Docosahexaenoic Acid (DHA) Supplementation in a Triglyceride Form Prevents from Polyglutamine-Induced Dysfunctions in Caenorhabditis elegans
Abstract
1. Introduction
2. Results
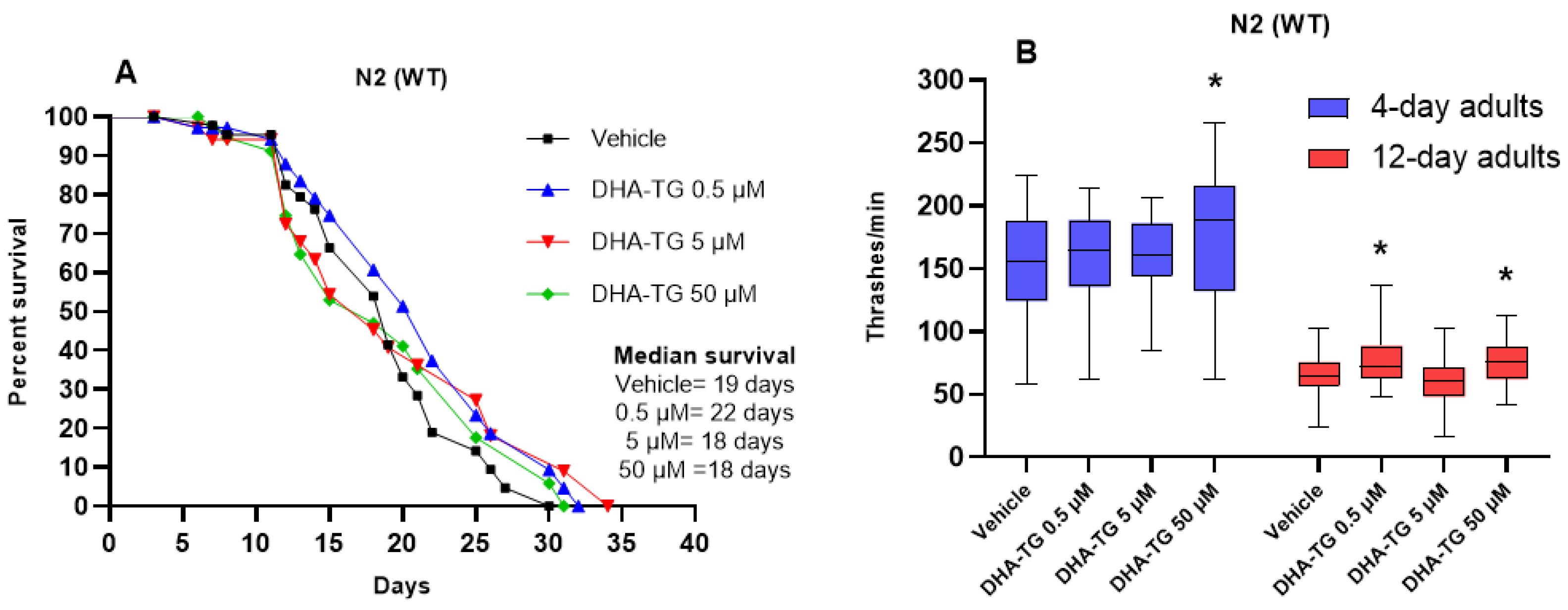
2.1. Determining DHA Concentration in WT Nematodes
2.2. Lifespan and Motility Promoting Effect of DHA-TG in AM101 (rmls110) Nematodes
2.3. PolyQ Reduction After DHA-TG Treatment in AM141 (rmIs133) Nematodes
2.4. Oxidative Stress Resistance of AM101 (rmls110) Nematodes After DHA-TG Treatment
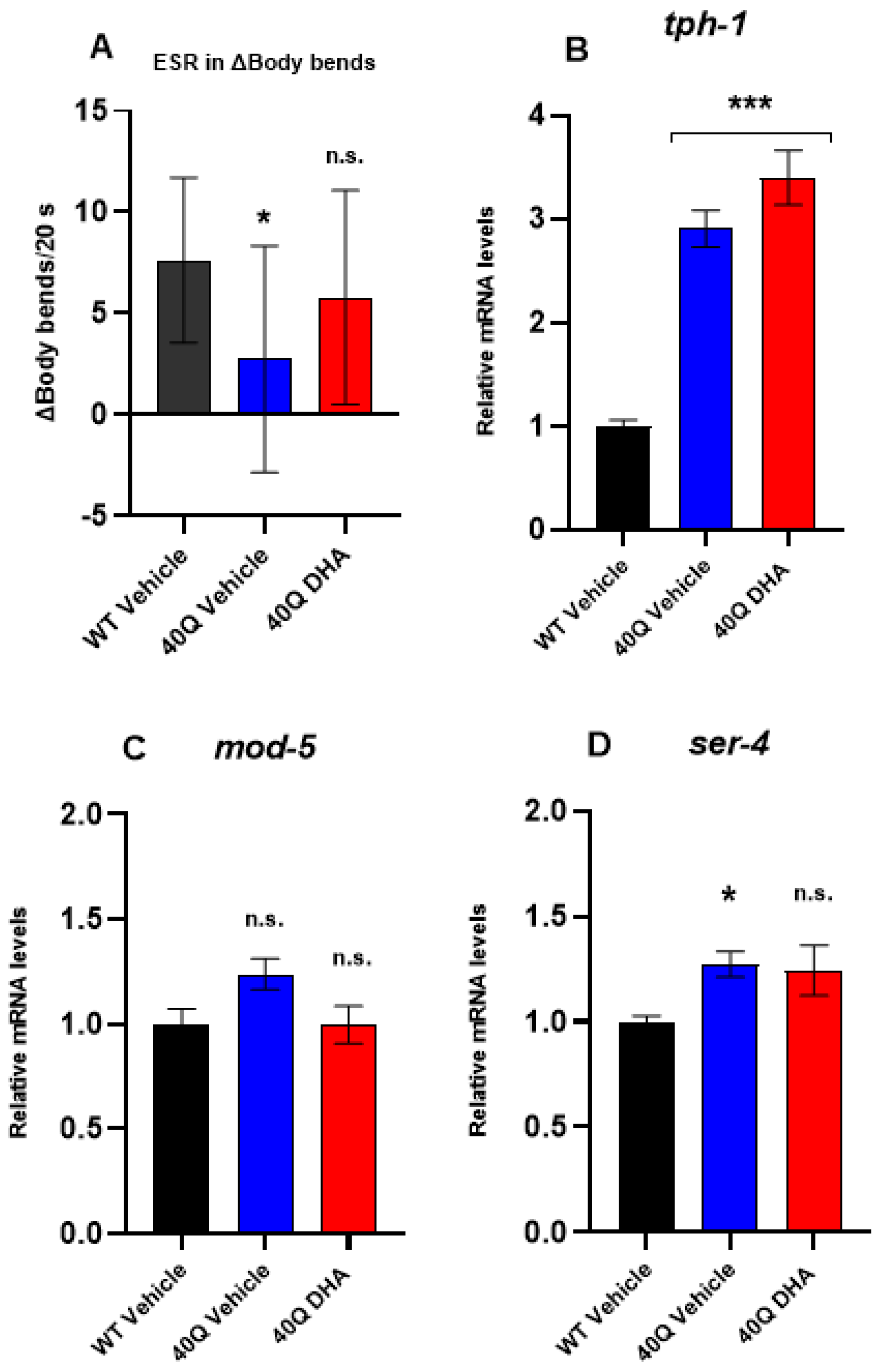
2.5. Neurobehavioral Function After DHA-TG Treatment of AM101 (rmls110) Nematodes
2.5.1. Dopaminergic Response
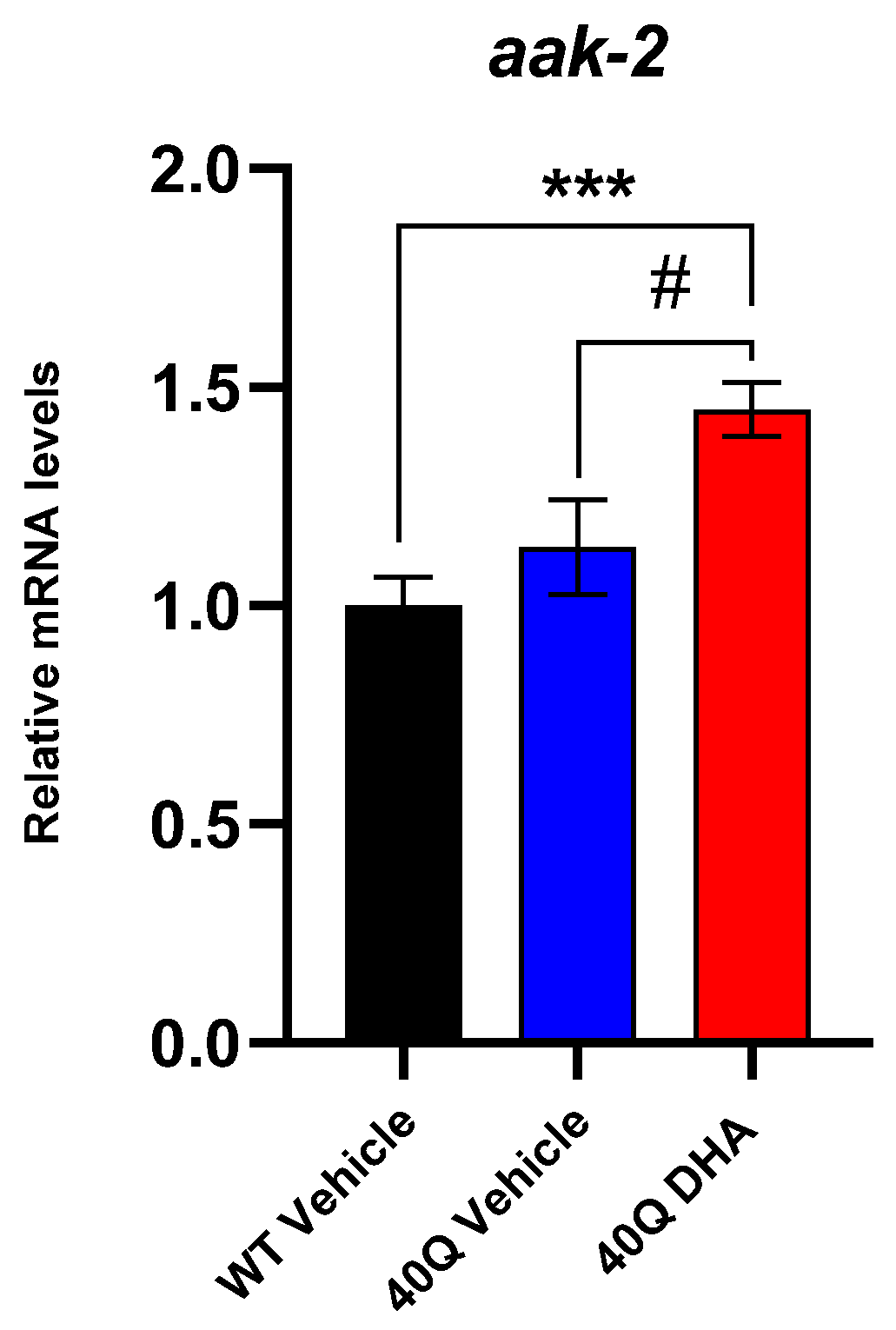
2.5.2. Serotonergic Response
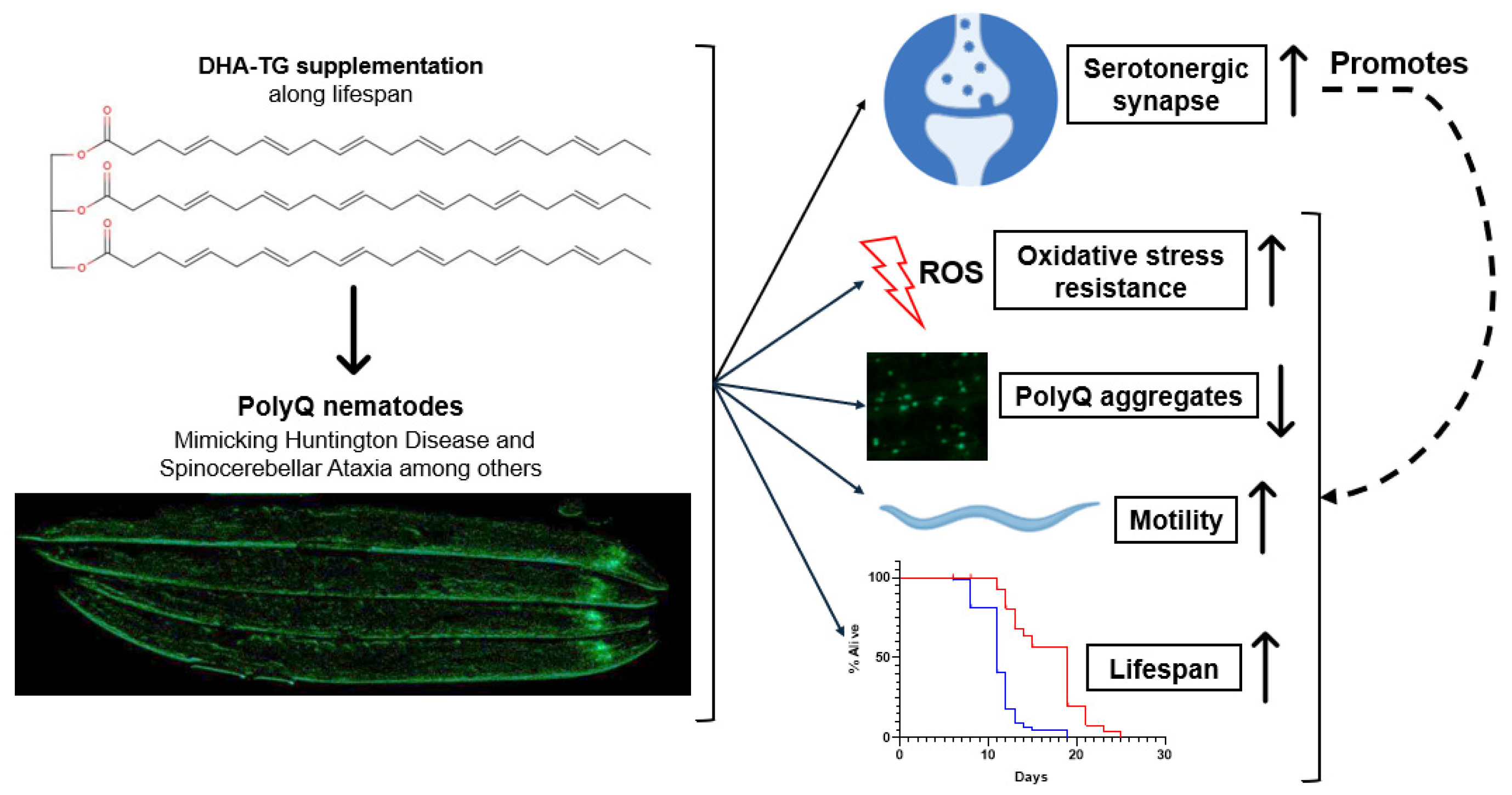
3. Discussion
4. Materials and Methods
4.1. C. elegans Strains and Maintenance
4.2. Preparation of DHA-TG Nanoemulsions
4.3. Lifespan Analysis
4.4. Motility Assay
4.5. Basal and Enhanced Slowing Response
4.6. Oxidative Stress Resistance Assay
4.7. In Vivo Scoring of polyQ in Muscle Cells
4.8. RNA Extraction, Retrotranscription, and (RT)-qPCR
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hipp, M.S.; Park, S.H.; Hartl, U.U. Proteostasis Impairment in Protein-Misfolding and -Aggregation Diseases. Trends Cell Biol. 2014, 24, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of Neuroinflammation in Neurodegeneration Development. Signal Transduct. Target. Ther. 2023, 8, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Daldin, M.; Fodale, V.; Cariulo, C.; Azzollini, L.; Verani, M.; Martufi, P.; Spiezia, M.C.; Deguire, S.M.; Cherubini, M.; MacDonald, D.; et al. Polyglutamine Expansion Affects Huntingtin Conformation in Multiple Huntington’s Disease Models. Sci. Rep. 2017, 7, 5070. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Del Río, C.; Tortajada-Pérez, J.; Gómez-Escribano, A.P.; Casterá, F.; Peiró, C.; Millán, J.M.; Herrero, M.J.; Vázquez-Manrique, R.P. Metformin to Treat Huntington Disease: A Pleiotropic Drug against a Multi-System Disorder. Mech. Ageing Dev. 2022, 204, 111670. [Google Scholar] [CrossRef]
- Kopito, R.R. Aggresomes, Inclusion Bodies and Protein Aggregation. Trends Cell Biol. 2000, 10, 524–530. [Google Scholar] [CrossRef]
- Lieberman, A.P.; Shakkottai, V.G.; Albin, R.L. Polyglutamine Repeats in Neurodegenerative Diseases. Annu. Rev. Pathol. Mech. Dis. 2019, 14, 1–27. [Google Scholar] [CrossRef]
- Jimenez-Sanchez, M.; Licitra, F.; Underwood, B.R.; Rubinsztein, D.C. Huntington’s Disease: Mechanisms of Pathogenesis and Therapeutic Strategies. Cold Spring Harb. Perspect. Med. 2017, 7, 1–22. [Google Scholar] [CrossRef]
