Treatment of Melanoma Cells with Chloroquine and Everolimus Activates the Apoptosis Process and Alters Lipid Redistribution
Abstract
1. Introduction
2. Results
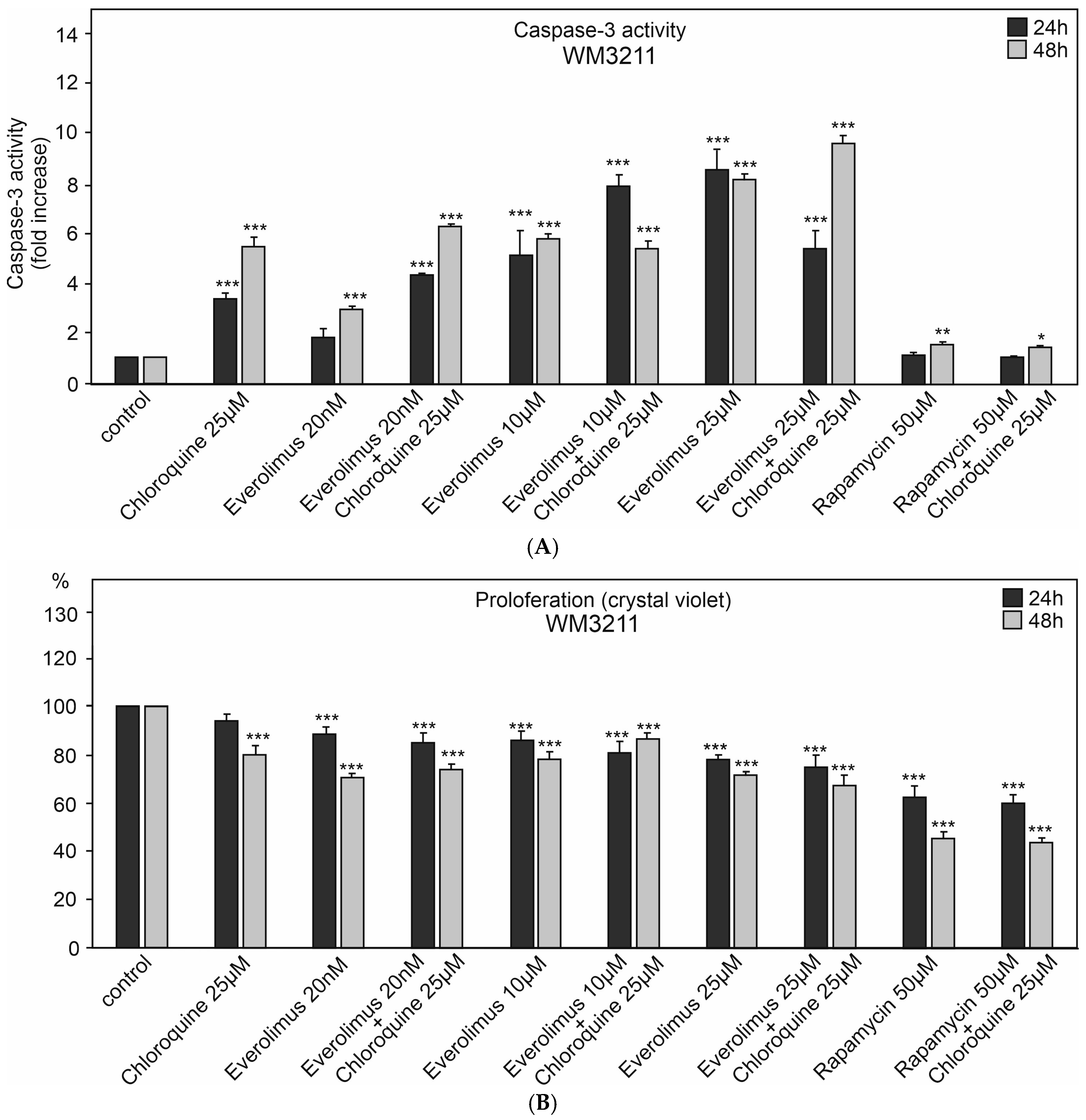
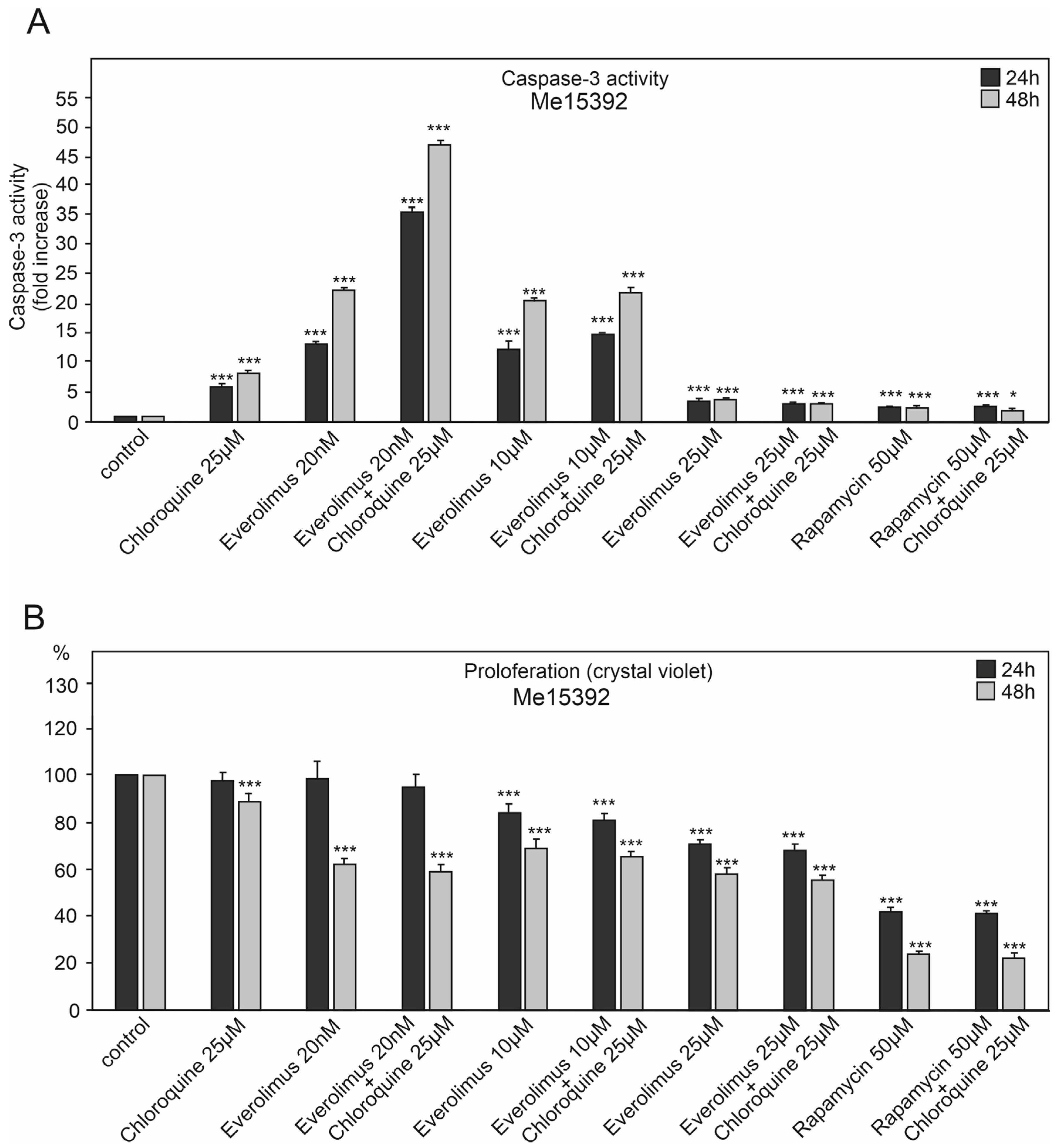
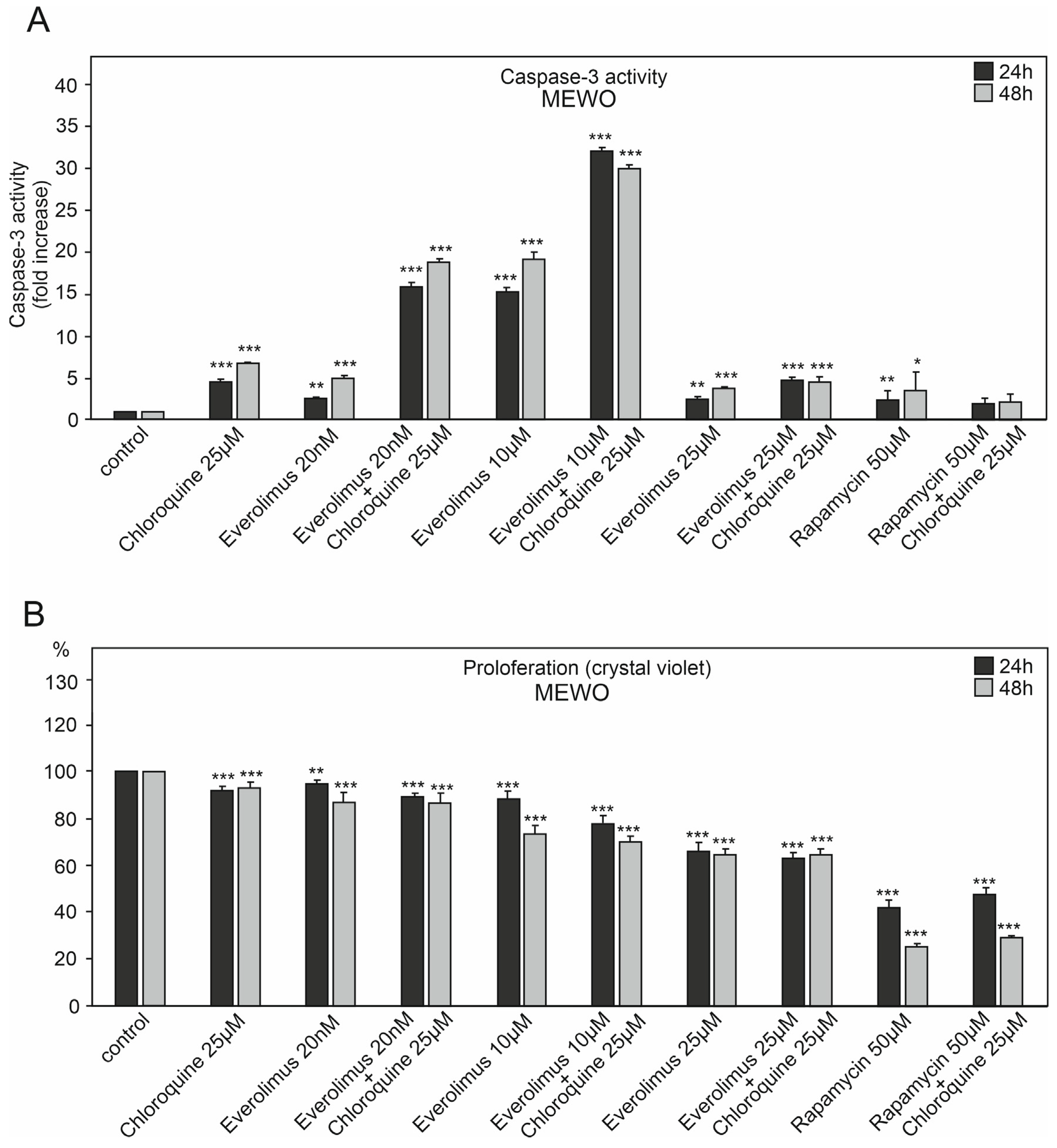
2.1. The Effect of Chloroquine and mTOR Kinase Inhibitor Everolimus on Caspase-3 Activity and Proliferation
2.2. Effect of Chloroquine and mTOR Kinase Inhibitor Everolimus on DNA Fragmentation ELISA Assay—Detection of Apoptosis
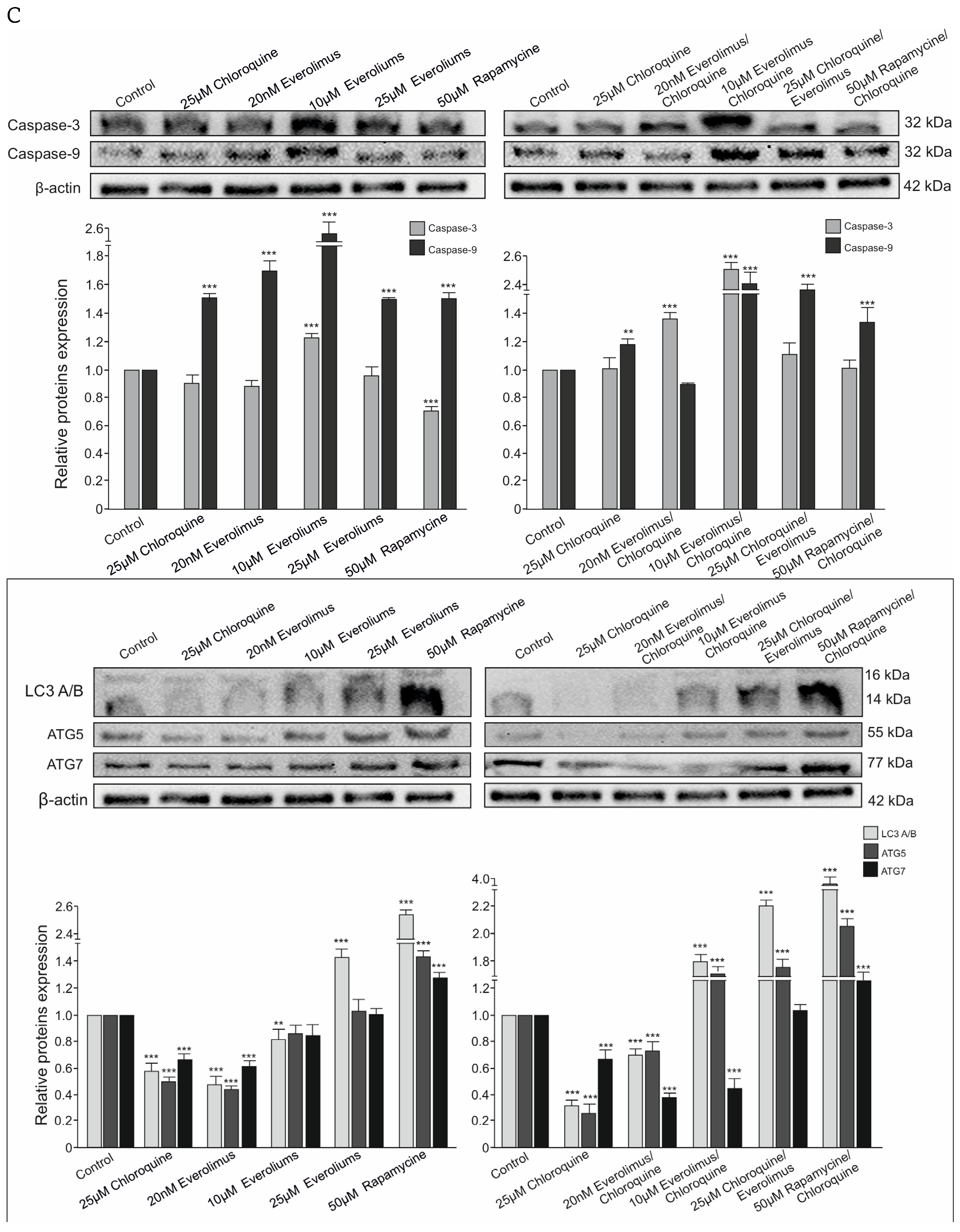
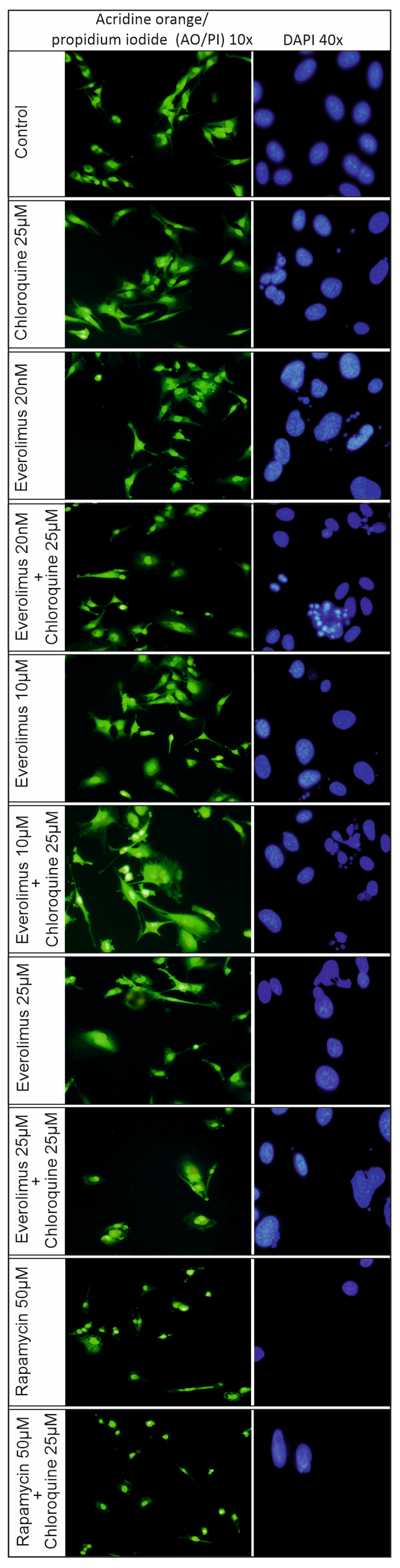
2.3. Analysis of the Process of Cellular Apoptosis and Autophagy Using Fluorescence Microscopy
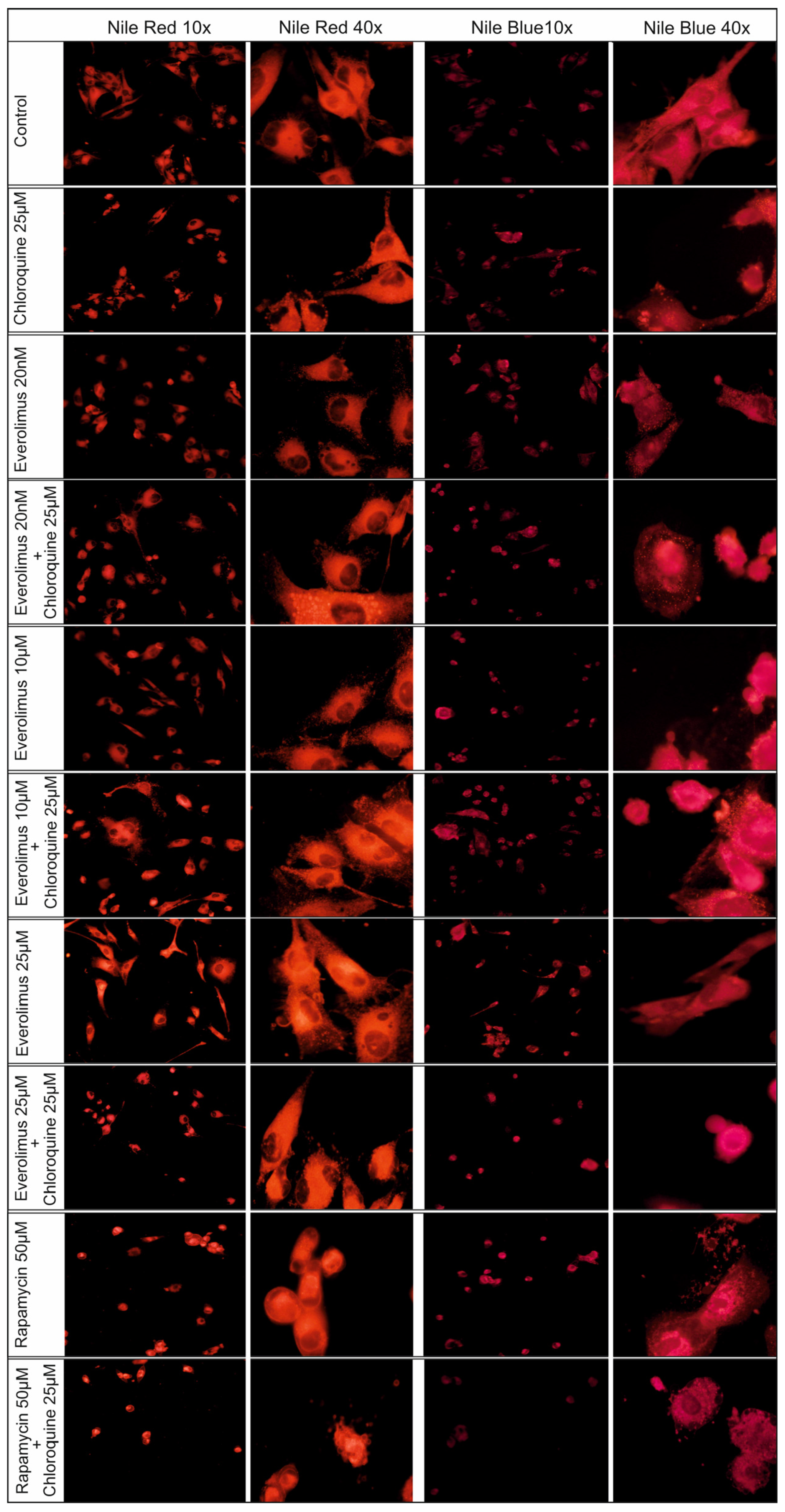
2.4. Changes Concerning Lipid Structures
2.5. Cytotoxicity Assay
3. Discussion
3.1. Chloroquine and Everolimus in Activating the Apoptosis Process and Inhibiting Proliferation in Melanoma Cells
3.2. Redistribution of Lipid Structures in Fluorescence Microscopy Images After Chloroquine and Everolimus Treatment
4. Materials and Methods
4.1. Cell Culture
