Backstage Heroes—Yeast in COVID-19 Research
Abstract
1. Introduction
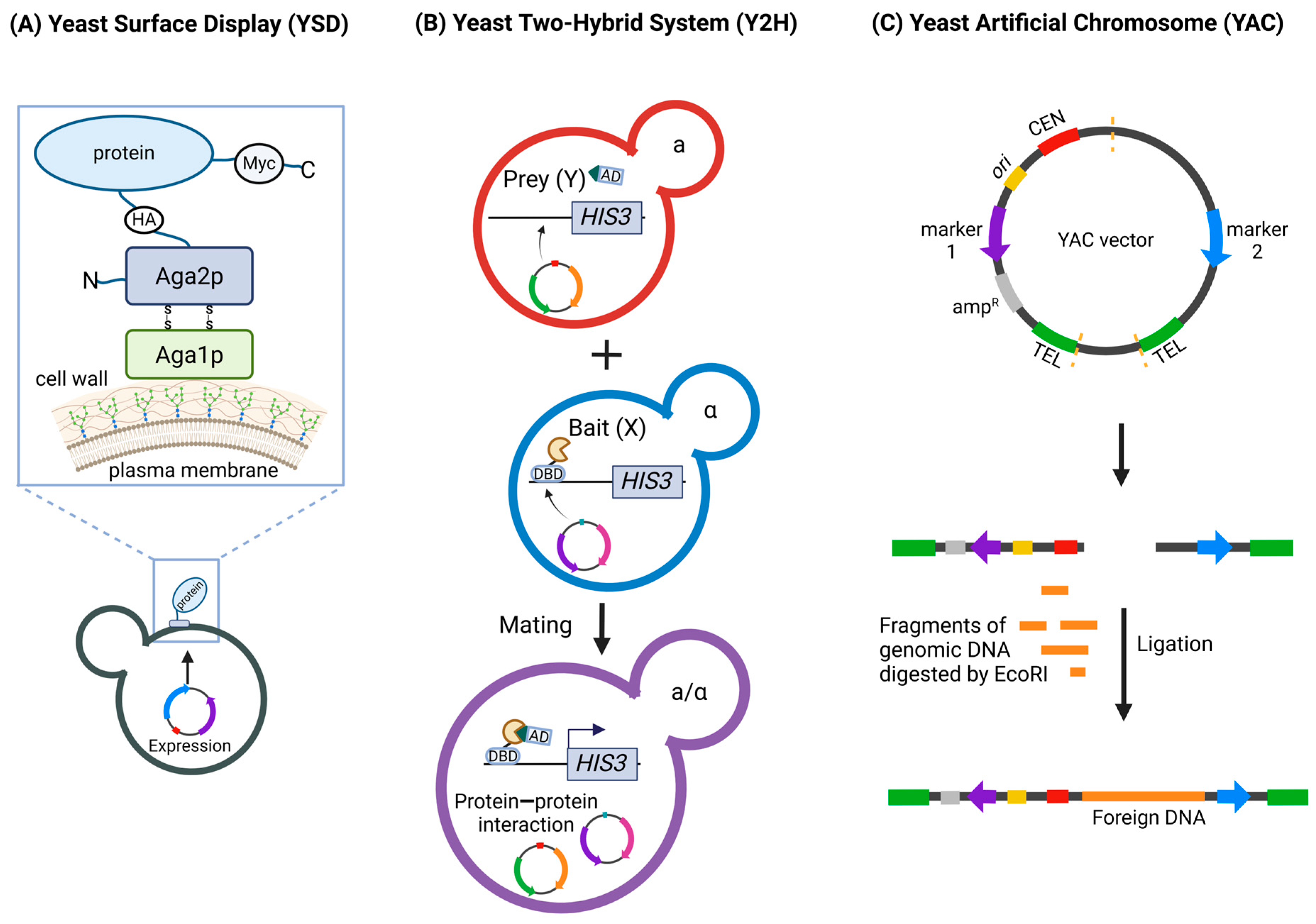
2. Overview of the Most Common Yeast Techniques Used in COVID-19 Studies
3. SARS-CoV-2 Interactions with Host Proteins
4. Vaccine Development
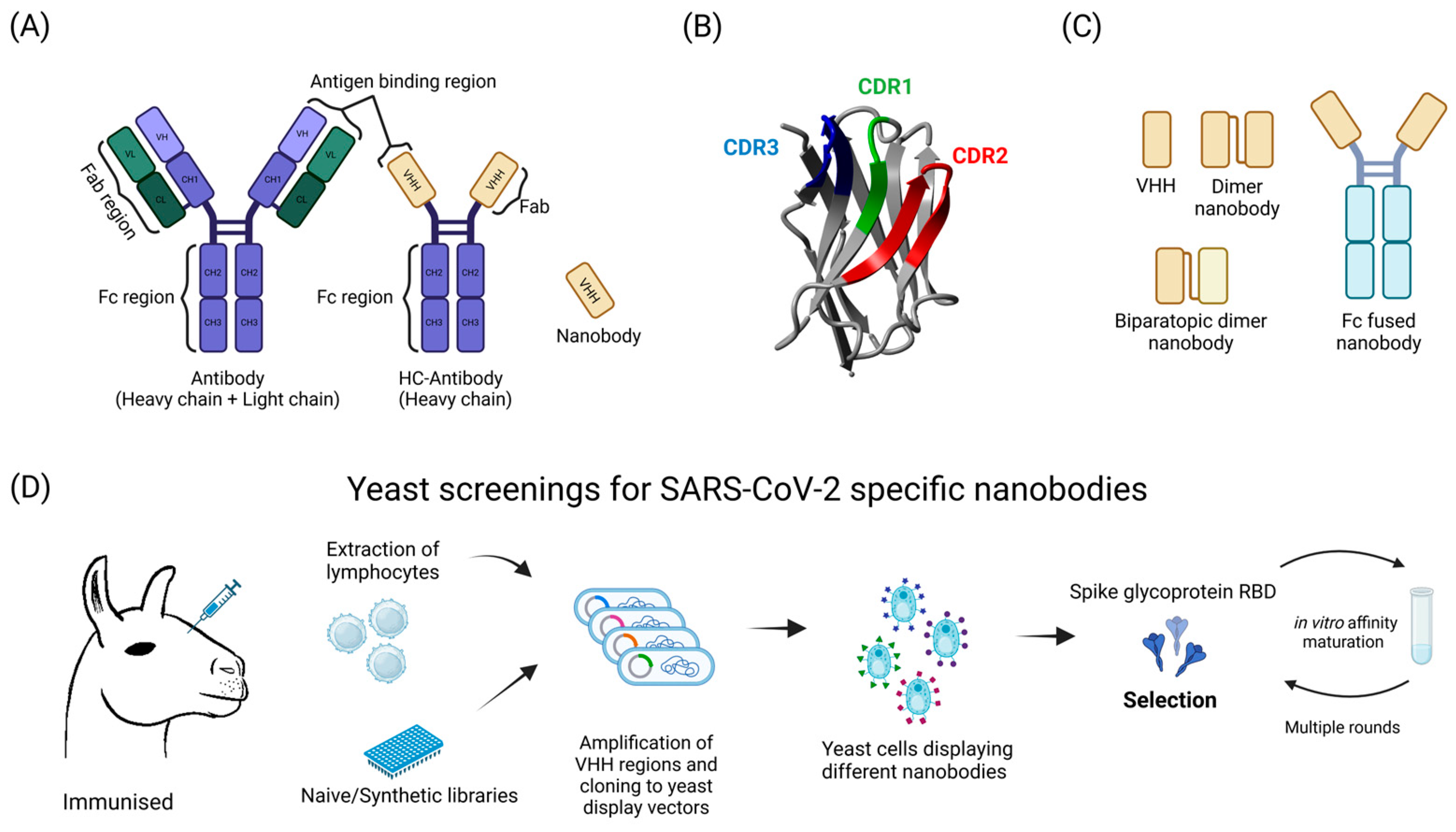
5. Antibody Discovery and Testing
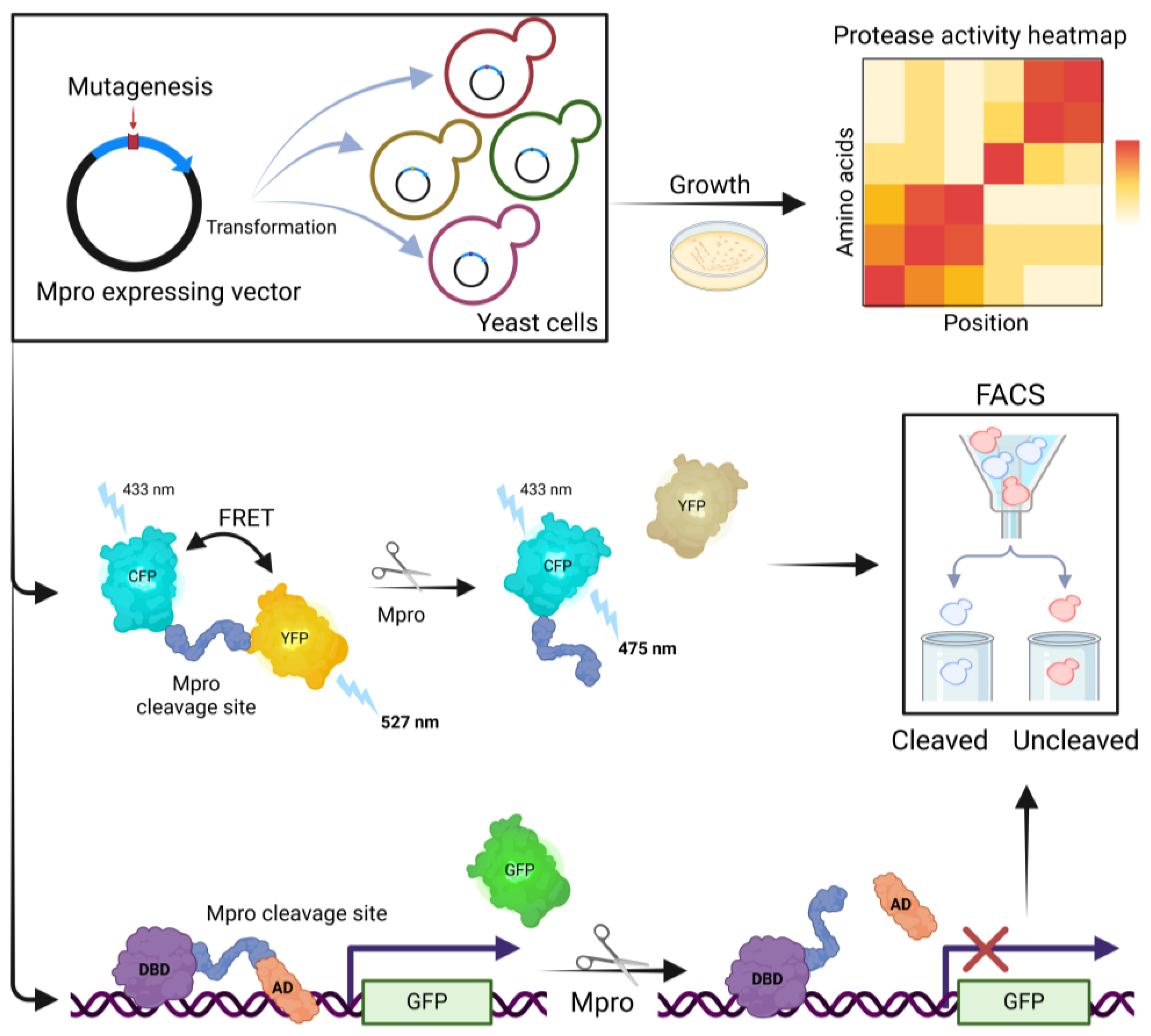
6. Mpro Activity Analysis
7. Synthetic Recombinant Viruses and Viral Replicons
8. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Kashani, N.R.; Azadbakht, J.; Ehteram, H.; Kashani, H.H.; Rajabi-Moghadam, H.; Ahmad, E.; Nikzad, H.; Hosseini, E.S. Molecular and Clinical Investigation of COVID-19: From Pathogenesis and Immune Responses to Novel Diagnosis and Treatment. Front. Mol. Biosci. 2022, 9, 770775. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, L.; Moore, A.; Micou, M.; Day, C.; Grossman, E.; Meaders, C. CRISPR in Your Kitchen: An At-Home CRISPR Kit to Edit Genes in Saccharomyces Cerevisiae Used during a Remote Lab Course. J. Microbiol. Biol. Educ. 2022, 23, e00321-21. [Google Scholar] [CrossRef] [PubMed]
- Gaikani, H.K.; Stolar, M.; Kriti, D.; Nislow, C.; Giaever, G. From Beer to Breadboards: Yeast as a Force for Biological Innovation. Genome Biol. 2024, 25, 10. [Google Scholar] [CrossRef] [PubMed]
- Gastelum, S.; Michael, A.F.; Bolger, T.A. Saccharomyces Cerevisiae as a Research Tool for RNA-Mediated Human Disease. Wiley Interdiscip. Rev. RNA 2023, 15, e1814. [Google Scholar] [CrossRef] [PubMed]
- Franco, L.V.R.; Bremner, L.; Barros, M.H. Human Mitochondrial Pathologies of the Respiratory Chain and ATP Synthase: Contributions from Studies of Saccharomyces Cerevisiae. Life 2020, 10, 304. [Google Scholar] [CrossRef]
- Cervelli, T.; Galli, A. Yeast as a Tool to Understand the Significance of Human Disease-Associated Gene Variants. Genes 2021, 12, 1303. [Google Scholar] [CrossRef]
- Cherf, G.M.; Cochran, J.R. Applications of Yeast Surface Display for Protein Engineering. In Yeast Surface Display: Methods in Molecular Biology; Humana Press: New York, NY, USA, 2015; Volume 1319, pp. 155–175. [Google Scholar] [CrossRef]
- Tsuda, M.; Nonaka, K. Recent Progress on Heterologous Protein Production in Methylotrophic Yeast Systems. World J. Microbiol. Biotechnol. 2024, 40, 200. [Google Scholar] [CrossRef]
- Zhu, T.; Sun, H.; Wang, M.; Li, Y. Pichia Pastoris as a Versatile Cell Factory for the Production of Industrial Enzymes and Chemicals: Current Status and Future Perspectives. Biotechnol. J. 2019, 14, e1800694. [Google Scholar] [CrossRef]
- Barrero, J.J.; Casler, J.C.; Valero, F.; Ferrer, P.; Glick, B.S. An Improved Secretion Signal Enhances the Secretion of Model Proteins from Pichia Pastoris. Microb. Cell Factories 2018, 17, 161. [Google Scholar] [CrossRef]
- Duan, G.; Ding, L.; Wei, D.; Zhou, H.; Chu, J.; Zhang, S.; Qian, J. Screening Endogenous Signal Peptides and Protein Folding Factors to Promote the Secretory Expression of Heterologous Proteins in Pichia Pastoris. J. Biotechnol. 2019, 306, 193–202. [Google Scholar] [CrossRef]
- Hamilton, S.R.; Bobrowicz, P.; Bobrowicz, B.; Davidson, R.C.; Li, H.; Mitchell, T.; Nett, J.H.; Rausch, S.; Stadheim, T.A.; Wischnewski, H.; et al. Production of Complex Human Glycoproteins in Yeast. Science 2003, 301, 1244–1246. [Google Scholar] [CrossRef]
