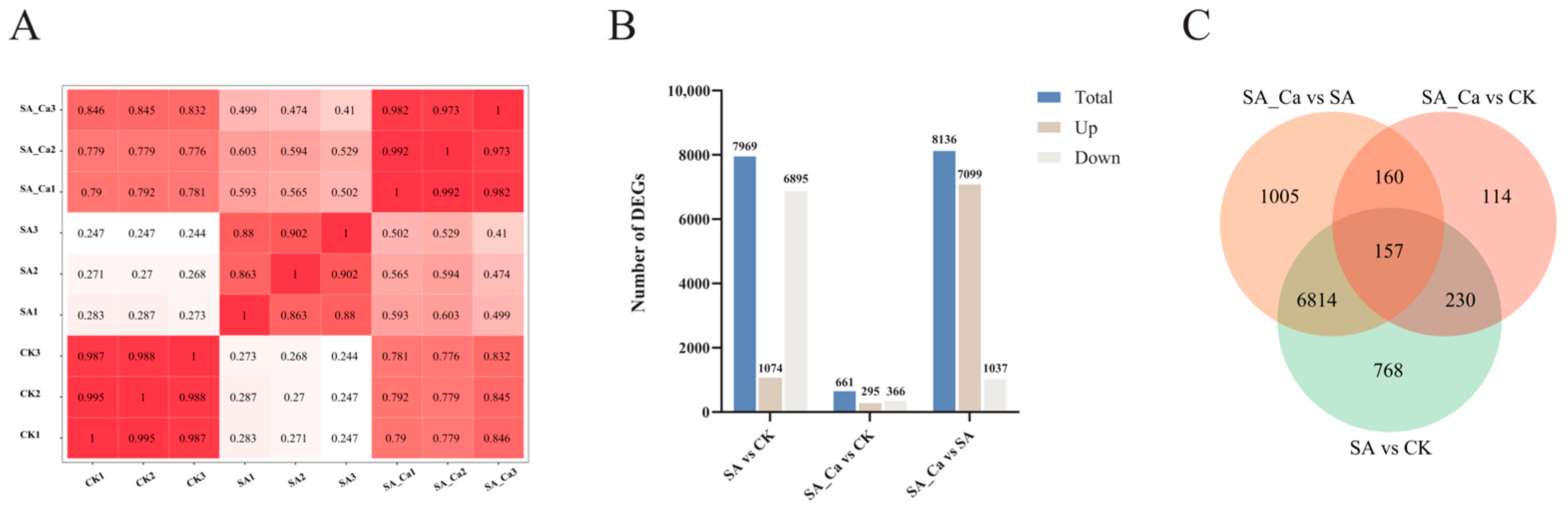
2.2. Transcriptome Sequencing and Differential Expression of Genes (DEGs) Identification
2.3. Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analysis of DEGs
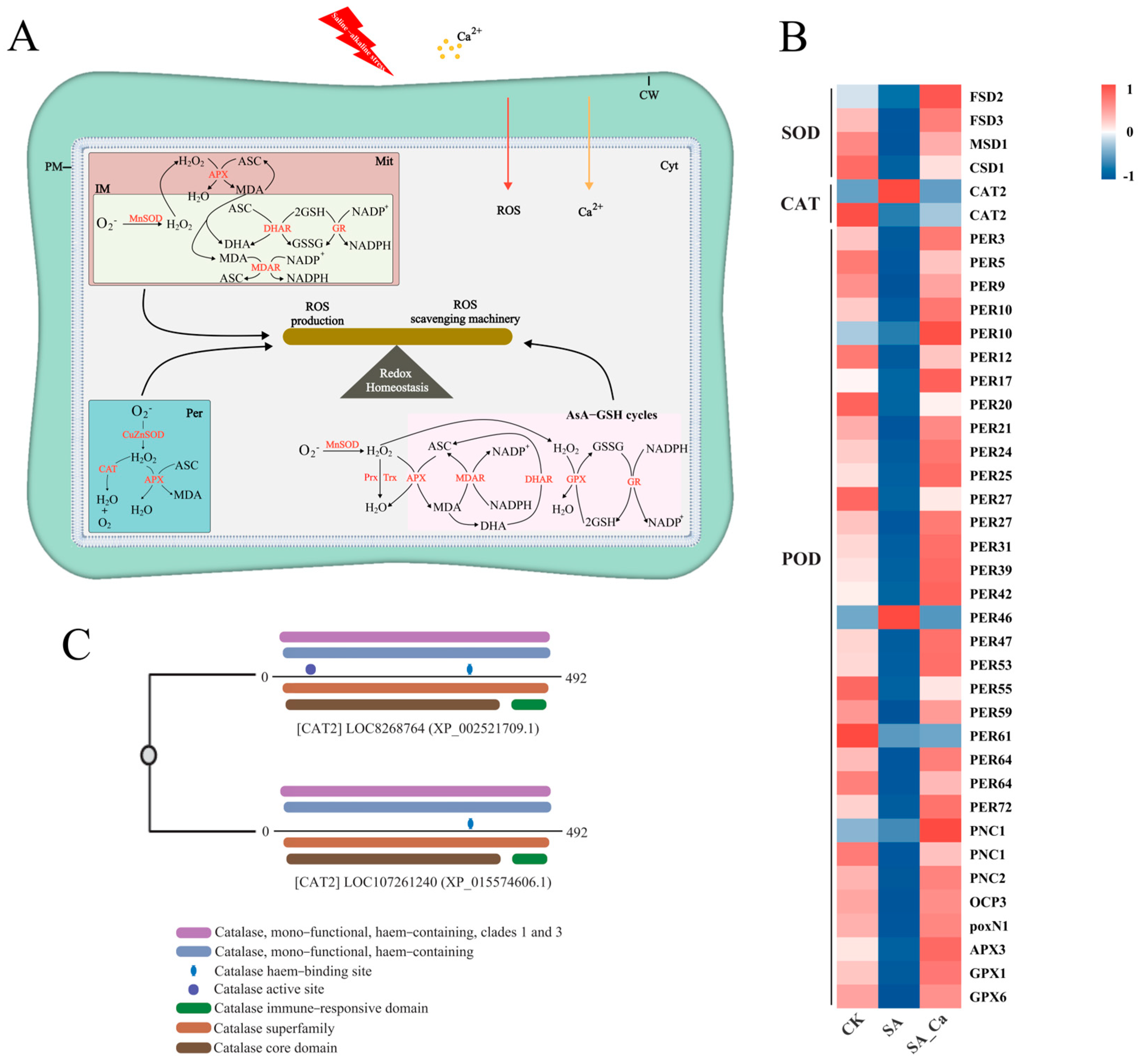
2.4. DEGs Associated with ROS Metabolism Under Different Treatments
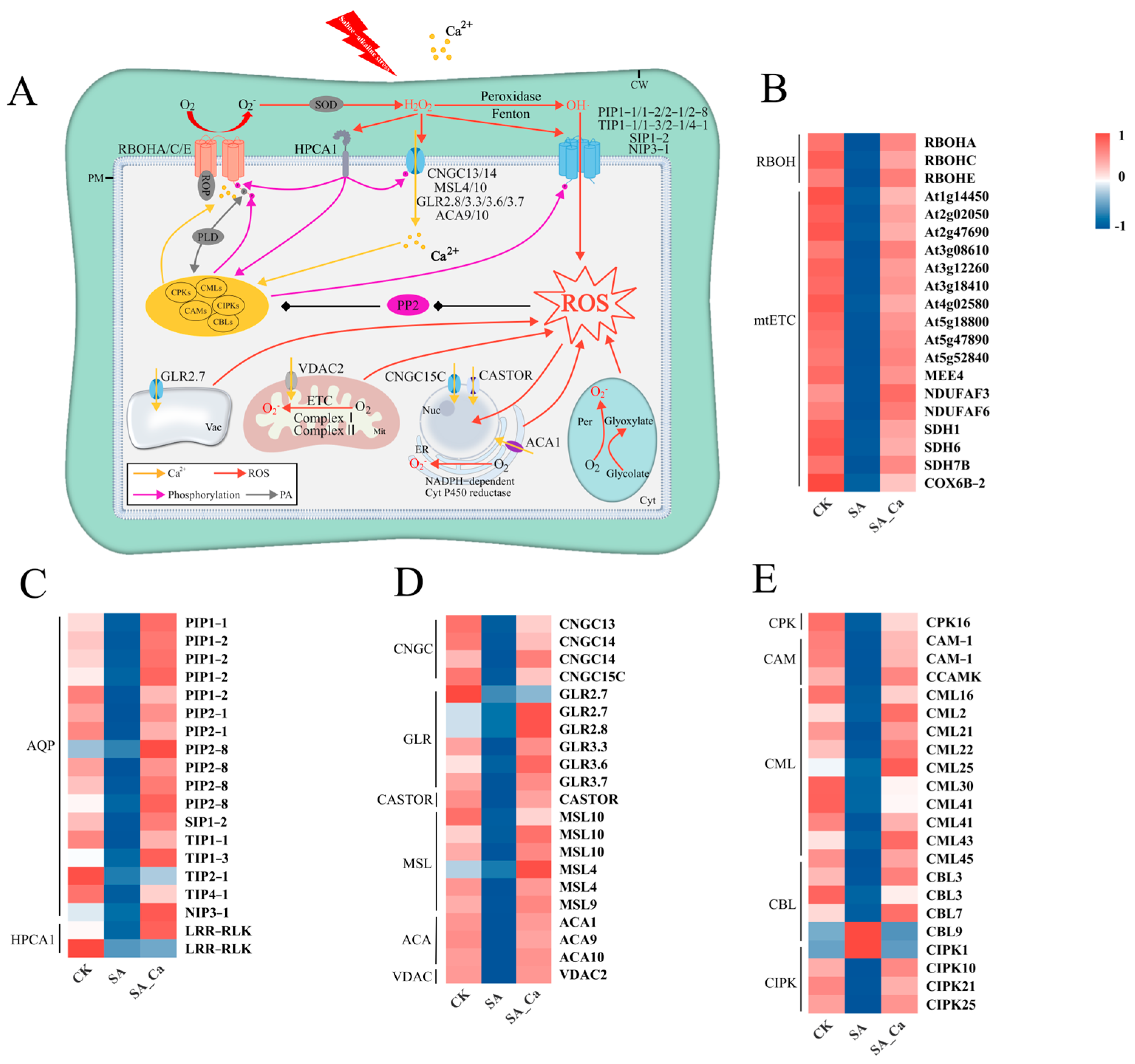
2.5. DEGs Associated with Signal Transduction Under Different Treatments
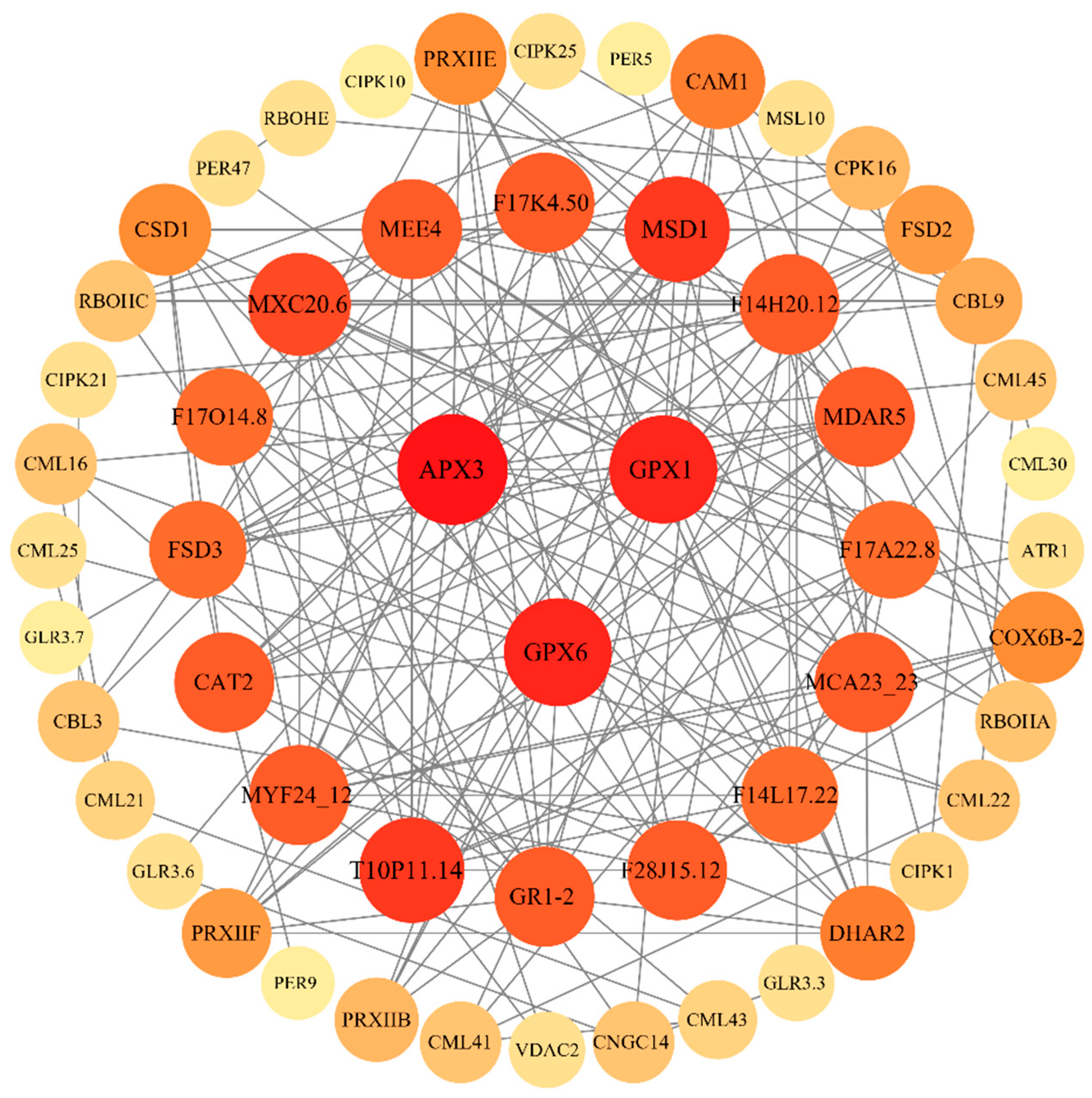
2.6. Protein-Protein Interaction Network Analysis (PPI)
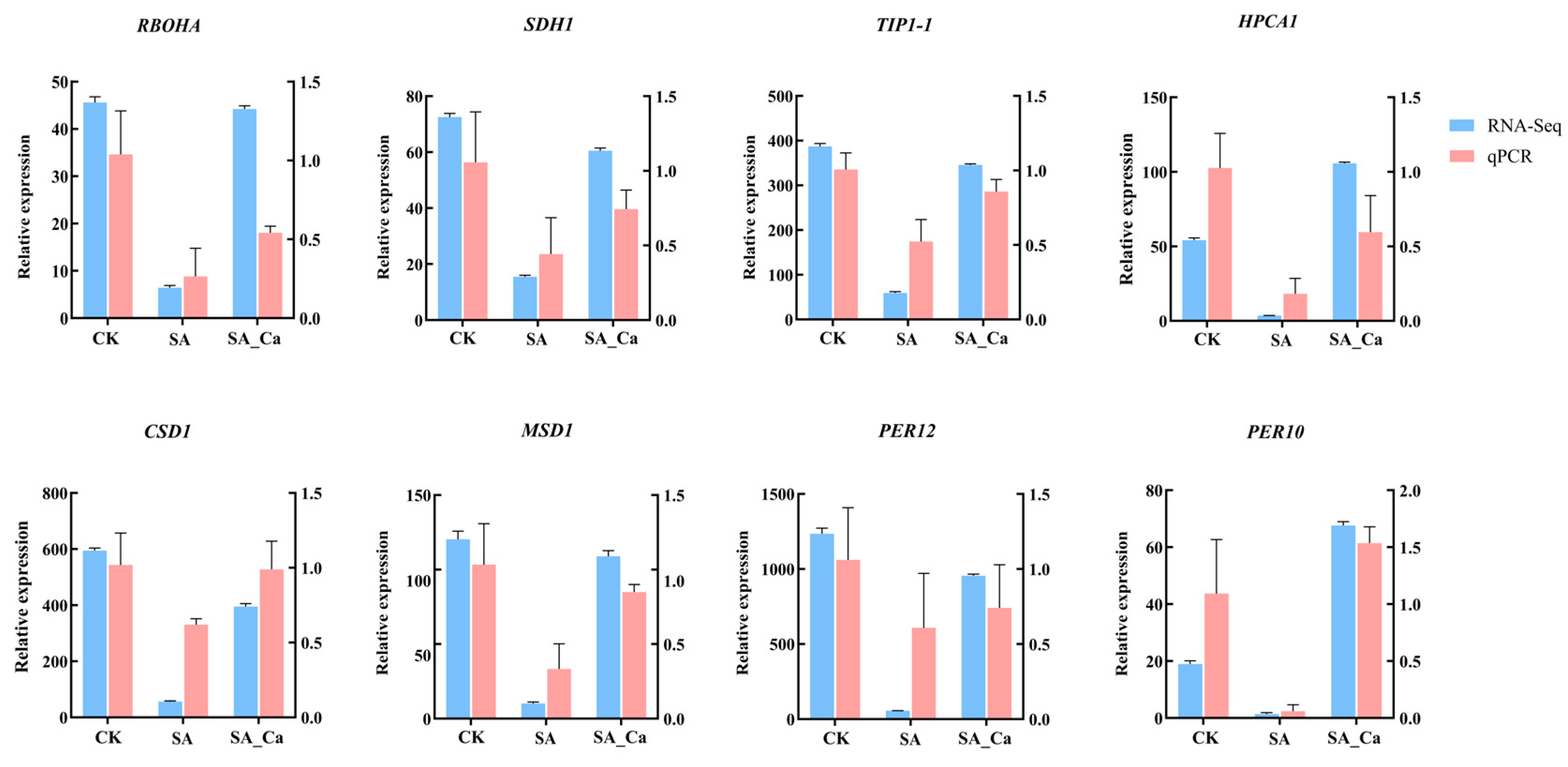
2.7. RT-qPCR
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Experimental Design
4.3. Physiological Index of Plants
4.4. RNA Isolation, Library Construction and RNA-Seq
4.5. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR) Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.; Pu, L.; Han, M.; Zhu, M.; Zhang, R.; Xiang, Y. Soil Salinization Research in China: Advances and Prospects. J. Geogr. Sci. 2014, 24, 943–960. [Google Scholar] [CrossRef]
- Fang, S.; Hou, X.; Liang, X. Response Mechanisms of Plants Under Saline-Alkali Stress. Front. Plant Sci. 2021, 12, 667458. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Shi, L.; Yan, C.; Zhong, X.; Gu, F.; Liu, Q.; Xia, X.; Li, H. Ionomic and Metabolic Responses to Neutral Salt or Alkaline Salt Stresses in Maize (Zea mays L.) Seedlings. BMC Plant Biol. 2017, 17, 41. [Google Scholar] [CrossRef]
- Song, L.; Yu, Y.; Chen, H.; Feng, Y.; Chen, S.; Zhang, H.; Zhou, H.; Meng, L.; Wang, Y. Response of Photosynthetic Characteristics and Antioxidant System in the Leaves of Safflower to NaCl and NaHCO3. Plant Cell Rep. 2024, 43, 146. [Google Scholar] [CrossRef]
- Yang, C.; Shi, D.; Wang, D. Comparative Effects of Salt and Alkali Stresses on Growth, Osmotic Adjustment and Ionic Balance of an Alkali-Resistant Halophyte Suaeda glauca (Bge.). Plant Growth Regul. 2008, 56, 179–190. [Google Scholar] [CrossRef]
- Chen, J.; Li, X.; Ye, X.; Guo, P.; Hu, Z.; Qi, G.; Cui, F.; Liu, S. An S-Ribonuclease Binding Protein EBS1 and Brassinolide Signaling Are Specifically Required for Arabidopsis Tolerance to Bicarbonate. J. Exp. Bot. 2021, 72, 1449–1459. [Google Scholar] [CrossRef]
- Li, N.; Shao, T.; Zhou, Y.; Cao, Y.; Hu, H.; Sun, Q.; Long, X.; Yue, Y.; Gao, X.; Rengel, Z. Effects of Planting L. on Soil Properties and Microbial Community in Saline-Alkali Soil. Land Degrad. Dev. 2021, 32, 2951–2961. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, C.; Liang, L.; Yi, Z.; Xue, S. Quantitative Assessment of the Potential for Soil Improvement by Planting Miscanthus on Saline-Alkaline Soil and the Underlying Microbial Mechanism. GCB Bioenergy 2021, 13, 1191–1205. [Google Scholar] [CrossRef]
