Spent Brewer’s Yeast Lysis Enables a Best Out of Waste Approach in the Beer Industry
Abstract
1. Introduction
2. Spent Brewer’s Yeast Composition
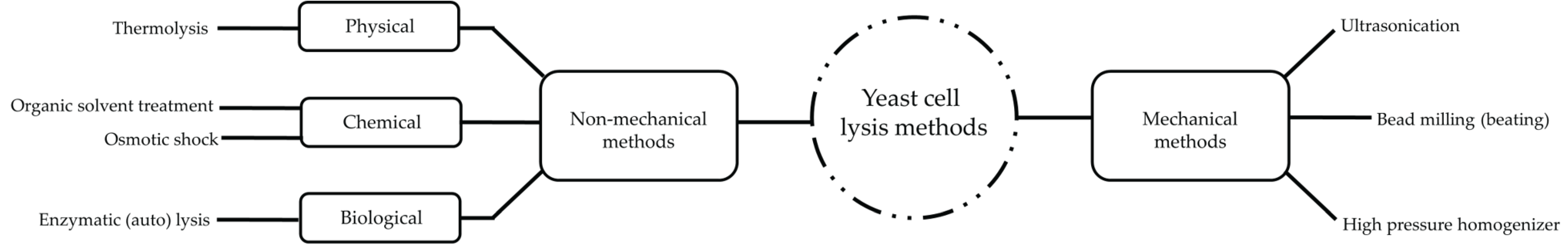
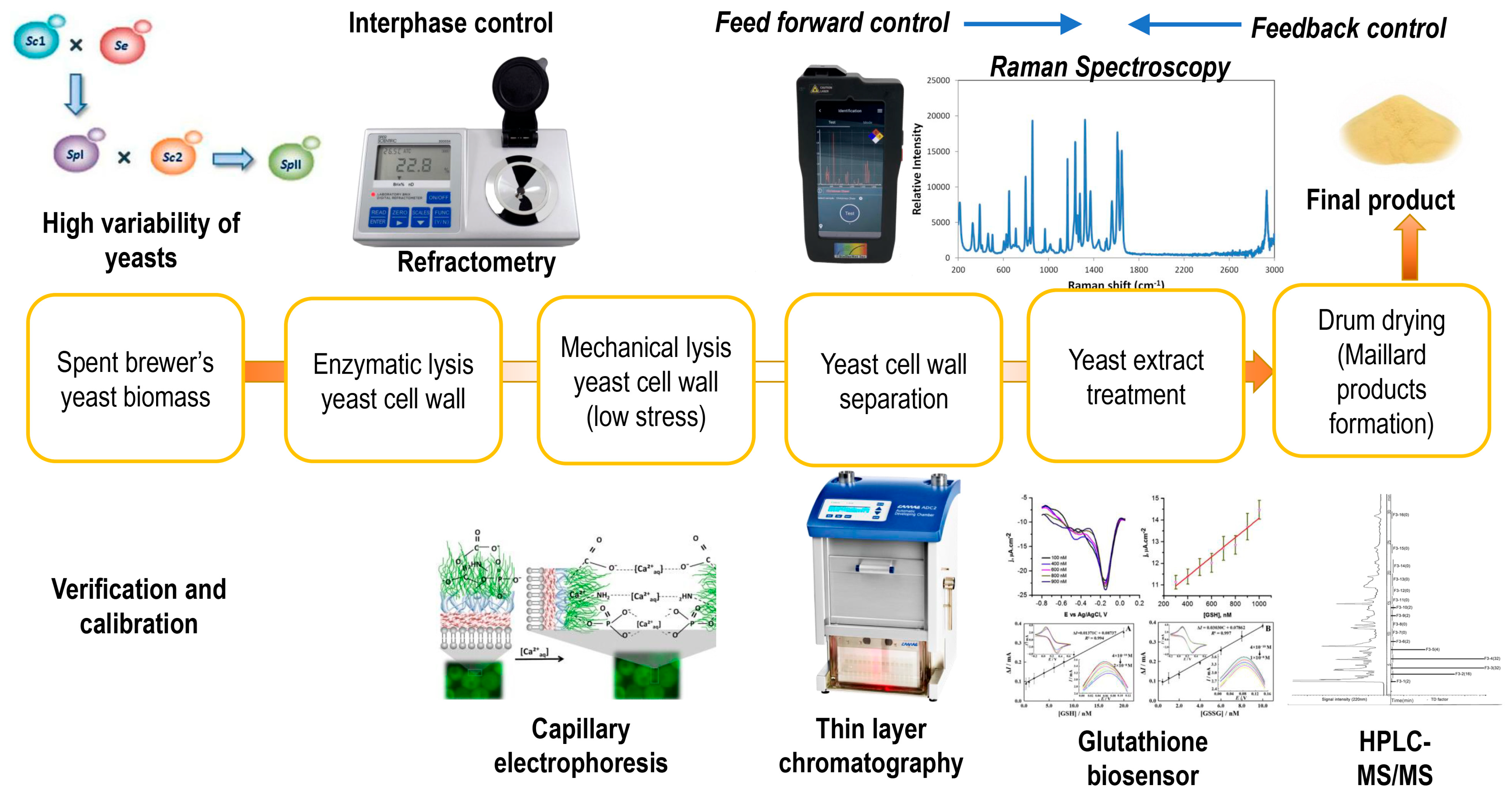
3. Spent Brewer’s Yeast Cell Lysis Methods
3.1. Non-Mechanical Lysis Methods
3.1.1. Autolysis
3.1.2. High-Temperature Lysis
3.1.3. Plasmolysis
3.1.4. Pulsed Electric Field Lysis
3.1.5. Enzymatic Hydrolysis
3.2. Mechanical Lysis Methods
3.2.1. Ultrasonication
3.2.2. Bead Milling (Beating)
3.2.3. High-Pressure Homogenization
3.3. Cascade and Combined Lysis Experiments for Disruption of Spent Brewer’s Yeast Cells
4. Bioactive Compounds from Yeast Cell Walls
4.1. Applications and Properties of YCW Components
4.1.1. Yeast β-Glucan Properties and Applications
4.1.2. Yeast Mannoprotein-Properties and Applications
4.1.3. Yeast Chitin–Properties and Applications
4.1.4. Potential Applications of Yeast Cell Walls
5. Yeast Extract
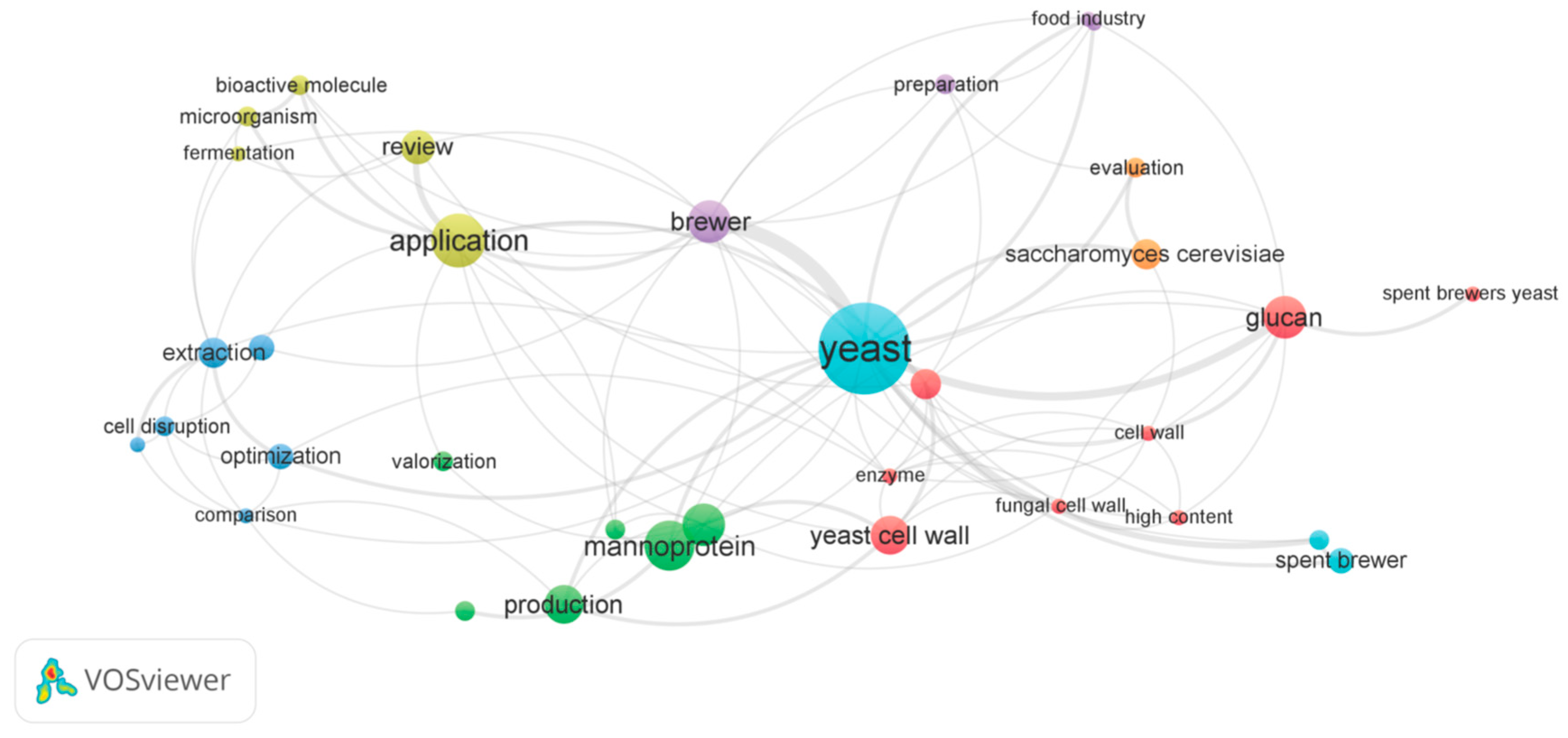
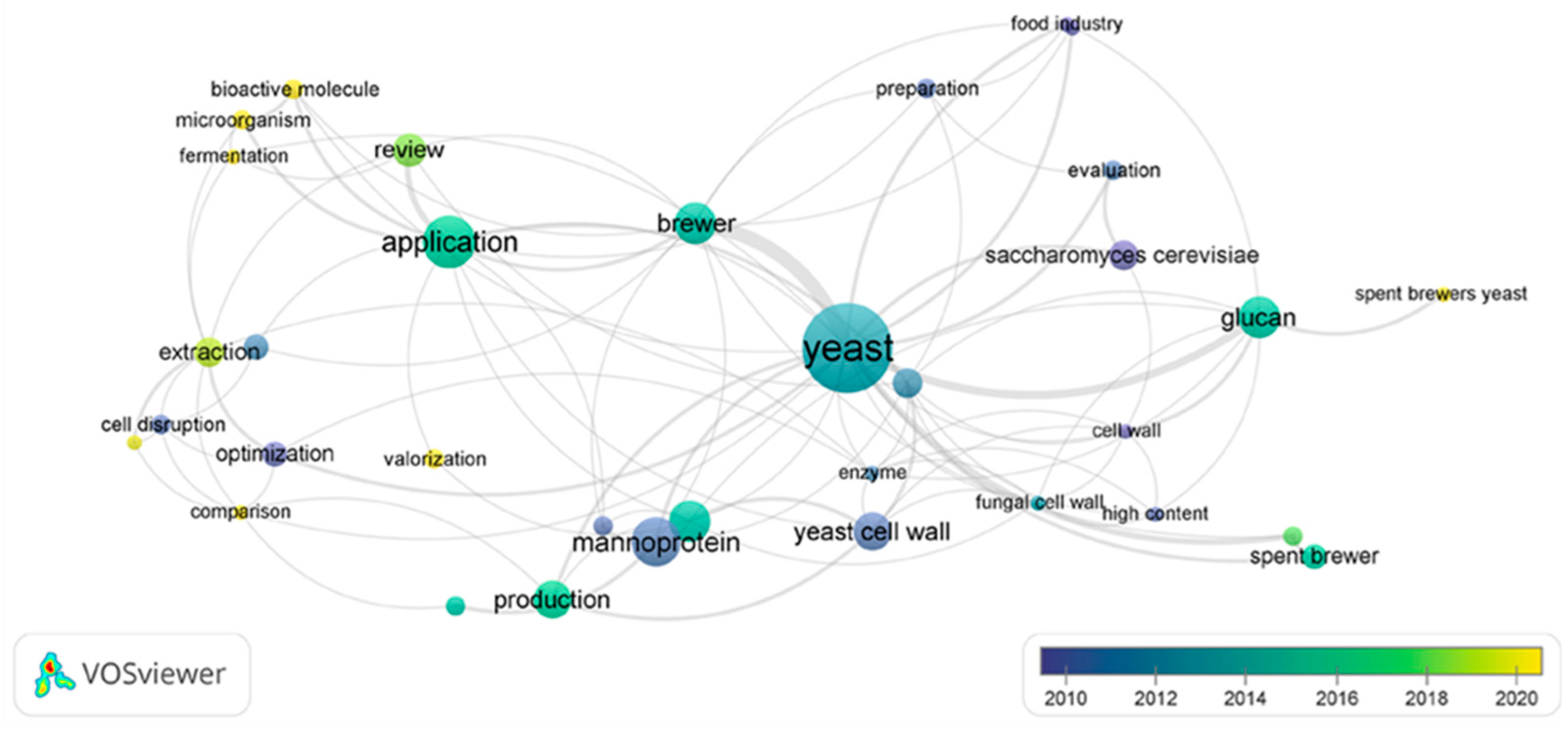
6. Network Bibliometric Analysis of the Main Keywords
