Network-Based Bioinformatics Highlights Broad Importance of Human Milk Hyaluronan
Abstract
1. Introduction
2. Results
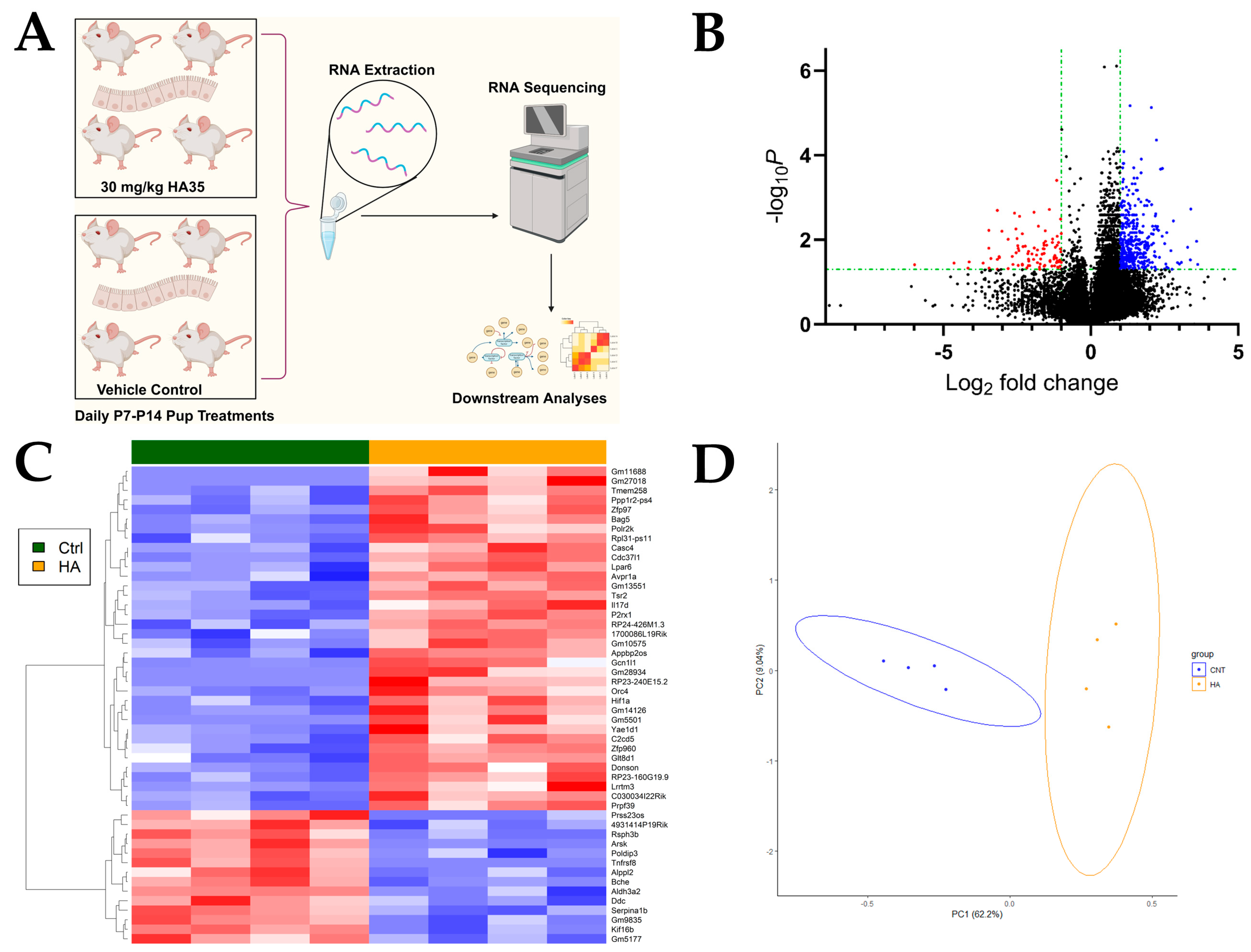
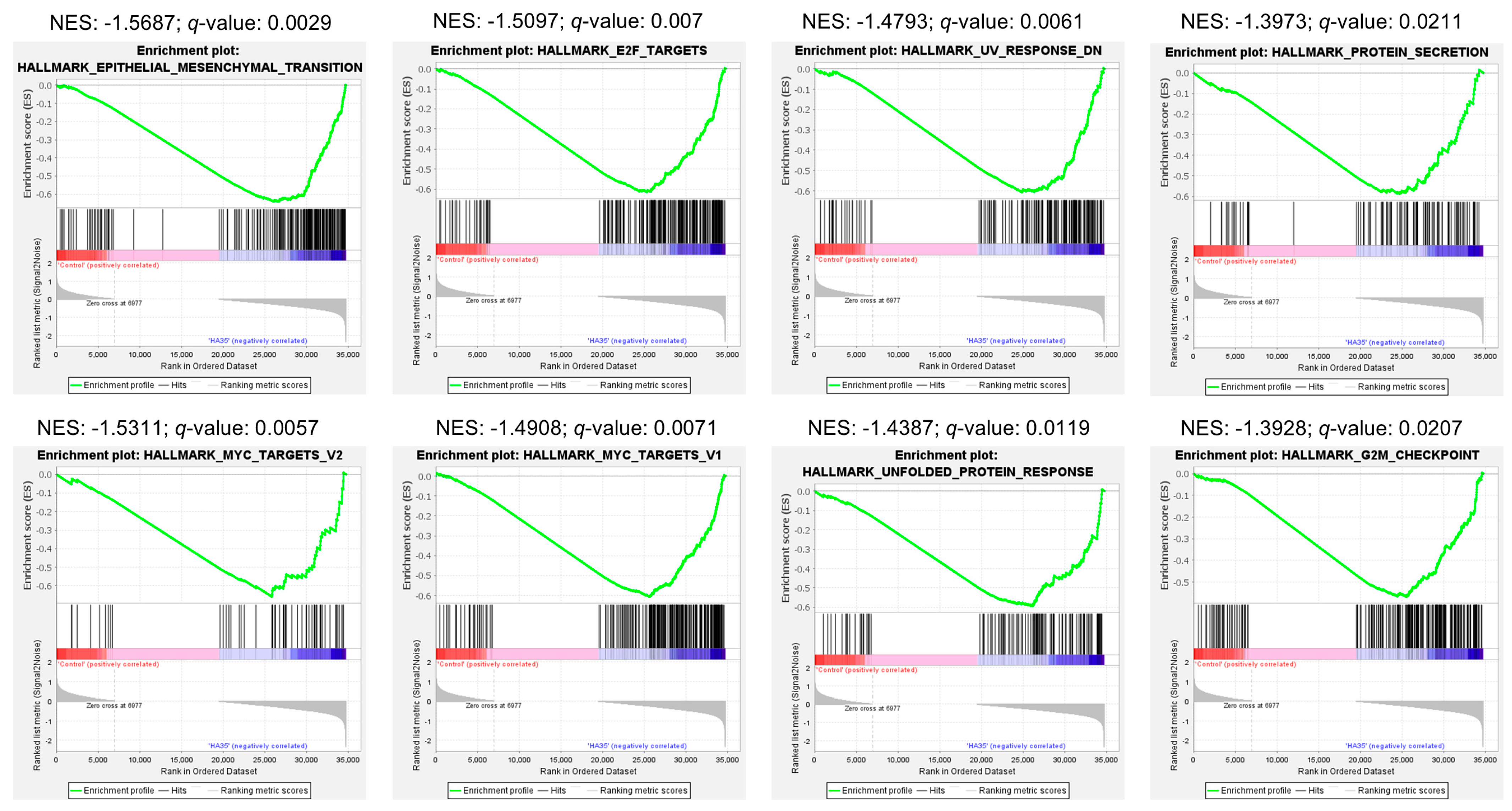
2.1. Oral HA35 Induces a Unique Transcriptional Response Within the Developing Ileum
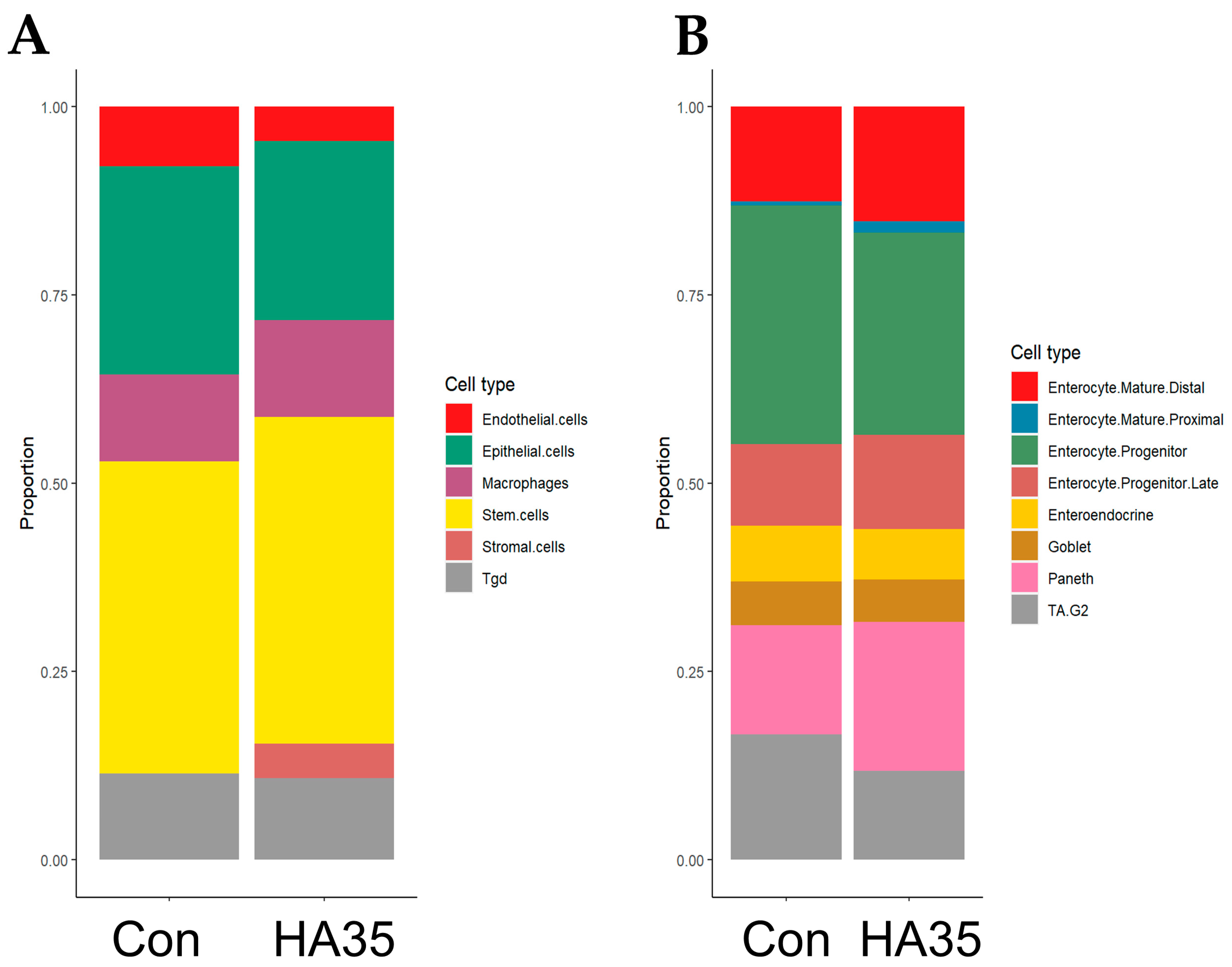
2.2. HA35 Induces Trend Toward Differentiation of Stromal, Mature Enterocytes, and Paneth Cells
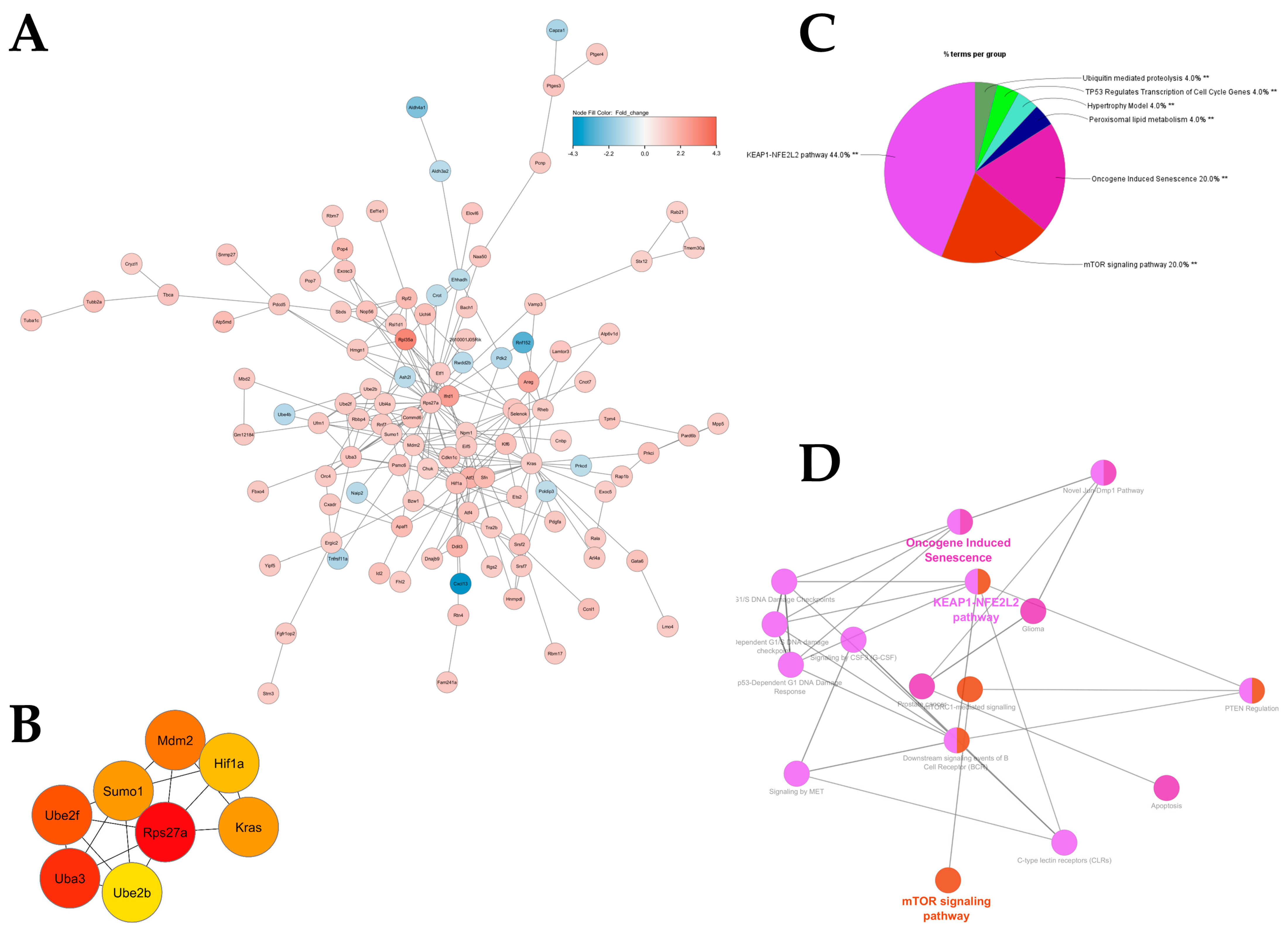
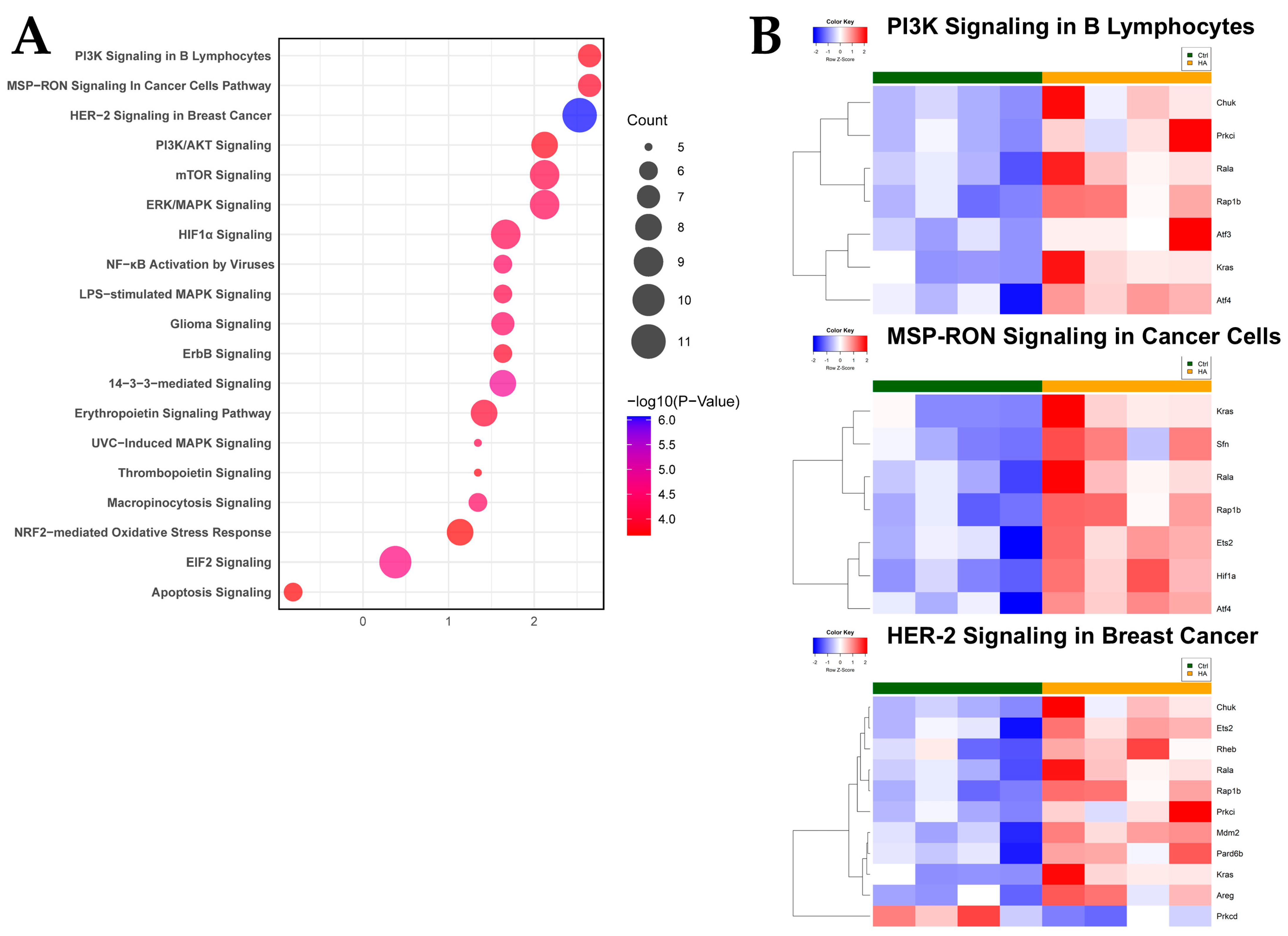
2.3. Antioxidant and Growth Signaling Characterize Functional Intestinal Response to HA35
3. Discussion
3.1. Role of HA35 in Differentiation of the Intestinal Epithelium
3.2. HA35 as an Inducer of the PI3K/Akt/mTOR Signaling Cascade
3.3. Activation of HIF-1α and VEGF Signaling Through HA35
3.4. HA35 and the NRF2-Mediated Response to Oxidative Stress
3.5. MSP-RON and Epithelial-Mesenchymal Transition (EMT) Signaling
3.6. The Role of HA35 as a Developmentally Appropriate Oncofetal Signal
4. Materials and Methods
4.1. Hyaluronic Acid 35 kDa (HA35)
4.2. Mouse Experiments
4.3. Bulk RNA-Sequencing
4.4. Analysis of Differentially Expressed Genes (DEGs)
4.5. Bioinformatic Analysis of DEGs
4.6. Protein–Protein Interaction (PPI) Networks
4.7. Ingenuity Pathway Analysis (IPA)
4.8. GSEA Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brockway, M.M.; Daniel, A.I.; Reyes, S.M.; Gauglitz, J.M.; Granger, M.; McDermid, J.M.; Chan, D.; Refvik, R.; Sidhu, K.K.; Musse, S.; et al. Human Milk Bioactive Components and Child Growth and Body Composition in the First 2 Years: A Systematic Review. Adv. Nutr. 2024, 15, 100127. [Google Scholar] [CrossRef] [PubMed]
- Grulee, C.G.; Sanford, H.N.; Herron, P.H. Breast and artificial feeding: Influence on morbidity and mortality of twenty thousand infants. JAMA J. Am. Med. Assoc. 1934, 103, 735–738. [Google Scholar] [CrossRef]
- Howie, P.W.; Forsyth, J.S.; Ogston, S.A.; Clark, A.; Florey, C.D. Protective effect of breast feeding against infection. BMJ (Clin. Res. Ed.) 1990, 300, 11–16. [Google Scholar] [CrossRef]
- He, Y.; Lawlor, N.T.; Newburg, D.S. Human Milk Components Modulate Toll-Like Receptor-Mediated Inflammation. Adv. Nutr. 2016, 7, 102–111. [Google Scholar] [CrossRef]
- Walker, W.A.; Iyengar, R.S. Breast milk, microbiota, and intestinal immune homeostasis. Pediatr. Res. 2015, 77, 220–228. [Google Scholar] [CrossRef]
- Maffei, D.; Schanler, R.J. Human milk is the feeding strategy to prevent necrotizing enterocolitis! Semin. Perinatol. 2017, 41, 36–40. [Google Scholar] [CrossRef]
- Westerbeek, E.A.; van den Berg, A.; Lafeber, H.N.; Fetter, W.P.; van Elburg, R.M. The effect of enteral supplementation of a prebiotic mixture of non-human milk galacto-, fructo- and acidic oligosaccharides on intestinal permeability in preterm infants. Br. J. Nutr. 2011, 105, 268–274. [Google Scholar] [CrossRef]
- van den Berg, A.; Fetter, W.P.; Westerbeek, E.A.; van der Vegt, I.M.; van der Molen, H.R.; van Elburg, R.M. The effect of glutamine-enriched enteral nutrition on intestinal permeability in very-low-birth-weight infants: A randomized controlled trial. JPEN J. Parenter. Enteral Nutr. 2006, 30, 408–414. [Google Scholar] [CrossRef]
- Foster, J.P.; Seth, R.; Cole, M.J. Oral immunoglobulin for preventing necrotizing enterocolitis in preterm and low birth weight neonates. Cochrane Database Syst. Rev. 2016, 4, Cd001816. [Google Scholar] [CrossRef]
