Proteomic Markers of Aging and Longevity: A Systematic Review
Abstract
1. Introduction
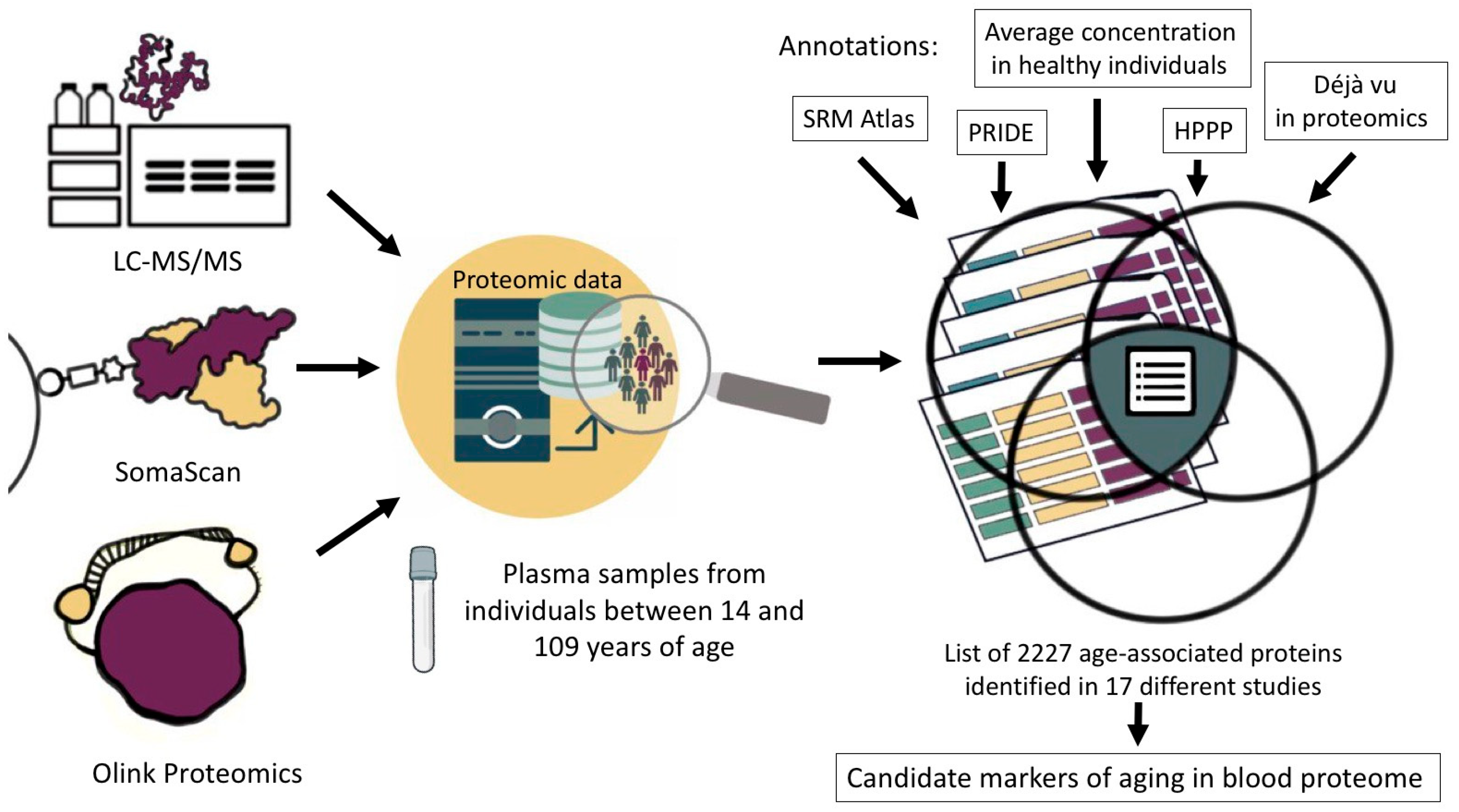
2. Materials and Methods
3. Results and Discussion
3.1. Proteomic Aging Clock
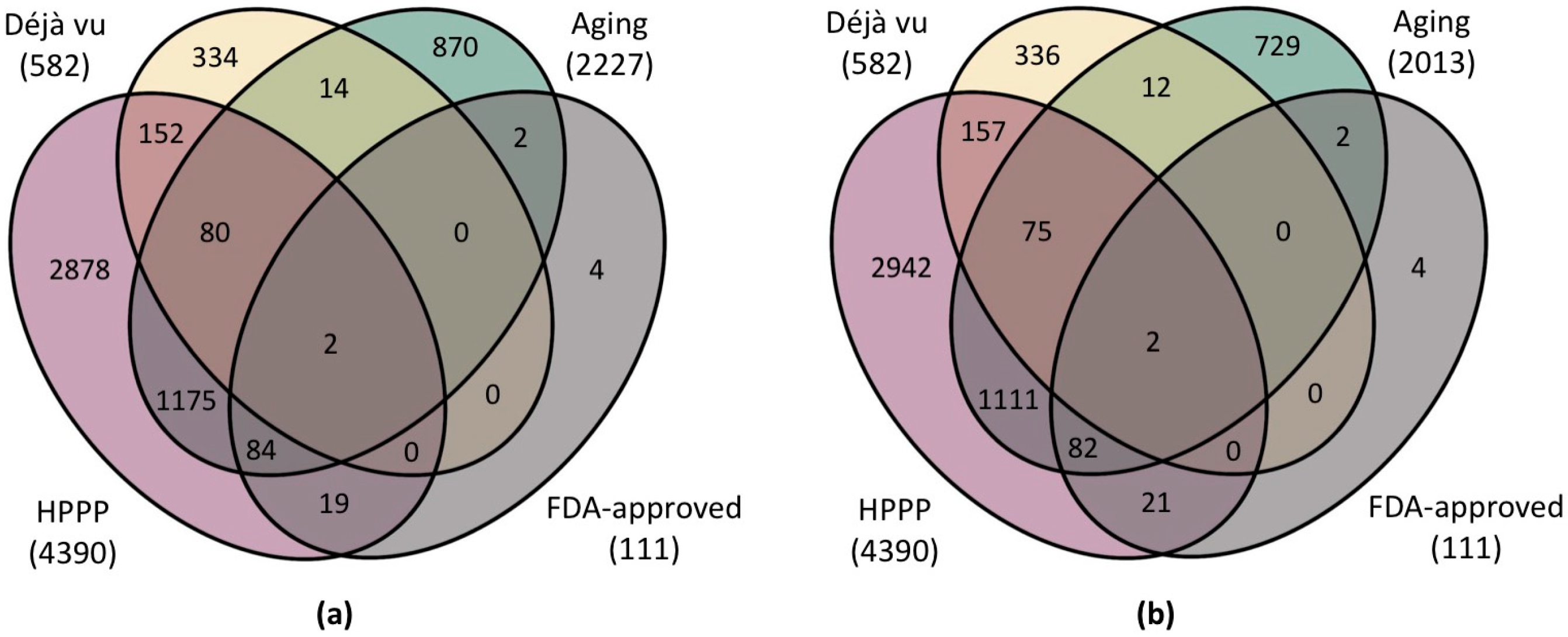
3.2. Most Frequent Proteins Associated with Aging and Age-Related Disease Development
3.3. Selection of a Panel of Proteins for Quantitative MS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Prattichizzo, F.; Frigé, C.; Pellegrini, V.; Scisciola, L.; Santoro, A.; Monti, D.; Rippo, M.R.; Ivanchenko, M.; Olivieri, F.; Franceschi, C. Organ-specific biological clocks: Ageotyping for personalized anti-aging medicine. Ageing Res. Rev. 2024, 96, 102253. [Google Scholar] [CrossRef] [PubMed]
- Sun, E.D.; Qian, Y.; Oppong, R.; Butler, T.J.; Zhao, J.; Chen, B.H.; Tanaka, T.; Kang, J.; Sidore, C.; Cucca, F.; et al. Predicting physiological aging rates from a range of quantitative traits using machine learning. Aging 2021, 13, 23471–23516. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Wang, C.; Zhou, X.; Zhou, W.; Hornburg, D.; Wu, S.; Snyder, M.P. Nonlinear dynamics of multi-omics profiles during human aging. Nat. Aging 2024, 4, 1619–1634. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.A.; Shokhirev, M.N. Contextualizing aging clocks and properly describing biological age. Aging Cell 2024, e14377. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xiong, M.; Fu, X.-H.; Fan, Y.; Dong, C.; Sun, X.; Zheng, F.; Wang, S.-W.; Liu, L.; Xu, M.; et al. Determining a multimodal aging clock in a cohort of Chinese women. Med 2023, 4, 825–848.e13. [Google Scholar] [CrossRef] [PubMed]
- Nie, C.; Li, Y.; Li, R.; Yan, Y.; Zhang, D.; Li, T.; Li, Z.; Sun, Y.; Zhen, H.; Ding, J.; et al. Distinct biological ages of organs and systems identified from a multi-omics study. Cell Rep. 2022, 38, 110459. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Fedintsev, A.; Moskalev, A. Stochastic non-enzymatic modification of long-lived macromolecules—A missing hallmark of aging. Ageing Res. Rev. 2020, 62, 101097. [Google Scholar] [CrossRef]