4.2. Caspase-3 Activation Assay
4.3. Western Blot Analysis
4.4. DNA Fragmentation ELISA Assay
4.5. Cell Proliferation
4.6. Cytotoxicity Assay
4.7. Fluorescence Microscopy
4.8. DAPI Staining Assay
4.9. Acridine Orange (AO)/Propidium Iodide (PI) Live and Dead Cell Co-Staining
4.10. Nile Red Staining Assay
4.11. Nile Blue Staining Assay
4.12. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, W.; Wang, H.; Yang, Y.; Chen, Z.S.; Zou, C.; Zhang, J. Chloroquine against malaria, cancers and viral diseases. Drug Discov. Today 2020, 25, 2012–2022. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, A.K.; Saadeldin, M.K.; Salem, A.H.; Ibrahim, S.A.; Shouman, S.; Abdel-Naim, A.B.; Orecchia, R. A Critical Review of Chloroquine and Hydroxychloroquine as Potential Adjuvant Agents for Treating People with Cancer. Future Pharmacol. 2022, 2, 431–443. [Google Scholar] [CrossRef]
- Burikhanov, R.; Hebbar, N.; Noothi, S.K.; Shukla, N.; Sledziona, J.; Araujo, N.; Kudrimoti, M.; Wang, Q.; Watt, D.S.; Welch, D.R.; et al. Chloroquine-Inducible Par-4 Secretion Is Essential for Tumor Cell Apoptosis and Inhibition of Metastasis. Cell Rep. 2017, 18, 508–519. [Google Scholar] [CrossRef] [PubMed]