- Dimmitt, R.A.; Staley, E.M.; Chuang, G.; Tanner, S.M.; Soltau, T.D.; Lorenz, R.G. Role of postnatal acquisition of the intestinal microbiome in the early development of immune function. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 262–273. [Google Scholar] [CrossRef]
- Pammi, M.; Cope, J.; Tarr, P.I.; Warner, B.B.; Morrow, A.L.; Mai, V.; Gregory, K.E.; Kroll, J.S.; McMurtry, V.; Ferris, M.J.; et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: A systematic review and meta-analysis. Microbiome 2017, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Samuels, N.; van de Graaf, R.A.; de Jonge, R.C.J.; Reiss, I.K.M.; Vermeulen, M.J. Risk factors for necrotizing enterocolitis in neonates: A systematic review of prognostic studies. BMC Pediatr. 2017, 17, 105. [Google Scholar] [CrossRef] [PubMed]
- Abrams, S.A.; Schanler, R.J.; Lee, M.L.; Rechtman, D.J. Greater mortality and morbidity in extremely preterm infants fed a diet containing cow milk protein products. Breastfeed. Med. Off. J. Acad. Breastfeed. Med. 2014, 9, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Sisk, P.M.; Lovelady, C.A.; Dillard, R.G.; Gruber, K.J.; O’Shea, T.M. Early human milk feeding is associated with a lower risk of necrotizing enterocolitis in very low birth weight infants. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2007, 27, 428–433. [Google Scholar] [CrossRef]
- Coppa, G.V.; Gabrielli, O.; Buzzega, D.; Zampini, L.; Galeazzi, T.; Maccari, F.; Bertino, E.; Volpi, N. Composition and structure elucidation of human milk glycosaminoglycans. Glycobiology 2011, 21, 295–303. [Google Scholar] [CrossRef]
- Kogan, G.; Soltés, L.; Stern, R.; Mendichi, R. Hyaluronic acid: A biopolymer with versatile physico-chemical and biological properties. In Handbook of Polymer Research: Monomers, Oligomers, Polymers and Composites; Pethrich, R.A., Antonio, B., Zaikov, G.E., Eds.; Nova Science Publishers Inc.: New York, NY, USA, 2007; pp. 393–439. [Google Scholar]
- Prehm, P. Release of hyaluronate from eukaryotic cells. Biochem. J. 1990, 267, 185–189. [Google Scholar] [CrossRef]
- Kim, Y.; Kessler, S.P.; Obery, D.R.; Homer, C.R.; McDonald, C.; de la Motte, C.A. Hyaluronan 35 kDa treatment protects mice from Citrobacter rodentium infection and induces epithelial tight junction protein ZO-1 in vivo. Matrix Biol. 2017, 62, 28–39. [Google Scholar] [CrossRef]
- Stern, R.; Asari, A.A.; Sugahara, K.N. Hyaluronan fragments: An information-rich system. Eur. J. Cell Biol. 2006, 85, 699–715. [Google Scholar] [CrossRef]
- Papakonstantinou, E.; Roth, M.; Karakiulakis, G. Hyaluronic acid: A key molecule in skin aging. Dermatoendocrinology 2012, 4, 253–258. [Google Scholar] [CrossRef]
- Monslow, J.; Govindaraju, P.; Puré, E. Hyaluronan—A functional and structural sweet spot in the tissue microenvironment. Front. Immunol. 2015, 6, 231. [Google Scholar] [CrossRef]
- Fallacara, A.; Baldini, E.; Manfredini, S.; Vertuani, S. Hyaluronic Acid in the Third Millennium. Polymers 2018, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Slevin, M.; Krupinski, J.; Gaffney, J.; Matou, S.; West, D.; Delisser, H.; Savani, R.C.; Kumar, S. Hyaluronan-mediated angiogenesis in vascular disease: Uncovering RHAMM and CD44 receptor signaling pathways. Matrix Biol. 2007, 26, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Jackson, D.G. Immunological functions of hyaluronan and its receptors in the lymphatics. Immunol. Rev. 2009, 230, 216–231. [Google Scholar] [CrossRef]
