Prognostic Significance of PD-L1 Expression on Circulating Myeloid-Derived Suppressor Cells in NSCLC Patients Treated with Anti-PD-1/PD-L1 Checkpoint Inhibitors
Abstract
1. Introduction
2. Results
2.1. Patients Characteristics
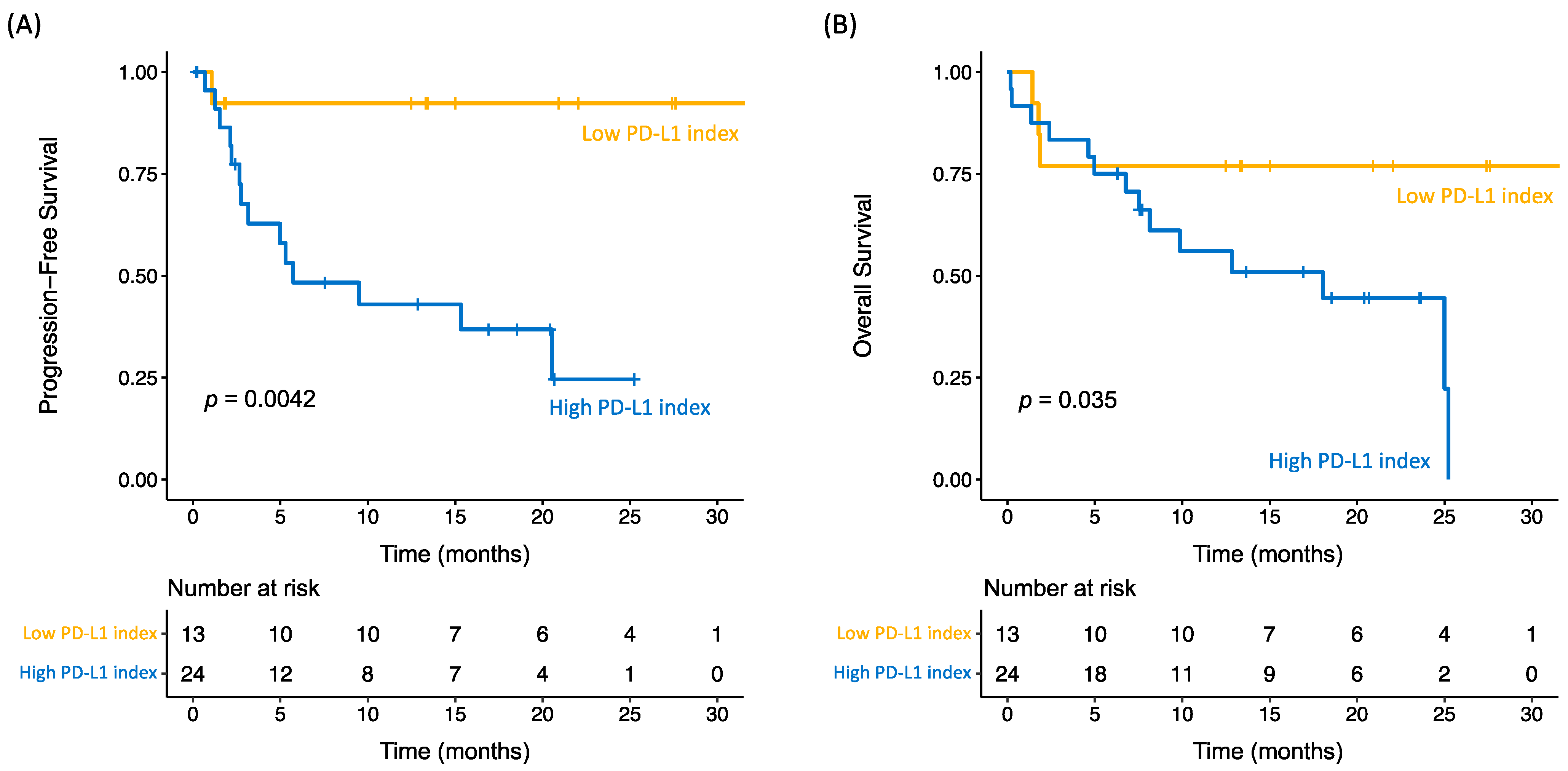
2.2. Clinical Outcome
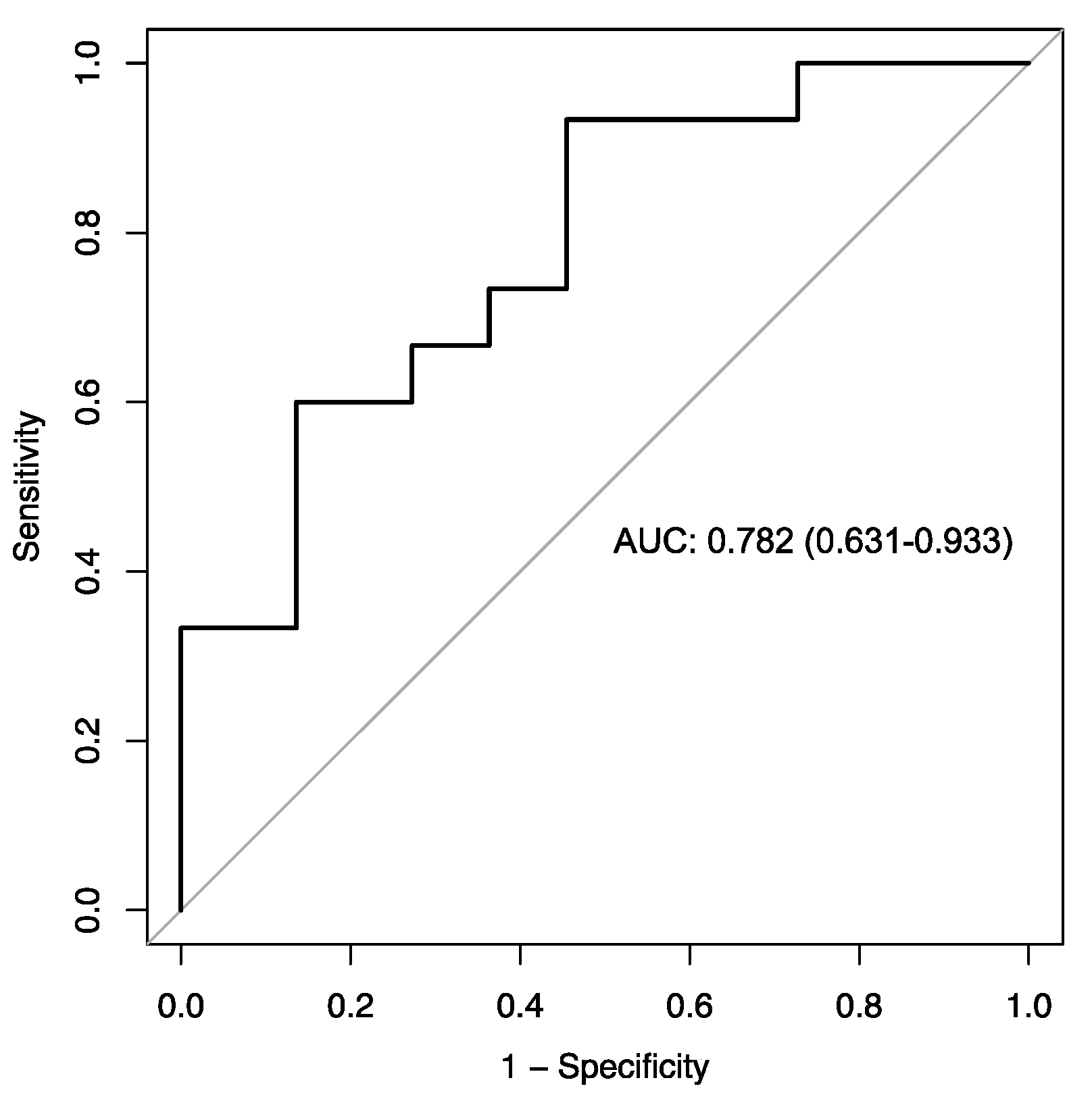
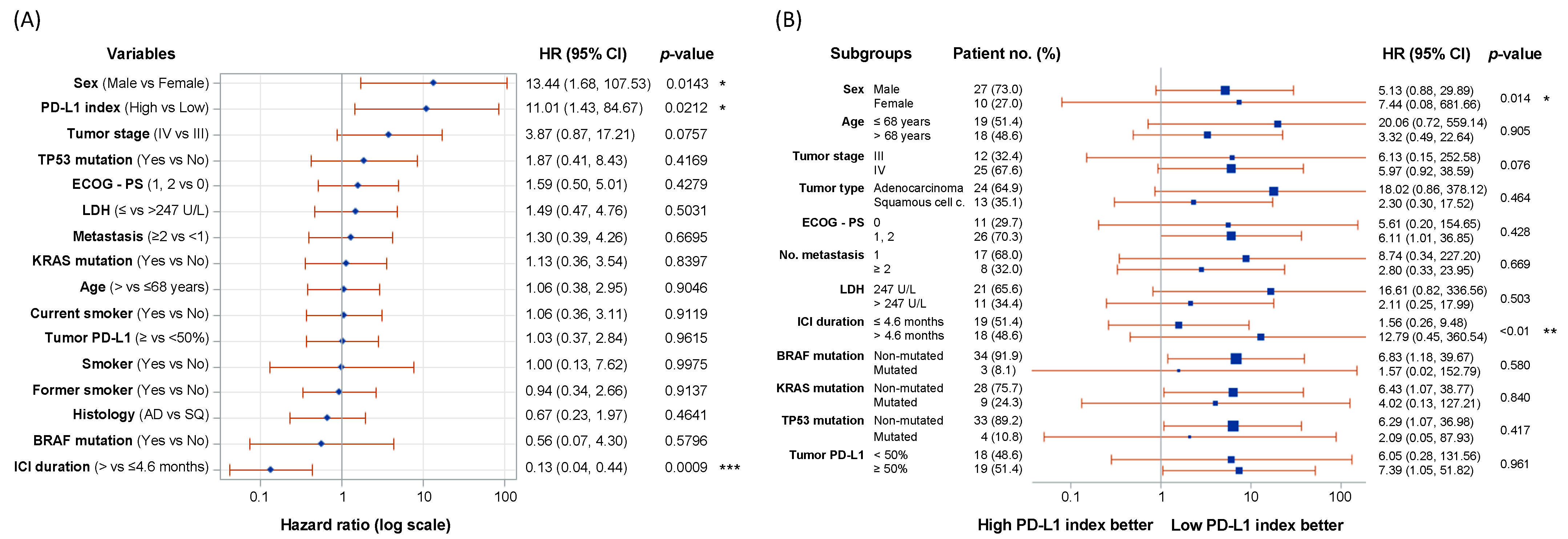
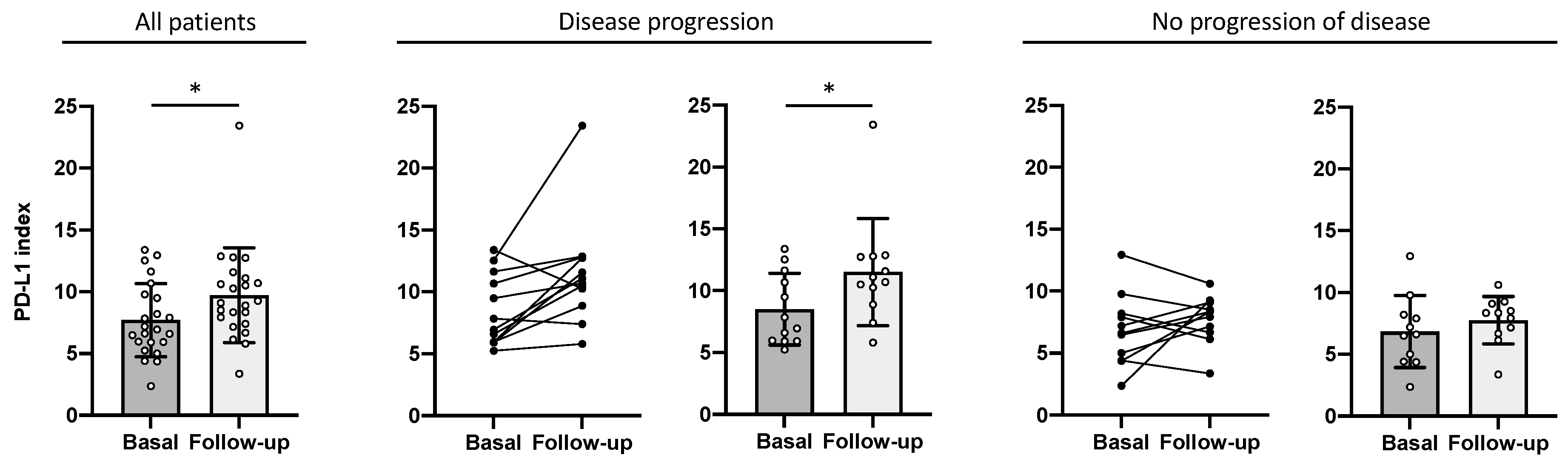
2.3. PD-L1 Index of Myeloid-Derived Suppressor Cells Is a Predictor of Clinical Outcome
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Sample Preparation and Flow Cytometry Data Analysis
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Howlader, N.; Forjaz, G.; Mooradian, M.J.; Meza, R.; Kong, C.Y.; Cronin, K.A.; Mariotto, A.B.; Lowy, D.R.; Feuer, E.J. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N. Engl. J. Med. 2020, 383, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Cronin, K.A.; Scott, S.; Firth, A.U.; Sung, H.; Henley, S.J.; Sherman, R.L.; Siegel, R.L.; Anderson, R.N.; Kohler, B.A.; Benard, V.B.; et al. Annual Report to the Nation on the Status of Cancer, Part 1: National Cancer Statistics. Cancer 2022, 128, 4251–4284. [Google Scholar] [CrossRef] [PubMed]
- Horn, L.; Spigel, D.R.; Vokes, E.E.; Holgado, E.; Ready, N.; Steins, M.; Poddubskaya, E.; Borghaei, H.; Felip, E.; Paz-Ares, L.; et al. Nivolumab versus Docetaxel in Previously Treated Patients with Advanced Non-Small-Cell Lung Cancer: Two-Year Outcomes from Two Randomized, Open-Label, Phase III Trials (CheckMate 017 and CheckMate 057). J. Clin. Oncol. 