- De Sanctis, J.B.; Charris, J.; Blanco, Z.; Ramírez, H.; Martínez, G.P.; Mijares, M.R. Molecular Mechanisms of Chloroquine and Hydroxychloroquine Used in Cancer Therapy. Anticancer Agents Med. Chem. 2023, 23, 1122–1144. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Meng, Y.; Zhang, J.; Gu, L.; Shen, S.; Zhu, Y.; Wang, J. Pharmacology Progresses and Applications of Chloroquine in Cancer Therapy. Int. J. Nanomed. 2024, 19, 6777–6809. [Google Scholar] [CrossRef]
- Varisli, L.; Cen, O.; Vlahopoulos, S. Dissecting pharmacological effects of chloroquine in cancer treatment: Interference with inflammatory signaling pathways. Immunology 2020, 159, 257–278. [Google Scholar] [CrossRef]
- Piffoux, M.; Eriau, E.; Cassier, P.A. Autophagy as a therapeutic target in pancreatic cancer. Br. J. Cancer 2021, 124, 333–344. [Google Scholar] [CrossRef]
- McCubrey, J.; Abrams, S.; Follo, M.; Manzoli, L.; Ratti, S.; Martelli, A.; Cervello, M. Effects of chloroquine and hydroxychloroquine on the sensitivity of pancreatic cancer cells to targeted therapies. Adv. Biol. Regul. 2023, 87, 100917. [Google Scholar] [CrossRef]