- Soltés, L.; Mendichi, R.; Kogan, G.; Schiller, J.; Stankovska, M.; Arnhold, J. Degradative action of reactive oxygen species on hyaluronan. Biomacromolecules 2006, 7, 659–668. [Google Scholar] [CrossRef]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan as an immune regulator in human diseases. Physiol. Rev. 2011, 91, 221–264. [Google Scholar] [CrossRef]
- Riehl, T.E.; Ee, X.; Stenson, W.F. Hyaluronic acid regulates normal intestinal and colonic growth in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2012, 303, G377–G388. [Google Scholar] [CrossRef]
- Slevin, M.; Kumar, S.; Gaffney, J. Angiogenic oligosaccharides of hyaluronan induce multiple signaling pathways affecting vascular endothelial cell mitogenic and wound healing responses. J. Biol. Chem. 2002, 277, 41046–41059. [Google Scholar] [CrossRef]
- Coppa, G.V.; Gabrielli, O.; Bertino, E.; Zampini, L.; Galeazzi, T.; Padella, L.; Santoro, L.; Marchesiello, R.L.; Galeotti, F.; Maccari, F.; et al. Human milk glycosaminoglycans: The state of the art and future perspectives. Ital. J. Pediatr. 2013, 39, 2. [Google Scholar] [CrossRef]
- Maccari, F.; Mantovani, V.; Gabrielli, O.; Carlucci, A.; Zampini, L.; Galeazzi, T.; Galeotti, F.; Coppa, G.V.; Volpi, N. Metabolic fate of milk glycosaminoglycans in breastfed and formula fed newborns. Glycoconj. J. 2016, 33, 181–188. [Google Scholar] [CrossRef]
- Hill, D.R.; Rho, H.K.; Kessler, S.P.; Amin, R.; Homer, C.R.; McDonald, C.; Cowman, M.K.; de la Motte, C.A. Human milk hyaluronan enhances innate defense of the intestinal epithelium. J. Biol. Chem. 2013, 288, 29090–29104. [Google Scholar] [CrossRef]
- Kessler, S.P.; Obery, D.R.; Nickerson, K.P.; Petrey, A.C.; McDonald, C.; de la Motte, C.A. Multifunctional Role of 35 Kilodalton Hyaluronan in Promoting Defense of the Intestinal Epithelium. J. Histochem. Cytochem. Off. J. Histochem. Soc. 2018, 66, 273–287. [Google Scholar] [CrossRef] [PubMed]
- Saikia, P.; Roychowdhury, S.; Bellos, D.; Pollard, K.A.; McMullen, M.R.; McCullough, R.L.; McCullough, A.J.; Gholam, P.; de la Motte, C.; Nagy, L.E. Hyaluronic acid 35 normalizes TLR4 signaling in Kupffer cells from ethanol-fed rats via regulation of microRNA291b and its target Tollip. Sci. Rep. 2017, 7, 15671. [Google Scholar] [CrossRef] [PubMed]
- Saikia, P.; Bellos, D.; McMullen, M.R.; Pollard, K.A.; de la Motte, C.; Nagy, L.E. MicroRNA 181b-3p and its target importin alpha5 regulate toll-like receptor 4 signaling in Kupffer cells and liver injury in mice in response to ethanol. Hepatology 2017, 66, 602–615. [Google Scholar] [CrossRef]
- Wilson, A.; Burge, K.; Eckert, J.; Chaaban, H. Effect of Hyaluronic Acid 35 kDa on an In Vitro Model of Preterm Small Intestinal Injury and Healing using Enteroid-derived Monolayers. J. Vis. Exp. 2022, 185, e63758. [Google Scholar] [CrossRef]
- Gunasekaran, A.; Eckert, J.; Burge, K.; Zheng, W.; Yu, Z.; Kessler, S.; de la Motte, C.; Chaaban, H. Hyaluronan 35 kDa enhances epithelial barrier function and protects against the development of murine necrotizing enterocolitis. Pediatr. Res. 2019, 87, 1177–1184. [Google Scholar] [CrossRef]
- Burge, K.; Eckert, J.; Wilson, A.; Trammell, M.; Lueschow, S.R.; McElroy, S.J.; Dyer, D.; Chaaban, H. Hyaluronic Acid 35 kDa Protects against a Hyperosmotic, Formula Feeding Model of Necrotizing Enterocolitis. Nutrients 2022, 14, 1779. [Google Scholar] [CrossRef]