- Gilroy, S.; Białasek, M.; Suzuki, N.; Górecka, M.; Devireddy, A.R.; Karpiński, S.; Mittler, R. ROS, Calcium, and Electric Signals: Key Mediators of Rapid Systemic Signaling in Plants. Plant Physiol. 2016, 171, 1606–1615. [Google Scholar] [CrossRef]
- Feng, D.; Wang, X.; Gao, J.; Zhang, C.; Liu, H.; Liu, P.; Sun, X. Exogenous Calcium: Its Mechanisms and Research Advances Involved in Plant Stress Tolerance. Front. Plant Sci. 2023, 14, 1143963. [Google Scholar] [CrossRef]
- Liang, C.; Zhang, B. Effect of Exogenous Calcium on Growth, Nutrients Uptake and Plasma Membrane H+-ATPase and Ca2+-ATPase Activities in Soybean (Glycine max) Seedlings under Simulated Acid Rain Stress. Ecotoxicol. Environ. Saf. 2018, 165, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Zhang, Y.; Ren, X. Calcium Regulates Antioxidative Isozyme Activity for Enhancing Rice Adaption to Acid Rain Stress. Plant Sci. 2021, 306, 110876. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Lin, S.; Yu, S.; Zhang, Y.; Su, L.; Geng, L.; Cheng, C.; Jiang, X. Exogenous Calcium Enhances the Physiological Status and Photosynthetic Capacity of Rose under Drought Stress. Hortic. Plant J. 2024, 10, 853–865. [Google Scholar] [CrossRef]
- Vafadar, F.; Amooaghaie, R.; Ehsanzadeh, P.; Ghanati, F.; Sajedi, R.H. Crosstalk between Melatonin and Ca2+/CaM Evokes Systemic Salt Tolerance in Dracocephalum kotschyi. J. Plant Physiol. 2020, 252, 153237. [Google Scholar] [CrossRef]
- Stael, S.; Wurzinger, B.; Mair, A.; Mehlmer, N.; Vothknecht, U.C.; Teige, M. Plant Organellar Calcium Signalling: An Emerging Field. J. Exp. Bot. 2012, 63, 1525–1542. [Google Scholar] [CrossRef]
- Valivand, M.; Amooaghaie, R.; Ahadi, A. Interplay between Hydrogen Sulfide and Calcium/Calmodulin Enhances Systemic Acquired Acclimation and Antioxidative Defense against Nickel Toxicity in Zucchini. Environ. Exp. Bot. 2019, 158, 40–50. [Google Scholar] [CrossRef]
- Wilkins, K.A.; Matthus, E.; Swarbreck, S.M.; Davies, J.M. Calcium-Mediated Abiotic Stress Signaling in Roots. Front. Plant Sci. 2016, 7, 1296. [Google Scholar] [CrossRef]
- Ranty, B.; Aldon, D.; Cotelle, V.; Galaud, J.-P.; Thuleau, P.; Mazars, C. Calcium Sensors as Key Hubs in Plant Responses to Biotic and Abiotic Stresses. Front. Plant Sci. 2016, 7, 327. [Google Scholar] [CrossRef]
