Recent Advances and Prospects in RNA Drug Development
Abstract
1. Introduction
1.1. Evolution of RNA Therapeutics
1.2. Versatility and Potential of RNA Therapeutics
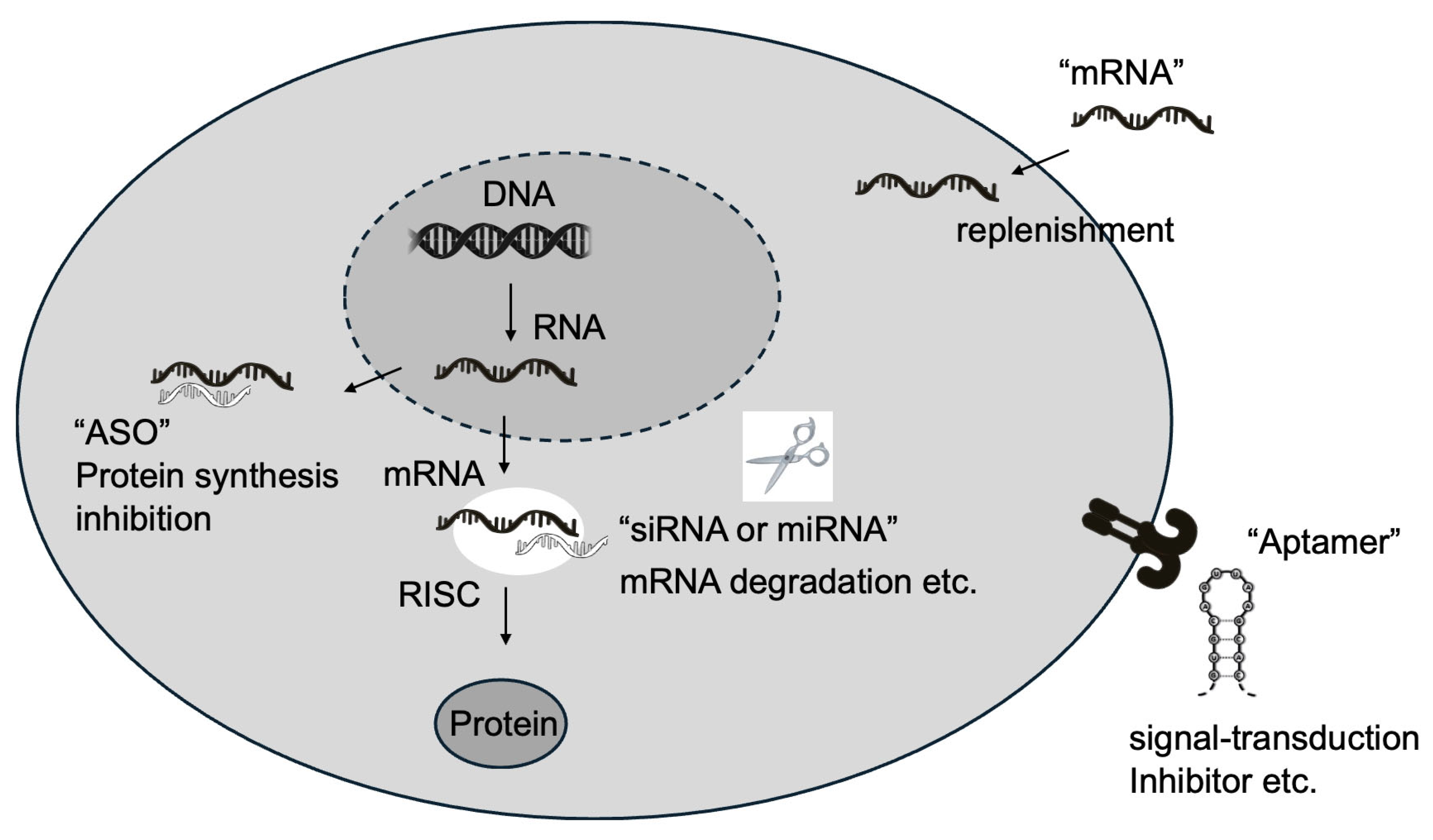
2. Types of RNA Therapeutics
2.1. Antisense Oligonucleotides (ASOs)
2.2. Small Interfering RNAs (siRNAs)
2.3. MicroRNAs (miRNAs)
2.4. Messenger RNAs (mRNAs)
2.5. Aptamers
3. Delivery Systems for RNA Drugs
3.1. Lipid Nanoparticles (LNPs)
3.2. Polymeric Nanoparticles (PNPs)
3.3. Conjugation Strategies
3.4. Viral Vectors
4. Clinical Applications and Approved RNA Drugs
4.1. Neurodegenerative Diseases
4.2. Genetic Disorders
4.3. Cancer Treatment
4.4. Infectious Diseases
4.5. Cardiovascular Diseases
5. Challenges in RNA Drug Development
5.1. Stability and Degradation
5.2. Off-Target Effects
5.3. Immunogenicity
5.4. Delivery to Target Tissues
6. Emerging Technologies and Approaches
6.1. Chemical Modifications
6.2. Novel Delivery Platforms
6.3. Combination Therapies
6.4. Personalized RNA Medicines
7. Regulatory Considerations and Clinical Trials
8. Future Perspectives and Conclusions
- (1)
- Personalized Medicine Revolution: RNA therapeutics are uniquely positioned to spearhead the era of truly personalized medicine. The ability to rapidly design and manufacture RNA-based drugs tailored to an individual’s genetic profile will likely lead to more effective treatments for rare genetic disorders and complex diseases like cancer. We may see the emergence of hospital-based RNA therapeutic platforms capable of producing patient-specific treatments on demand.