- Chaaban, H.; Burge, K.; Eckert, J.; Trammell, M.; Dyer, D.; Keshari, R.S.; Silasi, R.; Regmi, G.; Lupu, C.; Good, M.; et al. Acceleration of Small Intestine Development and Remodeling of the Microbiome Following Hyaluronan 35 kDa Treatment in Neonatal Mice. Nutrients 2021, 13, 2030. [Google Scholar] [CrossRef]
- Stanford, A.H.; Gong, H.; Noonan, M.; Lewis, A.N.; Gong, Q.; Lanik, W.E.; Hsieh, J.J.; Lueschow, S.R.; Frey, M.R.; Good, M.; et al. A direct comparison of mouse and human intestinal development using epithelial gene expression patterns. Pediatr. Res. 2020, 88, 66–76. [Google Scholar] [CrossRef]
- Han, X.; Wang, R.; Zhou, Y.; Fei, L.; Sun, H.; Lai, S.; Saadatpour, A.; Zhou, Z.; Chen, H.; Ye, F.; et al. Mapping the Mouse Cell Atlas by Microwell-Seq. Cell 2018, 172, 1091–1107.e1017. [Google Scholar] [CrossRef]
- Neu, J.; Walker, W.A. Necrotizing enterocolitis. N. Engl. J. Med. 2011, 364, 255–264. [Google Scholar] [CrossRef]
- Tanner, S.M.; Berryhill, T.F.; Ellenburg, J.L.; Jilling, T.; Cleveland, D.S.; Lorenz, R.G.; Martin, C.A. Pathogenesis of necrotizing enterocolitis: Modeling the innate immune response. Am. J. Pathol. 2015, 185, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Demers-Mathieu, V. The immature intestinal epithelial cells in preterm infants play a role in the necrotizing enterocolitis pathogenesis: A review. Health Sci. Rev. 2022, 4, 6. [Google Scholar] [CrossRef]
- Nanthakumar, N.; Meng, D.; Goldstein, A.M.; Zhu, W.; Lu, L.; Uauy, R.; Llanos, A.; Claud, E.C.; Walker, W.A. The mechanism of excessive intestinal inflammation in necrotizing enterocolitis: An immature innate immune response. PLoS ONE 2011, 6, e17776. [Google Scholar] [CrossRef] [PubMed]
- Thänert, R.; Keen, E.C.; Dantas, G.; Warner, B.B.; Tarr, P.I. Necrotizing Enterocolitis and the Microbiome: Current Status and Future Directions. J. Infect. Dis. 2021, 223, S257–S263. [Google Scholar] [CrossRef]
- Duchon, J.; Barbian, M.E.; Denning, P.W. Necrotizing Enterocolitis. Clin. Perinatol. 2021, 48, 229–250. [Google Scholar] [CrossRef]
- Nolan, L.S.; Parks, O.B.; Good, M. A Review of the Immunomodulating Components of Maternal Breast Milk and Protection Against Necrotizing Enterocolitis. Nutrients 2019, 12, 14. [Google Scholar] [CrossRef]
- Chong, C.Y.L.; Bloomfield, F.H.; O’Sullivan, J.M. Factors Affecting Gastrointestinal Microbiome Development in Neonates. Nutrients 2018, 10, 274. [Google Scholar] [CrossRef]
- Patel, A.L.; Kim, J.H. Human milk and necrotizing enterocolitis. Semin. Pediatr. Surg. 2018, 27, 34–38. [Google Scholar] [CrossRef]
- Cortez, J.; Makker, K.; Kraemer, D.F.; Neu, J.; Sharma, R.; Hudak, M.L. Maternal milk feedings reduce sepsis, necrotizing enterocolitis and improve outcomes of premature infants. J. Perinatol. Off. J. Calif. Perinat. Assoc. 2018, 38, 71–74. [Google Scholar] [CrossRef]
- Lewis, E.D.; Richard, C.; Larsen, B.M.; Field, C.J. The Importance of Human Milk for Immunity in Preterm Infants. Clin. Perinatol. 2017, 44, 23–47. [Google Scholar] [CrossRef]
- Shyer, A.E.; Tallinen, T.; Nerurkar, N.L.; Wei, Z.; Gil, E.S.; Kaplan, D.L.; Tabin, C.J.; Mahadevan, L. Villification: How the gut gets its villi. Science 2013, 342, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Chin, A.M.; Hill, D.R.; Aurora, M.; Spence, J.R. Morphogenesis and maturation of the embryonic and postnatal intestine. Semin. Cell Dev. Biol. 2017, 66, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Turner, J.R. Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 2009, 9, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H.C.; Bevins, C.L. Paneth cells: Maestros of the small intestinal crypts. Annu. Rev. Physiol. 2013, 75, 289–311. [Google Scholar] [CrossRef]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef]