- Solovev, I.; Sergeeva, A.; Geraskina, A.; Shaposhnikov, M.; Vedunova, M.; Borysova, O.; Moskalev, A. Aging and physiological barriers: Mechanisms of barrier integrity changes and implications for age-related diseases. Mol. Biol. Rep. 2024, 51, 917. [Google Scholar] [CrossRef]
- Jansen, R.; Han, L.K.; Verhoeven, J.E.; Aberg, K.A.; van den Oord, E.C.; Milaneschi, Y.; Penninx, B.W. An integrative study of five biological clocks in somatic and mental health. eLife 2021, 10, e66223. [Google Scholar] [CrossRef]
- Argentieri, M.A.; Xiao, S.; Bennett, D.; Winchester, L.; Nevado-Holgado, A.J.; Ghose, U.; Albukhari, A.; Yao, P.; Mazidi, M.; Lv, J.; et al. Proteomic aging clock predicts mortality and risk of common age-related diseases in diverse populations. Nat. Med. 2024, 30, 2450–2460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Liu, A.Y.; Loriaux, P.; Wollscheid, B.; Zhou, Y.; Watts, J.D.; Aebersold, R. Mass spectrometric detection of tissue proteins in plasma. Mol. Cell. Proteom. 2007, 6, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Kliuchnikova, A.A.; Novikova, S.E.; Ilgisonis, E.V.; Kiseleva, O.I.; Poverennaya, E.V.; Zgoda, V.G.; Moshkovskii, S.A.; Poroikov, V.V.; Lisitsa, A.V.; Archakov, A.I.; et al. Blood Plasma Proteome: A Meta-Analysis of the Results of Protein Quantification in Human Blood by Targeted Mass Spectrometry. Int. J. Mol. Sci. 2023, 24, 769. [Google Scholar] [CrossRef] [PubMed]
- Thul, P.J.; Lindskog, C. The human protein atlas: A spatial map of the human proteome. Protein Sci. 2018, 27, 233–244. [Google Scholar] [CrossRef]
- Enroth, S.; Enroth, S.B.; Johansson, Å.; Gyllensten, U. Protein profiling reveals consequences of lifestyle choices on predicted biological aging. Sci. Rep. 2015, 5, 17282. [Google Scholar] [CrossRef]