- Zou, Z.; Tao, T.; Li, H. mTOR signaling pathway and mTOR inhibitors in cancer: Progress and challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef]
- Kim, Y.C.; Guan, K.L. mTOR: A pharmacologic target for autophagy regulation. J. Clin. Investig. 2015, 125, 25–32. [Google Scholar] [CrossRef]
- Hage, A.; Dormond, O. Combining mTOR Inhibitors and T-cell-Based Immunotherapies in Cancer Treatment. Cancers 2021, 13, 1359. [Google Scholar] [CrossRef]
- Baroja-Mazo, A.; Revilla-Nuin, B.; Ramírez, P.; Pons, J.A. Immunosuppressive potency of mechanistic target of rapamycin inhibitors in solid-organ transplantation. World J. Transplant. 2016, 24, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.J.W.; Hodzic Kuerec, A.; Maier, A.B. Targeting ageing with rapamycin and its derivatives in humans: A systematic review. Lancet Healthy Longev. 2024, 5, e152–e162. [Google Scholar] [CrossRef]
- Ciołczyk-Wierzbicka, D.; Gil, D.; Laidler, P. Treatment of melanoma with selected inhibitors of signaling kinases effectively reduces proliferation and induces expression of cell cycle inhibitors. Med. Oncol. 2018, 35, 7. [Google Scholar] [CrossRef] [PubMed]
- Ciołczyk-Wierzbicka, D.; Gil, D.; Zarzycka, M.; Laidler, P. mTOR inhibitor everolimus reduces invasiveness of melanoma cells. Hum. Cell 2020, 33, 88–97. [Google Scholar] [CrossRef]
- Ciołczyk-Wierzbicka, D.; Zarzycka, M.; Gil, D.; Laidler, P. mTOR inhibitor Everolimus-induced apoptosis in melanoma cells. J. Cell Commun. Signal. 2019, 13, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Ciołczyk-Wierzbicka, D.; Krawczyk, A.; Zarzycka, M.; Zemanek, G.; Wierzbicki, K. Three generations of mTOR kinase inhibitors in the activation of the apoptosis process in melanoma cells. J. Cell Commun. Signal. 2023, 17, 975–989. [Google Scholar] [CrossRef] [PubMed]
- Fraser, S. Using Nile Blue and Nile Red to Visualize the Presence of Lipids in Cryosectioned Tissues. Posted by Stuart Fraser, on 14 September 2021. Available online: https://doi.org/10.1242/focalplane.6551 (accessed on 14 September 2021).