2017, 35, 3924–3933. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S.; Giaccone, G.; de Marinis, F.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1–Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef] [PubMed]
- Paz-Ares, L.; Luft, A.; Vicente, D.; Tafreshi, A.; Gümüş, M.; Mazières, J.; Hermes, B.; Çay Şenler, F.; Csőszi, T.; Fülöp, A.; et al. Pembrolizumab plus Chemotherapy for Squamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2018, 379, 2040–2051. [Google Scholar] [CrossRef]
- Antonia, S.J.; Villegas, A.; Daniel, D.; Vicente, D.; Murakami, S.; Hui, R.; Yokoi, T.; Chiappori, A.; Lee, K.H.; de Wit, M.; et al. Durvalumab after Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2017, 377, 1919–1929. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, X.Z.; Zhao, S.J.; Yang, Q.W. PD-L1 as a predictive factor for non-small-cell lung cancer prognosis. Lancet Oncol. 2024, 25, e233. [Google Scholar] [CrossRef]
- Davis, A.A.; Patel, V.G. The role of PD-L1 expression as a predictive biomarker: An analysis of all US Food and Drug Administration (FDA) approvals of immune checkpoint inhibitors. J. Immunother. Cancer 2019, 7, 278–285. [Google Scholar] [CrossRef]
- Evans, M.; O’Sullivan, B.; Smith, M.; Taniere, P. Predictive markers for anti-PD-1/PD-L1 therapy in non-small cell lung cancer—Where are we? Transl. Lung Cancer Res. 2018, 7, 682–690. [Google Scholar] [CrossRef]
- Nikolic, N.; Golubovic, A.; Ratkovic, A.; Pandurevic, S.; Kontic, M. Brief Report: Predictive value of PD-L1 Expression in non–Small-Cell Lung Cancer-Should we Set the Bar Higher for Monotherapy? Clin. Lung Cancer 2023, 24, e214–e218. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-Derived Suppressor Cells as Regulators of the Immune System. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Umansky, V.; Blattner, C.; Gebhardt, C.; Utikal, J. The Role of Myeloid-Derived Suppressor Cells (MDSC) in Cancer Progression. Vaccines 2016, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Youn, J.-I.; Collazo, M.; Shalova, I.N.; Biswas, S.K.; Gabrilovich, D.I. Characterization of the Nature of Granulocytic Myeloid-Derived Suppressor Cells in Tumor-Bearing Mice. J. Leukoc. Biol. 2011, 91, 167–181. [Google Scholar] [CrossRef] [PubMed]
- Veglia, F.; Perego, M.; Gabrilovich, D.I. Myeloid-Derived Suppressor Cells Coming of Age. Nat. Immunol. 2018, 19, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Zahran, A.M.; Hetta, H.F.; Zahran, Z.A.M.; Rashad, A.; Rayan, A.; Mohamed, D.O.; Elhameed, Z.A.A.; Khallaf, S.M.; Batiha, G.E.S.; Waheed, Y.; et al. Prognostic Role of Monocytic Myeloid-Derived Suppressor Cells in Advanced Non-Small-Cell Lung Cancer: Relation to Different Hematologic Indices. J. Immunol. Res. 2021, 2021, 3241150. [Google Scholar] [CrossRef]