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Peyrat, L.-A.; Tsafantakis, N.; Georgousaki, K.; Ouazzani, J.; Genilloud, O.; Trougakos, I.P.; Fokialakis, N. Terrestrial Microorganisms: Cell Factories of Bioactive Molecules with Skin Protecting Applications. Molecules 2019, 24, 1836. [Google Scholar] [CrossRef] [PubMed]
- Rani, A.; Saini, K.C.; Bast, F.; Mehariya, S.; Bhatia, S.K.; Lavecchia, R.; Zuorro, A. Microorganisms: A Potential Source of Bioactive Molecules for Antioxidant Applications. Molecules 2021, 26, 1142. [Google Scholar] [CrossRef] [PubMed]
- Perricone, M.; Gallo, M.; Corbo, M.R.; Sinigaglia, M.; Bevilacqua, A. Chapter 5—Yeasts. In The Microbiological Quality of Food; Bevilacqua, A., Corbo, M.R., Sinigaglia, M., Eds.; Woodhead Publishing: Sawston, UK, 2017; pp. 121–131. [Google Scholar]
- Liu, D.; Ding, L.; Sun, J.; Boussetta, N.; Vorobiev, E. Yeast cell disruption strategies for recovery of intracellular bio-active compounds—A review. Innov. Food Sci. Emerg. Technol. 2016, 36, 181–192. [Google Scholar] [CrossRef]
- Jon, C. Beer Production Worldwide from 1998 to 2023. Available online: https://www.statista.com/statistics/270275/worldwide-beer-production/ (accessed on 12 November 2024).