- López-Méndez, T.B.; Sánchez-Álvarez, M.; Trionfetti, F.; Pedraz, J.L.; Tripodi, M.; Cordani, M.; Strippoli, R.; González-Valdivieso, J. Nanomedicine for autophagy modulation in cancer therapy: A clinical perspective. Cell Biosci. 2023, 13, 44. [Google Scholar] [CrossRef]
- Biswas, U.; Roy, R.; Ghosh, S.; Chakrabarti, G. The interplay between autophagy and apoptosis: Its implication in lung cancer and therapeutics. Cancer Lett. 2024, 585, 216662. [Google Scholar] [CrossRef]
- Lopez, J.; Tait, S. Mitochondrial apoptosis: Killing cancer using the enemy within. Br. J. Cancer 2015, 112, 957–962. [Google Scholar] [CrossRef]
- Xi, H.; Wang, S.; Wang, B.; Hong, X.; Liu, X.; Li, M.; Shen, R.; Dong, Q. The role of interaction between autophagy and apoptosis in tumorigenesis. Oncol. Rep. 2020, 48, 208. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.L.; Wüstenberg, R.; Rübsam, A.; Schmitz-Salue, C.; Warnecke, G.; Bücker, E.M.; Pettkus, N.; Speidel, D.; Rohde, V.; Schulz-Schaeffer, W.; et al. Chloroquine activates the p53 pathway and induces apoptosis in human glioma cells. Neuro Oncol. 2010, 12, 389–400. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Nakamura, O.; Yamagami, Y.; Nishimura, H.; Fukuoka, N.; Yamamoto, T. Chloroquine Enhances Rapamycin-induced Apoptosis in MG63 Cells. Anticancer Res. 2019, 39, 649–654. [Google Scholar] [CrossRef]
- Doncel, J.P.; de la Cruz Ojeda, P.; OropesaÁvila, M.; Paz, M.V.; De Lavera, I.; La Mata, M.D.; Córdoba, M.Á.; Hidalgo, R.L.; Rivero, J.M.; Cotán, D. Cytoskeleton Rearrangements during the Execution Phase of Apoptosis [Internet]. In Cytoskeleton—Structure, Dynamics, Function and Disease; InTech: London, UK, 2017. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, X.; Gueydan, C.; Han, J. Plasma membrane changes during programmed cell deaths. Cell Res. 2018, 28, 9–21. [Google Scholar] [CrossRef]
- Grimaldi, A.; Santini, D.; Zappavigna, S.; Lombardi, A.; Misso, G.; Boccellino, M.; Desiderio, V.; Vitiello, P.P.; Di Lorenzo, G.; Zoccoli, A.; et al. Antagonistic effects of chloroquine on autophagy occurrence potentiate the anticancer effects of everolimus on renal cancer cells. Cancer Biol. Ther. 2015, 16, 567–579. [Google Scholar] [CrossRef]
- Haas, N.B.; Appleman, L.J.; Stein, M.; Redlinger, M.; Wilks, M.; Xu, X.; Onorati, A.; Kalavacharla, A.; Kim, T.; Zhen, C.J.; et al. Autophagy Inhibition to Augment mTOR Inhibition: A Phase I/II Trial of Everolimus and Hydroxychloroquine in Patients with Previously Treated Renal Cell Carcinoma. Clin. Cancer Res. 2019, 25, 2080–2087. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Han, L.; Weng, J.; Wang, K.; Chen, T. Rapamycin inhibits proliferation and induces autophagy in human neuroblastoma cells. Biosci. Rep. 2018, 38, BSR20181822. [Google Scholar] [CrossRef]
- Sotthibundhu, A.; McDonagh, K.; von Kriegsheim, A.; Garcia-Munoz, A.; Klawiter, A.; Thompson, K.; Chauhan, K.D.; Krawczyk, J.; McInerney, V.; Dockery, P.; et al. Rapamycin regulates autophagy and cell adhesion in induced pluripotent stem cells. Stem Cell Res. Ther. 2016, 7, 166. [Google Scholar] [CrossRef]
- Juricic, P.; Lu, Y.X.; Leech, T.; Drews, L.F.; Paulitz, J.; Lu, J.; Nespital, T.; Azami, S.; Regan, J.C.; Funk, E.; et al. Long-lasting geroprotection from brief rapamycin treatment in early adulthood by persistently increased intestinal autophagy. Nat. Aging 2022, 2, 824–836. [Google Scholar] [CrossRef]
- Elshazly, A.M.; Elzahed, A.A.; Gewirtz, D.A. The Cytoprotective and Cytotoxic Functions of Autophagy in Response to mTOR Inhibitors. Front. Biosci. 2024, 29, 231. [Google Scholar] [CrossRef]