- Riehl, T.E.; Santhanam, S.; Foster, L.; Ciorba, M.; Stenson, W.F. CD44 and TLR4 mediate hyaluronic acid regulation of Lgr5+ stem cell proliferation, crypt fission, and intestinal growth in postnatal and adult mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2015, 309, G874–G887. [Google Scholar] [CrossRef]
- Jacob, J.M.; Di Carlo, S.E.; Stzepourginski, I.; Lepelletier, A.; Ndiaye, P.D.; Varet, H.; Legendre, R.; Kornobis, E.; Benabid, A.; Nigro, G.; et al. PDGFRα-induced stromal maturation is required to restrain postnatal intestinal epithelial stemness and promote defense mechanisms. Cell Stem Cell 2022, 29, 856–868.e855. [Google Scholar] [CrossRef]
- Sanman, L.E.; Chen, I.W.; Bieber, J.M.; Steri, V.; Trentesaux, C.; Hann, B.; Klein, O.D.; Wu, L.F.; Altschuler, S.J. Transit-Amplifying Cells Coordinate Changes in Intestinal Epithelial Cell-Type Composition. Dev. Cell 2021, 56, 356–365.e359. [Google Scholar] [CrossRef]
- Coursodon, C.F.; Dvorak, B. Epidermal growth factor and necrotizing enterocolitis. Curr. Opin. Pediatr. 2012, 24, 160–164. [Google Scholar] [CrossRef]
- Austin, K.; Tsang, D.; Chalmers, J.A.; Maalouf, M.F.; Brubaker, P.L. Insulin-like growth factor-binding protein-4 inhibits epithelial growth and proliferation in the rodent intestine. Am. J. Physiol. Gastrointest. Liver Physiol. 2018, 315, G206–G219. [Google Scholar] [CrossRef]
- Fesler, Z.; Mitova, E.; Brubaker, P.L. GLP-2, EGF, and the Intestinal Epithelial IGF-1 Receptor Interactions in the Regulation of Crypt Cell Proliferation. Endocrinology 2020, 161, bqaa040. [Google Scholar] [CrossRef] [PubMed]
- Nair, R.R.; Warner, B.B.; Warner, B.W. Role of epidermal growth factor and other growth factors in the prevention of necrotizing enterocolitis. Semin. Perinatol. 2008, 32, 107–113. [Google Scholar] [CrossRef] [PubMed]
- de Jong, P.R.; Taniguchi, K.; Harris, A.R.; Bertin, S.; Takahashi, N.; Duong, J.; Campos, A.D.; Powis, G.; Corr, M.; Karin, M.; et al. ERK5 signalling rescues intestinal epithelial turnover and tumour cell proliferation upon ERK1/2 abrogation. Nat. Commun. 2016, 7, 11551. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. mTOR signaling at a glance. J. Cell Sci. 2009, 122, 3589–3594. [Google Scholar] [CrossRef] [PubMed]
- Panwar, V.; Singh, A.; Bhatt, M.; Tonk, R.K.; Azizov, S.; Raza, A.S.; Sengupta, S.; Kumar, D.; Garg, M. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduct. Target. Ther. 2023, 8, 375. [Google Scholar] [CrossRef]
- Rhoads, J.M.; Niu, X.; Odle, J.; Graves, L.M. Role of mTOR signaling in intestinal cell migration. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G510–G517. [Google Scholar] [CrossRef]
- Sheng, H.; Shao, J.; Townsend, C.M., Jr.; Evers, B.M. Phosphatidylinositol 3-kinase mediates proliferative signals in intestinal epithelial cells. Gut 2003, 52, 1472–1478. [Google Scholar] [CrossRef]
- Sampson, L.L.; Davis, A.K.; Grogg, M.W.; Zheng, Y. mTOR disruption causes intestinal epithelial cell defects and intestinal atrophy postinjury in mice. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2016, 30, 1263–1275. [Google Scholar] [CrossRef]