- Tanaka, T.; Basisty, N.; Fantoni, G.; Candia, J.; Moore, A.Z.; Biancotto, A.; Schilling, B.; Bandinelli, S.; Ferrucci, L. Plasma proteomic biomarker signature of age predicts health and life span. eLife 2020, 9, e61073. [Google Scholar] [CrossRef]
- Kuo, C.-L.; Chen, Z.; Liu, P.; Pilling, L.C.; Atkins, J.L.; Fortinsky, R.H.; Kuchel, G.A.; Diniz, B.S. Proteomic aging clock (PAC) predicts age-related outcomes in middle-aged and older adults. Aging Cell 2024, 23, e14195. [Google Scholar] [CrossRef]
- Ye, S.; Ma, L.; Zhang, R.; Liu, F.; Jiang, P.; Xu, J.; Cao, H.; Du, X.; Lin, F.; Cheng, L.; et al. Plasma proteomic and autoantibody profiles reveal the proteomic characteristics involved in longevity families in Bama, China. Clin. Proteom. 2019, 16, 22. [Google Scholar] [CrossRef]
- Santos-Lozano, A.; Valenzuela, P.L.; Llavero, F.; Lista, S.; Carrera-Bastos, P.; Hampel, H.; Pareja-Galeano, H.; Gálvez, B.G.; López, J.A.; Vázquez, J.; et al. Successful aging: Insights from proteome analyses of healthy centenarians. Aging 2020, 12, 3502–3515. [Google Scholar] [CrossRef]
- Lehallier, B.; Gate, D.; Schaum, N.; Nanasi, T.; Lee, S.E.; Yousef, H.; Moran Losada, P.; Berdnik, D.; Keller, A.; Verghese, J.; et al. Undulating changes in human plasma proteome profiles across the lifespan. Nat. Med. 2019, 25, 1843–1850. [Google Scholar] [CrossRef]
- Moqri, M.; Herzog, C.; Poganik, J.R.; Biomarkers of Aging Consortium; Justice, J.; Belsky, D.W.; Higgins-Chen, A.; Moskalev, A.; Fuellen, G.; Cohen, A.A.; et al. Biomarkers of aging for the identification and evaluation of longevity interventions. Cell 2023, 186, 3758–3775. [Google Scholar] [CrossRef] [PubMed]
- Page, M.; McKenzie, J.; Bossuyt, P.; Boutron, I.; Hoffmann, T.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Motschall, E.; Falck-Ytter, Y. Searching the MEDLINE literature database through PubMed: A short guide. Onkologie 2005, 28, 517–522. [Google Scholar] [CrossRef] [PubMed]
