Characterization and Safety Evaluation of Autoclaved Gut Commensal Parabacteroides goldsteinii RV-01
Abstract
1. Introduction
2. Results
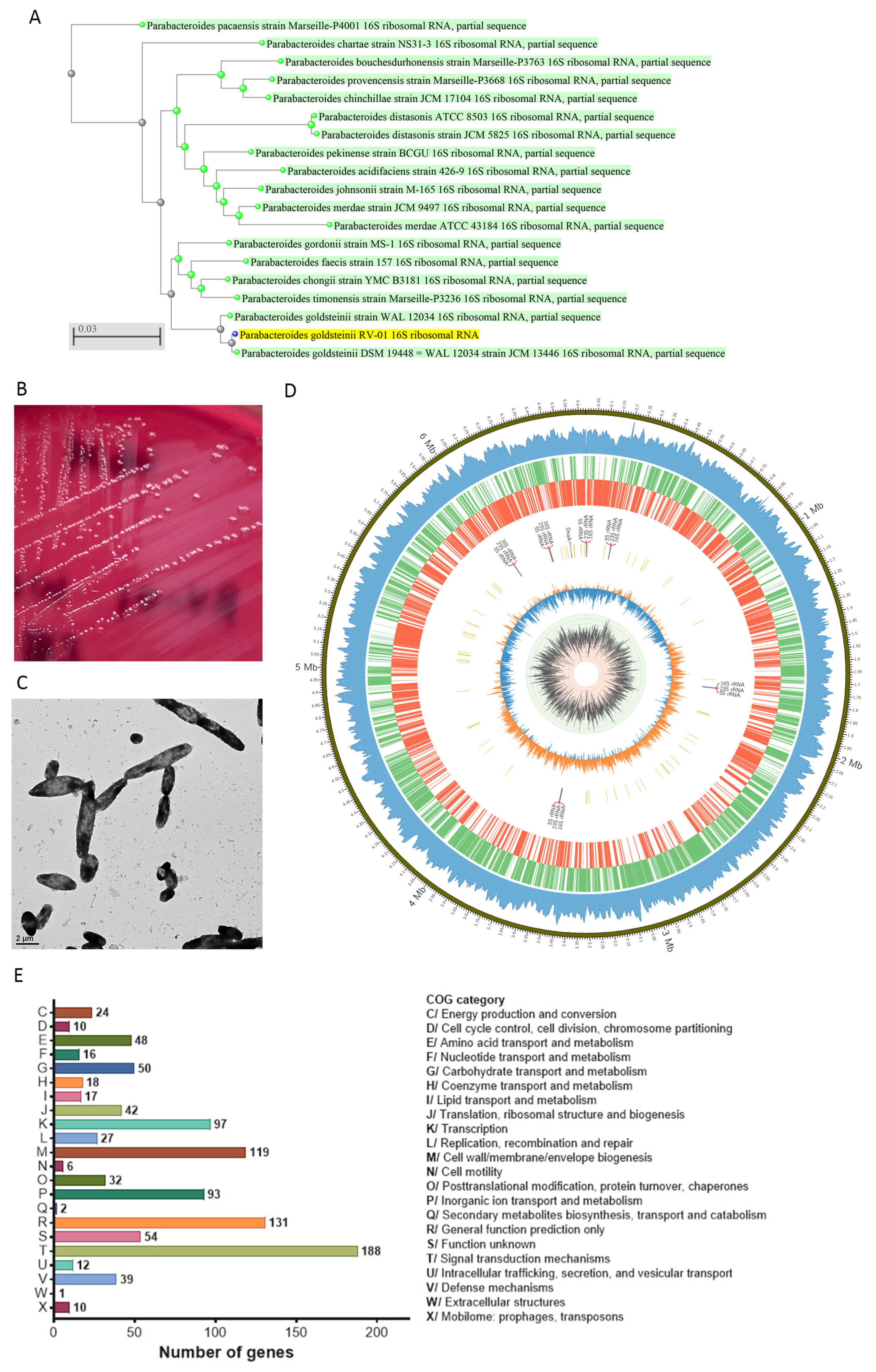
2.1. Descriptions of P. goldsteinii RV-01
2.2. Genomic and Phenotypic Characteristics for Safety Evaluation
2.2.1. Whole Genomic Characteristics
2.2.2. Minimum Inhibitory Concentration (MIC) Evaluation of Antibiotics
2.2.3. Bioinformatic Analyses for Antibiotic Resistance and Potential Virulence genes
2.2.4. Bioinformatic Analyses for Allergic Proteins
2.3. Bioinformatic Analysis of Genes and Traits Potentially Beneficial to Human Health
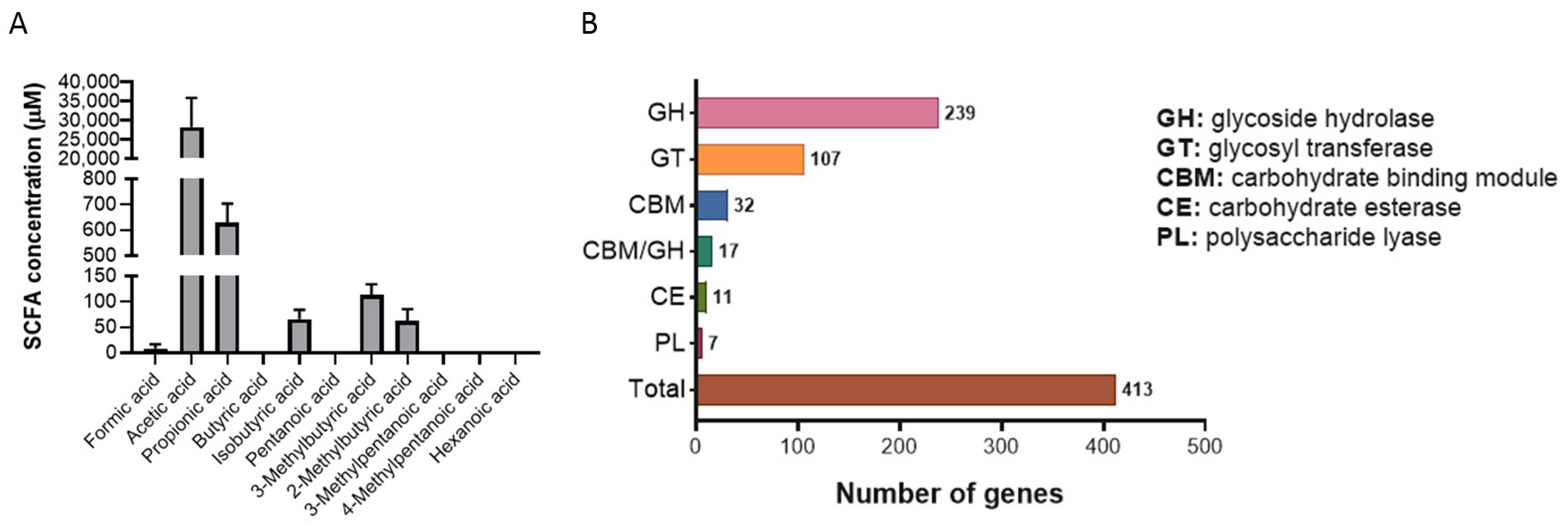
2.3.1. Production of Short-Chain Fatty Acids (SCFAs)
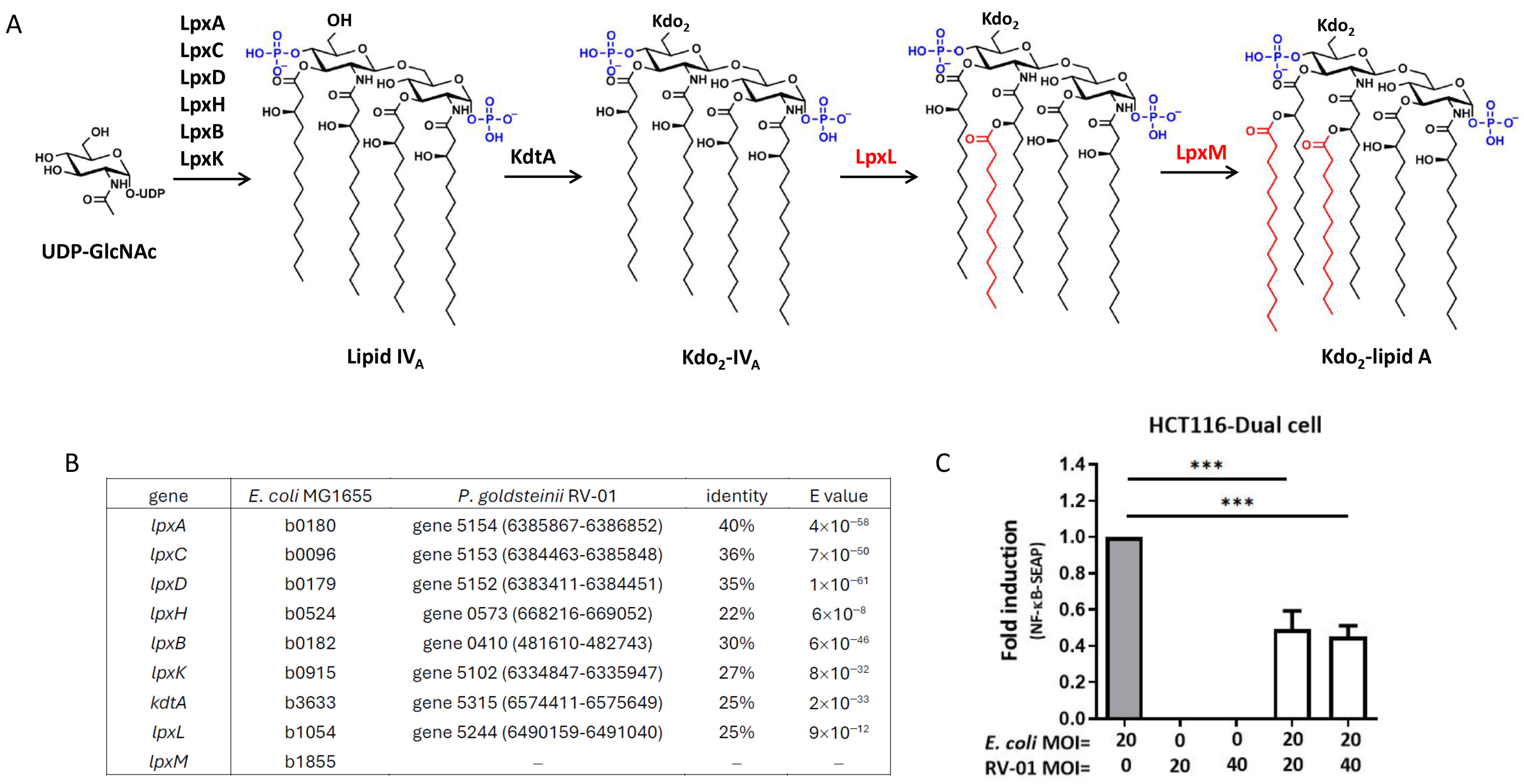
2.3.2. P. goldsteinii RV-01 Reduced TLR4-Related Inflammation
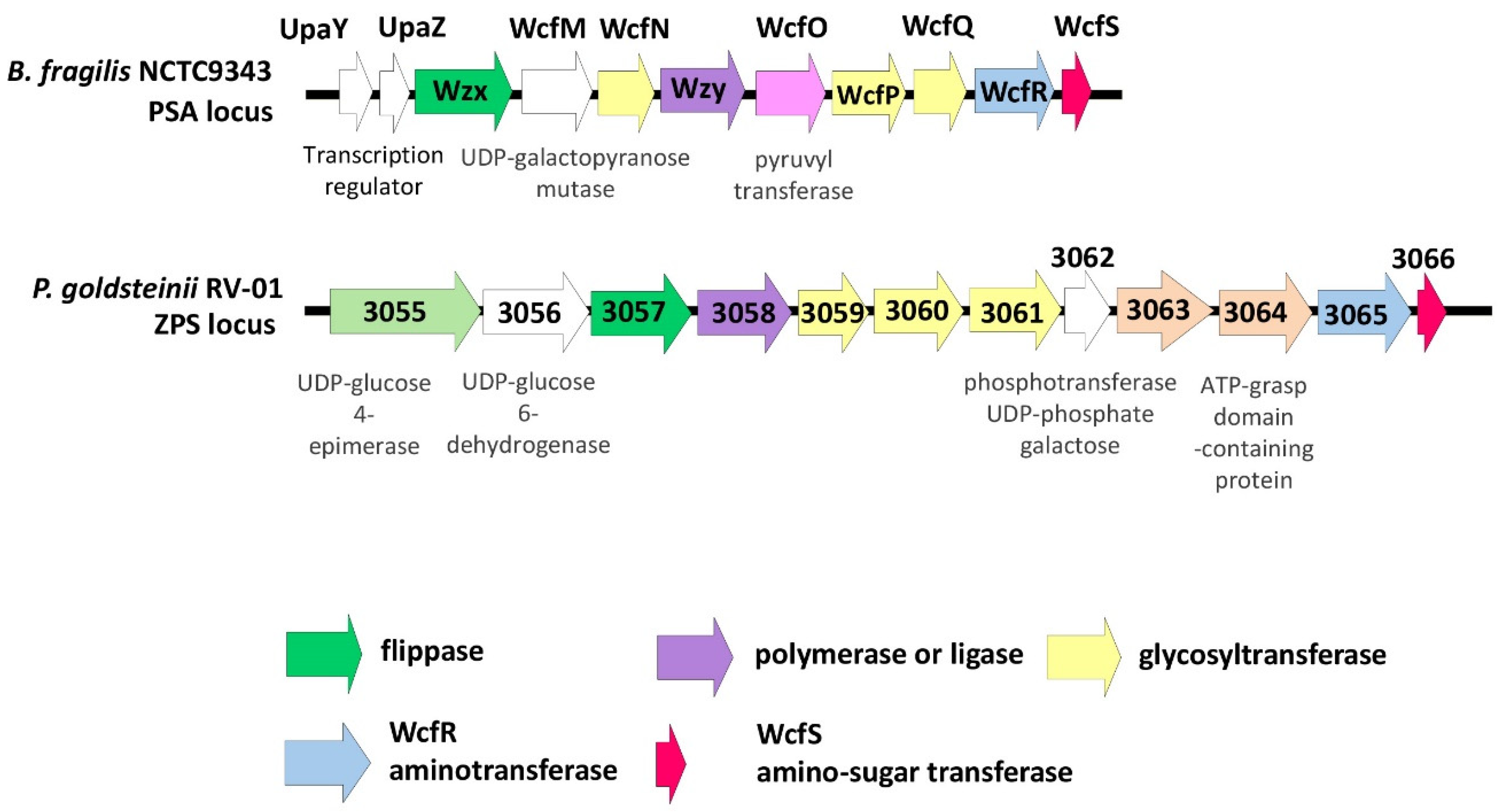
2.3.3. Bioinformatic Analyses for Zwitterionic Capsular Polysaccharide (ZPS)
2.3.4. Bioinformatic Analyses for Immune Regulatory Proteins
2.4. Toxicological Studies
2.4.1. HEK293 Cytotoxicity Assay
2.4.2. Bacterial Reverse Mutation Test (Ames Test)
2.4.3. In Vitro Mammalian Chromosomal Aberration Test
2.4.4. Rodent Micronucleus Test in Peripheral Blood
2.4.5. Twenty-Eight-Day Repeated Dose Oral Subacute Toxicity Study in Rats
2.4.6. Ninety-Day Repeated Dose Oral Subchronic Toxicity Study in Rats
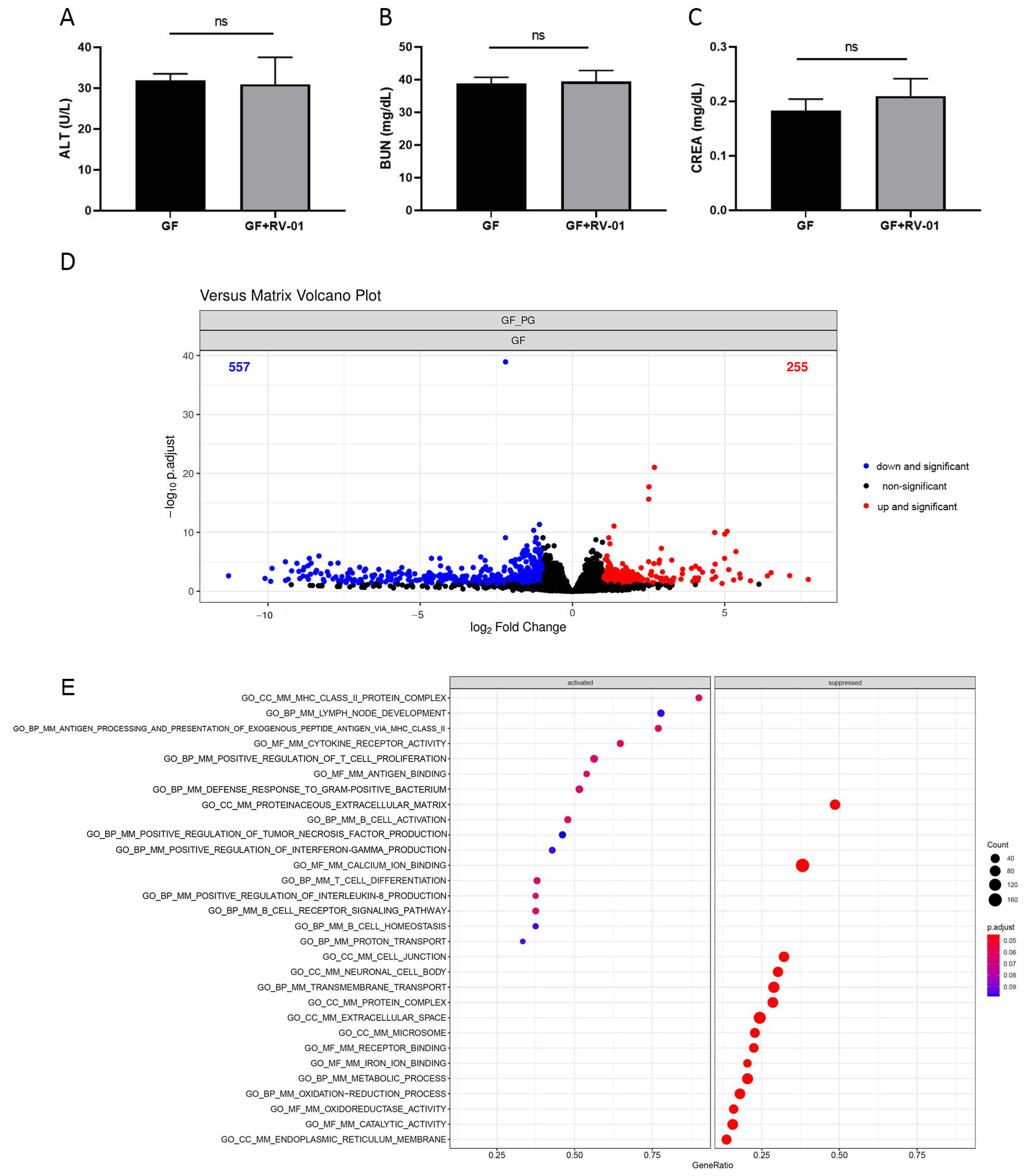
2.5. P. goldsteinii RV-01 Enhances Intestinal Immunity in Germ-Free (GF) Mice
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Isolation of P. goldsteinii RV-01
4.3. Production of P. goldsteinii RV-01 Ingredient
4.4. Genome Sequencing and Analysis
4.5. Determination of Minimum Inhibitory Concentration (MIC)
4.6. Quantification of Short-Chain Fatty Acid Production
4.7. Cell Activity
4.8. Cytotoxicity Assay
4.9. Toxicologic Studies
4.10. Colonization in Germ-Free Mice
4.11. Transcriptomic Analysis
4.12. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, Y.; Zhang, L.; Wang, X.; Yi, Y.; Shan, Y.; Liu, B.; Zhou, Y.; Lu, X. Roles of intestinal Parabacteroides in human health and diseases. FEMS Microbiol. Lett. 2022, 369, fnac072. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Tillotson, G.; MacKenzie, T.N.; Warren, C.A.; Wexler, H.M.; Goldstein, E.J.C. Bacteroides and related species: The keystone taxa of the human gut microbiota. Anaerobe 2024, 85, 102819. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.J.; Lin, T.L.; Tsai, Y.L.; Wu, T.R.; Lai, W.F.; Lu, C.C.; Lai, H.C. Next generation probiotics in disease amelioration. J. Food Drug Anal. 2019, 27, 615–622. [Google Scholar] [CrossRef]
- Ishioka, M.; Miura, K.; Minami, S.; Shimura, Y.; Ohnishi, H. Altered Gut Microbiota Composition and Immune Response in Experimental Steatohepatitis Mouse Models. Dig. Dis. Sci. 2017, 62, 396–406. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Xu, J.; Li, G.; Liu, T.; Guo, X.; Wang, H.; Luo, L. Ethanol extract of propolis prevents high-fat diet-induced insulin resistance and obesity in association with modulation of gut microbiota in mice. Food Res. Int. 2020, 130, 108939. [Google Scholar] [CrossRef]