- Jacobs, P.P.; Geysens, S.; Vervecken, W.; Contreras, R.; Callewaert, N. Engineering Complex-Type N-Glycosylation in Pichia Pastoris Using GlycoSwitch Technology. Nat. Protoc. 2009, 4, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Hirz, M.; Pichler, H.; Schwab, H. Protein Expression in Pichia Pastoris: Recent Achievements and Perspectives for Heterologous Protein Production. Appl. Microbiol. Biotechnol. 2014, 98, 5301–5317. [Google Scholar] [CrossRef] [PubMed]
- Boder, E.T.; Wittrup, K.D. Yeast Surface Display for Screening Combinatorial Polypeptide Libraries. Nat. Biotechnol. 1997, 15, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Kondo, A.; Ueda, M. Yeast Cell-Surface Display—Applications of Molecular Display. Appl. Microbiol. Biotechnol. 2004, 64, 28–40. [Google Scholar] [CrossRef]
- Fields, S.; Song, O. A Novel Genetic System to Detect Protein-Protein Interactions. Nature 1989, 340, 245–246. [Google Scholar] [CrossRef]
- Keegan, L.; Gill, G.; Ptashne, M. Separation of DNA Binding from the Transcription-Activating Function of a Eukaryotic Regulatory Protein. Science 1986, 231, 699–704. [Google Scholar] [CrossRef]
- Putz, U.; Skehel, P.; Kuhl, D. A Tri-Hybrid System for the Analysis and Detection of RNA—Protein Interactions. Nucleic Acids Res. 1996, 24, 4838–4840. [Google Scholar] [CrossRef]
- Ratushny, V.; Golemis, E. Resolving the Network of Cell Signaling Pathways Using the Evolving Yeast Two-Hybrid System. BioTechniques 2008, 44, 655–662. [Google Scholar] [CrossRef]
- Estojak, J.; Brent, R.; Golemis, E.A. Correlation of Two-Hybrid Affinity Data with in Vitro Measurements. Mol. Cell. Biol. 1995, 15, 5820–5829. [Google Scholar] [CrossRef]
- Li, B.; Fields, S. Identification of Mutations in P53 That Affect Its Binding to SV40 Large T Antigen by Using the Yeast Two-Hybrid System. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 1993, 7, 957–963. [Google Scholar] [CrossRef] [PubMed]
- Murray, A.W.; Szostak, J.W. Construction of Artificial Chromosomes in Yeast. Nature 1983, 305, 189–193. [Google Scholar] [CrossRef]
- Burke, D.T.; Carle, G.F.; Olson, M.V. Cloning of Large Segments of Exogenous DNA into Yeast by Means of Artificial Chromosome Vectors. Science 1987, 236, 806–812. [Google Scholar] [CrossRef]
- Kouprina, N.; Larionov, V. Selective Isolation of Genomic Loci from Complex Genomes by Transformation-Associated Recombination Cloning in the Yeast Saccharomyces Cerevisiae. Nat. Protoc. 2008, 3, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Y.; Wu, L.; Niu, S.; Song, C.; Zhang, Z.; Lu, G.; Qiao, C.; Hu, Y.; Yuen, K.-Y.; et al. Structural and Functional Basis of SARS-CoV-2 Entry by Using Human ACE2. Cell 2020, 181, 894–904.e9. [Google Scholar] [CrossRef]
- Ho, B.; Loll-Krippleber, R.; Brown, G.W. Yeast Goes Viral: Probing SARS-CoV-2 Biology Using S. Cerevisiae. Microb. Cell 2022, 9, 80–83. [Google Scholar] [CrossRef]
- Zahradník, J.; Marciano, S.; Shemesh, M.; Zoler, E.; Harari, D.; Chiaravalli, J.; Meyer, B.; Rudich, Y.; Li, C.; Marton, I.; et al. SARS-CoV-2 Variant Prediction and Antiviral Drug Design Are Enabled by RBD in Vitro Evolution. Nat. Microbiol. 2021, 6, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, A.; Glasgow, J.; Limonta, D.; Solomon, P.; Lui, I.; Zhang, Y.; Nix, M.A.; Rettko, N.J.; Zha, S.; Yamin, R.; et al. Engineered ACE2 Receptor Traps Potently Neutralize SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 28046–28055. [Google Scholar] [CrossRef] [PubMed]
- Heinzelman, P.; Greenhalgh, J.C.; Romero, P.A. Yeast Surface Display-Based Identification of ACE2 Mutations That Modulate SARS-CoV-2 Spike Binding across Multiple Mammalian Species. Protein Eng. Des. Sel. PEDS 2022, 35, gzab035. [Google Scholar] [CrossRef]