- Duran-Aniotz, C.; Moreno-Gonzalez, I.; Medinas, D.B.; Morales, R. Editorial: Protein Misfolding and Proteostasis Impairment in Aging and Neurodegeneration: From Spreading Studies to Therapeutic Approaches. Front. Aging Neurosci. 2021, 13, 830779. [Google Scholar] [CrossRef]
- Reza-Zaldívar, E.E.; Jacobo-Velázquez, D.A. Comprehensive Review of Nutraceuticals against Cognitive Decline Associated with Alzheimer’s Disease. ACS Omega 2023, 8, 35499–35522. [Google Scholar] [CrossRef]
- Kousparou, C.; Fyrilla, M.; Stephanou, A.; Patrikios, I. DHA/EPA (Omega-3) and LA/GLA (Omega-6) as Bioactive Molecules in Neurodegenerative Diseases. Int. J. Mol. Sci. 2023, 24, 10717. [Google Scholar] [CrossRef]
- Lafuente, M.; González-Herrero, M.E.R.; Villadóniga, S.R.; Domingo, J.C. Antioxidant Activity and Neuroprotective Role of Docosahexaenoic Acid (DHA) Supplementation in Eye Diseases That Can Lead to Blindness: A Narrative Review. Antioxidants 2021, 10, 386. [Google Scholar] [CrossRef] [PubMed]
- Troesch, B.; Eggersdorfer, M.; Laviano, A.; Rolland, Y.; Smith, A.D.; Warnke, I.; Weimann, A.; Calder, P.C. Expert Opinion on Benefits of Long-Chain Omega-3 Fatty Acids (DHA and EPA) in Aging and Clinical Nutrition. Nutrients 2020, 12, 2555. [Google Scholar] [CrossRef] [PubMed]
- Mora, I.; Arola, L.; Caimari, A.; Escoté, X.; Puiggròs, F. Structured Long-Chain Omega-3 Fatty Acids for Improvement of Cognitive Function during Aging. Int. J. Mol. Sci. 2022, 23, 3472. [Google Scholar] [CrossRef] [PubMed]
- Avallone, R.; Vitale, G.; Bertolotti, M. Omega-3 Fatty Acids and Neurodegenerative Diseases: New Evidence in Clinical Trials. Int. J. Mol. Sci. 2019, 20, 4256. [Google Scholar] [CrossRef] [PubMed]
- Vega, O.M.; Cepeda, C. Converging Evidence in Support of Omega-3 Polyunsaturated Fatty Acids as a Potential Therapy for Huntington’s Disease Symptoms. Rev. Neurosci. 2021, 32, 871–886. [Google Scholar] [CrossRef]
- Gómez-Escribano, A.P.; Bono-Yagüe, J.; García-Gimeno, M.A.; Sequedo, M.D.; Hervás, D.; Fornés-Ferrer, V.; Torres-Sánchez, S.C.; Millán, J.M.; Sanz, P.; Vázquez-Manrique, R.P. Synergistic Activation of AMPK Prevents from Polyglutamine-Induced Toxicity in Caenorhabditis elegans. Pharmacol. Res. 2020, 161, 105105. [Google Scholar] [CrossRef]
- Marza, E.; Lesa, G.M. Polyunsaturated Fatty Acids and Neurotransmission in Caenorhabditis elegans. Biochem. Soc. Trans. 2006, 34, 77–80. [Google Scholar] [CrossRef]
- Kosel, M.; Wild, W.; Bell, A.; Rothe, M.; Lindschau, C.; Steinberg, C.E.W.; Schunck, W.H.; Menzel, R. Eicosanoid Formation by a Cytochrome P450 Isoform Expressed in the Pharynx of Caenorhabditis elegans. Biochem. J. 2011, 435, 689–700. [Google Scholar] [CrossRef]