- Chang, C.J.; Lin, C.S.; Lu, C.C.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Tseng, S.F.; Wu, T.R.; Chen, Y.Y.; Young, J.D.; et al. Ganoderma lucidum reduces obesity in mice by modulating the composition of the gut microbiota. Nat. Commun. 2015, 6, 7489. [Google Scholar] [CrossRef]
- Wu, T.R.; Lin, C.S.; Chang, C.J.; Lin, T.L.; Martel, J.; Ko, Y.F.; Ojcius, D.M.; Lu, C.C.; Young, J.D.; Lai, H.C. Gut commensal Parabacteroides goldsteinii plays a predominant role in the anti-obesity effects of polysaccharides isolated from Hirsutella sinensis. Gut 2019, 68, 248–262. [Google Scholar] [CrossRef]
- Awadel-Kariem, F.M.; Patel, P.; Kapoor, J.; Brazier, J.S.; Goldstein, E.J.C. First report of Parabacteroides goldsteinii bacteraemia in a patient with complicated intra-abdominal infection. Anaerobe 2010, 16, 223–225. [Google Scholar] [CrossRef]
- Lai, H.C.; Lin, T.L.; Chen, T.W.; Kuo, Y.L.; Chang, C.J.; Wu, T.R.; Shu, C.C.; Tsai, Y.H.; Swift, S.; Lu, C.C. Gut microbiota modulates COPD pathogenesis: Role of anti-inflammatory Parabacteroides goldsteinii lipopolysaccharide. Gut 2022, 71, 309–321. [Google Scholar] [CrossRef]
- Ishibashi, R.; Furusawa, Y.; Honda, H.; Watanabe, Y.; Fujisaka, S.; Nishikawa, M.; Ikushiro, S.; Kurihara, S.; Tabuchi, Y.; Tobe, K.; et al. Isoliquiritigenin Attenuates Adipose Tissue Inflammation and Metabolic Syndrome by Modifying Gut Bacteria Composition in Mice. Mol. Nutr. Food Res. 2022, 66, e2101119. [Google Scholar] [CrossRef]
- Ishibashi, R.; Matsuhisa, R.; Nomoto, M.; Chudan, S.; Nishikawa, M.; Tabuchi, Y.; Ikushiro, S.; Nagai, Y.; Furusawa, Y. Effect of Oral Administration of Polyethylene Glycol 400 on Gut Microbiota Composition and Diet-Induced Obesity in Mice. Microorganisms 2023, 11, 1882. [Google Scholar] [CrossRef] [PubMed]
- Gerkins, C.; Oliero, M.; Hajjar, R.; Vennin Rendos, H.; Fragoso, G.; Calvé, A.; Diop, K.; Routy, B.; Santos, M.M. A271 the modulation of intestinal inflammation by Parabacteroides goldsteinii in experimental murine colitis. J. Can. Assoc. Gastroenterol. 2023, 6, 86–87. [Google Scholar] [CrossRef]
- Lai, C.H.; Lin, T.L.; Huang, M.Z.; Li, S.W.; Wu, H.Y.; Chiu, Y.F.; Yang, C.Y.; Chiu, C.H.; Lai, H.C. Gut Commensal Parabacteroides goldsteinii MTS01 Alters Gut Microbiota Composition and Reduces Cholesterol to Mitigate Helicobacter pylori-Induced Pathogenesis. Front. Immunol. 2022, 13, 916848. [Google Scholar] [CrossRef]
- Lin, T.L.; Lu, C.C.; Chen, T.W.; Huang, C.W.; Lu, J.J.; Lai, W.F.; Wu, T.S.; Lai, C.H.; Lai, H.C.; Chen, Y.L. Amelioration of Maternal Immune Activation-Induced Autism Relevant Behaviors by Gut Commensal Parabacteroides goldsteinii. Int. J. Mol. Sci. 2022, 23, 13070. [Google Scholar] [CrossRef]