- (2)
- Advanced Delivery Systems: The development of next-generation delivery systems will be crucial in overcoming current limitations. We can expect to see innovations in lipid nanoparticle technology, biomimetic nanoparticles, and cell-based delivery systems. These advancements will likely improve tissue-specific targeting, reduce off-target effects, and enhance the overall efficacy of RNA therapeutics.
- (3)
- Combination Therapies: The future will likely see an increase in combination therapies involving RNA drugs. This could include combinations of different RNA modalities (e.g., siRNA with miRNA) or RNA therapeutics paired with traditional small-molecule drugs. Such approaches may offer synergistic effects, particularly in complex diseases like cancer.
- (4)
- Expanded Applications: While current RNA therapeutics focus primarily on genetic disorders and certain cancers, future applications are likely to expand into areas such as regenerative medicine, immunomodulation, and even aging-related conditions. The versatility of RNA as a therapeutic modality will open new avenues for treating previously “undruggable” targets.
- (5)
- AI Integration: The integration of AI in RNA drug design and optimization will likely accelerate development timelines and improve efficacy. These technologies could help predict off-target effects, optimize delivery strategies, and even personalize treatment regimens.
- (6)
- Regulatory Evolution: As RNA therapeutics become more prevalent, regulatory frameworks will need to evolve to accommodate their unique characteristics. We may see the development of specialized regulatory pathways for RNA-based drugs, particularly for personalized therapies.
- (7)
- Manufacturing Innovations: Advancements in manufacturing technologies will be crucial to meet the growing demand for RNA therapeutics. This may include the development of more efficient and scalable production methods, as well as innovations in quality control and stability enhancement.
- (8)
- RNA Editing Technologies: The emergence of RNA editing technologies, such as CRISPR-based systems targeting RNA, could open new possibilities for transient and reversible genetic modifications, further expanding the therapeutic potential of RNA-based approaches.
Funding
Data Availability Statement
Conflicts of Interest
References
- Zamecnik, P.C.; Stephenson, M.L. Inhibition of Rous sarcoma virus replication and cell transformation by a specific oligodeoxynucleotide. Proc. Natl. Acad. Sci. USA 1978, 75, 280–284. [Google Scholar] [CrossRef]
- Wolff, J.A.; Malone, R.W.; Williams, P.; Chong, W.; Acsadi, G.; Jani, A.; Felgner, P.L. Direct gene transfer into mouse muscle in vivo. Science 1990, 247, 1465–1468. [Google Scholar] [CrossRef] [PubMed]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Gonzalez-Duarte, A.; O’Riordan, W.D.; Yang, C.C.; Ueda, M.; Kristen, A.V.; Tournev, I.; Schmidt, H.H.; Coelho, T.; Berk, J.L.; et al. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA COVID-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Zhou, Y.; Jiang, Y.; Chen, S.J. RNA-ligand molecular docking: Advances and challenges. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2022, 12, e1571. [Google Scholar] [CrossRef]
- Šponer, J.; Bussi, G.; Krepl, M.; Banáš, P.; Bottaro, S.; Cunha, R.A.; Gil-Ley, A.; Pinamonti, G.; Poblete, S.; Jurečka, P.; et al. RNA Structural Dynamics As Captured by Molecular Simulations: A Comprehensive Overview. Chem. Rev. 2018, 118, 4177–4338. [Google Scholar] [CrossRef]
- Pardi, N.; Hogan, M.J.; Porter, F.W.; Weissman, D. mRNA vaccines—A new era in vaccinology. Nat. Rev. Drug Discov. 2018, 17, 261–279. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, P.S.; Rudra, A.; Miao, L.; Anderson, D.G. Delivering the Messenger: Advances in Technologies for Therapeutic mRNA Delivery. Mol. Ther. 2019, 27, 710–728. [Google Scholar] [CrossRef]