- Mora, I.; Pérez-Santamaria, A.; Tortajada-Pérez, J.; Vázquez-Manrique, R.P.; Arola, L.; Puiggròs, F. Structured Docosahexaenoic Acid (DHA) Enhances Motility and Promotes the Antioxidant Capacity of Aged C. Elegans. Cells 2023, 12, 1932. [Google Scholar] [CrossRef]
- SenGupta, T.; Lefol, Y.; Lirussi, L.; Suaste, V.; Luders, T.; Gupta, S.; Aman, Y.; Sharma, K.; Fang, E.F.; Nilsen, H. Krill Oil Protects Dopaminergic Neurons from Age-Related Degeneration through Temporal Transcriptome Rewiring and Suppression of Several Hallmarks of Aging. Aging 2022, 14, 8661. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Ding, F.; Li, J.; Li, L.; Xu, Z.; Zhao, Y. N-3 Polyunsaturated Fatty Acids Attenuate Amyloid-Beta-Induced Toxicity in AD Transgenic Caenorhabditis elegans via Promotion of Proteasomal Activity and Activation of PPAR-Gamma. J. Nutr. Biochem. 2024, 127, 109603. [Google Scholar] [CrossRef] [PubMed]
- Chopra, M.; Reddy, A.S.; Abbott, N.L.; De Pablo, J.J. Folding of Polyglutamine Chains. J. Chem. Phys. 2008, 129, 135102. [Google Scholar] [CrossRef] [PubMed]
- Castejón, N.; Señoráns, F.J. Enzymatic Modification to Produce Health-Promoting Lipids from Fish Oil, Algae and Other New Omega-3 Sources: A Review. New Biotechnol. 2020, 57, 45–54. [Google Scholar] [CrossRef]
- Brunton, S.; Collins, N. Differentiating Prescription Omega-3-Acid Ethyl Esters (P-OM3) from Dietary-Supplement Omega-3 Fatty Acids. Curr. Med. Res. Opin. 2007, 23, 1139–1145. [Google Scholar] [CrossRef]
- Dyerberg, J.; Madsen, P.; Møller, J.M.; Aardestrup, I.; Schmidt, E.B. Bioavailability of Marine N-3 Fatty Acid Formulations. Prostaglandins Leukot. Essent. Fatty Acids 2010, 83, 137–141. [Google Scholar] [CrossRef]
- Kitson, A.P.; Metherel, A.H.; Chen, C.T.; Domenichiello, A.F.; Trépanier, M.O.; Berger, A.; Bazinet, R.P. Effect of Dietary Docosahexaenoic Acid (DHA) in Phospholipids or Triglycerides on Brain DHA Uptake and Accretion. J. Nutr. Biochem. 2016, 33, 91–102. [Google Scholar] [CrossRef]
- Schuchardt, J.P.; Hahn, A. Bioavailability of Long-Chain Omega-3 Fatty Acids. Prostaglandins Leukot. Essent. Fatty Acids 2013, 89, 1–8. [Google Scholar] [CrossRef]
- Chang, M.; Yang, J.; Guo, X.; Zhang, T.; Liu, R.; Jin, Q.; Wang, X. Medium / Long-Chain Structured Triglycerides Are Superior to Physical Mixtures Triglycerides in Caenorhabditis elegans Lifespan through an AMPK Modified Pathway. Food Biosci. 2021, 39, 100815. [Google Scholar] [CrossRef]
- Pietsch, K.; Saul, N.; Chakrabarti, S.; Stürzenbaum, S.R.; Menzel, R.; Steinberg, C.E.W. Hormetins, Antioxidants and Prooxidants: Defining Quercetin-, Caffeic Acid- and Rosmarinic Acid-Mediated Life Extension in C. elegans. Biogerontology 2011, 12, 329–347. [Google Scholar] [CrossRef]