- Kaur, H.; Moreau, R. Role of mTORC1 in intestinal epithelial repair and tumorigenesis. Cell Mol. Life Sci. 2019, 76, 2525–2546. [Google Scholar] [CrossRef]
- Lenart, M.; Rutkowska-Zapala, M.; Baj-Krzyworzeka, M.; Szatanek, R.; Węglarczyk, K.; Smallie, T.; Ziegler-Heitbrock, L.; Zembala, M.; Siedlar, M. Hyaluronan carried by tumor-derived microvesicles induces IL-10 production in classical (CD14++CD16−) monocytes via PI3K/Akt/mTOR-dependent signalling pathway. Immunobiology 2017, 222, 1–10. [Google Scholar] [CrossRef]
- Mitrousis, N.; Tam, R.Y.; Baker, A.E.G.; van der Kooy, D.; Shoichet, M.S. Hyaluronic Acid-Based Hydrogels Enable Rod Photoreceptor Survival and Maturation In Vitro through Activation of the mTOR Pathway. Adv. Funct. Mater. 2016, 26, 1975–1985. [Google Scholar] [CrossRef]
- Terabe, K.; Ohashi, Y.; Tsuchiya, S.; Ishizuka, S.; Knudson, C.B.; Knudson, W. Chondroprotective effects of 4-methylumbelliferone and hyaluronan synthase-2 overexpression involve changes in chondrocyte energy metabolism. J. Biol. Chem. 2019, 294, 17799–17817. [Google Scholar] [CrossRef] [PubMed]
- Kaplina, A.; Kononova, S.; Zaikova, E.; Pervunina, T.; Petrova, N.; Sitkin, S. Necrotizing Enterocolitis: The Role of Hypoxia, Gut Microbiome, and Microbial Metabolites. Int. J. Mol. Sci. 2023, 24, 2471. [Google Scholar] [CrossRef] [PubMed]
- Pral, L.P.; Fachi, J.L.; Corrêa, R.O.; Colonna, M.; Vinolo, M.A.R. Hypoxia and HIF-1 as key regulators of gut microbiota and host interactions. Trends Immunol. 2021, 42, 604–621. [Google Scholar] [CrossRef] [PubMed]
- Steiner, C.A.; Cartwright, I.M.; Taylor, C.T.; Colgan, S.P. Hypoxia-inducible factor as a bridge between healthy barrier function, wound healing, and fibrosis. Am. J. Physiol. Cell Physiol. 2022, 323, C866–C878. [Google Scholar] [CrossRef]
- Chanmee, T.; Ontong, P.; Izumikawa, T.; Higashide, M.; Mochizuki, N.; Chokchaitaweesuk, C.; Khansai, M.; Nakajima, K.; Kakizaki, I.; Kongtawelert, P.; et al. Hyaluronan Production Regulates Metabolic and Cancer Stem-like Properties of Breast Cancer Cells via Hexosamine Biosynthetic Pathway-coupled HIF-1 Signaling. J. Biol. Chem. 2016, 291, 24105–24120. [Google Scholar] [CrossRef]
- O’Neill, L.A. A feed-forward loop involving hyaluronic acid and toll-like receptor-4 as a treatment for colitis? Gastroenterology 2009, 137, 1889–1891. [Google Scholar] [CrossRef]
- Burge, K.Y.; Gunasekaran, A.; Makoni, M.M.; Mir, A.M.; Burkhart, H.M.; Chaaban, H. Clinical Characteristics and Potential Pathogenesis of Cardiac Necrotizing Enterocolitis in Neonates with Congenital Heart Disease: A Narrative Review. J. Clin. Med. 2022, 11, 3987. [Google Scholar] [CrossRef]
- Ma, D.; Gao, W.; Liu, J.; Kong, D.; Zhang, Y.; Qian, M. Mechanism of oxidative stress and Keap-1/Nrf2 signaling pathway in bronchopulmonary dysplasia. Medicine 2020, 99, e20433. [Google Scholar] [CrossRef]
- Wen, Z.; Liu, W.; Li, X.; Chen, W.; Liu, Z.; Wen, J.; Liu, Z. A Protective Role of the NRF2-Keap1 Pathway in Maintaining Intestinal Barrier Function. Oxid. Med. Cell Longev. 2019, 2019, 1759149. [Google Scholar] [CrossRef]
- Jin, W.; Wang, H.; Ji, Y.; Hu, Q.; Yan, W.; Chen, G.; Yin, H. Increased intestinal inflammatory response and gut barrier dysfunction in Nrf2-deficient mice after traumatic brain injury. Cytokine 2008, 44, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, A.; Kang, M.I.; Okawa, H.; Ohtsuji, M.; Zenke, Y.; Chiba, T.; Igarashi, K.; Yamamoto, M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004, 24, 7130–7139. [Google Scholar] [CrossRef] [PubMed]