- Deutsch, E.W.; Bandeira, N.; Perez-Riverol, Y.; Sharma, V.; Carver, J.J.; Mendoza, L.; Kundu, D.J.; Wang, S.; Bandla, C.; Kamatchinathan, S.; et al. The ProteomeXchange consortium at 10 years: 2023 update. Nucleic Acids Res. 2023, 51, D1539–D1548. [Google Scholar] [CrossRef] [PubMed]
- Kusebauch, U.; Campbell, D.S.; Deutsch, E.W.; Chu, C.S.; Spicer, D.A.; Brusniak, M.-Y.; Slagel, J.; Sun, Z.; Stevens, J.; Grimes, B.; et al. Human srmatlas: A resource of targeted assays to quantify the complete human proteome. Cell 2016, 166, 766–778. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Kawashima, M. KEGG mapping tools for uncovering hidden features in biological data. Protein Sci. 2022, 31, 47–53. [Google Scholar] [CrossRef]
- KEGG Mapper. Available online: https://www.genome.jp/kegg/mapper/ (accessed on 15 October 2024).
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the unification of biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Milacic, M.; Beavers, D.; Conley, P.; Gong, C.; Gillespie, M.; Griss, J.; Haw, R.; Jassal, B.; Matthews, L.; May, B.; et al. The reactome pathway knowledgebase 2024. Nucleic Acids Res. 2024, 52, D672–D678. [Google Scholar] [CrossRef]
- Omenn, G.S.; States, D.J.; Adamski, M.; Blackwell, T.W.; Menon, R.; Hermjakob, H.; Apweiler, R.; Haab, B.B.; Simpson, R.J.; Eddes, J.S.; et al. Overview of the HUPO Plasma Proteome Project: Results from the pilot phase with 35 collaborating laboratories and multiple analytical groups, generating a core dataset of 3020 proteins and a publicly-available database. Proteomics 2005, 5, 3226–3245. [Google Scholar] [CrossRef]
- Human Plasma Proteome-Project (HPPP). Available online: https://hupo.org/Human-Plasma-Proteome-Project-(HPPP) (accessed on 14 October 2024).