- Ferreira, I.; Pinho, O.; Vieira, E.; Tavarela, J. Brewer’s Saccharomyces yeast biomass: Characteristics and potential applications. Trends Food Sci. Technol. 2010, 21, 77–84. [Google Scholar] [CrossRef]
- Marson, G.V.; de Castro, R.J.S.; Belleville, M.P.; Hubinger, M.D. Spent brewer’s yeast as a source of high added value molecules: A systematic review on its characteristics, processing and potential applications. World J. Microbiol. Biotechnol. 2020, 36, 95. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Ferreira, C.; Pereira, J.O.; Pintado, M.E.; Carvalho, A.P. Valorisation of protein-rich extracts from spent brewer’s yeast (Saccharomyces cerevisiae): An overview. Biomass Convers. Biorefinery 2022. [Google Scholar] [CrossRef]
- San Martin, D.; Ibarruri, J.; Iñarra, B.; Luengo, N.; Ferrer, J.; Alvarez-Ossorio, C.; Bald, C.; Gutierrez, M.; Zufía, J. Valorisation of Brewer’s Spent Yeasts’ Hydrolysates as High-Value Bioactive Molecules. Sustainability 2021, 13, 6520. [Google Scholar] [CrossRef]
- Jaeger, A.; Arendt, E.; Zannini, E.; Sahin, A. Brewer’s Spent Yeast (BSY), an Underutilized Brewing By-Product. Fermentation 2020, 6, 123. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Ferreira, C.; Pereira, J.O.; Pintado, M.E.; Carvalho, A.P. Spent brewer’s yeast (Saccharomyces cerevisiae) as a potential source of bioactive peptides: An overview. Int. J. Biol. Macromol. 2022, 208, 1116–1126. [Google Scholar] [CrossRef]
- Censi, R.; Vargas Peregrina, D.; Gigliobianco, M.R.; Lupidi, G.; Angeloni, C.; Pruccoli, L.; Tarozzi, A.; Di Martino, P. New antioxidant ingredients from brewery by-products for cosmetic formulations. Cosmetics 2021, 8, 96. [Google Scholar] [CrossRef]
- Velez-Erazo, E.M.; Saturno, R.P.; Marson, G.V.; Hubinger, M.D. Spent brewer’s yeast proteins and cell debris as innovative emulsifiers and carrier materials for edible oil microencapsulation. Food Res. Int. 2021, 140, 109853. [Google Scholar] [CrossRef] [PubMed]
- Raikos, V.; Grant, S.B.; Hayes, H.; Ranawana, V. Use of β-glucan from spent brewer’s yeast as a thickener in skimmed yogurt: Physicochemical, textural, and structural properties related to sensory perception. J. Dairy Sci. 2018, 101, 5821–5831. [Google Scholar] [CrossRef] [PubMed]
- Sousa, P.; Tavares-Valente, D.; Pereira, C.F.; Pinto-Ribeiro, I.; Azevedo-Silva, J.; Madureira, R.; Ramos, Ó.L.; Pintado, M.; Fernandes, J.; Amorim, M. Circular economyeast: Saccharomyces cerevisiae as a sustainable source of glucans and its safety for skincare application. Int. J. Biol. Macromol. 2024, 265, 130933. [Google Scholar] [CrossRef] [PubMed]
- Capece, A.; Romaniello, R.; Siesto, G.; Romano, P. Conventional and Non-Conventional Yeasts in Beer Production. Fermentation 2018, 4, 38. [Google Scholar] [CrossRef]
- Gallone, B.; Steensels, J.; Prahl, T.; Soriaga, L.; Saels, V.; Herrera-Malaver, B.; Merlevede, A.; Roncoroni, M.; Voordeckers, K.; Miraglia, L. Domestication and divergence of Saccharomyces cerevisiae beer yeasts. Cell 2016, 166, 1397–1410.e16. [Google Scholar] [CrossRef]
- Okuno, M.; Kajitani, R.; Ryusui, R.; Morimoto, H.; Kodama, Y.; Itoh, T. Next-generation sequencing analysis of lager brewing yeast strains reveals the evolutionary history of interspecies hybridization. DNA Res. 2016, 23, 67–80. [Google Scholar] [CrossRef]
- Basso, R.F.; Alcarde, A.R.; Portugal, C.B. Could non-Saccharomyces yeasts contribute on innovative brewing fermentations? Food Res. Int. 2016, 86, 112–120. [Google Scholar] [CrossRef]
- Bellut, K.; Arendt, E.K. Chance and challenge: Non-saccharomyces yeasts in nonalcoholic and low alcohol beer brewing–A review. J. Am. Soc. Brew. Chem. 2019, 77, 77–91. [Google Scholar] [CrossRef]
- Miguel, G.A.; Carlsen, S.; Arneborg, N.; Saerens, S.M.; Laulund, S.; Knudsen, G.M. Non-Saccharomyces yeasts for beer production: Insights into safety aspects and considerations. Int. J. Food Microbiol. 2022, 383, 109951. [Google Scholar] [CrossRef]
- Delphine, N.; Micaël, B.E.; Christelle, A.I.; Charlotte, E.A. Nutritional Potential of Spent Brewer’s Yeast, A Residual By-Product of Beer Production in Breweries for Future Applications. J. Adv. Biol. Biotechnol. 2023, 26, 30–39. [Google Scholar] [CrossRef]
- Vieira, E.F.; Carvalho, J.; Pinto, E.; Cunha, S.; Almeida, A.A.; Ferreira, I.M.P.L.V.O. Nutritive value, antioxidant activity and phenolic compounds profile of brewer’s spent yeast extract. J. Food Compos. Anal. 2016, 52, 44–51. [Google Scholar] [CrossRef]
- Puligundla, P.; Mok, C.; Park, S. Advances in the valorization of spent brewer’s yeast. Innov. Food Sci. Emerg. Technol. 2020, 62, 102350. [Google Scholar] [CrossRef]
- León-González, M.E.; Gómez-Mejía, E.; Rosales-Conrado, N.; Madrid-Albarrán, Y. Residual brewing yeast as a source of polyphenols: Extraction, identification and quantification by chromatographic and chemometric tools. Food Chem. 2018, 267, 246–254. [Google Scholar] [CrossRef] [PubMed]
- Shahavi, M.H.; Jahanshahi, M. Advanced Downstream Processing in Biotechnology. In Biochemical Engineering and Biotechnology, 2nd ed.; Najafpour, G.D., Ed.; Elsevier Science Publishers: Amsterdam, The Netherlands, 2015; pp. 495–515. [Google Scholar]
- Kapoore, R.V.; Butler, T.O.; Pandhal, J.; Vaidyanathan, S. Microwave-Assisted Extraction for Microalgae: From Biofuels to Biorefinery. Biology 2018, 7, 18. [Google Scholar] [CrossRef]
- Shehadul Islam, M.; Aryasomayajula, A.; Selvaganapathy, P.R. A review on macroscale and microscale cell lysis methods. Micromachines 2017, 8, 83. [Google Scholar] [CrossRef]
- Bzducha-Wróbel, A.; Błażejak, S.; Kawarska, A.; Stasiak-Różańska, L.; Gientka, I.; Majewska, E. Evaluation of the efficiency of different disruption methods on yeast cell wall preparation for β-glucan isolation. Molecules 2014, 19, 20941–20961. [Google Scholar] [CrossRef]
- Harrison, S.T.L. 2.44—Cell Disruption. In Comprehensive Biotechnology, 2nd ed.; Moo-Young, M., Ed.; Academic Press: Burlington, MA, USA, 2011; pp. 619–640. [Google Scholar]
- Wang, D.; Yu, X.; Sheng, P.; Zhang, G. The Transcriptomic Mechanism of a Novel Autolysis Induced by a Recombinant Antibacterial Peptide from Chicken Expressed in Pichia pastoris. Molecules 2022, 27, 2029. [Google Scholar] [CrossRef]
- Alves, E.M.; Souza, J.F.d.; Oliva Neto, P.d. Advances in yeast autolysis technology—A faster and safer new bioprocess. Braz. J. Food Technol. 2021, 24, e2020249. [Google Scholar] [CrossRef]
- Alexandre, H. Autolysis of Yeasts. In Comprehensive Biotechnology; Elsevier: Amsterdam, The Netherlands, 2011; pp. 641–649. [Google Scholar]
- Fornairon-Bonnefond, C.; Camarasa, C.; Moutounet, M.; Salmon, J.-M. New trends on yeast autolysis and wine ageing on lees: A bibliographic review. OENO One 2002, 36, 49–69. [Google Scholar] [CrossRef]
- Vieira, E.F.; Melo, A.; Ferreira, I.M.P.L.V.O. Autolysis of intracellular content of Brewer’s spent yeast to maximize ACE-inhibitory and antioxidant activities. LWT-Food Sci. Technol. 2017, 82, 255–259. [Google Scholar] [CrossRef]
- Tanguler, H.; Erten, H. Utilisation of spent brewer’s yeast for yeast extract production by autolysis: The effect of temperature. Food Bioprod. Process. 2008, 86, 317–321. [Google Scholar] [CrossRef]