- Qin, Y.; Ashrafizadeh, M.; Mongiardini, V.; Grimaldi, B.; Crea, F.; Rietdorf, K.; Győrffy, B.; Klionsky, D.J.; Ren, J.; Zhang, W.; et al. Autophagy and cancer drug resistance in dialogue: Pre-clinical and clinical evidence. Cancer Lett. 2023, 570, 216307. [Google Scholar] [CrossRef] [PubMed]
- Mele, L.; Del Vecchio, V.; Liccardo, D.; Prisco, C.; Schwerdtfeger, M.; Robinson, N.; Desiderio, V.; Tirino, V.; Papaccio, G.; La Noce, M. The role of autophagy in resistance to targeted therapies. Cancer Treat. Rev. 2020, 88, 102043. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Das, D.; Taneja, R. Targeting Dysregulated Lipid Metabolism in Cancer with Pharmacological Inhibitors. Cancers 2024, 16, 1313. [Google Scholar] [CrossRef] [PubMed]
- Butler, L.M.; Perone, Y.; Dehairs, J.; Lupien, L.E.; de Laat, V.; Talebi, A.; Loda, M.; Kinlaw, W.B.; Swinnen, J.V. Lipids and cancer: Emerging roles in pathogenesis, diagnosis and therapeutic intervention. Adv. Drug Deliv. Rev. 2020, 159, 245–293. [Google Scholar] [CrossRef]
- Fu, Y.; Zou, T.; Shen, X.; Nelson, P.J.; Li, J.; Wu, C.; Yang, J.; Zheng, Y.; Bruns, C.; Zhao, Y.; et al. Lipid metabolism in cancer progression and therapeutic strategies. MedComm 2020, 24, 27–59. [Google Scholar] [CrossRef]
- Lin, Z.; Long, F.; Kang, R.; Klionsky, D.J.; Yang, M.; Tang, D. The lipid basis of cell death and autophagy. Autophagy 2024, 20, 469–488. [Google Scholar] [CrossRef]
- Schrezenmeier, E.; Dörner, T. Mechanisms of action of hydroxychloroquine and chloroquine: Implications for rheumatology. Nat. Rev. Rheumatol. 2020, 16, 155–166. [Google Scholar] [CrossRef]
- Bik, E.; Mateuszuk, L.; Orleanska, J.; Baranska, M.; Chlopicki, S.; Majzner, K. Chloroquine-Induced Accumulation of Autophagosomes and Lipids in the Endothelium. Int. J. Mol. Sci. 2021, 27, 2401. [Google Scholar] [CrossRef]
- Xu, F.; Tautenhahn, H.M.; Dirsch, O.; Dahmen, U. Blocking autophagy with chloroquine aggravates lipid accumulation and reduces intracellular energy synthesis in hepatocellular carcinoma cells, both contributing to its anti-proliferative effect. J. Cancer Res. Clin. Oncol. 2020, 148, 3243–3256. [Google Scholar] [CrossRef]
- Madsen, J.; Irene Canton, I.; Warren, N.; Themistou, E.; Blanazs, A.; Ustbas, B.; Tian, X.; Pearson, R.; Battaglia, G.; Lewis, A.L.; et al. Nile Blue-Based Nanosized pH Sensors for Simultaneous Far-Red and Near-Infrared Live Bioimaging. J. Am. Chem. Soc. 2013, 135, 14863–14870. [Google Scholar] [CrossRef]
- Laplante, M.; Sabatini, D.M. An Emerging Role of mTOR in Lipid Biosynthesis. Curr. Biol. 2009, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Panwar, V.; Singh, A.; Bhatt, M.; Tonk, R.K.; Azizov, S.; Raza, A.S.; Sengupta, S.; Kumar, D.; Garg, M. Multifaceted role of mTOR (mammalian target of rapamycin) signaling pathway in human health and disease. Signal Transduct. Target. Ther. 2023, 8, 375. [Google Scholar] [CrossRef] [PubMed]
- Ciołczyk-Wierzbicka, D.; Gil, D.; Laidler, P. The inhibition of cell proliferation using silencing of N-cadherin gene by siRNA process in human melanoma cell lines. Curr. Med. Chem. 2012, 19, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Boumelhem, B.B.; Pilgrim, C.; Zwicker, V.E.; Kolanowski, J.L.; Yeo, J.H.; Jolliffe, K.A.; New, E.J.; Day, M.L.; Assinder, S.J.; Stuart, T. Fraser; Intracellular flow cytometric lipid analysis—A multiparametric system to assess distinct lipid classes in live cells. J. Cell Sci. 2022, 135, jcs258322. [Google Scholar] [CrossRef]