- Chen, T.C.; Hsu, W.L.; Wu, C.Y.; Lai, Y.R.; Chao, H.R.; Chen, C.H.; Tsai, M.H. Effect of Omega-6 Linoleic Acid on Neurobehavioral Development in Caenorhabditis elegans. Prostaglandins Leukot. Essent. Fat. Acids 2023, 191, 102557. [Google Scholar] [CrossRef]
- Brignull, H.R.; Moore, F.E.; Tang, S.J.; Morimoto, R.I. Polyglutamine Proteins at the Pathogenic Threshold Display Neuron-Specific Aggregation in a Pan-Neuronal Caenorhabditis elegans Model. J. Neurosci. 2006, 26, 7597–7606. [Google Scholar] [CrossRef] [PubMed]
- Clifford, J.J.; Drago, J.; Natoli, A.L.; Wong, J.Y.F.; Kinsella, A.; Waddington, J.L.; Vaddadi, K.S. Essential Fatty Acids given from Conception Prevent Topographies of Motor Deficit in a Transgenic Model of Huntington’s Disease. Neuroscience 2002, 109, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, L.M.; Soares, M.V.; da Silva, A.F.; dos Santos, L.V.; de Souza, L.I.; da Silveira, T.L.; Baptista, F.B.O.; de Oliveira, G.V.; Pappis, C.; Dressler, V.L.; et al. Toxicity of Copper and Zinc Alone and in Combination in Caenorhabditis elegans Model of Huntington’s Disease and Protective Effects of Rutin. Neurotoxicology 2023, 97, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Shen, L.; Wang, Y. The Phenotypic and Behavioral Defects Can Be Transferred from Zinc-Exposed Nematodes to Their Progeny. Environ. Toxicol. Pharmacol. 2007, 24, 223–230. [Google Scholar] [CrossRef]
- Alex Parker, J.; Vazquez-Manrique, R.P.; Tourette, C.; Farina, F.; Offner, N.; Mukhopadhyay, A.; Orfila, A.M.; Darbois, A.; Menet, S.; Tissenbaum, H.A.; et al. Integration of β-Catenin, Sirtuin, and FOXO Signaling Protects from Mutant Huntingtin Toxicity. J. Neurosci. 2012, 32, 12630–22640. [Google Scholar] [CrossRef]
- Carling, D.; Thornton, C.; Woods, A.; Sanders, M.J. AMP-Activated Protein Kinase: New Regulation, New Roles? Biochem. J. 2012, 445, 11–27. [Google Scholar] [CrossRef]
- Vázquez-Manrique, R.P.; Farina, F.; Cambon, K.; Dolores Sequedo, M.; Parker, A.J.; Millán, J.M.; Weiss, A.; Déglon, N.; Neri, C. AMPK Activation Protects from Neuronal Dysfunction and Vulnerability across Nematode, Cellular and Mouse Models of Huntington’s Disease. Hum. Mol. Genet. 2016, 25, 1043–1058. [Google Scholar] [CrossRef]
- Machiela, E.; Dues, D.J.; Senchuk, M.M.; Van Raamsdonk, J.M. Oxidative Stress Is Increased in C. Elegans Models of Huntington’s Disease but Does Not Contribute to Polyglutamine Toxicity Phenotypes. Neurobiol. Dis. 2016, 96, 1–11. [Google Scholar] [CrossRef]
- Bicca Obetine Baptista, F.; Arantes, L.P.; Machado, M.L.; Da Silva, A.F.; Marafiga Cordeiro, L.; Da Silveira, T.L.; Soares, F.A.A. Diphenyl Diselenide Protects a Caenorhabditis elegans Model for Huntington’s Disease by Activation of the Antioxidant Pathway and a Decrease in Protein Aggregation. Metallomics 2020, 12, 1142–1158. [Google Scholar] [CrossRef]