- Sahin, U.; Karikó, K.; Türeci, Ö. mRNA-based therapeutics--developing a new class of drugs. Nat. Rev. Drug Discov. 2014, 13, 759–780. [Google Scholar] [CrossRef]
- Hwu, W.L. Gene therapy for ultrarare diseases: A geneticist’s perspective. J. Biomed. Sci. 2024, 31, 79. [Google Scholar] [CrossRef] [PubMed]
- Collotta, D.; Bertocchi, I.; Chiapello, E.; Collino, M. Antisense oligonucleotides: A novel Frontier in pharmacological strategy. Front. Pharmacol. 2023, 14, 1304342. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.H.; Lim, S.; Wong, W.S. Antisense oligonucleotides: From design to therapeutic application. Clin. Exp. Pharmacol. Physiol. 2006, 33, 533–540. [Google Scholar] [CrossRef]
- Liang, X.H.; Sun, H.; Nichols, J.G.; Crooke, S.T. RNase H1-Dependent Antisense Oligonucleotides Are Robustly Active in Directing RNA Cleavage in Both the Cytoplasm and the Nucleus. Mol. Ther. 2017, 25, 2075–2092. [Google Scholar] [CrossRef]
- Quemener, A.M.; Bachelot, L.; Forestier, A.; Donnou-Fournet, E.; Gilot, D.; Galibert, M.D. The powerful world of antisense oligonucleotides: From bench to bedside. Wiley Interdiscip. Rev. RNA 2020, 11, e1594. [Google Scholar] [CrossRef]
- Dhuri, K.; Bechtold, C.; Quijano, E.; Pham, H.; Gupta, A.; Vikram, A.; Bahal, R. Antisense Oligonucleotides: An Emerging Area in Drug Discovery and Development. J. Clin. Med. 2020, 9, 2004. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Li, Y.; Yu, W.; Fu, Y.; Zhang, J.; Cao, H. Recent Progress of Small Interfering RNA Delivery on the Market and Clinical Stage. Mol. Pharm. 2024, 21, 2081–2096. [Google Scholar] [CrossRef]
- Adams, D.; Tournev, I.L.; Taylor, M.S.; Coelho, T.; Planté-Bordeneuve, V.; Berk, J.L.; González-Duarte, A.; Gillmore, J.D.; Low, S.C.; Sekijima, Y.; et al. HELIOS-A Collaborators. Efficacy and safety of vutrisiran for patients with hereditary transthyretin-mediated amyloidosis with polyneuropathy: A randomized clinical trial. Amyloid. 2023, 30, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Pérez Marc, G.; Polack, F.P.; Zerbini, C.; et al. C4591001 Clinical Trial Group. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef]
- Ranasinghe, P.; Addison, M.L.; Dear, J.W.; Webb, D.J. Small interfering RNA: Discovery, pharmacology and clinical development-An introductory review. Br. J. Pharmacol. 2023, 180, 2697–2720. [Google Scholar] [CrossRef]
- Nair, J.K.; Willoughby, J.L.; Chan, A.; Charisse, K.; Alam, M.R.; Wang, Q.; Hoekstra, M.; Kandasamy, P.; Kel’in, A.V.; Milstein, S.; et al. Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J. Am. Chem. Soc. 2014, 136, 16958–16961. [Google Scholar] [CrossRef]
- Saliminejad, K.; Khorram Khorshid, H.R.; Soleymani Fard, S.; Ghaffari, S.H. An overview of microRNAs: Biology, functions, therapeutics, and analysis methods. J. Cell. Physiol. 2019, 234, 5451–5465. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M.; Kothari, S.; Gohir, W.; Camargo, J.F.; Husain, S. MicroRNAs in infectious diseases: Potential diagnostic biomarkers and therapeutic targets. Clin. Microbiol. Rev. 2023, 36, e0001523. [Google Scholar] [CrossRef]
- Gubu, A.; Su, W.; Zhao, X.; Zhang, X.; Fan, X.; Wang, J.; Wang, Q.; Tang, X. Circular Antisense Oligonucleotides for Specific RNase-H-Mediated microRNA Inhibition with Reduced Off-Target Effects and Nonspecific Immunostimulation. J. Med. Chem. 2021, 64, 16046–16055. [Google Scholar] [CrossRef] [PubMed]
- Parhiz, H.; Atochina-Vasserman, E.N.; Weissman, D. mRNA-based therapeutics: Looking beyond COVID-19 vaccines. Lancet 2024, 403, 1192–1204. [Google Scholar] [CrossRef] [PubMed]
- Youssef, M.; Hitti, C.; Puppin Chaves Fulber, J.; Kamen, A.A. Enabling mRNA Therapeutics: Current Landscape and Challenges in Manufacturing. Biomolecules 2023, 13, 1497. [Google Scholar] [CrossRef]