- Zhu, Y.; Wu, B.; Gao, X.; Guo, Y.; Peng, X.; Wang, L.; Tian, X.; Wang, J.; Yan, W.; Zhang, H.; et al. Fluorescence lifetime imaging microscopy for early diagnosis and severity prediction of preeclampsia with Nile Blue probe. Biosens. Bioelectron. 2024, 244, 115809. [Google Scholar] [CrossRef]


| Control | DMSO 1% | DMSO 2% | ||
|---|---|---|---|---|
| Wm3211 | 24 h | 1.1 ± 0.1 | 1.0 ± 0.1 | 1.1 ± 0.1 |
| 48 h | 1.5 ± 0.1 | 1.4 ± 0.1 | 1.6 ± 0.1 | |
| 72 h | 1.8 ± 0.1 | 1.8 ± 0.2 | 2.0 ± 0.1 | |
| Me15392 | 24 h | 2.1 ± 0.1 | 2.2 ± 0.1 | 2.1 ± 0.1 |
| 48 h | 2.3 ± 0.1 | 2.4 ± 0.1 | 2.1 ± 0.1 | |
| 72 h | 2.4 ± 0.1 | 2.5 ± 0.1 | 2.6 ± 0.2 | |
| MEWO | 24 h | 1.8 ± 0.1 | 2.0 ± 0.2 | 2.1 ± 0.1 |
| 48 h | 2.0 ± 0.1 | 2.2 ± 0.2 | 2.1 ± 0.2 | |
| 72 h | 2.1 ± 0.1 | 2.2 ± 0.2 | 2.3 ± 0.1 |
| Control | Everolimus 20 nM | Everolimus 10 μM | Everolimus 25 μM | Rapamycin 25 μM | Rapamycin 50 μM | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 25 μM Chloroquine | − | + | − | + | − | + | − | + | − | + | − | + | |
| Cytotoxicity [%] | |||||||||||||
| Wm3211 | 24 h | 1.1 ± 0.1 | 1.2 ± 0.1 | 1.5 ± 0.1 | 1.5 ± 0.2 | 2.1 ± 0.1 | 2.0 ± 0.1 | 2.3 ± 0.1 | 2.3 ± 0.1 | 3.2 ± 0.2 | 3.0 ± 0.2 | 6.2 ± 0.2 | 5.9 ± 0.2 |
| 48 h | 1.5 ± 0.1 | 1.4 ± 0.1 | 1.7 ± 0.2 | 1.7 ± 0.1 | 2.3 ± 0.1 | 2.1 ± 0.1 | 2.3 ± 0.2 | 2.3 ± 0.2 | 4.4 ± 0.2 | 4.0 ± 0.2 | 7.2 ± 0.2 | 7.0 ± 0.2 | |
| 72 h | 1.8 ± 0.1 | 1.7 ± 0.1 | 1.9 ± 0.2 | 2.0 ± 0.2 | 2.4 ± 0.1 | 2.4 ± 0.2 | 2.6 ± 0.2 | 2.4 ± 0.2 | 5.7 ± 0.2 | 5.2 ± 0.2 | 9.8 ± 0.2 | 9.1 ± 0.3 | |
| 24 h | 2.1 ± 0.1 | 2.2 ± 0.2 | 2.1 ± 0.1 | 2.4 ± 0.1 | 2.4 ± 0.1 | 2.1 ± 0.2 | 2.3 ± 0.1 | 2.1 ± 0.1 | 4.2 ± 0.2 | 3.5 ± 0.2 | 8.7 ± 0.2 | 8.1 ± 0.2 | |
| 48 h | 2.3 ± 0.1 | 2.1 ± 0.1 | 2.3 ± 0.1 | 2.1 ± 0.1 | 2.1 ± 0.1 | 2.0 ± 0.2 | 2.3 ± 0.1 | 2.2 ± 0.1 | 5.1 ± 0.2 | 4.5 ± 0.2 | 12 ± 0.3 | 11 ± 0.3 | |
| 72 h | 2.4 ± 0.1 | 2.3 ± 0.2 | 2.2 ± 0.2 | 2.2 ± 0.2 | 2.3 ± 0.1 | 2.4 ± 0.2 | 2.6 ± 0.1 | 2.2 ± 0.1 | 6.9 ± 0.2 | 6.2 ± 0.1 | 15 ± 0.3 | 14 ± 0.3 | |
| MEWO | 24 h | 1.8 ± 0.1 | 1.5 ± 0.1 | 2.1 ± 0.2 | 2.4 ± 0.1 | 2.2 ± 0.1 | 2.3 ± 0.2 | 2.4 ± 0.1 | 2.3 ± 0.2 | 4.8 ± 0.2 | 3.9 ± 0.2 | 9.1 ± 0.3 | 8 ± 0.2 |
| 48 h | 2.0 ± 0.1 | 1.8 ± 0.1 | 2.0 ± 0.2 | 2.4 ± 0.2 | 2.1 ± 0.1 | 2.1 ± 0.2 | 2.1 ± 0.2 | 2.2 ± 0.1 | 5.4 ± 0.1 | 4.8 ± 0.2 | 15 ± 0.3 | 13 ± 0.3 | |
| 72 h | 2.1 ± 0.1 | 2.2 ± 0.2 | 2.1 ± 0.2 | 2.3 ± 0.2 | 2.2 ± 0.1 | 2.2 ± 0.2 | 2.7 ± 0.2 | 2.1 ± 0.1 | 7.1 ± 0.2 | 6.0 ± 0.2 | 18 ± 0.3 | 15 ± 0.3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciołczyk-Wierzbicka, D.; Zarzycka, M.; Placha, W.; Zemanek, G.; Wierzbicki, K. Treatment of Melanoma Cells with Chloroquine and Everolimus Activates the Apoptosis Process and Alters Lipid Redistribution. Int. J. Mol. Sci. 2024, 25, 12278. https://doi.org/10.3390/ijms252212278
Ciołczyk-Wierzbicka D, Zarzycka M, Placha W, Zemanek G, Wierzbicki K. Treatment of Melanoma Cells with Chloroquine and Everolimus Activates the Apoptosis Process and Alters Lipid Redistribution. International Journal of Molecular Sciences. 2024; 25(22):12278. https://doi.org/10.3390/ijms252212278
Chicago/Turabian StyleCiołczyk-Wierzbicka, Dorota, Marta Zarzycka, Wojciech Placha, Grzegorz Zemanek, and Karol Wierzbicki. 2024. "Treatment of Melanoma Cells with Chloroquine and Everolimus Activates the Apoptosis Process and Alters Lipid Redistribution" International Journal of Molecular Sciences 25, no. 22: 12278. https://doi.org/10.3390/ijms252212278
APA StyleCiołczyk-Wierzbicka, D., Zarzycka, M., Placha, W., Zemanek, G., & Wierzbicki, K. (2024). Treatment of Melanoma Cells with Chloroquine and Everolimus Activates the Apoptosis Process and Alters Lipid Redistribution. International Journal of Molecular Sciences, 25(22), 12278. https://doi.org/10.3390/ijms252212278