- Starr, T.N.; Greaney, A.J.; Hilton, S.K.; Ellis, D.; Crawford, K.H.D.; Dingens, A.S.; Navarro, M.J.; Bowen, J.E.; Tortorici, M.A.; Walls, A.C.; et al. Deep Mutational Scanning of SARS-CoV-2 Receptor Binding Domain Reveals Constraints on Folding and ACE2 Binding. Cell 2020, 182, 1295–1310.e20. [Google Scholar] [CrossRef]
- Starr, T.N.; Greaney, A.J.; Addetia, A.; Hannon, W.W.; Choudhary, M.C.; Dingens, A.S.; Li, J.Z.; Bloom, J.D. Prospective Mapping of Viral Mutations That Escape Antibodies Used to Treat COVID-19. Science 2021, 371, 850–854. [Google Scholar] [CrossRef] [PubMed]
- Greaney, A.J.; Starr, T.N.; Gilchuk, P.; Zost, S.J.; Binshtein, E.; Loes, A.N.; Hilton, S.K.; Huddleston, J.; Eguia, R.; Crawford, K.H.D.; et al. Complete Mapping of Mutations to the SARS-CoV-2 Spike Receptor-Binding Domain That Escape Antibody Recognition. Cell Host Microbe 2021, 29, 44–57.e9. [Google Scholar] [CrossRef] [PubMed]
- Starr, T.N.; Greaney, A.J.; Dingens, A.S.; Bloom, J.D. Complete Map of SARS-CoV-2 RBD Mutations That Escape the Monoclonal Antibody LY-CoV555 and Its Cocktail with LY-CoV016. Cell Rep. Med. 2021, 2, 100255. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron Escapes the Majority of Existing SARS-CoV-2 Neutralizing Antibodies. Nature 2022, 602, 657–663. [Google Scholar] [CrossRef]
- Greaney, A.J.; Starr, T.N.; Barnes, C.O.; Weisblum, Y.; Schmidt, F.; Caskey, M.; Gaebler, C.; Cho, A.; Agudelo, M.; Finkin, S.; et al. Mapping Mutations to the SARS-CoV-2 RBD That Escape Binding by Different Classes of Antibodies. Nat. Commun. 2021, 12, 4196. [Google Scholar] [CrossRef]
- Francino-Urdaniz, I.M.; Steiner, P.J.; Kirby, M.B.; Zhao, F.; Haas, C.M.; Barman, S.; Rhodes, E.R.; Leonard, A.C.; Peng, L.; Sprenger, K.G.; et al. One-Shot Identification of SARS-CoV-2 S RBD Escape Mutants Using Yeast Screening. Cell Rep. 2021, 36, 109627. [Google Scholar] [CrossRef]
- Klemm, C.; Wood, H.; Thomas, G.H.; Ólafsson, G.; Torres, M.T.; Thorpe, P.H. Forced Association of SARS-CoV-2 Proteins with the Yeast Proteome Perturb Vesicle Trafficking. Microb. Cell 2021, 8, 280–296. [Google Scholar] [CrossRef]
- Santerre, M.; Arjona, S.P.; Allen, C.N.; Shcherbik, N.; Sawaya, B.E. Why Do SARS-CoV-2 NSPs Rush to the ER? J. Neurol. 2021, 268, 2013–2022. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Huarte, J.L.; Fita-Torró, J.; Viana, R.; Pascual-Ahuir, A.; Proft, M. Severe Acute Respiratory Syndrome Coronavirus-2 Accessory Proteins ORF3a and ORF7a Modulate Autophagic Flux and Ca2+ Homeostasis in Yeast. Front. Microbiol. 2023, 14, 1152249. [Google Scholar] [CrossRef]
- Miserey-Lenkei, S.; Trajkovic, K.; D’Ambrosio, J.M.; Patel, A.J.; Čopič, A.; Mathur, P.; Schauer, K.; Goud, B.; Albanèse, V.; Gautier, R.; et al. A Comprehensive Library of Fluorescent Constructs of SARS-CoV-2 Proteins and Their Initial Characterisation in Different Cell Types. Biol. Cell 2021, 113, 311–328. [Google Scholar] [CrossRef]
- Jiang, H.-W.; Zhang, H.-N.; Meng, Q.-F.; Xie, J.; Li, Y.; Chen, H.; Zheng, Y.-X.; Wang, X.-N.; Qi, H.; Zhang, J.; et al. SARS-CoV-2 Orf9b Suppresses Type I Interferon Responses by Targeting TOM70. Cell. Mol. Immunol. 2020, 17, 998–1000. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Q.; Cruz Cosme, R.S.; Gerzanich, V.; Tang, Q.; Simard, J.M.; Zhao, R.Y. Genome-Wide Characterization of SARS-CoV-2 Cytopathogenic Proteins in the Search of Antiviral Targets. mBio 2021, 13, e0016922. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Liu, Y.; Gupta, S.; Paramo, M.I.; Hou, Y.; Mao, C.; Luo, Y.; Judd, J.; Wierbowski, S.; Bertolotti, M.; et al. A Comprehensive SARS-CoV-2-Human Protein-Protein Interactome Reveals COVID-19 Pathobiology and Potential Host Therapeutic Targets. Nat. Biotechnol. 2023, 41, 128–139. [Google Scholar] [CrossRef]