- Bayarjargal, M.; Munkhbat, E.; Ariunsaikhan, T.; Odonchimeg, M.; Uurzaikh, T.; Gan-Erdene, T.; Regdel, D. Utilization of spent brewer’s yeast Saccharomyces cerevisiae for the production of yeast enzymatic hydrolysate. Mong. J. Chem. 2011, 12, 88–91. [Google Scholar] [CrossRef]
- Tessaro, G.; Biz, A.P.; Rigo, E.; Bertolo, A.P.; Bagatini, L.; Cavalheiro, D. Spent brewer’s yeast as an ingredient on the elaboration of cookies. Braz. J. Food Res. 2020, 11, 32–44. [Google Scholar] [CrossRef]
- Podpora, B.; Swiderski, F. Spent Brewer’s Yeast Autolysates as a New and Valuable Component of Functional Food and Dietary Supplements. J. Food Process. Technol. 2015, 6, 1000526. [Google Scholar] [CrossRef]
- Oliveira, A.S.; Odila Pereira, J.; Ferreira, C.; Faustino, M.; Durão, J.; Pereira, A.M.; Oliveira, C.M.; Pintado, M.E.; Carvalho, A.P. Spent Yeast Valorization for Food Applications: Effect of Different Extraction Methodologies. Foods 2022, 11, 4002. [Google Scholar] [CrossRef]
- Saksinchai, S.; Suphantharika, M.; Verduyn, C. Application of a simple yeast extract from spent brewer’s yeast for growth and sporulation of Bacillus thuringiensis subsp. kurstaki: A Physiological Study. World J. Microbiol. Biotechnol. 2001, 17, 307–316. [Google Scholar] [CrossRef]
- Thanardkit, P.; Khunrae, P.; Suphantharika, M.; Verduyn, C. Glucan from spent brewer’s yeast: Preparation, analysis and use as a potential immunostimulant in shrimp feed. World J. Microbiol. Biotechnol. 2002, 18, 527–539. [Google Scholar] [CrossRef]
- Bertolo, A.P.; Biz, A.P.; Kempka, A.P.; Rigo, E.; Cavalheiro, D. Yeast (Saccharomyces cerevisiae): Evaluation of cellular disruption processes, chemical composition, functional properties and digestibility. J. Food Sci. Technol. 2019, 56, 3697–3706. [Google Scholar] [CrossRef]
- Jacob, F.F.; Hutzler, M.; Methner, F.-J. Comparison of various industrially applicable disruption methods to produce yeast extract using spent yeast from top-fermenting beer production: Influence on amino acid and protein content. Eur. Food Res. Technol. 2019, 245, 95–109. [Google Scholar] [CrossRef]
- Beșliu, A.; Chiseliță, N.; Chiseliță, O.; Efremova, N.; Tofan, E.; Darie, G.; Sprincean, A.; Rotari, D. New Processes for Obtaining Mannoproteins from Beer Yeast Sediments and Their Biochemical Properties. Sci. Study Res. Chem. Chem. Eng. Biotechnol. Food Ind. 2022, 23, 41–48. [Google Scholar]
- Reed, G. Yeast Technology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Alves, E.M. Fractionation of Spent Brewer’s Yeast for High Value-Added Biomolecules Production; UNESP: Rio Claro, Brazil, 2020. [Google Scholar]
- Heringer, H.C.E.; Kuhn Marchioro, M.L.; Meneguzzi, D.; Barbosa-Dekker, A.M.; Dekker, R.F.H.; Alves da Cunha, M.A. Valorization of spent Brewers yeast in the integrated production of the fungal exopolysaccharide (1 → 6)-β-D-glucan (lasiodiplodan) and single-cell protein. Biocatal. Agric. Biotechnol. 2023, 54, 102971. [Google Scholar] [CrossRef]
- Gervais, P.; Martínez de Marañón, I.; Evrard, C.; Ferret, E.; Moundanga, S. Cell volume changes during rapid temperature shifts. J. Biotechnol. 2003, 102, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Levin David, E. Cell Wall Integrity Signaling in Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 2005, 69, 262–291. [Google Scholar] [CrossRef]
- Kakko, N.; Ivanona, N.; Rantasalo, A. Cell disruption methods. In CHEM-E3140 Bioprocess Technology II; Aalto University: Espoo, Finland, 2016; pp. 11–13. [Google Scholar]
- D’hondt, E.; Martin-Juarez, J.; Bolado, S.; Kasperoviciene, J.; Koreiviene, J.; Sulcius, S.; Elst, K.; Bastiaens, L. Cell disruption technologies. In Microalgae-Based Biofuels and Bioproducts; Elsevier: Amsterdam, The Netherlands, 2017; pp. 133–154. [Google Scholar]
- Beney, L.; Gervais, P. Influence of the fluidity of the membrane on the response of microorganisms to environmental stresses. Appl. Microbiol. Biotechnol. 2001, 57, 34–42. [Google Scholar]
- Koschorreck, K.; Wahrendorff, F.; Biemann, S.; Jesse, A.; Urlacher, V.B. Cell thermolysis—A simple and fast approach for isolation of bacterial laccases with potential to decolorize industrial dyes. Process Biochem. 2017, 56, 171–176. [Google Scholar] [CrossRef]
- Thakkar, A.; Barbera, E.; Sforza, E.; Bertucco, A.; Davis, R.; Kumar, S. Flash hydrolysis of yeast (Saccharomyces cerevisiae) for protein recovery. J. Supercrit. Fluids 2021, 173, 105240. [Google Scholar] [CrossRef]
- Khandual, S.; Pokharel, U.; Rathore, S.; Bonilla-Ahumada, F.; Kumar, S. A novel method of flash-hydrolysis assisted pigment extraction (carotenoids) from microalgae biomass. Biofuels Bioprod. Biorefin. 2024, 18, 1526–1540. [Google Scholar] [CrossRef]
- Farine, T.; Foss, M.A. Science in Nursing and Health Care; Routledge: London, UK, 2013. [Google Scholar]
- Alanazi, F.K.; Alsuwyeh, A.A.; Haq, N.; Salem-Bekhit, M.M.; Al-Dhfyan, A.; Shakeel, F. Vision of bacterial ghosts as drug carriers mandates accepting the effect of cell membrane on drug loading. Drug Dev. Ind. Pharm. 2020, 46, 1716–1725. [Google Scholar] [CrossRef]
- Yancey, P.H. Water Stress, Osmolytes and Proteins. Am. Zool. 2015, 41, 699–709. [Google Scholar] [CrossRef][Green Version]
- Wu, Y.; Li, P.; Jiang, Z.; Sun, X.; He, H.; Yan, P.; Xu, Y.; Liu, Y. Bioinspired yeast-based β-glucan system for oral drug delivery. Carbohydr. Polym. 2023, 319, 121163. [Google Scholar] [CrossRef]
- Coradello, G.; Tirelli, N. Yeast Cells in Microencapsulation. General Features and Controlling Factors of the Encapsulation Process. Molecules 2021, 26, 3123. [Google Scholar] [CrossRef] [PubMed]
- Pilizota, T.; Shaevitz, J.W. Plasmolysis and Cell Shape Depend on Solute Outer-Membrane Permeability during Hyperosmotic Shock in E. coli. Biophys. J. 2013, 104, 2733–2742. [Google Scholar] [CrossRef] [PubMed]
- de Jesus Costa, T.; Thomazini, M.; Cristina José, J.; Peres Brexó, R.; Martelli-Tosi, M.; Sílvia Favaro-Trindade, C. Impact of plasmolysis process on the enrichment of brewer’s spent yeast biomass with vitamin D3 by biosorption followed by spray-drying process. Food Res. Int. 2024, 191, 114677. [Google Scholar] [CrossRef]
- Qin, S.; Timoshkin, I.; Wilson, M.; Maclean, M.; MacGregor, S.; Given, M.; Anderson, J.; Wang, T. Pulsed electric field assisted treatment of microorganisms for lysis. In Proceedings of the 2013 19th IEEE Pulsed Power Conference (PPC), San Francisco, CA, USA, 16–21 June 2013; pp. 1–5. [Google Scholar] [CrossRef]
- Fu, L.; Wang, C.; Zhu, Y.; Wang, Y. Seafood allergy: Occurrence, mechanisms and measures. Trends Food Sci. Technol. 2019, 88, 80–92. [Google Scholar] [CrossRef]
- Ou, Q.X.; Nikolic-Jaric, M.; Gänzle, M. Mechanisms of inactivation of Candida humilis and Saccharomyces cerevisiae by pulsed electric fields. Bioelectrochem 2017, 115, 47–55. [Google Scholar] [CrossRef]
- Goettel, M.; Eing, C.; Gusbeth, C.; Straessner, R.; Frey, W. Pulsed electric field assisted extraction of intracellular valuables from microalgae. Algal Res. 2013, 2, 401–408. [Google Scholar] [CrossRef]