- Vafadar, F.; Amooaghaie, R.; Ehsanzadeh, P.; Ghanati, F.; Allakhverdiev, S. Melatonin Improves the Photosynthesis in Dracocephalum Kotschyi under Salinity Stress in a Ca2+/CaM-Dependent Manner. Funct. Plant Biol. 2021, 49, 89–101. [Google Scholar] [CrossRef]
- Hu, J.; Wang, B.; Yang, T.; Li, N.; Yang, H.; Yu, Q.; Wang, J. A Calcium-Dependent Protein Kinase Gene SpCPK33 from Solanum pennellii Associated with Increased Cold Tolerance in Tomato. J. Plant Physiol. 2022, 279, 153834. [Google Scholar] [CrossRef]
- Wu, X.; Zhu, J.; Zhu, L.; Tang, Y.; Hao, Z.; Zhang, J.; Shi, J.; Cheng, T.; Lu, L. Genome-Wide Analyses of Calmodulin and Calmodulin-like Proteins in the Halophyte Nitraria sibirica Reveal Their Involvement in Response to Salinity, Drought and Cold Stress. Int. J. Biol. Macromol. 2023, 253, 127442. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Liu, W.-C.; Han, C.; Wang, S.; Bai, M.-Y.; Song, C.-P. Reactive Oxygen Species: Multidimensional Regulators of Plant Adaptation to Abiotic Stress and Development. J. Integr. Plant Biol. 2024, 66, 330–367. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.E.; Postiglione, A.E.; Muday, G.K. Reactive Oxygen Species Function as Signaling Molecules in Controlling Plant Development and Hormonal Responses. Curr. Opin. Plant Biol. 2022, 69, 102293. [Google Scholar] [CrossRef] [PubMed]
- Peláez-Vico, M.Á.; Fichman, Y.; Zandalinas, S.I.; Foyer, C.H.; Mittler, R. ROS Are Universal Cell-to-Cell Stress Signals. Curr. Opin. Plant Biol. 2024, 79, 102540. [Google Scholar] [CrossRef]
- Chen, Z.; Xie, Y.; Gu, Q.; Zhao, G.; Zhang, Y.; Cui, W.; Xu, S.; Wang, R.; Shen, W. The AtrbohF-Dependent Regulation of ROS Signaling Is Required for Melatonin-Induced Salinity Tolerance in Arabidopsis. Free Radic. Biol. Med. 2017, 108, 465–477. [Google Scholar] [CrossRef]
- Xu, J.; Kang, Z.; Zhu, K.; Zhao, D.; Yuan, Y.; Yang, S.; Zhen, W.; Hu, X. RBOH1-Dependent H2O2 Mediates Spermine-Induced Antioxidant Enzyme System to Enhance Tomato Seedling Tolerance to Salinity–Alkalinity Stress. Plant Physiol. Biochem. 2021, 164, 237–246. [Google Scholar] [CrossRef]
- Ravi, B.; Foyer, C.H.; Pandey, G.K. The Integration of Reactive Oxygen Species (ROS) and Calcium Signalling in Abiotic Stress Responses. Plant Cell Environ. 2023, 46, 1985–2006. [Google Scholar] [CrossRef]
- Wang, T.; Chen, X.; Ju, C.; Wang, C. Calcium Signaling in Plant Mineral Nutrition: From Uptake to Transport. Plant Commun. 2023, 4, 100678. [Google Scholar] [CrossRef]