- Hajjar, R.; Gonzalez, E.; Fragoso, G.; Oliero, M.; Alaoui, A.A.; Calve, A.; Vennin Rendos, H.; Djediai, S.; Cuisiniere, T.; Laplante, P.; et al. Gut microbiota influence anastomotic healing in colorectal cancer surgery through modulation of mucosal proinflammatory cytokines. Gut 2023, 72, 1143–1154. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Fujii, T.; Yamakawa, S.; Yamada, C.; Fujiki, K.; Kondo, N.; Funasaka, K.; Hirooka, Y.; Tochio, T. Combined oral intake of short and long fructans alters the gut microbiota in food allergy model mice and contributes to food allergy prevention. BMC Microbiol. 2023, 23, 266. [Google Scholar] [CrossRef]
- Li, T.; Ding, N.; Guo, H.; Hua, R.; Lin, Z.; Tian, H.; Yu, Y.; Fan, D.; Yuan, Z.; Gonzalez, F.J.; et al. A gut microbiota-bile acid axis promotes intestinal homeostasis upon aspirin-mediated damage. Cell Host Microbe 2024, 32, 191–208 e199. [Google Scholar] [CrossRef]
- Chang, S.H.; Ko, Y.F.; Liau, J.C.; Wu, C.Y.; Hwang, T.L.; Ojcius, D.M.; Young, J.D.; Martel, J. Hirsutella sinensis polysaccharides and Parabacteroides goldsteinii reduce lupus severity in imiquimod-treated mice. Biomed. J. 2024, 100754. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ngo, V.L.; Zou, J.; Gewirtz, A.T. Commensal bacterial outer membrane protein A induces interleukin-22 production. Cell Rep. 2024, 43, 114292. [Google Scholar] [CrossRef]
- Cervantes-Barragan, L.; Colonna, M. A microbiota-derived antigen drives CD4(+) intraepithelial lymphocyte (CD4IEL) development. Trends Immunol. 2022, 43, 858–860. [Google Scholar] [CrossRef]
- Bousbaine, D.; Fisch, L.I.; London, M.; Bhagchandani, P.; Rezende de Castro, T.B.; Mimee, M.; Olesen, S.; Reis, B.S.; VanInsberghe, D.; Bortolatto, J.; et al. A conserved Bacteroidetes antigen induces anti-inflammatory intestinal T lymphocytes. Science 2022, 377, 660–666. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhang, X.; Fu, J.; Duan, Z.; Qiu, W.; Cai, Y.; Ma, W.; Zhou, H.; Chen, Y.; Zheng, J.; et al. Sex- and Age-Dependent Associations between Parabacteroides and Obesity: Evidence from Two Population Cohort. Microorganisms 2023, 11, 2087. [Google Scholar] [CrossRef] [PubMed]
- Bharti, B.; Li, H.; Ren, Z.; Zhu, R.; Zhu, Z. Recent advances in sterilization and disinfection technology: A review. Chemosphere 2022, 308, 136404. [Google Scholar] [CrossRef]
- Part 170—Food additives. §170.22—Safety factors to be considered. In US Code of Federal Regulations (CFR); Title 21: Food and drugs; Food and Drug Administration: Washington, DC, USA; US Government Printing Office (GPO): Washington, DC, USA, 2024. [Google Scholar]
- Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes. Off. J. Eur. Union 2020, 53, 33–79.