- Yan, L.-G.; He, L.; Xi, J. High intensity pulsed electric field as an innovative technique for extraction of bioactive compounds—A review. Crit. Rev. Food Sci. Nutr. 2017, 57, 2877–2888. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, M.; Lin, S.; Liu, J.; Yang, Y.; Jin, Y. Optimization of extraction parameters for protein from beer waste brewing yeast treated by pulsed electric fields (PEF). Afr. J. Microbiol. Res. 2012, 6, 4739–4746. [Google Scholar]
- Berzosa, A.; Marín-Sánchez, J.; Delso, C.; Sanz, J.; Álvarez, I.; Sánchez-Gimeno, C.; Raso, J. Sequential extraction optimization of compounds of interest from spent brewer’s yeast biomass treated by Pulsed Electric Fields. Innov. Food Sci. Emerg. Technol. 2024, 94, 103705. [Google Scholar] [CrossRef]
- Salazar, O.; Asenjo, J.A. Enzymatic lysis of microbial cells. Biotechnol. Lett. 2007, 29, 985–994. [Google Scholar] [CrossRef]
- Vogelsang-O’Dwyer, M.; Sahin, A.W.; Arendt, E.K.; Zannini, E. Enzymatic Hydrolysis of Pulse Proteins as a Tool to Improve Techno-Functional Properties. Foods 2022, 11, 1307. [Google Scholar] [CrossRef] [PubMed]
- Noman, A.; Xu, Y.; Al-Bukhaiti, W.Q.; Abed, S.M.; Ali, A.H.; Ramadhan, A.H.; Xia, W. Influence of enzymatic hydrolysis conditions on the degree of hydrolysis and functional properties of protein hydrolysate obtained from Chinese sturgeon (Acipenser sinensis) by using papain enzyme. Process Biochem. 2018, 67, 19–28. [Google Scholar] [CrossRef]
- Liu, L.-C.; Prokopakis, G.J.; Asenjo, J.A. Optimization of enzymatic lysis of yeast. Biotechnol. Bioeng. 1988, 32, 1113–1127. [Google Scholar] [CrossRef] [PubMed]
- Takalloo, Z.; Nikkhah, M.; Nemati, R.; Jalilian, N.; Sajedi, R.H. Autolysis, plasmolysis and enzymatic hydrolysis of baker’s yeast (Saccharomyces cerevisiae): A comparative study. World J. Microbiol. Biotechnol. 2020, 36, 68. [Google Scholar] [CrossRef] [PubMed]
- Jung, E.Y.; Lee, H.S.; Choi, J.W.; Ra, K.S.; Kim, M.R.; Suh, H.J. Glucose tolerance and antioxidant activity of spent brewer’s yeast hydrolysate with a high content of Cyclo-His-Pro (CHP). J. Food Sci. 2011, 76, C272–C278. [Google Scholar] [CrossRef]
- Tsaroucha, C.; Oikonomopoulou, V.; Kritsotakis, K.; Topakas, E.; Krokida, M. Isolation of proteins from by-products and residues of the brewing industry. In Proceedings of the Corfu 2022 9th International Conference on Sustainable Solid Waste Management, Corfu, Greece, 15–18 June 2022. [Google Scholar]
- Marson, G.V.; Machado, M.T.C.; de Castro, R.J.S.; Hubinger, M.D. Sequential hydrolysis of spent brewer’s yeast improved its physico-chemical characteristics and antioxidant properties: A strategy to transform waste into added-value biomolecules. Process Biochem. 2019, 84, 91–102. [Google Scholar] [CrossRef]
- Tam, T.M.; Duy, N.Q.; Minh, P.N.; Dong, T.A.D. Optimization of Beta-glucan Extracion from Waste Brewer’s Yeast Saccharomyces cerevisiae Using Autolysis, Enzyme, Ultrasonic and Combined Enzyme-Ultrasonic Treatment. Am. J. Res. Commun. 2013, 1, 149–158. [Google Scholar]
- Shojaeiarani, J.; Bajwa, D.; Holt, G. Sonication amplitude and processing time influence the cellulose nanocrystals morphology and dispersion. Nanocomposites 2020, 6, 41–46. [Google Scholar] [CrossRef]
- Vyas, S.; Ting, Y.-P. A Review of the Application of Ultrasound in Bioleaching and Insights from Sonication in (Bio)Chemical Processes. Resources 2017, 7, 3. [Google Scholar] [CrossRef]
- Grogan, S.P.; Mount, C.A. Ultrasound Physics and Instrumentation. In StatPearls; StatPearls Publishing Copyright © 2022; StatPearls Publishing LLC: Treasure Island, FL, USA, 2022. [Google Scholar]
- Chisti, Y.; Moo-young, M. Disruption of microbial cells for intracellular products. Enzym. Microb. Technol. 1986, 8, 194–204. [Google Scholar] [CrossRef]
- Ciobanu, L.T.; Constantinescu-Aruxandei, D.; Tritean, N.; Lupu, C.; Negrilă, R.N.; Farcasanu, I.C.; Oancea, F. Valorization of Spent Brewer’s Yeast Bioactive Components via an Optimized Ultrasonication Process. Fermentation 2023, 9, 952. [Google Scholar] [CrossRef]
- Jacob, F.F.; Striegel, L.; Rychlik, M.; Hutzler, M.; Methner, F.-J. Spent Yeast from Brewing Processes: A Biodiverse Starting Material for Yeast Extract Production. Fermentation 2019, 5, 51. [Google Scholar] [CrossRef]
- de Carvalho, J.C.; Magalhães, A.I.; de Melo Pereira, G.V.; Medeiros, A.B.P.; Sydney, E.B.; Rodrigues, C.; Aulestia, D.T.M.; de Souza Vandenberghe, L.P.; Soccol, V.T.; Soccol, C.R. Microalgal biomass pretreatment for integrated processing into biofuels, food, and feed. Bioresour. Technol. 2020, 300, 122719. [Google Scholar] [CrossRef]
- Corrêa, P.S.; Morais Júnior, W.G.; Martins, A.A.; Caetano, N.S.; Mata, T.M. Microalgae Biomolecules: Extraction, Separation and Purification Methods. Processes 2021, 9, 10. [Google Scholar] [CrossRef]
- Nemer, G.; Louka, N.; Vorobiev, E.; Salameh, D.; Nicaud, J.-M.; Maroun, R.G.; Koubaa, M. Mechanical Cell Disruption Technologies for the Extraction of Dyes and Pigments from Microorganisms: A Review. Fermentation 2021, 7, 36. [Google Scholar] [CrossRef]
- Burden, D.; Brangs, H.; Gibbons, L. Bead Beating: A Primer; OPS Diagnostics, LLC: Lebanon, NJ, USA, 2014. [Google Scholar]
- Passos, F.; Uggetti, E.; Carrère, H.; Ferrer, I. Chapter 11—Algal Biomass: Physical Pretreatments. In Pretreatment of Biomass; Pandey, A., Negi, S., Binod, P., Larroche, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 195–226. [Google Scholar]
- Quesada-Salas, M.C.; Delfau-Bonnet, G.; Willig, G.; Préat, N.; Allais, F.; Ioannou, I. Optimization and Comparison of Three Cell Disruption Processes on Lipid Extraction from Microalgae. Processes 2021, 9, 369. [Google Scholar] [CrossRef]
- Caballero-Córdoba, G.M.; Sgarbieri, V.C. Nutritional and toxicological evaluation of yeast (Saccharomyces cerevisiae) biomass and a yeast protein concentrate. J. Sci. Food Agric. 2000, 80, 341–351. [Google Scholar] [CrossRef]
- Avramia, I.; Amariei, S. A Simple and Efficient Mechanical Cell Disruption Method Using Glass Beads to Extract β-Glucans from Spent Brewer’s Yeast. Appl. Sci. 2022, 12, 648. [Google Scholar] [CrossRef]
- Pancrazio, G.; Cunha, S.C.; de Pinho, P.G.; Loureiro, M.; Meireles, S.; Ferreira, I.M.P.L.V.O.; Pinho, O. Spent brewer’s yeast extract as an ingredient in cooked hams. Meat Sci. 2016, 121, 382–389. [Google Scholar] [CrossRef]
- Håkansson, A.; Trägårdh, C.; Bergenståhl, B. Studying the effects of adsorption, recoalescence and fragmentation in a high pressure homogenizer using a dynamic simulation model. Food Hydrocoll. 2009, 23, 1177–1183. [Google Scholar] [CrossRef]
- Donsì, F.; Ferrari, G.; Maresca, P. High-Pressure Homogenisation for Food Sanitisation. In Proceedings of the IUFoST 13th World Congress of Food Sciences Technology, Nantes, France, 17–21 September 2006. [Google Scholar]
- Middelberg, A. 2 Microbial Cell Disruption by High-Pressure Homogenization. In Downstream Processing of Proteins: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2008; Volume 9, pp. 11–21. [Google Scholar]
- Garcia-Ortega, X.; Reyes, C.; Montesinos, J.L.; Valero, F. Overall Key Performance Indicator to Optimizing Operation of High-Pressure Homogenizers for a Reliable Quantification of Intracellular Components in Pichia pastoris. Front. Bioeng. Biotechnol. 2015, 3, 107. [Google Scholar] [CrossRef]