- Wu, F.; Chi, Y.; Jiang, Z.; Xu, Y.; Xie, L.; Huang, F.; Wan, D.; Ni, J.; Yuan, F.; Wu, X.; et al. Hydrogen Peroxide Sensor HPCA1 Is an LRR Receptor Kinase in Arabidopsis. Nature 2020, 578, 577–581. [Google Scholar] [CrossRef]
- Landoni, M.; Bertagnon, G.; Ghidoli, M.; Cassani, E.; Adani, F.; Pilu, R. Opportunities and Challenges of Castor Bean (Ricinus communis L.) Genetic Improvement. Agronomy 2023, 13, 2076. [Google Scholar] [CrossRef]
- Wang, Y.; Peng, X.; Salvato, F.; Wang, Y.; Yan, X.; Zhou, Z.; Lin, J. Salt-Adaptive Strategies in Oil Seed Crop Ricinus Communis Early Seedlings (Cotyledon vs. True Leaf) Revealed from Proteomics Analysis. Ecotoxicol. Environ. Saf. 2019, 171, 12–25. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, W.H.; Lv, Z.W.; Zhang, S.L.; Hidema, J.; Shi, F.M.; Liu, L.L. Abscisic Acid Is Involved in the Response of Arabidopsis Mutant Sad2-1 to Ultraviolet-B Radiation by Enhancing Antioxidant Enzymes. S. Afr. J. Bot. 2013, 85, 79–86. [Google Scholar] [CrossRef]
- Aghaei, K.; Ehsanpour, A.A.; Komatsu, S. Potato Responds to Salt Stress by Increased Activity of Antioxidant Enzymes. J. Integr. Plant Biol. 2009, 51, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Manquián-Cerda, K.; Cruces, E.; Escudey, M.; Zúñiga, G.; Calderón, R. Interactive Effects of Aluminum and Cadmium on Phenolic Compounds, Antioxidant Enzyme Activity and Oxidative Stress in Blueberry (Vaccinium corymbosum L.) Plantlets Cultivated In Vitro. Ecotoxicol. Environ. Saf. 2018, 150, 320–326. [Google Scholar] [CrossRef]
- Guo, J.; Qin, S.; Rengel, Z.; Gao, W.; Nie, Z.; Liu, H.; Li, C.; Zhao, P. Cadmium Stress Increases Antioxidant Enzyme Activities and Decreases Endogenous Hormone Concentrations More in Cd-Tolerant than Cd-Sensitive Wheat Varieties. Ecotoxicol. Environ. Saf. 2019, 172, 380–387. [Google Scholar] [CrossRef]
- Ji, Y.; Zhou, Y.; Ma, C.; Feng, Y.; Hao, Y.; Rui, Y.; Wu, W.; Gui, X.; Le, V.N.; Han, Y.; et al. Jointed Toxicity of TiO2 NPs and Cd to Rice Seedlings: NPs Alleviated Cd Toxicity and Cd Promoted NPs Uptake. Plant Physiol. Biochem. 2017, 110, 82–93. [Google Scholar] [CrossRef]
- Huang, F.; Jiang, Y.; Zhang, S.; Liu, S.; Eh, T.-J.; Meng, F.; Lei, P. A Comparative Analysis on Morphological and Physiological Characteristics between Castor Varieties (Ricinus communis L.) under Salt Stress. Sustainability 2022, 14, 10032. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Y.; Liu, W.; Huang, T.; Yang, Y.; Mao, Y.; Meng, Y. Combined Transcriptomics and Metabolomics to Analyse the Response of Cuminum cyminum L. under Pb Stress. Sci. Total Environ. 2024, 923, 171497. [Google Scholar] [CrossRef]