- Liu, Y.; Li, H.; Li, Y.; Yang, M.; Wang, X.; Peng, Y. Velvet Antler Methanol Extracts Ameliorate Parkinson’s Disease by Inhibiting Oxidative Stress and Neuroinflammation: From C. elegans to Mice. Oxid. Med. Cell Longev. 2021, 2021, 8864395. [Google Scholar] [CrossRef]
- Cepeda, C.; Murphy, K.P.S.; Parent, M.; Levine, M.S. The Role of Dopamine in Huntington’s Disease. Prog. Brain Res. 2014, 211, 235–254. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, F.A.; Edwards, R.H.; Fonnum, F. Vesicular Neurotransmitter Transporters as Targets for Endogenous and Exogenous Toxic Substances. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 277–301. [Google Scholar] [CrossRef] [PubMed]
- Schwab, L.C.; Garas, S.N.; Drouin-Ouellet, J.; Mason, S.L.; Stott, S.R.; Barker, R.A. Dopamine and Huntington’s Disease. Expert. Rev. Neurother. 2015, 15, 445–458. [Google Scholar] [CrossRef] [PubMed]
- Chitre, N.M.; Wood, B.J.; Ray, A.; Moniri, N.H.; Murnane, K.S. Docosahexaenoic Acid Protects Motor Function and Increases Dopamine Synthesis in a Rat Model of Parkinson’s Disease via Mechanisms Associated with Increased Protein Kinase Activity in the Striatum. Neuropharmacology 2020, 167, 107976. [Google Scholar] [CrossRef]
- Miller, G.W.; Gainetdinov, R.R.; Levey, A.I.; Caron, M.G. Dopamine Transporters and Neuronal Injury. Trends Pharmacol. Sci. 1999, 20, 424–429. [Google Scholar] [CrossRef]
- Rivard, L.; Srinivasan, J.; Stone, A.; Ochoa, S.; Sternberg, P.W.; Loer, C.M. A Comparison of Experience-Dependent Locomotory Behaviors and Biogenic Amine Neurons in Nematode Relatives of Caenorhabditis elegans. BMC Neurosci. 2010, 11, 22. [Google Scholar] [CrossRef]
- Zhang, Y.; Lu, H.; Bargmann, C.I. Pathogenic Bacteria Induce Aversive Olfactory Learning in Caenorhabditis elegans. Nature 2005, 438, 179–184. [Google Scholar] [CrossRef]
- Di Matteo, V.; Pierucci, M.; Esposito, E.; Crescimanno, G.; Benigno, A.; Di Giovanni, G. Serotonin Modulation of the Basal Ganglia Circuitry: Therapeutic Implication for Parkinson’s Disease and Other Motor Disorders. Prog. Brain Res. 2008, 172, 423–463. [Google Scholar] [CrossRef]
- Yohrling IV, G.J.; Jiang, G.C.T.; DeJohn, M.M.; Robertson, D.J.; Vrana, K.E.; Cha, J.H.J. Inhibition of Tryptophan Hydroxylase Activity and Decreased 5-HT1A Receptor Binding in a Mouse Model of Huntington’s Disease. J. Neurochem. 2002, 82, 1416–1423. [Google Scholar] [CrossRef]
- Dag, U.; Nwabudike, I.; Kang, D.; Gomes, M.A.; Kim, J.; Atanas, A.A.; Bueno, E.; Estrem, C.; Pugliese, S.; Wang, Z.; et al. Dissecting the Functional Organization of the C. elegans Serotonergic System at Whole-Brain Scale. Cell 2023, 186, 2574–2592.e20. [Google Scholar] [CrossRef]