- Patrignani, F.; Lanciotti, R. Applications of High and Ultra High Pressure Homogenization for Food Safety. Front. Microbiol. 2016, 7, 1132. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Y.; Wang, Q.; Cui, S.W.; Liu, H.-Z. A new isolation method of β-d-glucans from spent yeast Saccharomyces cerevisiae. Food Hydrocoll. 2008, 22, 239–247. [Google Scholar] [CrossRef]
- Thammakiti, S.; Suphantharika, M.; Phaesuwan, T.; Verduyn, C. Preparation of spent brewer’s yeast β-glucans for potential applications in the food industry. Int. J. Food Sci. Technol. 2004, 39, 21–29. [Google Scholar] [CrossRef]
- Tian, X.; Yang, P.; Jiang, W. Effect of Alkali Treatment Combined with High Pressure on Extraction Efficiency of β-d-Glucan from Spent Brewer’s Yeast. Waste Biomass Valorization 2019, 10, 1131–1140. [Google Scholar] [CrossRef]
- Barbieru, O.-G.; Trică, B.; Tritean, N.; Negrilă, R.; Constantinescu Aruxandei, D.; Oancea, F. Maximizing total protein extraction from spent brewer’s yeast using high-pressure homogenization. Sci. Bull. 2021, 25, 75–81. [Google Scholar]
- Sombutyanuchit, P.; Suphantharika, M.; Verduyn, C. Preparation of 5′-GMP-rich yeast extracts from spent brewer’s yeast. World J. Microbiol. Biotechnol. 2001, 17, 163–168. [Google Scholar] [CrossRef]
- Amorim, M.; Pereira, J.O.; Gomes, D.; Pereira, C.D.; Pinheiro, H.; Pintado, M. Nutritional ingredients from spent brewer’s yeast obtained by hydrolysis and selective membrane filtration integrated in a pilot process. J. Food Eng. 2016, 185, 42–47. [Google Scholar] [CrossRef]
- Amorim, M.; Pinheiro, H.; Pintado, M. Valorization of spent brewer’s yeast: Optimization of hydrolysis process towards the generation of stable ACE-inhibitory peptides. LWT 2019, 111, 77–84. [Google Scholar] [CrossRef]
- Aguilar-Uscanga, B.; François, J.M. A study of the yeast cell wall composition and structure in response to growth conditions and mode of cultivation. Lett. Appl. Microbiol. 2003, 37, 268–274. [Google Scholar] [CrossRef]
- Aimanianda, V.; Clavaud, C.; Simenel, C.; Fontaine, T.; Delepierre, M.; Latgé, J.P. Cell wall beta-(1,6)-glucan of Saccharomyces cerevisiae: Structural characterization and in situ synthesis. J. Biol. Chem. 2009, 284, 13401–13412. [Google Scholar] [CrossRef] [PubMed]
- Korolenko, T.A.; Bgatova, N.P.; Vetvicka, V. Glucan and Mannan-Two Peas in a Pod. Int. J. Mol. Sci. 2019, 20, 3189. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, J.; Farkaš, V.; Sanz, A.B.; Cabib, E. ‘Strengthening the fungal cell wall through chitin–glucan cross-links: Effects on morphogenesis and cell integrity’. Cell. Microbiol. 2016, 18, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Moreno, J.; Peinado, R. Chapter 6—Sugars: Structure and Classification. In Enological Chemistry; Moreno, J., Peinado, R., Eds.; Academic Press: San Diego, CA, USA, 2012; pp. 77–93. [Google Scholar]
- Xie, X.; Lipke, P.N. On the evolution of fungal and yeast cell walls. Yeast 2010, 27, 479–488. [Google Scholar] [CrossRef]
- Kollár, R.; Reinhold, B.B.; Petráková, E.; Yeh, H.J.C.; Ashwell, G.; Drgonová, J.; Kapteyn, J.C.; Klis, F.M.; Cabib, E. Fractionation of spent brewer’s yeast for high value-added biomolecules production Architecture of the Yeast Cell Wall: β(1→6)-glucan interconnects mannoprotein, β(1→3)-glucan, and chitin. J. Biol. Chem. 1997, 272, 17762–17775. [Google Scholar] [CrossRef]
- Klis, F.M.; Mol, P.; Hellingwerf, K.; Brul, S. Dynamics of cell wall structure in Saccharomyces cerevisiae. FEMS Microbiol. Rev. 2002, 26, 239–256. [Google Scholar] [CrossRef]
- Narusaka, M.; Minami, T.; Iwabuchi, C.; Hamasaki, T.; Takasaki, S.; Kawamura, K.; Narusaka, Y. Yeast cell wall extract induces disease resistance against bacterial and fungal pathogens in Arabidopsis thaliana and Brassica crop. PLoS ONE 2015, 10, e0115864. [Google Scholar] [CrossRef]
- Bastos, R.; Oliveira, P.G.; Gaspar, V.M.; Mano, J.F.; Coimbra, M.A.; Coelho, E. Brewer’s yeast polysaccharides—A review of their exquisite structural features and biomedical applications. Carbohydr. Polym. 2022, 277, 118826. [Google Scholar] [CrossRef]
- Kasai, T.; Eguchi, T.; Ishiwaki, N.; Kaneshige, J.; Ozeki, T.; Yuasa, H. Application of acid-treated yeast cell wall (AYC) as a pharmaceutical additive I. AYC as a novel coating material. Int. J. Pharm. 2000, 204, 53–59. [Google Scholar] [CrossRef]
- Petravic-Tominac, V.; Krpan, V.; Grba, S.; Srečec, S.; Krbavčić, I.; Vidovic, L. Biological effects of yeast β-glucans. Agric. Conspec. Sci. 2010, 75, 149–158. [Google Scholar]
- Murphy, E.J.; Rezoagli, E.; Major, I.; Rowan, N.J.; Laffey, J.G. β-Glucan Metabolic and Immunomodulatory Properties and Potential for Clinical Application. J. Fungi 2020, 6, 356. [Google Scholar] [CrossRef] [PubMed]
- Stier, H.; Ebbeskotte, V.; Gruenwald, J. Immune-modulatory effects of dietary Yeast Beta-1,3/1,6-D-glucan. Nutr. J. 2014, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Krpan, V.; Vlatka, P.-T.; Krbavčić, I.; Slobodan, G.; Katarina, B. Potential Application of Yeast β-Glucans in Food Industry. Agric. Conspec. Sci. (ACS) 2009, 74, 277–282. [Google Scholar]
- Avramia, I.; Amariei, S. Spent Brewer’s Yeast as a Source of Insoluble β-Glucans. Int. J. Mol. Sci. 2021, 22, 825. [Google Scholar] [CrossRef]
- Caruso, M.A.; Piermaria, J.A.; Abraham, A.G.; Medrano, M. β-glucans obtained from beer spent yeasts as functional food grade additive: Focus on biological activity. Food Hydrocoll. 2022, 133, 107963. [Google Scholar] [CrossRef]
- Silva Araújo, V.B.d.; Melo, A.N.F.d.; Costa, A.G.; Castro-Gomez, R.H.; Madruga, M.S.; Souza, E.L.d.; Magnani, M. Followed extraction of β-glucan and mannoprotein from spent brewer’s yeast (Saccharomyces uvarum) and application of the obtained mannoprotein as a stabilizer in mayonnaise. Innov. Food Sci. Emerg. Technol. 2014, 23, 164–170. [Google Scholar] [CrossRef]
- de Melo, A.N.F.; de Souza, E.L.; da Silva Araujo, V.B.; Magnani, M. Stability, nutritional and sensory characteristics of French salad dressing made with mannoprotein from spent brewer’s yeast. LWT-Food Sci. Technol. 2015, 62, 771–774. [Google Scholar] [CrossRef]
- Dikit, P.; Maneerat, S.; Musikasang, H.; H-kittikun, A. Emulsifier properties of the mannoprotein extract from yeast isolated from sugar palm wine. ScienceAsia 2010, 36, 312–318. [Google Scholar] [CrossRef]
- Cameron, D.R.; Cooper, D.G.; Neufeld, R.J. The mannoprotein of Saccharomyces cerevisiae is an effective bioemulsifier. Appl. Environ. Microbiol. 1988, 54, 1420–1425. [Google Scholar] [CrossRef]
- Juega, M.; Núñez, Y.; Carrascosa, A.; Martínez-Rodríguez, A. Influence of Yeast Mannoproteins in the Aroma Improvement of White Wines. J. Food Sci. 2012, 77, M499–M504. [Google Scholar] [CrossRef]
- Ribeiro, T.; Fernandes, C.; Nunes, F.M.; Filipe-Ribeiro, L.; Cosme, F. Influence of the structural features of commercial mannoproteins in white wine protein stabilization and chemical and sensory properties. Food Chem. 2014, 159, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Guan, Y.; Zhong, Q. Yeast mannoproteins improve thermal stability of anthocyanins at pH 7.0. Food Chem. 2015, 172, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.A.; Asiri, S.M.M. Green synthesis, antimicrobial, antibiofilm and antitumor activities of superparamagnetic γ-Fe2O3 NPs and their molecular docking study with cell wall mannoproteins and peptidoglycan. Int. J. Biol. Macromol. 2021, 171, 44–58. [Google Scholar] [CrossRef]