- Bronte, G.; Petracci, E.; De Matteis, S.; Canale, M.; Zampiva, I.; Priano, I.; Cravero, P.; Andrikou, K.; Burgio, M.A.; Ulivi, P.; et al. High Levels of Circulating Monocytic Myeloid-Derived Suppressive-Like Cells Are Associated with the Primary Resistance to Immune Checkpoint Inhibitors in Advanced Non-Small Cell Lung Cancer: An Exploratory Analysis. Front. Immunol. 2022, 13, 866561. [Google Scholar] [CrossRef]
- Koh, J.; Kim, Y.; Lee, K.Y.; Hur, J.Y.; Kim, M.S.; Kim, B.; Cho, H.J.; Lee, Y.C.; Bae, Y.H.; Ku, B.M.; et al. MDSC Subtypes and CD39 Expression on CD8+ T Cells Predict the Efficacy of Anti-PD-1 Immunotherapy in Patients with Advanced NSCLC. Eur. J. Immunol. 2020, 50, 1810–1819. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the Treatment of Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef]
- Reck, M.; Rodríguez-Abreu, D.; Robinson, A.G.; Hui, R.; Csőszi, T.; Fülöp, A.; Gottfried, M.; Peled, N.; Tafreshi, A.; Cuffe, S.; et al. Pembrolizumab versus Chemotherapy for PD-L1–Positive Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2016, 375, 1823–1833. [Google Scholar] [CrossRef]
- Woodford, R.; Zhou, D.; Lord, S.J.; Marschner, I.; Cooper, W.A.; Lewis, C.R.; John, T.; Yang, J.C.H.; Lee, C.K. PD-L1 Expression as a Prognostic Marker in Patients Treated with Chemotherapy for Metastatic Non-Small-Cell Lung Cancer. Future Oncol. 