- Kim, S.; Yee, E.; Miller, E.A.; Hao, Y.; Tay, D.M.Y.; Sung, K.-J.; Jia, H.; Johnson, J.M.; Saeed, M.; Mace, C.R.; et al. Developing a SARS-CoV-2 Antigen Test Using Engineered Affinity Proteins. ACS Appl. Mater. Interfaces 2021, 13, 38990–39002. [Google Scholar] [CrossRef]
- Heaton Penny, M. The Covid-19 Vaccine-Development Multiverse. N. Engl. J. Med. 2020, 383, 1986–1988. [Google Scholar] [CrossRef]
- Keech, C.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Zhu, M.; Cloney-Clark, S.; Zhou, H.; et al. Phase 1-2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N. Engl. J. Med. 2020, 383, 2320–2332. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Wang, W.; Chen, Z.; Lu, S.; Yang, F.; Bi, Z.; Bao, L.; Mo, F.; Li, X.; Huang, Y.; et al. A Vaccine Targeting the RBD of the S Protein of SARS-CoV-2 Induces Protective Immunity. Nature 2020, 586, 572–577. [Google Scholar] [CrossRef]
- Nagar, G.; Jain, S.; Rajurkar, M.; Lothe, R.; Rao, H.; Majumdar, S.; Gautam, M.; Rodriguez-Aponte, S.A.; Crowell, L.E.; Love, J.C.; et al. Large-Scale Purification and Characterization of Recombinant Receptor-Binding Domain (RBD) of SARS-CoV-2 Spike Protein Expressed in Yeast. Vaccines 2023, 11, 1602. [Google Scholar] [CrossRef] [PubMed]
- Argentinian AntiCovid Consortium. Structural and Functional Comparison of SARS-CoV-2-Spike Receptor Binding Domain Produced in Pichia Pastoris and Mammalian Cells. Sci. Rep. 2020, 10, 21779. [Google Scholar] [CrossRef]
- Chen, W.-H.; Wei, J.; Kundu, R.T.; Adhikari, R.; Liu, Z.; Lee, J.; Versteeg, L.; Poveda, C.; Keegan, B.; Villar, M.J.; et al. Genetic Modification to Design a Stable Yeast-Expressed Recombinant SARS-CoV-2 Receptor Binding Domain as a COVID-19 Vaccine Candidate. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129893. [Google Scholar] [CrossRef]
- Pollet, J.; Chen, W.-H.; Versteeg, L.; Keegan, B.; Zhan, B.; Wei, J.; Liu, Z.; Lee, J.; Kundu, R.; Adhikari, R.; et al. SARS-CoV-2 RBD219-N1C1: A Yeast-Expressed SARS-CoV-2 Recombinant Receptor-Binding Domain Candidate Vaccine Stimulates Virus Neutralizing Antibodies and T-Cell Immunity in Mice. Hum. Vaccines Immunother. 2021, 17, 2356–2366. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Liu, Z.; Chen, W.-H.; Wei, J.; Kundu, R.; Adhikari, R.; Rivera, J.A.; Gillespie, P.M.; Strych, U.; Zhan, B.; et al. Process Development and Scale-up Optimization of the SARS-CoV-2 Receptor Binding Domain-Based Vaccine Candidate, RBD219-N1C1. Appl. Microbiol. Biotechnol. 2021, 105, 4153–4165. [Google Scholar] [CrossRef] [PubMed]
- Pollet, J.; Strych, U.; Chen, W.-H.; Versteeg, L.; Keegan, B.; Zhan, B.; Wei, J.; Liu, Z.; Lee, J.; Kundu, R.; et al. Receptor-Binding Domain Recombinant Protein on Alum-CpG Induces Broad Protection against SARS-CoV-2 Variants of Concern. Vaccine 2022, 40, 3655–3663. [Google Scholar] [CrossRef] [PubMed]
- Hotez, P.J.; Adhikari, R.; Chen, W.-H.; Chen, Y.-L.; Gillespie, P.; Islam, N.Y.; Keegan, B.; Tyagi Kundu, R.; Lee, J.; Liu, Z.; et al. From Concept to Delivery: A Yeast-Expressed Recombinant Protein-Based COVID-19 Vaccine Technology Suitable for Global Access. Expert Rev. Vaccines 2023, 22, 495–500. [Google Scholar] [CrossRef]