- Sakamoto, M.; Benno, Y. Reclassification of Bacteroides distasonis, Bacteroides goldsteinii and Bacteroides merdae as Parabacteroides distasonis gen. nov., comb. nov., Parabacteroides goldsteinii comb. nov. and Parabacteroides merdae comb. nov. Int. J. Syst. Evol. Microbiol. 2006, 56, 1599–1605. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Lim, J.; Kwon, S.; Chun, J. A large-scale evaluation of algorithms to calculate average nucleotide identity. Antonie Van Leeuwenhoek 2017, 110, 1281–1286. [Google Scholar] [CrossRef]
- Additives, E.P.o.; Feed, P.o.S.u.i.A. Guidance on the assessment of bacterial susceptibility to antimicrobials of human and veterinary importance. EFSA J. 2012, 10, 2740. [Google Scholar] [CrossRef]
- Chen, L.; Yang, J.; Yu, J.; Yao, Z.; Sun, L.; Shen, Y.; Jin, Q. VFDB: A reference database for bacterial virulence factors. Nucleic Acids Res. 2005, 33, D325–D328. [Google Scholar] [CrossRef] [PubMed]
- Alcock, B.P.; Raphenya, A.R.; Lau, T.T.Y.; Tsang, K.K.; Bouchard, M.; Edalatmand, A.; Huynh, W.; Nguyen, A.V.; Cheng, A.A.; Liu, S.; et al. CARD 2020: Antibiotic resistome surveillance with the comprehensive antibiotic resistance database. Nucleic Acids Res. 2020, 48, D517–D525. [Google Scholar] [CrossRef]
- European Food Safety, A. EFSA statement on the requirements for whole genome sequence analysis of microorganisms intentionally used in the food chain. EFSA J. 2021, 19, e06506. [Google Scholar] [CrossRef]
- Fiers, M.W.; Kleter, G.A.; Nijland, H.; Peijnenburg, A.A.; Nap, J.P.; van Ham, R.C. Allermatch, a webtool for the prediction of potential allergenicity according to current FAO/WHO Codex alimentarius guidelines. BMC Bioinform. 2004, 5, 133. [Google Scholar] [CrossRef] [PubMed]
- Pascale, A.; Marchesi, N.; Marelli, C.; Coppola, A.; Luzi, L.; Govoni, S.; Giustina, A.; Gazzaruso, C. Microbiota and metabolic diseases. Endocrine 2018, 61, 357–371. [Google Scholar] [CrossRef] [PubMed]
- Cantarel, B.L.; Coutinho, P.M.; Rancurel, C.; Bernard, T.; Lombard, V.; Henrissat, B. The Carbohydrate-Active EnZymes database (CAZy): An expert resource for Glycogenomics. Nucleic Acids Res. 2009, 37, D233–D238. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Yohe, T.; Huang, L.; Entwistle, S.; Wu, P.; Yang, Z.; Busk, P.K.; Xu, Y.; Yin, Y. dbCAN2: A meta server for automated carbohydrate-active enzyme annotation. Nucleic Acids Res. 2018, 46, W95–W101. [Google Scholar] [CrossRef]
- Raetz, C.R.; Reynolds, C.M.; Trent, M.S.; Bishop, R.E. Lipid A modification systems in gram-negative bacteria. Annu. Rev. Biochem. 2007, 76, 295–329. [Google Scholar] [CrossRef]
- Erturk-Hasdemir, D.; Kasper, D.L. Finding a needle in a haystack: Bacteroides fragilis polysaccharide A as the archetypical symbiosis factor. Ann. N. Y. Acad. Sci. 2018, 1417, 116–129. [Google Scholar] [CrossRef]
- Neff, C.P.; Rhodes, M.E.; Arnolds, K.L.; Collins, C.B.; Donnelly, J.; Nusbacher, N.; Jedlicka, P.; Schneider, J.M.; McCarter, M.D.; Shaffer, M.; et al. Diverse Intestinal Bacteria Contain Putative Zwitterionic Capsular Polysaccharides with Anti-inflammatory Properties. Cell Host Microbe 2016, 20, 535–547. [Google Scholar] [CrossRef]