- Jurcau, A.; Jurcau, C.M. Mitochondria in Huntington’s Disease: Implications in Pathogenesis and Mitochondrial-Targeted Therapeutic Strategies. Neural Regen. Res. 2023, 18, 1472. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, L.M.; Soares, M.V.; da Silva, A.F.; Machado, M.L.; Bicca Obetine Baptista, F.; da Silveira, T.L.; Arantes, L.P.; Soares, F.A.A. Neuroprotective Effects of Rutin on ASH Neurons in Caenorhabditis elegans Model of Huntington’s Disease. Nutr. Neurosci. 2022, 25, 2288–2301. [Google Scholar] [CrossRef] [PubMed]
- Bono-Yagüe, J.; Gómez-Escribano, A.P.; Millán, J.M.; Vázquez-Manrique, R.P. Reactive Species in Huntington Disease: Are They Really the Radicals You Want to Catch? Antioxidants 2020, 9, 577. [Google Scholar] [CrossRef] [PubMed]
- Sanchis, A.; García-Gimeno, M.A.; Cañada-Martínez, A.J.; Sequedo, M.D.; Millán, J.M.; Sanz, P.; Vázquez-Manrique, R.P. Metformin Treatment Reduces Motor and Neuropsychiatric Phenotypes in the ZQ175 Mouse Model of Huntington Disease. Exp. Mol. Med. 2019, 51, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Landon, G.; Whitney, W.; Priya, R.; Mindy, F. Glucose Effects on Polyglutamine-Induced Proteotoxic Stress in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2020, 522, 709–715. [Google Scholar] [CrossRef]
- Lesa, G.M.; Palfreyman, M.; Hall, D.H.; Clandinin, M.T.; Rudolph, C.; Jorgensen, E.M.; Schiavo, G. Long Chain Polyunsaturated Fatty Acids Are Required for Efficient Neurotransmission in C. elegans. J. Cell Sci. 2003, 116, 4965–4975. [Google Scholar] [CrossRef]
- Hardaker, L.A.; Singer, E.; Kerr, R.; Zhou, G.; Schafer, W.R. Serotonin Modulates Locomotory Behavior and Coordinates Egg-Laying and Movement in Caenorhabditis elegans. J. Neurobiol. 2001, 49, 303–313. [Google Scholar] [CrossRef]
- Berendzen, K.M.; Durieux, J.; Shao, L.W.; Tian, Y.; Kim, H.; Wolff, S.; Liu, Y.; Dillin, A. Neuroendocrine Coordination of Mitochondrial Stress Signaling and Proteostasis. Cell 2016, 166, 1553. [Google Scholar] [CrossRef]
- Naß, J.; Abdelfatah, S.; Efferth, T. Induction of Stress Resistance and Extension of Lifespan in Chaenorhabditis elegans Serotonin-Receptor Knockout Strains by Withanolide A. Phytomedicine 2021, 84, 153482. [Google Scholar] [CrossRef]
- Tatum, M.C.; Ooi, F.K.; Chikka, M.R.; Chauve, L.; Martinez-Velazquez, L.A.; Steinbusch, H.W.M.; Morimoto, R.I.; Prahlad, V. Neuronal Serotonin Release Triggers the Heat Shock Response in C. elegans in the Absence of Temperature Increase. Curr. Biol. 2015, 25, 163–174. [Google Scholar] [CrossRef]