- Liu, Y.; Li, M.; Cui, T.; Chen, Z.; Xu, L.; Li, W.; Peng, Q.; Li, X.; Zhao, D.; Valencia, C.A.; et al. A Superior Heterologous Prime-Boost Vaccination Strategy against COVID-19: A Bivalent Vaccine Based on Yeast-Derived RBD Proteins Followed by a Heterologous Vaccine. J. Med. Virol. 2024, 96, e29454. [Google Scholar] [CrossRef]
- Wang, T.; Zheng, J.; Xu, H.; Wang, Z.; Sun, P.; Hou, X.; Gong, X.; Zhang, B.; Wu, J.; Liu, B. A Delta-Omicron Bivalent Subunit Vaccine Elicited Antibody Responses in Mice against Both Ancestral and Variant Strains of SARS-CoV-2. Vaccines 2023, 11, 1539. [Google Scholar] [CrossRef]
- Xu, H.; Wang, T.; Sun, P.; Hou, X.; Gong, X.; Zhang, B.; Wu, J.; Liu, B. A Bivalent Subunit Vaccine Efficiently Produced in Pichia Pastoris against SARS-CoV-2 and Emerging Variants. Front. Microbiol. 2022, 13, 1093080. [Google Scholar] [CrossRef]
- Gao, T.; Ren, Y.; Li, S.; Lu, X.; Lei, H. Immune Response Induced by Oral Administration with a Saccharomyces Cerevisiae-Based SARS-CoV-2 Vaccine in Mice. Microb. Cell Factories 2021, 20, 95. [Google Scholar] [CrossRef]
- Zhang, L.; Yao, L.; Guo, Y.; Li, X.; Ma, L.; Sun, R.; Han, X.; Liu, J.; Huang, J. Oral SARS-CoV-2 Spike Protein Recombinant Yeast Candidate Prompts Specific Antibody and Gut Microbiota Reconstruction in Mice. Front. Microbiol. 2022, 13, 792532. [Google Scholar] [CrossRef]
- Lei, Z.; Zhu, L.; Pan, P.; Ruan, Z.; Gu, Y.; Xia, X.; Wang, S.; Ge, W.; Yao, Y.; Luo, F.; et al. A Vaccine Delivery System Promotes Strong Immune Responses against SARS-CoV-2 Variants. J. Med. Virol. 2023, 95, e28475. [Google Scholar] [CrossRef] [PubMed]
- Goodridge, H.S.; Wolf, A.J.; Underhill, D.M. Beta-Glucan Recognition by the Innate Immune System. Immunol. Rev. 2009, 230, 38–50. [Google Scholar] [CrossRef]
- Ramanjulu, J.M.; Pesiridis, G.S.; Yang, J.; Concha, N.; Singhaus, R.; Zhang, S.-Y.; Tran, J.-L.; Moore, P.; Lehmann, S.; Eberl, H.C.; et al. Design of Amidobenzimidazole STING Receptor Agonists with Systemic Activity. Nature 2018, 564, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Kelley, B.; De Moor, P.; Douglas, K.; Renshaw, T.; Traviglia, S. Monoclonal Antibody Therapies for COVID-19: Lessons Learned and Implications for the Development of Future Products. Curr. Opin. Biotechnol. 2022, 78, 102798. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; Franchini, M.; Maggi, F.; Shoham, S. COVID-19 Therapeutics. Clin. Microbiol. Rev. 2024, 37, e0011923. [Google Scholar] [CrossRef] [PubMed]
- Focosi, D.; McConnell, S.; Sullivan, D.J.; Casadevall, A. Analysis of SARS-CoV-2 Mutations Associated with Resistance to Therapeutic Monoclonal Antibodies That Emerge after Treatment. Drug Resist. Updat. Rev. Comment. Antimicrob. Anticancer Chemother. 2023, 71, 100991. [Google Scholar] [CrossRef]
- Mitchell, L.S.; Colwell, L.J. Comparative Analysis of Nanobody Sequence and Structure Data. Proteins 2018, 86, 697–706. [Google Scholar] [CrossRef]
- Czajka, T.F.; Vance, D.J.; Mantis, N.J. Slaying SARS-CoV-2 One (Single-Domain) Antibody at a Time. Trends Microbiol. 2021, 29, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, F.; Du, L. Therapeutic Nanobodies against SARS-CoV-2 and Other Pathogenic Human Coronaviruses. J. Nanobiotechnology 2024, 22, 304. [Google Scholar] [CrossRef]
- McMahon, C.; Baier, A.S.; Pascolutti, R.; Wegrecki, M.; Zheng, S.; Ong, J.X.; Erlandson, S.C.; Hilger, D.; Rasmussen, S.G.F.; Ring, A.M.; et al. Yeast Surface Display Platform for Rapid Discovery of Conformationally Selective Nanobodies. Nat. Struct. Mol. Biol. 2018, 25, 289–296. [Google Scholar] [CrossRef]