- Arnolds, K.L.; Yamada, E.; Neff, C.P.; Schneider, J.M.; Palmer, B.E.; Lozupone, C.A. Disruption of Genes Encoding Putative Zwitterionic Capsular Polysaccharides of Diverse Intestinal Bacteroides Reduces the Induction of Host Anti-Inflammatory Factors. Microb. Ecol. 2023, 85, 1620–1629. [Google Scholar] [CrossRef]
- OECD. Test. No. 471: Bacterial Reverse Mutation Test; OECD: Paris, France, 2020. [Google Scholar] [CrossRef]
- OECD. Test. No. 473: In Vitro Mammalian Chromosomal Aberration Test; OECD: Paris, France, 2016. [Google Scholar] [CrossRef]
- OECD. Test. No. 474: Mammalian Erythrocyte Micronucleus Test; OECD: Paris, France, 2014. [Google Scholar] [CrossRef]
- OECD. Test. No. 407: Repeated Dose 28-day Oral. Toxicity Study in Rodents; OECD: Paris, France, 2008. [Google Scholar] [CrossRef]
- OECD. Test. No. 408: Repeated Dose 90-Day Oral. Toxicity Study in Rodents; OECD: Paris, France, 2018. [Google Scholar] [CrossRef]
- Muller, P.A.; Schneeberger, M.; Matheis, F.; Wang, P.; Kerner, Z.; Ilanges, A.; Pellegrino, K.; Del Marmol, J.; Castro, T.B.R.; Furuichi, M.; et al. Microbiota modulate sympathetic neurons via a gut-brain circuit. Nature 2020, 583, 441–446. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- US FDA. Guidance for Industry and Other Stakeholders: Toxicological Principles for the Safety Assessment of Food Ingredients: Redbook 2000 [Updated to July 2007]; US Food and Drug Administration (US FDA), Center for Food Safety and Applied Nutrition (CFSAN): Silver Spring, MD, USA, 2020. [Google Scholar]
- Douillard, F.P.; de Vos, W.M. Functional genomics of lactic acid bacteria: From food to health. Microb. Cell Fact. 2014, 13 (Suppl. S1), S8. [Google Scholar] [CrossRef] [PubMed]
- O’Toole, P.W.; Marchesi, J.R.; Hill, C. Next-generation probiotics: The spectrum from probiotics to live biotherapeutics. Nat. Microbiol. 2017, 2, 17057. [Google Scholar] [CrossRef] [PubMed]
- Kumari, M.; Singh, P.; Nataraj, B.H.; Kokkiligadda, A.; Naithani, H.; Azmal Ali, S.; Behare, P.V.; Nagpal, R. Fostering next-generation probiotics in human gut by targeted dietary modulation: An emerging perspective. Food Res. Int. 2021, 150, 110716. [Google Scholar] [CrossRef] [PubMed]
- Vallianou, N.G.; Kounatidis, D.; Tsilingiris, D.; Panagopoulos, F.; Christodoulatos, G.S.; Evangelopoulos, A.; Karampela, I.; Dalamaga, M. The Role of Next-Generation Probiotics in Obesity and Obesity-Associated Disorders: Current Knowledge and Future Perspectives. Int. J. Mol. Sci. 2023, 24, 6755. [Google Scholar] [CrossRef] [PubMed]
- Shetty, S.A.; Zuffa, S.; Bui, T.P.N.; Aalvink, S.; Smidt, H.; De Vos, W.M. Reclassification of Eubacterium hallii as Anaerobutyricum hallii gen. nov., comb. nov., and description of Anaerobutyricum soehngenii sp. nov., a butyrate and propionate-producing bacterium from infant faeces. Int. J. Syst. Evol. Microbiol. 2018, 68, 3741–3746. [Google Scholar] [CrossRef]