- Önder, D.G.; Önder, S.; Uysal, A.T.; Karakurt, Y. Impact of Postharvest Hot Water, 1-MCP and CaCl2 Treatments on Antioxidant Enzymes and Related Genes during Cold Storage in Sweet Cherry (Prunus avium L.). J. Food Meas. Charact. 2021, 15, 5744–5758. [Google Scholar] [CrossRef]
- Xu, H.; Li, Z.; Jiang, P.-F.; Zhao, L.; Qu, C.; Van de Peer, Y.; Liu, Y.-J.; Zeng, Q.-Y. Divergence of active site motifs among different classes of Populus glutaredoxins results in substrate switches. Plant J. 2022, 110, 129–146. [Google Scholar] [CrossRef]
- Kudla, J.; Batistič, O.; Hashimoto, K. Calcium Signals: The Lead Currency of Plant Information Processing. Plant Cell 2010, 22, 541–563. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Wang, C.; Gao, Q.; Li, L.; Luan, S. Calcium Spikes, Waves and Oscillations in Plant Development and Biotic Interactions. Nat. Plants 2020, 6, 750–759. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.-Y.; Xu, Z.-S.; He, G.-Y.; Yang, G.-X.; Chen, M.; Li, L.-C.; Ma, Y. The Voltage-Dependent Anion Channel 1 (AtVDAC1) Negatively Regulates Plant Cold Responses during Germination and Seedling Development in Arabidopsis and Interacts with Calcium Sensor CBL1. Int. J. Mol. Sci. 2013, 14, 701–713. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, E.S.; Schlegel, A.M.; Haswell, E.S. United in Diversity: Mechanosensitive Ion Channels in Plants. Annu. Rev. Plant Biol. 2015, 66, 113–137. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Yan, J.; Du, X.; Hua, J. Overlapping and Differential Roles of Plasma Membrane Calcium ATPases in Arabidopsis Growth and Environmental Responses. J. Exp. Bot. 2018, 69, 2693–2703. [Google Scholar] [CrossRef]
- Duszyn, M.; Świeżawska, B.; Szmidt-Jaworska, A.; Jaworski, K. Cyclic Nucleotide Gated Channels (CNGCs) in Plant Signalling—Current Knowledge and Perspectives. J. Plant Physiol. 2019, 241, 153035. [Google Scholar] [CrossRef]
- Yan, C.; Gao, Q.; Yang, M.; Shao, Q.; Xu, X.; Zhang, Y.; Luan, S. Ca2+/Calmodulin-Mediated Desensitization of Glutamate Receptors Shapes Plant Systemic Wound Signalling and Anti-Herbivore Defence. Nat. Plants 2024, 10, 145–160. [Google Scholar] [CrossRef]
- Liu, Q.; Feng, Z.; Xu, W.; Vetukuri, R.R.; Xu, X. Exogenous Melatonin-Stimulated Transcriptomic Alterations of Davidia Involucrata Seedlings under Drought Stress. Trees 2021, 35, 1025–1038. [Google Scholar] [CrossRef]