- Aceti, A.; Beghetti, I.; Martini, S.; Faldella, G.; Corvaglia, L. Oxidative Stress and Necrotizing Enterocolitis: Pathogenetic Mechanisms, Opportunities for Intervention, and Role of Human Milk. Oxid. Med. Cell Longev. 2018, 2018, 7397659. [Google Scholar] [CrossRef] [PubMed]
- Khor, T.O.; Huang, M.T.; Kwon, K.H.; Chan, J.Y.; Reddy, B.S.; Kong, A.N. Nrf2-deficient mice have an increased susceptibility to dextran sulfate sodium-induced colitis. Cancer Res. 2006, 66, 11580–11584. [Google Scholar] [CrossRef] [PubMed]
- Rockwell, C.E.; Zhang, M.; Fields, P.E.; Klaassen, C.D. Th2 skewing by activation of Nrf2 in CD4+ T cells. J. Immunol. 2012, 188, 1630–1637. [Google Scholar] [CrossRef]
- Jia, X.; Shi, M.; Wang, Q.; Hui, J.; Shofaro, J.H.; Erkhembayar, R.; Hui, M.; Gao, C.; Gantumur, M.A. Anti-Inflammatory Effects of the 35kDa Hyaluronic Acid Fragment (B-HA/HA35). J. Inflamm. Res. 2023, 16, 209–224. [Google Scholar] [CrossRef]
- Onodera, Y.; Teramura, T.; Takehara, T.; Fukuda, K. Hyaluronic acid regulates a key redox control factor Nrf2 via phosphorylation of Akt in bovine articular chondrocytes. FEBS Open Bio 2015, 5, 476–484. [Google Scholar] [CrossRef]
- Li, R.; Wilson, K.F.; Cerione, R.A. Elucidation of an mTORC2-PKC-NRF2 pathway that sustains the ATF4 stress response and identification of Sirt5 as a key ATF4 effector. Cell Death Discov. 2022, 8, 357. [Google Scholar] [CrossRef]
- Yetkin-Arik, B.; Jansen, S.A.; Varderidou-Minasian, S.; Westendorp, B.; Skarp, K.P.; Altelaar, M.; Lindemans, C.A.; Lorenowicz, M.J. Mesenchymal stromal/stem cells promote intestinal epithelium regeneration after chemotherapy-induced damage. Stem Cell Res. Ther. 2024, 15, 125. [Google Scholar] [CrossRef]
- Neal, M.D.; Sodhi, C.P.; Dyer, M.; Craig, B.T.; Good, M.; Jia, H.; Yazji, I.; Afrazi, A.; Richardson, W.M.; Beer-Stolz, D.; et al. A critical role for TLR4 induction of autophagy in the regulation of enterocyte migration and the pathogenesis of necrotizing enterocolitis. J. Immunol. 2013, 190, 3541–3551. [Google Scholar] [CrossRef]
- Yao, H.P.; Zhou, Y.Q.; Zhang, R.; Wang, M.H. MSP-RON signalling in cancer: Pathogenesis and therapeutic potential. Nat. Rev. Cancer 2013, 13, 466–481. [Google Scholar] [CrossRef] [PubMed]
- Weng, T.; Yan, D.; Shi, D.; Zhu, M.; Liu, Y.; Wu, Z.; Tang, T.; Zhu, L.; Zhang, H.; Yao, H.; et al. The MSP-RON pathway regulates liver fibrosis through transforming growth factor beta-dependent epithelial-mesenchymal transition. Liver Int. 2021, 41, 1956–1968. [Google Scholar] [CrossRef] [PubMed]
- Mao, T.; Li, J.; Liu, L.; Zhao, W.; Liu, Y.; Gao, K.; Guo, Y.; Xie, T.; Li, N.; Shi, R. Qingchang Wenzhong Decoction Attenuates DSS-Induced Colitis in Rats by Reducing Inflammation and Improving Intestinal Barrier Function via Upregulating the MSP/RON Signalling Pathway. Evid. Based Complement. Alternat. Med. 2017, 2017, 4846876. [Google Scholar] [CrossRef]
- Huang, L.; Fang, X.; Shi, D.; Yao, S.; Wu, W.; Fang, Q.; Yao, H. MSP-RON Pathway: Potential Regulator of Inflammation and Innate Immunity. Front. Immunol. 2020, 11, 569082. [Google Scholar] [CrossRef]