- Trivedi, V.; Yang, C.; Klippel, K.; Yegorov, O.; von Roemeling, C.; Hoang-Minh, L.; Fenton, G.; Ogando-Rivas, E.; Castillo, P.; Moore, G.; et al. mRNA-based precision targeting of neoantigens and tumor-associated antigens in malignant brain tumors. Genome Med. 2024, 16, 17. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, W.; Qi, H. Recent Advancements in mRNA Vaccines: From Target Selection to Delivery Systems. Vaccines 2024, 12, 873. [Google Scholar] [CrossRef]
- Mayer, G. The chemical biology of aptamers. Angew. Chem. Int. Ed. Engl. 2009, 48, 2672–2689. [Google Scholar] [CrossRef]
- Subramanian, N.; Kanwar, J.R.; Kanwar, R.K.; Krishnakumar, S. Targeting Cancer Cells Using LNA-Modified Aptamer-siRNA Chimeras. Nucleic Acid. Ther. 2015, 25, 317–322. [Google Scholar] [CrossRef]
- Zhou, J.; Rossi, J. Aptamers as targeted therapeutics: Current potential and challenges. Nat. Rev. Drug Discov. 2017, 16, 181–202. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Li, Y.; Wu, W. Aptamers: Promising Reagents in Biomedicine Application. Adv. Biol. 2024, 8, e2300584. [Google Scholar] [CrossRef] [PubMed]
- Kielpinski, L.J.; Hagedorn, P.H.; Lindow, M.; Vinther, J. RNase H sequence preferences influence antisense oligonucleotide efficiency. Nucleic Acids Res. 2017, 45, 12932–12944. [Google Scholar] [CrossRef]
- Patel, S.; Ashwanikumar, N.; Robinson, E.; Darcsi, A.; Grover, N.; Liang, X.; Olson, D.; Martinez, J.; Choi, Y.; Subramanian, M.; et al. Naturally-occurring cholesterol analogues in lipid nanoparticles induce polymorphic shape and enhance intracellular delivery of mRNA. Nat. Commun. 2020, 11, 983. [Google Scholar] [CrossRef] [PubMed]
- Yanez Arteta, M.; Kjellman, T.; Bartesaghi, S.; Wallin, S.; Wu, X.; Kvist, A.J.; Dabkowska, A.; Székely, N.; Radulescu, A.; Bergenholtz, J.; et al. Successful reprogramming of cellular protein production through mRNA delivered by functionalized lipid nanoparticles. Proc. Natl. Acad. Sci. USA 2018, 115, E3351–E3360. [Google Scholar] [CrossRef]
- Miao, L.; Li, L.; Huang, Y.; Delcassian, D.; Chahal, J.; Han, J.; Shi, Y.; Sadtler, K.; Gao, W.; Lin, J.; et al. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat. Biotechnol. 2019, 37, 1174–1185. [Google Scholar] [CrossRef]
- Wu, Y.; Yu, S.; de Lázaro, I. Advances in lipid nanoparticle mRNA therapeutics beyond COVID-19 vaccines. Nanoscale 2024, 16, 6820–6836. [Google Scholar] [CrossRef]
- Sristi; Almalki, W. H.; Karwasra, R.; Gupta, G.; Singh, S.; Sharma, A.; Sahebkar, A.; Kesharwani, P. Advances in the polymeric nanoparticulate delivery systems for RNA therapeutics. Prog. Mol. Biol. Transl. Sci. 2024, 204, 219–248. [Google Scholar]
- Yousefi, A.S.; Hanrahan, J.W.; Kakkar, A. mRNA Delivery: Challenges and Advances through Polymeric Soft Nanoparticles. Int. J. Mol. Sci. 2024, 25, 1739. [Google Scholar] [CrossRef]
- Yang, W.; Mixich, L.; Boonstra, E.; Cabral, H. Polymer-Based mRNA Delivery Strategies for Advanced Therapies. Adv. Healthc. Mater. 2023, 12, e2202688. [Google Scholar] [CrossRef]
- Segel, M.; Lash, B.; Song, J.; Ladha, A.; Liu, C.C.; Jin, X.; Mekhedov, S.L.; Macrae, R.K.; Koonin, E.V.; Zhang, F. Mammalian retrovirus-like protein PEG10 packages its own mRNA and can be pseudotyped for mRNA delivery. Science 2021, 373, 882–889. [Google Scholar] [CrossRef]
- Cui, H.; Zhu, X.; Li, S.; Wang, P.; Fang, J. Liver-Targeted Delivery of Oligonucleotides with N-Acetylgalactosamine Conjugation. ACS Omega 2021, 6, 16259–16265. [Google Scholar] [CrossRef]
- Dugal-Tessier, J.; Thirumalairajan, S.; Jain, N. Antibody-Oligonucleotide Conjugates: A Twist to Antibody-Drug Conjugates. J. Clin. Med. 2021, 10, 838. [Google Scholar] [CrossRef]
- Osborn, M.F.; Khvorova, A. Improving siRNA Delivery In Vivo Through Lipid Conjugation. Nucleic Acid Ther. 2018, 28, 128–136. [Google Scholar] [CrossRef]
- Verdonckt, T.W.; Vanden Broeck, J. Methods for the Cost-Effective Production of Bacteria-Derived Double-Stranded RNA for in vitro Knockdown Studies. Front. Physiol. 2022, 13, 836106. [Google Scholar] [CrossRef] [PubMed]