- Martin, R.; Rios-Covian, D.; Huillet, E.; Auger, S.; Khazaal, S.; Bermudez-Humaran, L.G.; Sokol, H.; Chatel, J.M.; Langella, P. Faecalibacterium: A bacterial genus with promising human health applications. FEMS Microbiol. Rev. 2023, 47, fuad039. [Google Scholar] [CrossRef]
- Li, L.; Li, M.; Chen, Y.; Yu, Z.; Cheng, P.; Yu, Z.; Cheng, W.; Zhang, W.; Wang, Z.; Gao, X.; et al. Function and therapeutic prospects of next-generation probiotic Akkermansia muciniphila in infectious diseases. Front. Microbiol. 2024, 15, 1354447. [Google Scholar] [CrossRef]
- Ignatyeva, O.; Tolyneva, D.; Kovalyov, A.; Matkava, L.; Terekhov, M.; Kashtanova, D.; Zagainova, A.; Ivanov, M.; Yudin, V.; Makarov, V.; et al. Christensenella minuta, a new candidate next-generation probiotic: Current evidence and future trajectories. Front. Microbiol. 2023, 14, 1241259. [Google Scholar] [CrossRef]
- Zhang, H.; Duan, Y.; Cai, F.; Cao, D.; Wang, L.; Qiao, Z.; Hong, Q.; Li, N.; Zheng, Y.; Su, M.; et al. Next-Generation Probiotics: Microflora Intervention to Human Diseases. Biomed. Res. Int. 2022, 2022, 5633403. [Google Scholar] [CrossRef]
- Kazmierczak-Siedlecka, K.; Skonieczna-Zydecka, K.; Hupp, T.; Duchnowska, R.; Marek-Trzonkowska, N.; Polom, K. Next-generation probiotics-do they open new therapeutic strategies for cancer patients? Gut Microbes 2022, 14, 2035659. [Google Scholar] [CrossRef]
- Al-Fakhrany, O.M.; Elekhnawy, E. Next-generation probiotics: The upcoming biotherapeutics. Mol. Biol. Rep. 2024, 51, 505. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhao, L.; Islam, M.Z.; Pham, L.D.; Corsi, M.S.; Xavier, R.J.; Benoist, C.; Kostic, A.D. Extracellular Vesicles from a Gut Symbiont Mediate Adenosinergic Responses to Promote Immune Tolerance. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Pique, N.; Berlanga, M.; Minana-Galbis, D. Health Benefits of Heat-Killed (Tyndallized) Probiotics: An Overview. Int. J. Mol. Sci. 2019, 20, 2534. [Google Scholar] [CrossRef]
- EFSA Panel on Dietetic Products, Nutrition and Allergies (NDA). Scientific Opinion on the safety of ‘heat-treated milk products fermented with Bacteroides xylanisolvens DSM 23964’ as a novel food. EFSA J. 2015, 13, 3956. [Google Scholar] [CrossRef]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Castenmiller, J.; De Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Safety of heat-killed Mycobacterium setense manresensis as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2019, 17, e05824. [Google Scholar] [CrossRef]
- Ghaderi, F.; Sotoodehnejadnematalahi, F.; Hajebrahimi, Z.; Fateh, A.; Siadat, S.D. Effects of active, inactive, and derivatives of Akkermansia muciniphila on the expression of the endocannabinoid system and PPARs genes. Sci. Rep. 2022, 12, 10031. [Google Scholar] [CrossRef]
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667. [Google Scholar] [CrossRef]