- Kauder, S.E.; Santell, L.; Mai, E.; Wright, L.Y.; Luis, E.; N’Diaye, E.N.; Lutman, J.; Ratti, N.; Sa, S.M.; Maun, H.R.; et al. Functional consequences of the macrophage stimulating protein 689C inflammatory bowel disease risk allele. PLoS ONE 2013, 8, e83958. [Google Scholar] [CrossRef]
- Manzanares, D.; Monzon, M.E.; Savani, R.C.; Salathe, M. Apical oxidative hyaluronan degradation stimulates airway ciliary beating via RHAMM and RON. Am. J. Respir. Cell Mol. Biol. 2007, 37, 160–168. [Google Scholar] [CrossRef]
- West, R.C.; Bouma, G.J.; Winger, Q.A. Shifting perspectives from “oncogenic” to oncofetal proteins; how these factors drive placental development. Reprod. Biol. Endocrinol. 2018, 16, 101. [Google Scholar] [CrossRef]
- Fey, S.K.; Vaquero-Siguero, N.; Jackstadt, R. Dark force rising: Reawakening and targeting of fetal-like stem cells in colorectal cancer. Cell Rep. 2024, 43, 114270. [Google Scholar] [CrossRef]
- Wang, C.; Lang, Y.; Li, Q.; Jin, X.; Li, G.; Yu, G. Glycosaminoglycanomic profiling of human milk in different stages of lactation by liquid chromatography-tandem mass spectrometry. Food Chem. 2018, 258, 231–236. [Google Scholar] [CrossRef]
- Coppa, G.V.; Gabrielli, O.; Zampini, L.; Galeazzi, T.; Maccari, F.; Buzzega, D.; Galeotti, F.; Bertino, E.; Volpi, N. Glycosaminoglycan content in term and preterm milk during the first month of lactation. Neonatology 2012, 101, 74–76. [Google Scholar] [CrossRef]
- Baird, N.L.; Bowlin, J.L.; Cohrs, R.J.; Gilden, D.; Jones, K.L. Comparison of varicella-zoster virus RNA sequences in human neurons and fibroblasts. J. Virol. 2014, 88, 5877–5880. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.D.; Nacu, S. Fast and SNP-tolerant detection of complex variants and splicing in short reads. Bioinformatics 2010, 26, 873–881. [Google Scholar] [CrossRef] [PubMed]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Doncheva, N.T.; Morris, J.H.; Gorodkin, J.; Jensen, L.J. Cytoscape StringApp: Network Analysis and Visualization of Proteomics Data. J. Proteome Res. 2019, 18, 623–632. [Google Scholar] [CrossRef]
- Bindea, G.; Mlecnik, B.; Hackl, H.; Charoentong, P.; Tosolini, M.; Kirilovsky, A.; Fridman, W.H.; Pagès, F.; Trajanoski, Z.; Galon, J. ClueGO: A Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics 2009, 25, 1091–1093. [Google Scholar] [CrossRef]
- Chin, C.H.; Chen, S.H.; Wu, H.H.; Ho, C.W.; Ko, M.T.; Lin, C.Y. cytoHubba: Identifying hub objects and sub-networks from complex interactome. BMC Syst. Biol. 2014, 8 (Suppl. 4), S11. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burge, K.Y.; Zhong, H.; Wilson, A.P.; Chaaban, H. Network-Based Bioinformatics Highlights Broad Importance of Human Milk Hyaluronan. Int. J. Mol. Sci. 2024, 25, 12679. https://doi.org/10.3390/ijms252312679
Burge KY, Zhong H, Wilson AP, Chaaban H. Network-Based Bioinformatics Highlights Broad Importance of Human Milk Hyaluronan. International Journal of Molecular Sciences. 2024; 25(23):12679. https://doi.org/10.3390/ijms252312679
Chicago/Turabian StyleBurge, Kathryn Y., Hua Zhong, Adam P. Wilson, and Hala Chaaban. 2024. "Network-Based Bioinformatics Highlights Broad Importance of Human Milk Hyaluronan" International Journal of Molecular Sciences 25, no. 23: 12679. https://doi.org/10.3390/ijms252312679
APA StyleBurge, K. Y., Zhong, H., Wilson, A. P., & Chaaban, H. (2024). Network-Based Bioinformatics Highlights Broad Importance of Human Milk Hyaluronan. International Journal of Molecular Sciences, 25(23), 12679. https://doi.org/10.3390/ijms252312679