- Schoof, M.; Faust, B.; Saunders, R.A.; Sangwan, S.; Rezelj, V.; Hoppe, N.; Boone, M.; Billesbølle, C.B.; Puchades, C.; Azumaya, C.M.; et al. An Ultrapotent Synthetic Nanobody Neutralizes SARS-CoV-2 by Stabilizing Inactive Spike. Science 2020, 370, 1473–1479. [Google Scholar] [CrossRef]
- Zupancic, J.M.; Desai, A.A.; Schardt, J.S.; Pornnoppadol, G.; Makowski, E.K.; Smith, M.D.; Kennedy, A.A.; Garcia de Mattos Barbosa, M.; Cascalho, M.; Lanigan, T.M.; et al. Directed Evolution of Potent Neutralizing Nanobodies against SARS-CoV-2 Using CDR-Swapping Mutagenesis. Cell Chem. Biol. 2021, 28, 1379–1388.e7. [Google Scholar] [CrossRef] [PubMed]
- Wellner, A.; McMahon, C.; Gilman, M.S.A.; Clements, J.R.; Clark, S.; Nguyen, K.M.; Ho, M.H.; Hu, V.J.; Shin, J.-E.; Feldman, J.; et al. Rapid Generation of Potent Antibodies by Autonomous Hypermutation in Yeast. Nat. Chem. Biol. 2021, 17, 1057–1064. [Google Scholar] [CrossRef]
- Pymm, P.; Redmond, S.J.; Dolezal, O.; Mordant, F.; Lopez, E.; Cooney, J.P.; Davidson, K.C.; Haycroft, E.R.; Tan, C.W.; Seneviratna, R.; et al. Biparatopic Nanobodies Targeting the Receptor Binding Domain Efficiently Neutralize SARS-CoV-2. iScience 2022, 25, 105259. [Google Scholar] [CrossRef]
- Schepens, B.; van Schie, L.; Nerinckx, W.; Roose, K.; Van Breedam, W.; Fijalkowska, D.; Devos, S.; Weyts, W.; De Cae, S.; Vanmarcke, S.; et al. An Affinity-Enhanced, Broadly Neutralizing Heavy Chain-Only Antibody Protects against SARS-CoV-2 Infection in Animal Models. Sci. Transl. Med. 2021, 13, eabi7826. [Google Scholar] [CrossRef] [PubMed]
- Cross, F.R.; Fridy, P.C.; Ketaren, N.E.; Mast, F.D.; Li, S.; Olivier, J.P.; Pecani, K.; Chait, B.T.; Aitchison, J.D.; Rout, M.P. Expanding and Improving Nanobody Repertoires Using a Yeast Display Method: Targeting SARS-CoV-2. J. Biol. Chem. 2023, 299, 102954. [Google Scholar] [CrossRef]
- Wang, B.; DeKosky, B.J.; Timm, M.R.; Lee, J.; Normandin, E.; Misasi, J.; Kong, R.; McDaniel, J.R.; Delidakis, G.; Leigh, K.E.; et al. Functional Interrogation and Mining of Natively Paired Human VH:VL Antibody Repertoires. Nat. Biotechnol. 2018, 36, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Banach, B.B.; Cerutti, G.; Fahad, A.S.; Shen, C.-H.; Oliveira De Souza, M.; Katsamba, P.S.; Tsybovsky, Y.; Wang, P.; Nair, M.S.; Huang, Y.; et al. Paired Heavy- and Light-Chain Signatures Contribute to Potent SARS-CoV-2 Neutralization in Public Antibody Responses. Cell Rep. 2021, 37, 109771. [Google Scholar] [CrossRef]
- Madan, B.; Reddem, E.R.; Wang, P.; Casner, R.G.; Nair, M.S.; Huang, Y.; Fahad, A.S.; de Souza, M.O.; Banach, B.B.; López Acevedo, S.N.; et al. Antibody Screening at Reduced pH Enables Preferential Selection of Potently Neutralizing Antibodies Targeting SARS-CoV-2. AIChE J. Am. Inst. Chem. Eng. 2021, 67, e17440. [Google Scholar] [CrossRef]
- de Souza, M.O.; Madan, B.; Teng, I.-T.; Huang, A.; Liu, L.; Fahad, A.S.; Lopez Acevedo, S.N.; Pan, X.; Sastry, M.; Gutierrez-Gonzalez, M.; et al. Mapping Monoclonal Anti-SARS-CoV-2 Antibody Repertoires against Diverse Coronavirus Antigens. Front. Immunol. 2022, 13, 977064. [Google Scholar] [CrossRef]
- Bell, B.N.; Powell, A.E.; Rodriguez, C.; Cochran, J.R.; Kim, P.S. Neutralizing Antibodies Targeting the SARS-CoV-2 Receptor Binding Domain Isolated from a Naïve Human Antibody Library. Protein Sci. Publ. Protein Soc. 2021, 30, 716–727. [Google Scholar] [CrossRef]