- Wang, B.; Xue, P.; Zhang, Y.; Zhan, X.; Wu, W.; Yu, P.; Chen, D.; Fu, J.; Hong, Y.; Shen, X.; et al. OsCPK12 Phosphorylates OsCATA and OsCATC to Regulate H2O2 Homeostasis and Improve Oxidative Stress Tolerance in Rice. Plant Commun. 2024, 5, 100780. [Google Scholar] [CrossRef]
- You, Z.; Guo, S.; Li, Q.; Fang, Y.; Huang, P.; Ju, C.; Wang, C. The CBL1/9-CIPK1 Calcium Sensor Negatively Regulates Drought Stress by Phosphorylating the PYLs ABA Receptor. Nat. Commun. 2023, 14, 5886. [Google Scholar] [CrossRef]
- Fichman, Y.; Zandalinas, S.I.; Peck, S.; Luan, S.; Mittler, R. HPCA1 Is Required for Systemic Reactive Oxygen Species and Calcium Cell-to-Cell Signaling and Plant Acclimation to Stress. Plant Cell 2022, 34, 4453–4471. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Su, N.; Huang, X.; Cui, J.; Shabala, L.; Zhou, M.; Yu, M.; Shabala, S. Hypoxia-Induced Increase in GABA Content Is Essential for Restoration of Membrane Potential and Preventing ROS-Induced Disturbance to Ion Homeostasis. Plant Commun. 2021, 2, 100188. [Google Scholar] [CrossRef] [PubMed]
- Bienert, G.P.; Møller, A.L.B.; Kristiansen, K.A.; Schulz, A.; Møller, I.M.; Schjoerring, J.K.; Jahn, T.P. Specific Aquaporins Facilitate the Diffusion of Hydrogen Peroxide across Membranes. J. Biol. Chem. 2007, 282, 1183–1192. [Google Scholar] [CrossRef]
- Han, Y.; Luo, F.; Liang, A.; Xu, D.; Zhang, H.; Liu, T.; Qi, H. Aquaporin CmPIP2; 3 Links H2O2 Signal and Antioxidation to Modulate Trehalose-Induced Cold Tolerance in Melon Seedlings. Plant Physiol. 2024, kiae477. [Google Scholar] [CrossRef]
- Zhang, T.; Xiao, J.; Zhao, Y.; Zhang, Y.; Jie, Y.; Shen, D.; Yue, C.; Huang, J.; Hua, Y.; Zhou, T. Comparative Physiological and Transcriptomic Analyses Reveal Ascorbate and Glutathione Coregulation of Cadmium Toxicity Resistance in Wheat Genotypes. BMC Plant Biol. 2021, 21, 459. [Google Scholar] [CrossRef]
- Mardani-Korrani, F.; Amooaghaie, R.; Ahadi, A.; Ghanadian, M. RBOH-Dependent Signaling Is Involved in He-Ne Laser-Induced Salt Tolerance and Production of Rosmarinic Acid and Carnosol in Salvia Officinalis. BMC Plant Biol. 2024, 24, 798. [Google Scholar] [CrossRef]
- Tiwari, B.S.; Belenghi, B.; Levine, A. Oxidative Stress Increased Respiration and Generation of Reactive Oxygen Species, Resulting in ATP Depletion, Opening of Mitochondrial Permeability Transition, and Programmed Cell Death. Plant Physiol. 2002, 128, 1271–1281. [Google Scholar] [CrossRef]
- Dahal, K.; Vanlerberghe, G.C. Alternative Oxidase Respiration Maintains Both Mitochondrial and Chloroplast Function during Drought. New Phytol. 2017, 213, 560–571. [Google Scholar] [CrossRef]