- PeptideAtlas. Available online: https://db.systemsbiology.net/sbeams/cgi/PeptideAtlas/GetProteins?atlas_build_id=559&organism_id=2&redundancy_constraint=4&presence_level_constraint=1&action=QUERY (accessed on 14 October 2024).
- Petrak, J.; Ivanek, R.; Toman, O.; Cmejla, R.; Cmejlova, J.; Vyoral, D.; Zivny, J.; Vulpe, C.D. Déjà vu in proteomics. A hit parade of repeatedly identified differentially expressed proteins. Proteomics 2008, 8, 1744–1749. [Google Scholar] [CrossRef]
- Anderson, N.L. The clinical plasma proteome: A survey of clinical assays for proteins in plasma and serum. Clin. Chem. 2010, 56, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhao, T.; Zeng, J.; Zhang, R.; Pu, L.; Qian, S.; Xu, S.; Jiang, Y.; Pan, L.; Dai, X.; et al. Methods and clinical biomarker discovery for targeted proteomics using Olink technology. Proteom. Clin. Appl. 2024, 18, e2300233. [Google Scholar] [CrossRef] [PubMed]
- Gold, L.; Ayers, D.; Bertino, J.; Bock, C.; Bock, A.; Brody, E.N.; Carter, J.; Dalby, A.B.; Eaton, B.E.; Fitzwater, T.; et al. Aptamer-based multiplexed proteomic technology for biomarker discovery. PLoS ONE 2010, 5, e15004. [Google Scholar] [CrossRef] [PubMed]
- Kuh, D.; Karunananthan, S.; Bergman, H.; Cooper, R. A life-course approach to healthy ageing: Maintaining physical capability. Proc. Nutr. Soc. 2014, 73, 237–248. [Google Scholar] [CrossRef]
- Tanaka, T.; Biancotto, A.; Moaddel, R.; Moore, A.Z.; Gonzalez-Freire, M.; Aon, M.A.; Candia, J.; Zhang, P.; Cheung, F.; Fantoni, G.; et al. Plasma proteomic signature of age in healthy humans. Aging Cell 2018, 17, e12799. [Google Scholar] [CrossRef]
- Menni, C.; Kiddle, S.J.; Mangino, M.; Viñuela, A.; Psatha, M.; Steves, C.; Sattlecker, M.; Buil, A.; Newhouse, S.; Nelson, S.; et al. Circulating proteomic signatures of chronological age. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 809–816. [Google Scholar] [CrossRef]
- Lehallier, B.; Shokhirev, M.N.; Wyss-Coray, T.; Johnson, A.A. Data mining of human plasma proteins generates a multitude of highly predictive aging clocks that reflect different aspects of aging. Aging Cell 2020, 19, e13256. [Google Scholar] [CrossRef]
- Orwoll, E.S.; Wiedrick, J.; Jacobs, J.; Baker, E.S.; Piehowski, P.; Petyuk, V.; Gao, Y.; Shi, T.; Smith, R.D.; Bauer, D.C.; et al. Osteoporotic Fractures in Men Study (MrOS) Research Group High-throughput serum proteomics for the identification of protein biomarkers of mortality in older men. Aging Cell 2018, 17, e12717. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, X.; Shen, J.; Zhao, E.-F.; He, D.; Shen, H.; Liu, H.; Zhou, Y. Quantitative iTRAQ-based proteomic analysis of differentially expressed proteins in aging in human and monkey. BMC Genom. 2019, 20, 725. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, R.; Liu, F.; Jiang, P.; Xu, J.; Cao, H.; Du, X.; Ma, L.; Lin, F.; Cheng, L.; et al. TMT-Based Quantitative Proteomic Analysis Reveals Proteomic Changes Involved in Longevity. Proteom. Clin. Appl. 2019, 13, e1800024. [Google Scholar] [CrossRef]