- Haslwanter, D.; Dieterle, M.E.; Wec, A.Z.; O’Brien, C.M.; Sakharkar, M.; Florez, C.; Tong, K.; Rappazzo, C.G.; Lasso, G.; Vergnolle, O.; et al. A Combination of Receptor-Binding Domain and N-Terminal Domain Neutralizing Antibodies Limits the Generation of SARS-CoV-2 Spike Neutralization-Escape Mutants. mBio 2021, 12, e0247321. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Morales, J.; Vanella, R.; Utzinger, T.; Schittny, V.; Hirsiger, J.; Osthoff, M.; Berger, C.T.; Guri, Y.; Nash, M.A. Multiplexed On-Yeast Serological Assay for Immune Escape Screening of SARS-CoV-2 Variants. iScience 2023, 26, 106648. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of Mpro from SARS-CoV-2 and Discovery of Its Inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Verschueren, K.H.G.; Anand, K.; Shen, J.; Yang, M.; Xu, Y.; Rao, Z.; Bigalke, J.; Heisen, B.; Mesters, J.R.; et al. pH-Dependent Conformational Flexibility of the SARS-CoV Main Proteinase (M(pro)) Dimer: Molecular Dynamics Simulations and Multiple X-Ray Structure Analyses. J. Mol. Biol. 2005, 354, 25–40. [Google Scholar] [CrossRef] [PubMed]
- Kneller, D.W.; Phillips, G.; O’Neill, H.M.; Jedrzejczak, R.; Stols, L.; Langan, P.; Joachimiak, A.; Coates, L.; Kovalevsky, A. Structural Plasticity of SARS-CoV-2 3CL Mpro Active Site Cavity Revealed by Room Temperature X-Ray Crystallography. Nat. Commun. 2020, 11, 3202. [Google Scholar] [CrossRef]
- Ullrich, S.; Nitsche, C. The SARS-CoV-2 Main Protease as Drug Target. Bioorg. Med. Chem. Lett. 2020, 30, 127377. [Google Scholar] [CrossRef]
- Agbowuro, A.A.; Huston, W.M.; Gamble, A.B.; Tyndall, J.D.A. Proteases and Protease Inhibitors in Infectious Diseases. Med. Res. Rev. 2018, 38, 1295–1331. [Google Scholar] [CrossRef]
- Dömling, A.; Gao, L. Chemistry and Biology of SARS-CoV-2. Chem 2020, 6, 1283–1295. [Google Scholar] [CrossRef] [PubMed]
- Paciaroni, A.; Libera, V.; Ripanti, F.; Orecchini, A.; Petrillo, C.; Francisci, D.; Schiaroli, E.; Sabbatini, S.; Gidari, A.; Bianconi, E.; et al. Stabilization of the Dimeric State of SARS-CoV-2 Main Protease by GC376 and Nirmatrelvir. Int. J. Mol. Sci. 2023, 24, 6062. [Google Scholar] [CrossRef]
- Citarella, A.; Dimasi, A.; Moi, D.; Passarella, D.; Scala, A.; Piperno, A.; Micale, N. Recent Advances in SARS-CoV-2 Main Protease Inhibitors: From Nirmatrelvir to Future Perspectives. Biomolecules 2023, 13, 1339. [Google Scholar] [CrossRef]
- Li, X.; Song, Y. Structure and Function of SARS-CoV and SARS-CoV-2 Main Proteases and Their Inhibition: A Comprehensive Review. Eur. J. Med. Chem. 2023, 260, 115772. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.M.; Zvornicanin, S.N.; Tsepal, T.; Shaqra, A.M.; Kurt Yilmaz, N.; Jia, W.; Moquin, S.; Dovala, D.; Schiffer, C.A.; Bolon, D.N.A. Contributions of Hyperactive Mutations in Mpro from SARS-CoV-2 to Drug Resistance. ACS Infect. Dis. 2024, 10, 1174–1184. [Google Scholar] [CrossRef] [PubMed]
- Flynn, J.M.; Huang, Q.Y.J.; Zvornicanin, S.N.; Schneider-Nachum, G.; Shaqra, A.M.; Yilmaz, N.K.; Moquin, S.A.; Dovala, D.; Schiffer, C.A.; Bolon, D.N.A. Systematic Analyses of the Resistance Potential of Drugs Targeting SARS-CoV-2 Main Protease. ACS Infect. Dis. 2023, 9, 1372–1386. [Google Scholar] [CrossRef]
- Flynn, J.M.; Samant, N.; Schneider-Nachum, G.; Barkan, D.T.; Yilmaz, N.K.; Schiffer, C.A.; Moquin, S.A.; Dovala, D.; Bolon, D.N.A. Comprehensive Fitness Landscape of SARS-CoV-2 Mpro Reveals Insights into Viral Resistance Mechanisms. eLife 2022, 11, e77433. [Google Scholar] [CrossRef]