- Oh, G.G.K.; O’Leary, B.M.; Signorelli, S.; Millar, A.H. Alternative Oxidase (AOX) 1a and 1d Limit Proline-Induced Oxidative Stress and Aid Salinity Recovery in Arabidopsis. Plant Physiol. 2022, 188, 1521–1536. [Google Scholar] [CrossRef]
- Sako, K.; Futamura, Y.; Shimizu, T.; Matsui, A.; Hirano, H.; Kondoh, Y.; Muroi, M.; Aono, H.; Tanaka, M.; Honda, K.; et al. Inhibition of Mitochondrial Complex I by the Novel Compound FSL0260 Enhances High Salinity-Stress Tolerance in Arabidopsis Thaliana. Sci. Rep. 2020, 10, 8691. [Google Scholar] [CrossRef]
- Belt, K.; Huang, S.; Thatcher, L.F.; Casarotto, H.; Singh, K.B.; Van Aken, O.; Millar, A.H. Salicylic Acid-Dependent Plant Stress Signaling via Mitochondrial Succinate Dehydrogenase. Plant Physiol. 2017, 173, 2029–2040. [Google Scholar] [CrossRef]
- Jardim-Messeder, D.; Caverzan, A.; Rauber, R.; de Souza Ferreira, E.; Margis-Pinheiro, M.; Galina, A. Succinate Dehydrogenase (Mitochondrial Complex II) Is a Source of Reactive Oxygen Species in Plants and Regulates Development and Stress Responses. New Phytol. 2015, 208, 776–789. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.-K. Abiotic Stress Signaling and Responses in Plants. Cell 2016, 167, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Mura, A.; Medda, R.; Longu, S.; Floris, G.; Rinaldi, A.C.; Padiglia, A. A Ca2+/Calmodulin-Binding Peroxidase from Euphorbia Latex: Novel Aspects of Calcium−Hydrogen Peroxide Cross-Talk in the Regulation of Plant Defenses. Biochemistry 2005, 44, 14120–14130. [Google Scholar] [CrossRef]
- Dong, X.; Gao, Y.; Bao, X.; Wang, R.; Ma, X.; Zhang, H.; Liu, Y.; Jin, L.; Lin, G. Multi-Omics Revealed Peanut Root Metabolism Regulated by Exogenous Calcium under Salt Stress. Plants 2023, 12, 3130. [Google Scholar] [CrossRef]
- Wang, X.; Yin, J.; Wang, J.; Li, J. Integrative Analysis of Transcriptome and Metabolome Revealed the Mechanisms by Which Flavonoids and Phytohormones Regulated the Adaptation of Alfalfa Roots to NaCl Stress. Front. Plant Sci. 2023, 14, 1117868. [Google Scholar] [CrossRef]
- Wu, Y.; von Tiedemann, A. Impact of Fungicides on Active Oxygen Species and Antioxidant Enzymes in Spring Barley (Hordeum vulgare L.) Exposed to Ozone. Environ. Pollut. 2002, 116, 37–47. [Google Scholar] [CrossRef]