- Salignon, J.; Faridani, O.R.; Miliotis, T.; Janssens, G.E.; Chen, P.; Zarrouki, B.; Sandberg, R.; Davidsson, P.; Riedel, C.G. Age prediction from human blood plasma using proteomic and small RNA data: A comparative analysis. Aging 2023, 15, 5240–5265. [Google Scholar] [CrossRef] [PubMed]
- Coenen, L.; Lehallier, B.; de Vries, H.E.; Middeldorp, J. Markers of aging: Unsupervised integrated analyses of the human plasma proteome. Front. Aging 2023, 4, 1112109. [Google Scholar] [CrossRef] [PubMed]
- Johnson, A.A.; Shokhirev, M.N.; Wyss-Coray, T.; Lehallier, B. Systematic review and analysis of human proteomics aging studies unveils a novel proteomic aging clock and identifies key processes that change with age. Ageing Res. Rev. 2020, 60, 101070. [Google Scholar] [CrossRef]
- Moaddel, R.; Ubaida-Mohien, C.; Tanaka, T.; Lyashkov, A.; Basisty, N.; Schilling, B.; Semba, R.D.; Franceschi, C.; Gorospe, M.; Ferrucci, L. Proteomics in aging research: A roadmap to clinical, translational research. Aging Cell 2021, 20, e13325. [Google Scholar] [CrossRef]
- Kalyakulina, A.; Yusipov, I.; Kondakova, E.; Bacalini, M.G.; Franceschi, C.; Vedunova, M.; Ivanchenko, M. Small immunological clocks identified by deep learning and gradient boosting. Front. Immunol. 2023, 14, 1177611. [Google Scholar] [CrossRef]
- Yusipov, I.; Kondakova, E.; Kalyakulina, A.; Krivonosov, M.; Lobanova, N.; Bacalini, M.G.; Franceschi, C.; Vedunova, M.; Ivanchenko, M. Accelerated epigenetic aging and inflammatory/immunological profile (ipAGE) in patients with chronic kidney disease. Geroscience 2022, 44, 817–834. [Google Scholar] [CrossRef]
- Conte, M.; Ostan, R.; Fabbri, C.; Santoro, A.; Guidarelli, G.; Vitale, G.; Mari, D.; Sevini, F.; Capri, M.; Sandri, M.; et al. Human aging and longevity are characterized by high levels of mitokines. J. Gerontol. A Biol. Sci. Med. Sci. 2019, 74, 600–607. [Google Scholar] [CrossRef]
- Hanks, L.J.; Gutiérrez, O.M.; Bamman, M.M.; Ashraf, A.; McCormick, K.L.; Casazza, K. Circulating levels of fibroblast growth factor-21 increase with age independently of body composition indices among healthy individuals. J. Clin. Transl. Endocrinol. 2015, 2, 77–82. [Google Scholar] [CrossRef]
- Villarroya, J.; Gallego-Escuredo, J.M.; Delgado-Anglés, A.; Cairó, M.; Moure, R.; Gracia Mateo, M.; Domingo, J.C.; Domingo, P.; Giralt, M.; Villarroya, F. Aging is associated with increased FGF21 levels but unaltered FGF21 responsiveness in adipose tissue. Aging Cell 2018, 17, e12822. [Google Scholar] [CrossRef]
- Gao, W.; Yang, J.; Liu, R.; Yan, Y.; Xie, C.; Yu, J.; Tang, K. FGF-21 biomarker detection at the sub-nanogram per mL level in human serum using normal-flow liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2020, 34, e8817. [Google Scholar] [CrossRef]