- Iketani, S.; Hong, S.J.; Sheng, J.; Bahari, F.; Culbertson, B.; Atanaki, F.F.; Aditham, A.K.; Kratz, A.F.; Luck, M.I.; Tian, R.; et al. Functional Map of SARS-CoV-2 3CL Protease Reveals Tolerant and Immutable Sites. Cell Host Microbe 2022, 30, 1354–1362.e6. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Lewandowski, E.M.; Hu, Y.; Lipinski, A.A.; Aljasser, A.; Colon-Ascanio, M.; Morgan, R.T.; Jacobs, L.M.C.; Zhang, X.; Bikowitz, M.J.; et al. A Yeast-Based System to Study SARS-CoV-2 Mpro Structure and to Identify Nirmatrelvir Resistant Mutations. PLoS Pathog. 2023, 19, e1011592. [Google Scholar] [CrossRef]
- Alalam, H.; Sigurdardóttir, S.; Bourgard, C.; Tiukova, I.; King, R.D.; Grøtli, M.; Sunnerhagen, P. A Genetic Trap in Yeast for Inhibitors of SARS-CoV-2 Main Protease. mSystems 2021, 6, e0108721. [Google Scholar] [CrossRef]
- Grabiński, W.; Kicińska, A.; Kosicka, E.; Baranek-Grabińska, M.; Hejenkowska, E.D.; Budzik, J.; Śliska, P.; Śliwińska, W.; Karachitos, A. Meisoindigo: An Effective Inhibitor of SARS-CoV-2 Main Protease Revealed by Yeast System. bioRxiv 2023. [Google Scholar] [CrossRef]
- Gao, Q.; Liu, S.; Zhou, Y.; Fan, J.; Ke, S.; Zhou, Y.; Fan, K.; Wang, Y.; Zhou, Y.; Xia, Z.; et al. Discovery of Meisoindigo Derivatives as Noncovalent and Orally Available Mpro Inhibitors: Their Therapeutic Implications in the Treatment of COVID-19. Eur. J. Med. Chem. 2024, 273, 116498. [Google Scholar] [CrossRef]
- Thi Nhu Thao, T.; Labroussaa, F.; Ebert, N.; V’kovski, P.; Stalder, H.; Portmann, J.; Kelly, J.; Steiner, S.; Holwerda, M.; Kratzel, A.; et al. Rapid Reconstruction of SARS-CoV-2 Using a Synthetic Genomics Platform. Nature 2020, 582, 561–565. [Google Scholar] [CrossRef]
- Wang, B.; Zhang, C.; Lei, X.; Ren, L.; Zhao, Z.; Wang, J.; Huang, H. Construction of Non-Infectious SARS-CoV-2 Replicons and Their Application in Drug Evaluation. Virol. Sin. 2021, 36, 890–900. [Google Scholar] [CrossRef]
- Ricardo-Lax, I.; Luna, J.M.; Thao, T.T.N.; Le Pen, J.; Yu, Y.; Hoffmann, H.-H.; Schneider, W.M.; Razooky, B.S.; Fernandez-Martinez, J.; Schmidt, F.; et al. Replication and Single-Cycle Delivery of SARS-CoV-2 Replicons. Science 2021, 374, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Vora, S.M.; Fontana, P.; Mao, T.; Leger, V.; Zhang, Y.; Fu, T.M.; Lieberman, J.; Gehrke, L.; Shi, M.; Wang, L.; et al. Targeting stem-loop 1 of the SARS-CoV-2 5’ UTR to suppress viral translation and Nsp1 evasion. Proc. Natl. Acad. Sci. USA 2022, 119, e2117198119. [Google Scholar] [CrossRef] [PubMed]
- Kachroo, A.H.; Vandeloo, M.; Greco, B.M.; Abdullah, M. Humanized yeast to model human biology, disease and evolution. Dis. Model Mech. 2022, 15, dmm049309. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grabiński, W.; Karachitos, A.; Kicińska, A. Backstage Heroes—Yeast in COVID-19 Research. Int. J. Mol. Sci. 2024, 25, 12661. https://doi.org/10.3390/ijms252312661
Grabiński W, Karachitos A, Kicińska A. Backstage Heroes—Yeast in COVID-19 Research. International Journal of Molecular Sciences. 2024; 25(23):12661. https://doi.org/10.3390/ijms252312661
Chicago/Turabian StyleGrabiński, Wojciech, Andonis Karachitos, and Anna Kicińska. 2024. "Backstage Heroes—Yeast in COVID-19 Research" International Journal of Molecular Sciences 25, no. 23: 12661. https://doi.org/10.3390/ijms252312661
APA StyleGrabiński, W., Karachitos, A., & Kicińska, A. (2024). Backstage Heroes—Yeast in COVID-19 Research. International Journal of Molecular Sciences, 25(23), 12661. https://doi.org/10.3390/ijms252312661