- Lundstrom, K. Are Viral Vectors Any Good for RNAi Antiviral Therapy? Viruses 2020, 12, 1189. [Google Scholar] [CrossRef] [PubMed]
- Moreno, A.M.; Fu, X.; Zhu, J.; Katrekar, D.; Shih, Y.V.; Marlett, J.; Cabotaje, J.; Tat, J.; Naughton, J.; Lisowski, L.; et al. In Situ Gene Therapy via AAV-CRISPR-Cas9-Mediated Targeted Gene Regulation. Mol. Ther. 2018, 26, 1818–1827. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, J.C.; Kowalski, P.S.; Anderson, D.G. Advances in the delivery of RNA therapeutics: From concept to clinical reality. Genome Med. 2017, 9, 60. [Google Scholar] [CrossRef] [PubMed]
- Ayala, Y.M.; Nguyen, A.D. RNA-Based Therapies for Neurodegenerative Diseases. Mo. Med. 2021, 118, 340–345. [Google Scholar]
- Wild, E.J.; Tabrizi, S.J. Therapies targeting DNA and RNA in Huntington’s disease. Lancet Neurol. 2017, 16, 837–847. [Google Scholar] [CrossRef] [PubMed]
- Katrekar, D.; Yen, J.; Xiang, Y.; Saha, A.; Meluzzi, D.; Savva, Y.; Mali, P. Efficient in vitro and in vivo RNA editing via recruitment of endogenous ADARs using circular guide RNAs. Nat. Biotechnol. 2022, 40, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Clarke, L.A.; Amaral, M.D. What Can RNA-Based Therapy Do for Monogenic Diseases? Pharmaceutics 2023, 15, 260. [Google Scholar] [CrossRef] [PubMed]
- Traber, G.M.; Yu, A.M. The Growing Class of Novel RNAi Therapeutics. Mol. Pharmacol. 2024, 106, 13–20. [Google Scholar] [CrossRef] [PubMed]
- An, D.; Schneller, J.L.; Frassetto, A.; Liang, S.; Zhu, X.; Park, J.S.; Theisen, M.; Hong, S.J.; Zhou, J.; Rajendran, R.; et al. Systemic Messenger RNA Therapy as a Treatment for Methylmalonic Acidemia. Cell Rep. 2018, 24, 2520. [Google Scholar] [CrossRef]
- Sayour, E.J.; Boczkowski, D.; Mitchell, D.A.; Nair, S.K. Cancer mRNA vaccines: Clinical advances and future opportunities. Nat. Rev. Clin. Oncol. 2024, 21, 489–500. [Google Scholar] [CrossRef]
- Van Hoecke, L.; Verbeke, R.; Dewitte, H.; Lentacker, I.; Vermaelen, K.; Breckpot, K.; Van Lint, S. mRNA in cancer immunotherapy: Beyond a source of antigen. Mol. Cancer 2021, 20, 48. [Google Scholar] [CrossRef] [PubMed]
- Weber, J.S.; Carlino, M.S.; Khattak, A.; Meniawy, T.; Ansstas, G.; Taylor, M.H.; Kim, K.B.; McKean, M.; Long, G.V.; Sullivan, R.J.; et al. Individualised neoantigen therapy mRNA-4157 (V940) plus pembrolizumab versus pembrolizumab monotherapy in resected melanoma (KEYNOTE-942): A randomised, phase 2b study. Lancet 2024, 403, 632–644. [Google Scholar] [CrossRef]
- Kang, H.; Ga, Y.J.; Kim, S.H.; Cho, Y.H.; Kim, J.W.; Kim, C.; Yeh, J.Y. Small interfering RNA (siRNA)-based therapeutic applications against viruses: Principles, potential, and challenges. J. Biomed. Sci. 2023, 30, 88. [Google Scholar] [CrossRef]
- Tarn, W.Y.; Cheng, Y.; Ko, S.H.; Huang, L.M. Antisense Oligonucleotide-Based Therapy of Viral Infections. Pharmaceutics 2021, 13, 2015. [Google Scholar] [CrossRef]
- Chia, S.P.S.; Pang, J.K.S.; Soh, B.S. Current RNA strategies in treating cardiovascular diseases. Mol. Ther. 2024, 32, 580–608. [Google Scholar] [CrossRef]
- Raal, F.J.; Kallend, D.; Ray, K.K.; Turner, T.; Koenig, W.; Wright, R.S.; Wijngaard, P.L.J.; Curcio, D.; Jaros, M.J.; Leiter, L.A.; et al. ORION-9 Investigators. Inclisiran for the Treatment of Heterozygous Familial Hypercholesterolemia. N. Engl. J. Med. 2020, 382, 1520–1530. [Google Scholar] [CrossRef] [PubMed]
- Rohner, E.; Yang, R.; Foo, K.S.; Goedel, A.; Chien, K.R. Unlocking the promise of mRNA therapeutics. Nat. Biotechnol. 2022, 40, 1586–1600. [Google Scholar] [CrossRef] [PubMed]
- Ardiana, M.; Fadila, A.N.; Zuhra, Z.; Kusuma, N.M.; Surya Erlangga Rurus, M.E.; Oceandy, D. Non-coding RNA therapeutics in cardiovascular diseases and risk factors: Systematic review. Noncoding RNA Res. 2023, 8, 487–506. [Google Scholar] [CrossRef]