- Pena-Cearra, A.; Palacios, A.; Pellon, A.; Castelo, J.; Pasco, S.T.; Seoane, I.; Barriales, D.; Martin, J.E.; Pascual-Itoiz, M.A.; Gonzalez-Lopez, M.; et al. Akkermansia muciniphila-induced trained immune phenotype increases bacterial intracellular survival and attenuates inflammation. Commun. Biol. 2024, 7, 192. [Google Scholar] [CrossRef]
- Yoon, S.A.; Lim, Y.; Byeon, H.R.; Jung, J.; Ma, S.; Hong, M.G.; Kim, D.; Song, E.J.; Nam, Y.D.; Seo, J.G.; et al. Heat-killed Akkermansia muciniphila ameliorates allergic airway inflammation in mice. Front. Microbiol. 2024, 15, 1386428. [Google Scholar] [CrossRef]
- Maehata, H.; Arai, S.; Iwabuchi, N.; Abe, F. Immuno-modulation by heat-killed Lacticaseibacillus paracasei MCC1849 and its application to food products. Int. J. Immunopathol. Pharmacol. 2021, 35, 20587384211008291. [Google Scholar] [CrossRef]
- Wang, S.; Ahmadi, S.; Nagpal, R.; Jain, S.; Mishra, S.P.; Kavanagh, K.; Zhu, X.; Wang, Z.; McClain, D.A.; Kritchevsky, S.B.; et al. Lipoteichoic acid from the cell wall of a heat killed Lactobacillus paracasei D3-5 ameliorates aging-related leaky gut, inflammation and improves physical and cognitive functions: From C. elegans to mice. Geroscience 2020, 42, 333–352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Pu, F.; Cheng, R.; Guo, J.; Shen, X.; Wang, S.; Zhu, H.; Zhang, X.; Cheng, G.; Li, M.; et al. Effect of heat-inactivated Lactobacillus paracasei N1115 on microbiota and gut-brain axis related molecules. Biosci. Microbiota Food Health 2020, 39, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Nishida, K.; Sawada, D.; Kuwano, Y.; Tanaka, H.; Rokutan, K. Health Benefits of Lactobacillus gasseri CP2305 Tablets in Young Adults Exposed to Chronic Stress: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients 2019, 11, 1859. [Google Scholar] [CrossRef] [PubMed]
- Maehata, H.; Kobayashi, Y.; Mitsuyama, E.; Kawase, T.; Kuhara, T.; Xiao, J.Z.; Tsukahara, T.; Toyoda, A. Heat-killed Lactobacillus helveticus strain MCC1848 confers resilience to anxiety or depression-like symptoms caused by subchronic social defeat stress in mice. Biosci. Biotechnol. Biochem. 2019, 83, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Kambe, J.; Watcharin, S.; Makioka-Itaya, Y.; Inoue, R.; Watanabe, G.; Yamaguchi, H.; Nagaoka, K. Heat-killed Enterococcus fecalis (EC-12) supplement alters the expression of neurotransmitter receptor genes in the prefrontal cortex and alleviates anxiety-like behavior in mice. Neurosci. Lett. 2020, 720, 134753. [Google Scholar] [CrossRef]
- Landete, J.M.; Gaya, P.; Rodriguez, E.; Langa, S.; Peiroten, A.; Medina, M.; Arques, J.L. Probiotic Bacteria for Healthier Aging: Immunomodulation and Metabolism of Phytoestrogens. Biomed. Res. Int. 2017, 2017, 5939818. [Google Scholar] [CrossRef]
- ISO 19344:2015; Milk and Milk Products—Starter Cultures, Probiotics and Fermented Products—Quantification of Lactic Acid Bacteria by Flow Cytometry. International Organization for Standardization (ISO): Geneva, Switzerland, 2015.