- Ganan, M.; Carrascosa, A.V.; de Pascual-Teresa, S.; Martinez-Rodriguez, A.J. Effect of mannoproteins on the growth, gastrointestinal viability, and adherence to Caco-2 cells of lactic acid bacteria. J. Food Sci. 2012, 77, M176–M180. [Google Scholar] [CrossRef]
- Abdolshahi, A.; Monjazeb Marvdashti, L.; Salehi, B.; Sharifi-Rad, M.; Ghobakhloo, S.; Iriti, M.; Sharifi-Rad, J. Antifungal activities of coating incorporated with Saccharomyces cerevisiae cell wall mannoprotein on Aspergillus flavus growth and aflatoxin production in pistachio (Pistacia vera L.). J. Food Saf. 2018, 39, e12608. [Google Scholar] [CrossRef]
- Harish Prashanth, K.V.; Tharanathan, R.N. Chitin/chitosan: Modifications and their unlimited application potential—An overview. Trends Food Sci. Technol. 2007, 18, 117–131. [Google Scholar] [CrossRef]
- Barikani, M.; Oliaei, E.; Seddiqi, H.; Honarkar, H. Preparation and application of chitin and its derivatives: A review. Iran. Polym. J. 2014, 23, 307–326. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Sun, C.; Fu, D.; Jin, L.; Chen, M.; Zheng, X.; Yu, T. Chitin isolated from yeast cell wall induces the resistance of tomato fruit to Botrytis cinerea. Carbohydr. Polym. 2018, 199, 341–352. [Google Scholar] [CrossRef]
- Roca, C.; Chagas, B.; Farinha, I.; Freitas, F.; Mafra, L.; Aguiar, F.; Oliveira, R.; Reis, M.A.M. Production of yeast chitin–glucan complex from biodiesel industry byproduct. Process Biochem. 2012, 47, 1670–1675. [Google Scholar] [CrossRef]
- Chagas, B.; Farinha, I.; Galinha, C.F.; Freitas, F.; Reis, M.A.M. Chitin–glucan complex production by Komagataella (Pichia) pastoris: Impact of cultivation pH and temperature on polymer content and composition. New Biotechnol. 2014, 31, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Araújo, D.; Alves, V.D.; Lima, S.A.C.; Reis, S.; Freitas, F.; Reis, M.A.M. Novel hydrogels based on yeast chitin-glucan complex: Characterization and safety assessment. Int. J. Biol. Macromol. 2020, 156, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Malerba, M.; Cerana, R. Recent Applications of Chitin- and Chitosan-Based Polymers in Plants. Polymers 2019, 11, 839. [Google Scholar] [CrossRef]
- Yuasa, H.; Kaneshige, J.; Ozeki, T.; Kasai, T.; Eguchi, T.; Ishiwaki, N. Application of acid-treated yeast cell wall (AYC) as a pharmaceutical additive. II: Effects of curing on the medicine release from AYC-coated tablets. Int. J. Pharm. 2000, 209, 69–77. [Google Scholar] [CrossRef]
- Yuasa, H.; Kaneshige, J.; Ozeki, T.; Kasai, T.; Eguchi, T.; Ishiwaki, N. Application of acid-treated yeast cell wall (AYC) as a pharmaceutical additive. III. AYC aqueous coating onto granules and film formation mechanism of AYC. Int. J. Pharm. 2002, 237, 15–22. [Google Scholar] [CrossRef]
- Almeida, S.; Raposo, A.; Almeida-González, M.; Carrascosa, C. Bisphenol A: Food Exposure and Impact on Human Health. Compr. Rev. Food Sci. Food Saf. 2018, 17, 1503–1517. [Google Scholar] [CrossRef]
- Rajmohan, K.V.S.; Ramya, C.; Raja Viswanathan, M.; Varjani, S. Plastic pollutants: Effective waste management for pollution control and abatement. Curr. Opin. Environ. Sci. Health 2019, 12, 72–84. [Google Scholar] [CrossRef]
- Peltzer, M.A.; Salvay, A.G.; Delgado, J.F.; de la Osa, O.; Wagner, J.R. Use of Residual Yeast Cell Wall for New Biobased Materials Production: Effect of Plasticization on Film Properties. Food Bioprocess Technol. 2018, 11, 1995–2007. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Boller, T.; Felix, G. A renaissance of elicitors: Perception of microbe-associated molecular patterns and danger signals by pattern-recognition receptors. Annu. Rev. Plant Biol. 2009, 60, 379–406. [Google Scholar] [CrossRef] [PubMed]
- Falcón-Rodríguez, A.; Costales, D.; Rogers, H.; Diosdado, E.; González, S.; Cabrera, G.; González, L.; Cabrera, J.; Wégria, G.; Wattiez, R. Practical use of oligosaccharins in agriculture. Acta Hortic. 2012, 1009, 195–212. [Google Scholar] [CrossRef]
- De Miccolis Angelini, R.M.; Rotolo, C.; Gerin, D.; Abate, D.; Pollastro, S.; Faretra, F. Global transcriptome analysis and differentially expressed genes in grapevine after application of the yeast-derived defense inducer cerevisane. Pest Manag. Sci. 2019, 75, 2020–2033. [Google Scholar] [CrossRef] [PubMed]
- Pujos, P. Protection of Plants Against Their Pathogenic Agents. EP1965649 (B1), 28 August 2013. [Google Scholar]
- Sun, C.; Lin, M.; Fu, D.; Yang, J.; Huang, Y.; Zheng, X.; Yu, T. Yeast cell wall induces disease resistance against Penicillium expansum in pear fruit and the possible mechanisms involved. Food Chem. 2018, 241, 301–307. [Google Scholar] [CrossRef]
- Pereyra, C.M.; Gil, S.; Cristofolini, A.; Bonci, M.; Makita, M.; Monge, M.P.; Montenegro, M.A.; Cavaglieri, L.R. The production of yeast cell wall using an agroindustrial waste influences the wall thickness and is implicated on the aflatoxin B1 adsorption process. Food Res. Int. 2018, 111, 306–313. [Google Scholar] [CrossRef]
- Liu, N.; Wang, J.Q.; Jia, S.C.; Chen, Y.K.; Wang, J.P. Effect of yeast cell wall on the growth performance and gut health of broilers challenged with aflatoxin B1 and necrotic enteritis. Poult. Sci. 2018, 97, 477–484. [Google Scholar] [CrossRef]
- Tao, Z.; Yuan, H.; Liu, M.; Liu, Q.; Zhang, S.; Liu, H.; Jiang, Y.; Huang, D.; Wang, T. Yeast extract: Characteristics, production, applications and future perspectives. J. Microbiol. Biotechnol. 2023, 33, 151. [Google Scholar] [CrossRef]
- Tomé, D. Yeast Extracts: Nutritional and Flavoring Food Ingredients. ACS Food Sci. Technol. 2021, 1, 487–494. [Google Scholar] [CrossRef]
- Kurihara, K. Umami the Fifth Basic Taste: History of Studies on Receptor Mechanisms and Role as a Food Flavor. BioMed Res. Int. 2015, 2015, 189402. [Google Scholar] [CrossRef]
- Rhyu, M.-R.; Song, A.-Y.; Kim, E.-Y.; Son, H.-J.; Kim, Y.; Mummalaneni, S.; Qian, J.; Grider, J.R.; Lyall, V. Kokumi Taste Active Peptides Modulate Salt and Umami Taste. Nutrients 2020, 12, 1198. [Google Scholar] [CrossRef]
- Ueda, Y.; Sakaguchi, M.; Hirayama, K.; Miyajima, R.; Kimizuka, A. Characteristic flavor constituents in water extract of garlic. Agric. Biol. Chem. 1990, 54, 163–169. [Google Scholar]
- Yang, J.; Huang, Y.R.; Cui, C.; Dong, H.; Zeng, X.F.; Bai, W.D. Umami-enhancing effect of typical kokumi-active gamma-glutamyl peptides evaluated via sensory analysis and molecular modeling approaches. Food Chem. 2021, 338, 8. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Zhang, L.; Lametsch, R. Current progress in kokumi-active peptides, evaluation and preparation methods: A review. Crit. Rev. Food Sci. Nutr. 2020, 62, 1230–1241. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.S.; Ma, Y.; Ahmed, Z.; Geng, W.T.; Tang, W.; Liu, Y.; Jin, H.H.; Jiang, F.; Wang, J.J.; Wang, Y.P. Purification and identification of kokumi-enhancing peptides from chicken protein hydrolysate. Int. J. Food Sci. Technol. 2019, 54, 2151–2158. [Google Scholar] [CrossRef]