- Halloy, F.; Biscans, A.; Bujold, K.E.; Debacker, A.; Hill, A.C.; Lacroix, A.; Luige, O.; Strömberg, R.; Sundstrom, L.; Vogel, J.; et al. Innovative developments and emerging technologies in RNA therapeutics. RNA Biol. 2022, 19, 313–332. [Google Scholar] [CrossRef] [PubMed]
- Yu, A.M.; Choi, Y.H.; Tu, M.J. RNA Drugs and RNA Targets for Small Molecules: Principles, Progress, and Challenges. Pharmacol. Rev. 2020, 72, 862–898. [Google Scholar] [CrossRef]
- Seok, H.; Lee, H.; Jang, E.S.; Chi, S.W. Evaluation and control of miRNA-like off-target repression for RNA interference. Cell. Mol. Life Sci. 2018, 75, 797–814. [Google Scholar] [CrossRef]
- Neumeier, J.; Meister, G. siRNA Specificity: RNAi Mechanisms and Strategies to Reduce Off-Target Effects. Front. Plant Sci. 2021, 11, 526455. [Google Scholar] [CrossRef]
- Yi, Z.; Qu, L.; Tang, H.; Liu, Z.; Liu, Y.; Tian, F.; Wang, C.; Zhang, X.; Feng, Z.; Yu, Y.; et al. Engineered circular ADAR-recruiting RNAs increase the efficiency and fidelity of RNA editing in vitro and in vivo. Nat. Biotechnol. 2022, 40, 946–955. [Google Scholar] [CrossRef]
- Verbeke, R.; Hogan, M.J.; Loré, K.; Pardi, N. Innate immune mechanisms of mRNA vaccines. Immunity 2022, 55, 1993–2005. [Google Scholar] [CrossRef]
- Huang, E.; Frydman, C.; Xiao, X. Navigating the landscape of epitranscriptomics and host immunity. Genome Res. 2024, 34, 515–529. [Google Scholar] [CrossRef] [PubMed]
- Wan, J.; Wang, Z.; Wang, L.; Wu, L.; Zhang, C.; Zhou, M.; Fu, Z.F.; Zhao, L. Circular RNA vaccines with long-term lymph node-targeting delivery stability after lyophilization induce potent and persistent immune responses. mBio 2024, 15, e0177523. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Qi, J.; Wang, J.; Hu, W.; Zhou, W.; Wang, Y.; Li, T. Nanoparticle technology for mRNA: Delivery strategy, clinical application and developmental landscape. Theranostics 2024, 14, 738–760. [Google Scholar] [CrossRef] [PubMed]
- Yuan, M.; Han, Z.; Liang, Y.; Sun, Y.; He, B.; Chen, W.; Li, F. mRNA nanodelivery systems: Targeting strategies and administration routes. Biomater. Res. 2023, 27, 90. [Google Scholar] [CrossRef]
- Xiang, Y.; Katrekar, D.; Mali, P. Methods for recruiting endogenous and exogenous ADAR enzymes for site-specific RNA editing. Methods 2022, 205, 158–166. [Google Scholar] [CrossRef]
- Ho, L.L.Y.; Schiess, G.H.A.; Miranda, P.; Weber, G.; Astakhova, K. Pseudouridine and N1-methylpseudouridine as potent nucleotide analogues for RNA therapy and vaccine development. RSC Chem. Biol. 2024, 5, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Prakash, T.P.; Bhat, B. 2′-Modified oligonucleotides for antisense therapeutics. Curr. Top. Med. Chem. 2007, 7, 641–649. [Google Scholar] [CrossRef]
- Crooke, S.T.; Vickers, T.A.; Liang, X.H. Phosphorothioate modified oligonucleotide-protein interactions. Nucleic Acids Res. 2020, 48, 5235–5253. [Google Scholar] [CrossRef]
- Liu, A.; Wang, X. The Pivotal Role of Chemical Modifications in mRNA Therapeutics. Front. Cell Dev. Biol. 2022, 10, 901510. [Google Scholar] [CrossRef]
- Dad, H.A.; Gu, T.W.; Zhu, A.Q.; Huang, L.Q.; Peng, L.H. Plant Exosome-like Nanovesicles: Emerging Therapeutics and Drug Delivery Nanoplatforms. Mol. Ther. 2021, 29, 13–31. [Google Scholar] [CrossRef]
- Paunovska, K.; Loughrey, D.; Dahlman, J.E. Drug delivery systems for RNA therapeutics. Nat. Rev. Genet. 2022, 23, 265–280. [Google Scholar] [CrossRef]
- Truong, L.B.; Medina-Cruz, D.; Mostafavi, E. Current state of RNA delivery using lipid nanoparticles to extrahepatic tissues: A review towards clinical translation. Int. J. Biol. Macromol. 2023, 242, 125185. [Google Scholar] [CrossRef]
- Cecchin, R.; Troyer, Z.; Witwer, K.; Morris, K.V. Extracellular vesicles: The next generation in gene therapy delivery. Mol. Ther. 2023, 31, 1225–1230. [Google Scholar] [CrossRef]