- Liang, B.; Moussaif, M.; Kuan, C.J.; Gargus, J.J.; Sze, J.Y. Serotonin Targets the DAF-16/FOXO Signaling Pathway to Modulate Stress Responses. Cell Metab. 2006, 4, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhou, Y.; Liang, Y.; Chen, L.; Liu, L.; Wei, F.; Li, G. Fluoxetine Promotes Longevity via Reactive Oxygen Species in Caenorhabditis elegans. J. Gerontol. Ser. A 2024, 79, glad220. [Google Scholar] [CrossRef]
- Menzel, R.; Zhang, X.; Pietrucik, T.; Bathelt, A.; Ruess, L. Omega-3 PUFA and the Fitness and Cognition of the Nematode Caenorhabditis elegans under Different Environmental Conditions. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2024, 270, 110925. [Google Scholar] [CrossRef]
- Jacobs, M.L.; Faizi, H.A.; Peruzzi, J.A.; Vlahovska, P.M.; Kamat, N.P. EPA and DHA Differentially Modulate Membrane Elasticity in the Presence of Cholesterol. Biophys. J. 2021, 120, 2317–2329. [Google Scholar] [CrossRef]
- Caires, R.; Sierra-Valdez, F.J.; Millet, J.R.M.; Herwig, J.D.; Roan, E.; Vásquez, V.; Cordero-Morales, J.F. Omega-3 Fatty Acids Modulate TRPV4 Function through Plasma Membrane Remodeling. Cell Rep. 2017, 21, 246–258. [Google Scholar] [CrossRef]
- Zhang, Y.; Yin, J.; Yan, H.; Yan, L.; Li, Y.; Zhang, C.; Li, Y.; Liu, B.; Lin, J.; Zhang, L.; et al. Correlations between Omega-3 Fatty Acids and Inflammatory/Glial Abnormalities: The Involvement of the Membrane and Neurotransmitter Dysfunction in Schizophrenia. Front. Cell Neurosci. 2023, 17, 1163764. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Noormohammadi, A.; Koyuncu, S.; Calculli, G.; Simic, M.S.; Herholz, M.; Trifunovic, A.; Vilchez, D. Prostaglandin Signals from Adult Germline Stem Cells Delay Somatic Ageing of Caenorhabditis elegans. Nat. Metab. 2019, 1, 790–810. [Google Scholar] [CrossRef] [PubMed]
- Bird, J.K.; Troesch, B.; Warnke, I.; Calder, P.C. The Effect of Long Chain Omega-3 Polyunsaturated Fatty Acids on Muscle Mass and Function in Sarcopenia: A Scoping Systematic Review and Meta-Analysis. Clin. Nutr. ESPEN 2021, 46, 73–86. [Google Scholar] [CrossRef]
- Cross, A.J.; Reynolds, G.P.; Hewitt, L.M.; Slater, P. Brain Serotonin Receptors in Huntington’s Disease. Neurochem. Int. 1986, 9, 431–435. [Google Scholar] [CrossRef]
- Pang, T.Y.C.; Du, X.; Zajac, M.S.; Howard, M.L.; Hannan, A.J. Altered Serotonin Receptor Expression Is Associated with Depression-Related Behavior in the R6/1 Transgenic Mouse Model of Huntington’s Disease. Hum. Mol. Genet. 2009, 18, 753–766. [Google Scholar] [CrossRef]
- Brenner, S. The Genetics of Caenorhabditis elegans. Genetics 1974, 77, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Porta-de-la-Riva, M.; Fontrodona, L.; Villanueva, A.; Cerón, J. Basic Caenorhabditis elegans Methods: Synchronization and Observation. J. Vis. Exp. 2012, 64, e4019. [Google Scholar] [CrossRef]