- Foglia, C.; Allesina, S.; Amoruso, A.; De Prisco, A.; Pane, M. New insights in enumeration methodologies of probiotic cells in finished products. J. Microbiol. Methods 2020, 175, 105993. [Google Scholar] [CrossRef]
- Caron, G.N.V.; Badley, R.A. Viability assessment of bacteria in mixed populations using flow cytometry. J. Microsc. 1995, 179, 55–66. [Google Scholar] [CrossRef]
- Koren, S.; Walenz, B.P.; Berlin, K.; Miller, J.R.; Bergman, N.H.; Phillippy, A.M. Canu: Scalable and accurate long-read assembly via adaptive k-mer weighting and repeat separation. Genome Res. 2017, 27, 722–736. [Google Scholar] [CrossRef]
- Kolmogorov, M.; Yuan, J.; Lin, Y.; Pevzner, P.A. Assembly of long, error-prone reads using repeat graphs. Nat. Biotechnol. 2019, 37, 540–546. [Google Scholar] [CrossRef]
- Vaser, R.; Sovic, I.; Nagarajan, N.; Sikic, M. Fast and accurate de novo genome assembly from long uncorrected reads. Genome Res. 2017, 27, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.T.; Liu, P.Y.; Shih, P.W. Homopolish: A method for the removal of systematic errors in nanopore sequencing by homologous polishing. Genome Biol. 2021, 22, 95. [Google Scholar] [CrossRef] [PubMed]
- Seemann, T. Prokka: Rapid prokaryotic genome annotation. Bioinformatics 2014, 30, 2068–2069. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]

| Parameter | Typical Range | Method of Analysis |
|---|---|---|
| Protein | 2.5–3.0 g/100 g | AOAC 992.15 (1992) |
| Fat | 0.4–0.9 g/100 g | CNS5036 |
| Ash | 0.2–0.3 g/100 g | CNS5034 |
| Carbohydrates | 95.1–96.3 g/100 g | 100 − (protein + fat + moisture + ash) |
| Energy value | 397.8–401.7 Kcal/100 g | [4 × carbohydrates + 4 × protein + 9 × fat] Kcal |
| ANI Value (%) | ||||||
|---|---|---|---|---|---|---|
| P. goldsteinii MTS01 | P. goldsteinii DSM19448 | P. distasonis ATCC8503 | P. merdae ATCC43184 | P. gordonii DSM23371 | P. faecis DSM102983 | |
| P. goldsteinii RV-01 | 98.17 | 98.44 | 73.83 | 76.77 | 82.57 | 83.96 |
| Antibiotic Agent | MIC Range (μg/mL) | CLSI 2020 MIC Breakpoint for S (μg/mL) | |||||
|---|---|---|---|---|---|---|---|
| B. fragilis ATCC25285 | P. goldsteinii RV-01 | P. goldsteinii MTS01 | P. goldsteinii JCM13446 | P. distasonis ATCC8503 | P. merdae ATCC43184 | ||
| Ampicillin | 128 | 4 | 16 | 16 | 2 | 4 | ≤0.5 |
| Vancomycin | 32 | 16 | >256 | 32 | >256 | 32 | NA |
| Gentamycin | >256 | >256 | >256 | >256 | >256 | >256 | NA |
| Kanamycin | >256 | >256 | >256 | >256 | >256 | >256 | NA |
| Streptomycin | >256 | >256 | >256 | >256 | >256 | >256 | NA |
| Erythromycin | 8 | 8 | 8 | 8 | 1 | 8 | NA |
| Clindamycin | 0.5 | 2 | 4 | 0.25 | <0.125 | 0.25 | ≤2 |
| Tetracycline | 0.25 | 4 | 0.5 | 0.25 | 0.25 | 8 | ≤4 |
| Chloramphenicol | 8 | 4 | 8 | 8 | 4 | 4 | ≤8 |
| Tylosine | 2 | 0.5 | 1 | 0.5 | 0.5 | 0.5 | NA |
| Apramycin | >256 | >256 | >256 | >256 | >256 | >256 | NA |
| Nalidixic acid | 256 | 128 | 128 | 128 | 128 | 128 | NA |
| Sulfonamide | >256 | >256 | >256 | >256 | >256 | >256 | NA |
| Trimethoprim | 32 | >256 | >256 | >256 | 128 | >256 | NA |
| Test | Type of Study | Test System | Dose |
|---|---|---|---|
| 1 | Bacterial reverse mutation test (GLP, OECD test No. 471) [40] | S. Typhimurium TA97a, TA98, TA100, TA102, and TA1535 | Up to 5 mg/plate (in absence and presence of S9 mix) |
| 2 | In vitro mammalian chromosomal aberration test (GLP, OECD test No. 473) [41] | Chinese hamster ovary cells (CHO-K1, BCRC No. 60006) | Up to 5 mg/mL for 3 h (in the absence and presence of S9 mix); up to 5 mg/mL for 18 h (in the absence of S9 mix) |
| 3 | Rodent micronucleus test in peripheral blood (GLP, OECD test No. 474) [42] | Imprinting control region mice (ICR mice) | Up to 2000 mg/kg |
| 4 | 28-day repeated dose oral toxicity (GLP, OECD 407) [43] | Sprague–Dawley rats | Up to 1500 mg/kg bw per day (8.109 × 1010 TFU/kg bw per day) |
| 5 | 90-day repeated dose oral toxicity (GLP, OECD TG408) [44] | Sprague–Dawley rats | Up to 1500 mg/kg bw per day (8.109 × 1010 TFU/kg bw per day) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, T.-L.; Chen, W.-J.; Hung, C.-M.; Wong, Y.-L.; Lu, C.-C.; Lai, H.-C. Characterization and Safety Evaluation of Autoclaved Gut Commensal Parabacteroides goldsteinii RV-01. Int. J. Mol. Sci. 2024, 25, 12660. https://doi.org/10.3390/ijms252312660
Lin T-L, Chen W-J, Hung C-M, Wong Y-L, Lu C-C, Lai H-C. Characterization and Safety Evaluation of Autoclaved Gut Commensal Parabacteroides goldsteinii RV-01. International Journal of Molecular Sciences. 2024; 25(23):12660. https://doi.org/10.3390/ijms252312660
Chicago/Turabian StyleLin, Tzu-Lung, Wan-Jiun Chen, Chien-Min Hung, Yea-Lin Wong, Chia-Chen Lu, and Hsin-Chih Lai. 2024. "Characterization and Safety Evaluation of Autoclaved Gut Commensal Parabacteroides goldsteinii RV-01" International Journal of Molecular Sciences 25, no. 23: 12660. https://doi.org/10.3390/ijms252312660
APA StyleLin, T.-L., Chen, W.-J., Hung, C.-M., Wong, Y.-L., Lu, C.-C., & Lai, H.-C. (2024). Characterization and Safety Evaluation of Autoclaved Gut Commensal Parabacteroides goldsteinii RV-01. International Journal of Molecular Sciences, 25(23), 12660. https://doi.org/10.3390/ijms252312660