- Damase, T.R.; Sukhovershin, R.; Boada, C.; Taraballi, F.; Pettigrew, R.I.; Cooke, J.P. The Limitless Future of RNA Therapeutics. Front. Bioeng. Biotechnol. 2021, 9, 628137. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, M.; Jung, E.J.; Shah, M.Y.; Lu, C.; Spizzo, R.; Shimizu, M.; Han, H.D.; Ivan, C.; Rossi, S.; Zhang, X.; et al. Therapeutic synergy between microRNA and siRNA in ovarian cancer treatment. Cancer Discov. 2013, 3, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Rani, V.; Mishra, M.; Chawla, R. New paradigm in combination therapy of siRNA with chemotherapeutic drugs for effective cancer therapy. Curr. Res. Pharmacol. Drug Discov. 2022, 3, 100103. [Google Scholar] [CrossRef]
- Bogaert, B.; Sauvage, F.; Guagliardo, R.; Muntean, C.; Nguyen, V.P.; Pottie, E.; Wels, M.; Minnaert, A.K.; De Rycke, R.; Yang, Q.; et al. A lipid nanoparticle platform for mRNA delivery through repurposing of cationic amphiphilic drugs. J. Control. Release 2022, 350, 256–270. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.H.; Androsavich, J.R.; So, N.; Jenkins, M.P.; MacCormack, D.; Prigodich, A.; Welch, V.; True, J.M.; Dolsten, M. Breaking the mold with RNA-a “RNAissance” of life science. NPJ Genom. Med. 2024, 9, 2. [Google Scholar] [CrossRef]
- Yao, R.; Xie, C.; Xia, X. Recent progress in mRNA cancer vaccines. Hum. Vaccines Immunother. 2024, 20, 2307187. [Google Scholar] [CrossRef]
- Adachi, H.; Hengesbach, M.; Yu, Y.T.; Morais, P. From Antisense RNA to RNA Modification: Therapeutic Potential of RNA-Based Technologies. Biomedicines 2021, 9, 550. [Google Scholar] [CrossRef] [PubMed]
- Sankar, A.; Kumar, Y.S.R.; Singh, A.; Roy, R.; Shukla, R.; Verma, B. Next-generation therapeutics for rare genetic disorders. Mutagenesis 2024, 39, 157–171. [Google Scholar] [CrossRef]
- Mollocana-Lara, E.C.; Ni, M.; Agathos, S.N.; Gonzales-Zubiate, F.A. The infinite possibilities of RNA therapeutics. J. Ind. Microbiol. Biotechnol. 2021, 48, kuab063. [Google Scholar] [CrossRef] [PubMed]
- Naik, R.; Peden, K. Regulatory Considerations on the Development of mRNA Vaccines. Curr. Top. Microbiol. Immunol. 2022, 440, 187–205. [Google Scholar]
- Stewart, J.M. RNA nanotechnology on the horizon: Self-assembly, chemical modifications, and functional applications. Curr. Opin. Chem. Biol. 2024, 81, 102479. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.Y.; Wong, M.S.; Liu, J. Aptamer-functionalized liposomes for drug delivery. Biomed. J. 2024, 47, 100685. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zhu, L.; Wang, X.; Jin, H. RNA-based therapeutics: An overview and prospectus. Cell Death Dis. 2022, 13, 644. [Google Scholar] [CrossRef]
- Yokoyama, S.; Muto, H.; Honda, T.; Kurokawa, Y.; Ogawa, H.; Nakajima, R.; Kawashima, H.; Tani, H. Identification of Two Long Noncoding RNAs, Kcnq1ot1 and Rmst, as Biomarkers in Chronic Liver Diseases in Mice. Int. J. Mol. Sci. 2024, 25, 8927. [Google Scholar] [CrossRef]



| RNA Therapeutic Type | Mechanism | Key Applications | API Example |
|---|---|---|---|
| Antisense oligonucleotides (ASOs) | Modulate gene expression through complementary binding | Neurodegenerative diseases, genetic disorders | Nusinersen (Spinraza), inotersen (Tegsedi), golodirsen (Vyondys 53) |
| Small interfering RNAs (siRNAs) | Gene silencing through RNA interference | Cancer, genetic disorders, cardiovascular diseases | Patisiran (Onpattro), givosiran (Givlaari), inclisiran (Leqvio) |
| MicroRNAs (miRNAs) | Regulate gene networks | Cancer, cardiovascular diseases | Cobomarsen (MRG-106) |
| Messenger RNAs (mRNAs) | Protein replacement therapy | Genetic disorders, infectious diseases (vaccines) | Moderna and Pfizer-BioNTech COVID-19 vaccines |
| Aptamers | Bind specific targets with high affinity | Diagnostic tools, targeted drug delivery | Pegaptanib (Macugen) |
| CRISPR–Cas9 guide RNAs | Direct genome editing | Genetic disorders, cancer | Alt-R CRISPR–Cas9 System, CRISPR Therapeutics’ CTX001 |
| Delivery System | Advantages | Disadvantages | Examples of Marked APIs |
|---|---|---|---|