- Mitchell, D.H.; Stiles, J.W.; Santelli, J.; Rao Sanadi, D. Synchronous Growth and Aging of Caenorhabditis elegans in the Presence of Fluorodeoxyuridine. J. Gerontol. 1979, 34, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Colmenares, D.; Sun, Q.; Shen, P.; Yue, Y.; McClements, D.J.; Park, Y. Delivery of Dietary Triglycerides to Caenorhabditis elegans Using Lipid Nanoparticles: Nanoemulsion-Based Delivery Systems. Food Chem. 2016, 202, 451–457. [Google Scholar] [CrossRef]
- Lionaki, E.; Tavernarakis, N. Assessing Aging and Senescent Decline in Caenorhabditis elegans: Cohort Survival Analysis. Methods Mol. Biol. 2013, 965, 473–484. [Google Scholar] [CrossRef]
- Sawin, E.R.; Ranganathan, R.; Horvitz, H.R. C. Elegans Locomotory Rate Is Modulated by the Environment through a Dopaminergic Pathway and by Experience through a Serotonergic Pathway. Neuron 2000, 26, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Possik, E.; Pause, A. Measuring Oxidative Stress Resistance of Caenorhabditis elegans in 96-Well Microtiter Plates. J. Vis. Exp. 2015, 2015, e52746. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 Years of Image Analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Saul, N.; Dhondt, I.; Kuokkanen, M.; Perola, M.; Verschuuren, C.; Wouters, B.; von Chrzanowski, H.; De Vos, W.H.; Temmerman, L.; Luyten, W.; et al. Identification of Healthspan-Promoting Genes in Caenorhabditis elegans Based on a Human GWAS Study. Biogerontology 2022, 23, 431–452. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mora, I.; Teixidó, A.; Vázquez-Manrique, R.P.; Puiggròs, F.; Arola, L. Docosahexaenoic Acid (DHA) Supplementation in a Triglyceride Form Prevents from Polyglutamine-Induced Dysfunctions in Caenorhabditis elegans. Int. J. Mol. Sci. 2024, 25, 12594. https://doi.org/10.3390/ijms252312594
Mora I, Teixidó A, Vázquez-Manrique RP, Puiggròs F, Arola L. Docosahexaenoic Acid (DHA) Supplementation in a Triglyceride Form Prevents from Polyglutamine-Induced Dysfunctions in Caenorhabditis elegans. International Journal of Molecular Sciences. 2024; 25(23):12594. https://doi.org/10.3390/ijms252312594
Chicago/Turabian StyleMora, Ignasi, Alex Teixidó, Rafael P. Vázquez-Manrique, Francesc Puiggròs, and Lluís Arola. 2024. "Docosahexaenoic Acid (DHA) Supplementation in a Triglyceride Form Prevents from Polyglutamine-Induced Dysfunctions in Caenorhabditis elegans" International Journal of Molecular Sciences 25, no. 23: 12594. https://doi.org/10.3390/ijms252312594
APA StyleMora, I., Teixidó, A., Vázquez-Manrique, R. P., Puiggròs, F., & Arola, L. (2024). Docosahexaenoic Acid (DHA) Supplementation in a Triglyceride Form Prevents from Polyglutamine-Induced Dysfunctions in Caenorhabditis elegans. International Journal of Molecular Sciences, 25(23), 12594. https://doi.org/10.3390/ijms252312594