- Li, H.; Xu, L.; Jiang, W.; Qiu, X.; Xu, H.; Zhu, F.; Hu, Y.; Liang, S.; Cai, C.; Qiu, W.; et al. Pleiotrophin ameliorates age-induced adult hippocampal neurogenesis decline and cognitive dysfunction. Cell Rep. 2023, 42, 113022. [Google Scholar] [CrossRef] [PubMed]
- Hugosson, F.; Sjögren, C.; Birve, A.; Hedlund, L.; Eriksson, T.; Palmer, R.H. The Drosophila midkine/pleiotrophin homologues Miple1 and Miple2 affect adult lifespan but are dispensable for alk signaling during embryonic gut formation. PLoS ONE 2014, 9, e112250. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-T.; Pamir, N.; Liu, N.-C.; Kirk, E.A.; Averill, M.M.; Becker, L.; Larson, I.; Hagman, D.K.; Foster-Schubert, K.E.; van Yserloo, B.; et al. Macrophage metalloelastase (MMP12) regulates adipose tissue expansion, insulin sensitivity, and expression of inducible nitric oxide synthase. Endocrinology 2014, 155, 3409–3420. [Google Scholar] [CrossRef] [PubMed]
- Traylor, M.; Mäkelä, K.-M.; Kilarski, L.L.; Holliday, E.G.; Devan, W.J.; Nalls, M.A.; Wiggins, K.L.; Zhao, W.; Cheng, Y.-C.; Achterberg, S.; et al. A novel MMP12 locus is associated with large artery atherosclerotic stroke using a genome-wide age-at-onset informed approach. PLoS Genet. 2014, 10, e1004469. [Google Scholar] [CrossRef]
- Krim, S.R.; Vivo, R.P.; de Lemos, J.A. B-type natriuretic peptides in acute coronary syndromes: Implications in an aging population. Clin. Cardiol. 2012, 35, 682–685. [Google Scholar] [CrossRef]
- Gassanov, N.; Biesenbach, E.; Caglayan, E.; Nia, A.; Fuhr, U.; Er, F. Natriuretic peptides in therapy for decompensated heart failure. Eur. J. Clin. Pharmacol. 2012, 68, 223–230. [Google Scholar] [CrossRef]
- Fried, L.P.; Ferrucci, L.; Darer, J.; Williamson, J.D.; Anderson, G. Untangling the concepts of disability, frailty, and comorbidity: Implications for improved targeting and care. J. Gerontol. A Biol. Sci. Med. Sci. 2004, 59, 255–263. [Google Scholar] [CrossRef]
- Li, J.; Yi, X.; Yao, Z.; Chakkalakal, J.V.; Xing, L.; Boyce, B.F. TNF Receptor-Associated Factor 6 Mediates TNFα-Induced Skeletal Muscle Atrophy in Mice During Aging. J. Bone Miner. Res. 2020, 35, 1535–1548. [Google Scholar] [CrossRef]
- Takahashi, S.; Futatsugi-Yumikura, S.; Fukuoka, A.; Yoshimoto, T.; Nakanishi, K.; Yonehara, S. Fas deficiency in mice with the Balb/c background induces blepharitis with allergic inflammation and hyper-IgE production in conjunction with severe autoimmune disease. Int. Immunol. 2013, 25, 287–293. [Google Scholar] [CrossRef]
- Hong, S.W.; Kang, J.-H. Growth differentiation factor-15 as a modulator of bone and muscle metabolism. Front. Endocrinol. 2022, 13, 948176. [Google Scholar] [CrossRef]
- Wang, X.; Chrysovergis, K.; Kosak, J.; Kissling, G.; Streicker, M.; Moser, G.; Li, R.; Eling, T.E. hNAG-1 increases lifespan by regulating energy metabolism and insulin/IGF-1/mTOR signaling. Aging 2014, 6, 690–704. [Google Scholar] [CrossRef] [PubMed]
- PeptideAtlas—HPPP Data Central. Available online: https://peptideatlas.org/hupo/hppp/ (accessed on 25 October 2024).