| Lipid Nanoparticles (LNPs) | Efficient encapsulation of RNA, enhanced cellular uptake, improved stability, versatile and tunable, proven success in clinical applications | Limited biodistribution beyond liver, potential immunogenicity, manufacturing and quality control challenges, storage and transportation stability issues | Moderna and Pfizer-BioNTech COVID-19 vaccines (mRNA), patisiran (Onpattro) for hereditary, transthyretin-mediated amyloidosis (siRNA) |
| Polymeric Nanoparticles (PNPs) | High tailoring ability, biodegradability, ease of functionalization, good drug release profile, potential for stimuli-responsive delivery | Potential toxicity of cationic polymers, challenges in large-scale production, complex polymer–RNA interactions | No FDA-approved RNA therapeutics yet, several candidates in clinical trials |
| Conjugation Strategies | Enhanced stability, improved cellular uptake, targeted delivery, reduced immunogenicity | Limited to specific tissues/cell types, potential alteration of RNA activity, complex synthesis and characterization | Givosiran (Givlaari) for acute hepatic porphyria (siRNA–GalNAc conjugate), inclisiran (Leqvio) for hypercholesterolemia (siRNA–GalNAc conjugate) |
| Viral Vectors | High transduction efficiency, long-term gene expression (for some vectors), tissue-specific targeting | Immunogenicity concerns, limited payload capacity, potential for insertional mutagenesis | No FDA-approved RNA therapeutics yet, several candidates in clinical trials for gene therapy |
| Disease | RNA Therapeutic Approaches | Examples/Progress | Challenges/Prospects |
|---|---|---|---|
| Neurodegenerative Diseases | ASOs, RNAi | Promising clinical trials for spinal muscular atrophy and Huntington’s disease | Delivery across blood–brain barrier, widespread distribution in CNS |
| Genetic Disorders | ASOs, RNAi, mRNA therapy | Patisiran (Onpattro) and vutrisiran (Amvuttra), mRNA therapy for metabolic disorders | Potential to treat a wide range of genetic conditions |
| Cancer Treatment | mRNA vaccines, ASOs. RNAi, mRNA therapy | Tumor-specific antigen-encoding mRNA vaccines, silencing oncogenes, restoring tumor suppressor genes | Combination with existing treatments, overcoming drug resistance |
| Infectious Diseases | mRNA vaccines, RNAi, ASOs, RNA aptamers | COVID-19 mRNA vaccines, siRNAs targeting viral genes | Rapid response to emerging threats, potential solutions for antibiotic-resistant bacteria |
| Cardiovascular Diseases | RNAi, ASOs, mRNA therapy, microRNA therapeutics | siRNAs for hypertension and LDL cholesterol management, VEGF mRNA therapy for myocardial revascularization | Targeting lipid metabolism genes, potential treatments for atherosclerosis |
| Challenge | Description | Strategies to Overcome |
|---|---|---|
| Stability | RNA degradation in biological fluids | Chemical modifications, nanoparticle encapsulation |
| Off-target effects | Unintended gene modulation | Careful sequence design, chemical modifications |
| Immunogenicity | Immune system activation | Modified nucleosides, optimized delivery systems |
| Delivery to target tissues | Difficulty reaching specific organs/cells | Lipid nanoparticles, conjugation strategies, targeted delivery |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tani, H. Recent Advances and Prospects in RNA Drug Development. Int. J. Mol. Sci. 2024, 25, 12284. https://doi.org/10.3390/ijms252212284
Tani H. Recent Advances and Prospects in RNA Drug Development. International Journal of Molecular Sciences. 2024; 25(22):12284. https://doi.org/10.3390/ijms252212284
Chicago/Turabian StyleTani, Hidenori. 2024. "Recent Advances and Prospects in RNA Drug Development" International Journal of Molecular Sciences 25, no. 22: 12284. https://doi.org/10.3390/ijms252212284
APA StyleTani, H. (2024). Recent Advances and Prospects in RNA Drug Development. International Journal of Molecular Sciences, 25(22), 12284. https://doi.org/10.3390/ijms252212284