- Kang, L.; Alim, A.; Song, H.L. Identification and characterization of flavor precursor peptide from beef enzymatic hydrolysate by Maillard reaction. J. Chromatogr. B 2019, 1104, 176–181. [Google Scholar] [CrossRef]
- Murata, M. Browning and pigmentation in food through the Maillard reaction. Glycoconj. J. 2021, 38, 283–292. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Elfalleh, W.; He, S.D.; Tang, M.M.; Zhao, J.L.; Wu, Z.Y.; Wang, J.H.; Sun, H.J. Heating and cysteine effect on physicochemical and flavor properties of soybean peptide Maillard reaction products. Int. J. Biol. Macromol. 2018, 120, 2137–2146. [Google Scholar] [CrossRef]
- Diez-Simon, C.; Eichelsheim, C.; Mumm, R.; Hall, R.D. Chemical and Sensory Characteristics of Soy Sauce: A Review. J. Agric. Food Chem. 2020, 68, 11612–11630. [Google Scholar] [CrossRef]
- Qiu, Z.C.; Zheng, Z.J.; Zhang, B.; Sun-Waterhouse, D.; Qiao, X.G. Formation, nutritional value, and enhancement of characteristic components in black garlic: A review for maximizing the goodness to humans. Compr. Rev. Food Sci. Food Saf. 2020, 19, 801–834. [Google Scholar] [CrossRef]
- Maruyama, Y.; Yasuda, R.; Kuroda, M.; Eto, Y. Kokumi substances, enhancers of basic tastes, induce responses in calcium-sensing receptor expressing taste cells. PLoS ONE 2012, 7, e34489. [Google Scholar] [CrossRef]
- He, F.J.; Campbell, N.R.; Woodward, M.; MacGregor, G.A. Salt reduction to prevent hypertension: The reasons of the controversy. Eur. Heart J. 2021, 42, 2501–2505. [Google Scholar] [CrossRef] [PubMed]
- Ojangba, T.; Boamah, S.; Miao, Y.; Guo, X.; Fen, Y.; Agboyibor, C.; Yuan, J.; Dong, W. Comprehensive effects of lifestyle reform, adherence, and related factors on hypertension control: A review. J. Clin. Hypertens. 2023, 25, 509–520. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yi, H.-B.; Wang, P.; Wang, S.-N.; Liu, H.; Zhang, N. Current Advances in the Salt Reduction Properties of Kokumi γ-Glutamyl Peptides: A Review. Food Rev. Int. 2024, 1–21. [Google Scholar] [CrossRef]
- Magi, S.; Piccirillo, S.; Amoroso, S. The dual face of glutamate: From a neurotoxin to a potential survival factor—Metabolic implications in health and disease. Cell. Mol. Life Sci. 2019, 76, 1473–1488. [Google Scholar] [CrossRef] [PubMed]
- Lao, H.; Chang, J.; Zhuang, H.; Song, S.; Sun, M.; Yao, L.; Wang, H.; Liu, Q.; Xiong, J.; Li, P. Novel kokumi peptides from yeast extract and their taste mechanism via an in silico study. Food Funct. 2024, 15, 2459–2473. [Google Scholar] [CrossRef]
- Shotipruk, A.; Kittianong, P.; Suphantharika, M.; Muangnapoh, C. Application of rotary microfiltration in debittering process of spent brewer’s yeast. Bioresour. Technol. 2005, 96, 1851–1859. [Google Scholar] [CrossRef]
- Suzuki, H.; Nakafuji, Y.; Tamura, T. New method to produce kokumi seasoning from protein hydrolysates using bacterial enzymes. J. Agric. Food Chem. 2017, 65, 10514–10519. [Google Scholar] [CrossRef]
| Yeast Concentration (% w/v) | Temperature (°C) | Time (h) | Analyzed Fraction | Content (% w/w) | Reference |
|---|---|---|---|---|---|
| 15 | 50 | 24 | YE | Proteins: 48.70% Carbohydrates: 13.9% α-amino nitrogen: 3.9% | Tanguler et al., 2008 [36] |
| 50 | 55 | 24 | YE | Proteins: 45.30% Carbohydrates: 29.36% Lipids: 0.97% | Tessaro et al., 2020 [38] |
| 15.5–16.5 * | 47 | 48 | YE | Proteins: 7.53% Amino acids: 77.5% Main AA: Phe + Tyr, Val, Leu + Nle Polyphenols: 228.3–336.1 mg GAE/100 mL | Swiderski et al., 2015 [39] |
| 7 * | 50 | 24 | YE | Main AA: Leu, Lys, Val, Thr | Jacob et al., 2019 [44] |
| 50 | 50 | 24 | YE | Proteins: 54.50% Carbohydrates: 14.8% Main AA: Leu, Val, Lys, Thr Main minerals: P, K | Oliveira et al., 2022 [40] |
| 15 | 50 | 20 | YE | Amino nitrogen: 4.5% Carbohydrate (as glucose): 26.8% | Saksinchai et al., 2001 [41] |
| 18 * | 50 | 20 | YCW | Proteins: 29% Carbohydrates: 57% | Thanardkit et al., 2022 [42] |
| 50 | 55 | 24 | whole autolyzed yeast | Proteins: 39.32% Carbohydrates: 46.48% Lipids: 1.25% | Bertolo et al., 2019 [43] |
| Yeast Sample Concentration (% w/v) | Temperature (°C) | Time (h) | Enzyme/ Enzyme Mix | Enzyme Concentration (% w/w) | pH | Type of Analyzed Fraction | Content (% w/w) | References |
|---|---|---|---|---|---|---|---|---|
| 10 [37] | 45 | 5 | Pancreatin | 2.5% (w/v) | 8 | YE | Proteins: 55.9% α-amino nitrogen: 1.8% | Bayarjargal et al., 2011 [37] |
| 8 [76] | 50 | 48 | Flavourzyme | 1% (w/w) E:S | 7 | YE | Proteins: 64.9% Carbohydrates: 26.9% Lipids: 0.8% α-amino nitrogen: 5.81% Main AA: Glu, Leu, Lys, Asp | Jung et al., 2011 [76] |
| 100% (undiluted SBY) * [78] | 60 | 5.5 | Brauzyn | 10% (w/w) E:S | YE | Proteins: 54.3% Carbohydrates: 40.9% | Marson et al., 2019 [78] | |
| 15 [79] | - | 5 | Alcalase | 0.86% (w/w) E:S | 7 | YCW | Proteins: 23.33% β-glucan: 60.32% Lipids: 1.33% | Tam et al., 2013 [79] |
| 5 [77] | 50 | 24 | Alcalase | - | 7 | YE | Proteins: 2.015% | Tsaroucha et al., 2022 [77] |
| Cascade Lysis Experiment * | Aim of the Experiment | References |
|---|---|---|
| Thermolysis, HPH Organic solvent treatment, EH | Extraction of β-glucan | Liu et al., 2008 [100] |
| Autolysis, HPH | Thammakiti et al., 2004 [101] | |
| Alkaline treatment, HPH | Tian et al., 2019 [102] | |
| HPH | Maximizing protein extraction | Barbieru et al., 2021 [103] |
| Autolysis, EH | Obtaining 5′-GMP-rich YE | Sombutyanuchit et al., 2001 [104] |
| Field | YCW Uses | YCW Effects | References |
|---|---|---|---|
| Pharmaceutical | Drug coating agent | Control of the release time of the active ingredient | Kasai et al., 2000 [117] Yuasa et al., 2000 [142] Yuasa et al., 2002 [143] |
| Materials science | Base matrix for biodegradable films | Forming sustainable materials for food packaging | Peltzer et al., 2018 [146] |
| Agricultural crop management | Bio stimulant | Enhances plant resistance to pathogens Exerts antifungal and antibacterial activity | Narusaka et al., 2015 [115] |
| Veterinary | Mycotoxin adsorbent | Improves survival rate and health of aflatoxin-infected broilers | Pereyra et al., 2018 [154] Liu et al., 2018 [155] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciobanu, L.T.; Constantinescu-Aruxandei, D.; Farcasanu, I.C.; Oancea, F. Spent Brewer’s Yeast Lysis Enables a Best Out of Waste Approach in the Beer Industry. Int. J. Mol. Sci. 2024, 25, 12655. https://doi.org/10.3390/ijms252312655
Ciobanu LT, Constantinescu-Aruxandei D, Farcasanu IC, Oancea F. Spent Brewer’s Yeast Lysis Enables a Best Out of Waste Approach in the Beer Industry. International Journal of Molecular Sciences. 2024; 25(23):12655. https://doi.org/10.3390/ijms252312655
Chicago/Turabian StyleCiobanu, Livia Teodora, Diana Constantinescu-Aruxandei, Ileana Cornelia Farcasanu, and Florin Oancea. 2024. "Spent Brewer’s Yeast Lysis Enables a Best Out of Waste Approach in the Beer Industry" International Journal of Molecular Sciences 25, no. 23: 12655. https://doi.org/10.3390/ijms252312655
APA StyleCiobanu, L. T., Constantinescu-Aruxandei, D., Farcasanu, I. C., & Oancea, F. (2024). Spent Brewer’s Yeast Lysis Enables a Best Out of Waste Approach in the Beer Industry. International Journal of Molecular Sciences, 25(23), 12655. https://doi.org/10.3390/ijms252312655