- He, J.; Qin, J.; Long, L.; Ma, Y.; Li, H.; Li, K.; Jiang, X.; Liu, T.; Polle, A.; Liang, Z.; et al. Net Cadmium Flux and Accumulation Reveal Tissue-Specific Oxidative Stress and Detoxification in Populus × Canescens. Physiol. Plant. 2011, 143, 50–63. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide Dismutases: I. Occurrence in Higher Plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Enhanced Superoxide Radical Production in Roots of Zinc-Deficient Plants. J. Exp. Bot. 1988, 39, 1449–1460. [Google Scholar] [CrossRef]
- Bradford, M.M. A Rapid and Sensitive Method for the Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein-Dye Binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Han, B.; Liu, A.; Xu, W. Integrated Lipidomic and Transcriptomic Analysis Reveals Triacylglycerol Accumulation in Castor Bean Seedlings under Heat Stress. Ind. Crops Prod. 2022, 180, 114702. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “One for All, All for One” Bioinformatics Platform for Biological Big-Data Mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Blum, M.; Chang, H.-Y.; Chuguransky, S.; Grego, T.; Kandasaamy, S.; Mitchell, A.; Nuka, G.; Paysan-Lafosse, T.; Qureshi, M.; Raj, S.; et al. The InterPro Protein Families and Domains Database: 20 Years On. Nucleic Acids Res. 2021, 49, D344–D354. [Google Scholar] [CrossRef] [PubMed]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, F.; Cui, Z.; Dong, X.; Gao, Y.; Wang, R.; Zhang, H.; Lin, G. Exogenous Calcium Enhances Castor Tolerance to Saline–Alkaline Stress by Regulating Antioxidant Enzyme Activity and Activating Ca2+ and ROS Signaling Crosstalk. Int. J. Mol. Sci. 2024, 25, 12717. https://doi.org/10.3390/ijms252312717
Hao F, Cui Z, Dong X, Gao Y, Wang R, Zhang H, Lin G. Exogenous Calcium Enhances Castor Tolerance to Saline–Alkaline Stress by Regulating Antioxidant Enzyme Activity and Activating Ca2+ and ROS Signaling Crosstalk. International Journal of Molecular Sciences. 2024; 25(23):12717. https://doi.org/10.3390/ijms252312717
Chicago/Turabian StyleHao, Fei, Zhigang Cui, Xuan Dong, Yan Gao, Rongjin Wang, Hui Zhang, and Guolin Lin. 2024. "Exogenous Calcium Enhances Castor Tolerance to Saline–Alkaline Stress by Regulating Antioxidant Enzyme Activity and Activating Ca2+ and ROS Signaling Crosstalk" International Journal of Molecular Sciences 25, no. 23: 12717. https://doi.org/10.3390/ijms252312717
APA StyleHao, F., Cui, Z., Dong, X., Gao, Y., Wang, R., Zhang, H., & Lin, G. (2024). Exogenous Calcium Enhances Castor Tolerance to Saline–Alkaline Stress by Regulating Antioxidant Enzyme Activity and Activating Ca2+ and ROS Signaling Crosstalk. International Journal of Molecular Sciences, 25(23), 12717. https://doi.org/10.3390/ijms252312717