- Vavilov, N.; Ilgisonis, E.; Lisitsa, A.; Ponomarenko, E.; Farafonova, T.; Tikhonova, O.; Zgoda, V.; Archakov, A. Number of detected proteins as the function of the sensitivity of proteomic technology in human liver cells. Curr. Protein Pept. Sci. 2022, 23, 290–298. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Jiang, Z.; Qiu, Y.; Yu, J.; Zhang, Y.; Wang, J.; Ye, B.; Huang, Y.; Gu, W.; Huang, Y.; et al. High blood galectin-3 level associated with risk of frailty in aging. Front. Endocrinol. 2023, 14, 1189192. [Google Scholar] [CrossRef] [PubMed]
- Fraser, H.C.; Kuan, V.; Johnen, R.; Zwierzyna, M.; Hingorani, A.D.; Beyer, A.; Partridge, L. Biological mechanisms of aging predict age-related disease co-occurrence in patients. Aging Cell 2022, 21, e13524. [Google Scholar] [CrossRef]

| # | Uniprot ID | Gene Name | Protein Name | Count (Out of 17 Datasets) | MS-Data | Concentration, nmol/L |
|---|---|---|---|---|---|---|
| 1 | P15692 * | VEGFA | Vascular endothelial growth factor A | 10 | 598 | n/a |
| 2 | P01034 | CST3 | Cystatin-C | 9 | 334 | 34.7 |
| 3 | P14151 | SELL | L-selectin | 8 | 878 | 76.0 |
| 4 | P01011 | SERPINA3 | α1-Antichymotrypsin | 8 | 714 | 5270.0 |
| 5 | P02765 | AHSG | Alpha-2-HS-glycoprotein | 8 | 544 | 1982.7 |
| 6 | P18065 | IGFBP2 | Insulin-like growth factor-binding protein 2 | 7 | 553 | 8.5 |
| 7 | P02751 | FINC | Fibronectin | 7 | 395 | 1252.0 |
| 8 | P00742 | F10 | Coagulation factor X | 7 | 330 | 170.3 |
| 9 | P02778 * | CXCL10 | C-X-C motif chemokine 10 | 7 | 239 | n/a |
| 10 | P08697 | A2AP | Alpha-2-antiplasmin | 7 | 68 | 875.4 |
| 11 | P01031 | CO5 | Complement C5 | 6 | 702 | 150.8 |
| 12 | P02679 | FIBG | Fibrinogen gamma chain | 6 | 588 | 5384.0 |
| 13 | P02768 | ALB | Albumin, serum | 6 | 583 | 1,101,000.0 |
| 14 | P17931 * | LGALS3 | Galectin-3 | 6 | 408 | n/a |
| 15 | P02748 | C9 | Complement component C9 | 6 | 368 | 259.0 |
| 16 | O14791 | APOL1 | Apolipoprotein L1 | 6 | 355 | 692.5 |
| 17 | P05155 | IC1 | Plasma protease C1 inhibitor | 6 | 344 | 399.5 |
| 18 | P00747 | PLMN | Plasminogen | 6 | 267 | 661.5 |
| 19 | P48740 | MASP1 | Mannan-binding lectin serine protease 1 | 6 | 123 | 89.9 |
| 20 | Q07325 * | CXCL9 | C-X-C motif chemokine 9 | 6 | 12 | n/a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kliuchnikova, A.A.; Ilgisonis, E.V.; Archakov, A.I.; Ponomarenko, E.A.; Moskalev, A.A. Proteomic Markers of Aging and Longevity: A Systematic Review. Int. J. Mol. Sci. 2024, 25, 12634. https://doi.org/10.3390/ijms252312634
Kliuchnikova AA, Ilgisonis EV, Archakov AI, Ponomarenko EA, Moskalev AA. Proteomic Markers of Aging and Longevity: A Systematic Review. International Journal of Molecular Sciences. 2024; 25(23):12634. https://doi.org/10.3390/ijms252312634
Chicago/Turabian StyleKliuchnikova, Anna A., Ekaterina V. Ilgisonis, Alexander I. Archakov, Elena A. Ponomarenko, and Alexey A. Moskalev. 2024. "Proteomic Markers of Aging and Longevity: A Systematic Review" International Journal of Molecular Sciences 25, no. 23: 12634. https://doi.org/10.3390/ijms252312634
APA StyleKliuchnikova, A. A., Ilgisonis, E. V., Archakov, A. I., Ponomarenko, E. A., & Moskalev, A. A. (2024). Proteomic Markers of Aging and Longevity: A Systematic Review. International Journal of Molecular Sciences, 25(23), 12634. https://doi.org/10.3390/ijms252312634