2022, 18, 1793–1799. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Ding, K.; Yi, M.; Liang, H.; Li, Z.; Zhang, Y. Efficacy Comparison of Immune Treating Strategies for NSCLC Patients with Negative PD-L1 Expression. Expert Rev. Clin. Immunol. 2022, 18, 759–771. [Google Scholar] [CrossRef] [PubMed]
- Borghaei, H.; Langer, C.J.; Paz-Ares, L.; Rodríguez-Abreu, D.; Halmos, B.; Garassino, M.C.; Houghton, B.; Kurata, T.; Cheng, Y.; Lin, J.; et al. Pembrolizumab plus Chemotherapy versus Chemotherapy Alone in Patients with Advanced Non–Small Cell Lung Cancer without Tumor PD-L1 Expression: A Pooled Analysis of 3 Randomized Controlled Trials. Cancer 2020, 126, 4867–4877. [Google Scholar] [CrossRef] [PubMed]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef]
- Zhao, Y.; Shi, F.; Zhou, Q.; Li, Y.; Wu, J.; Wang, R.; Song, Q.; Shishodia, G. Prognostic Significance of PD-L1 in Advanced Non-Small Cell Lung Carcinoma. Medicine 2020, 99, E23172. [Google Scholar] [CrossRef]
- McLaughlin, J.; Han, G.; Schalper, K.A.; Carvajal-Hausdorf, D.; Pelakanou, V.; Rehman, J.; Velcheti, V.; Herbst, R.; LoRusso, P.; Rimm, D.L. Quantitative Assessment of the Heterogeneity of PD-L1 Expression in Non-Small Cell Lung Cancer (NSCLC). JAMA Oncol. 2016, 2, 46–54. [Google Scholar] [CrossRef]
- Rico, L.G.; Aguilar Hernández, A.; Ward, M.D.; Bradford, J.A.; Juncà, J.; Rosell, R.; Petriz, J. Unmasking the Expression of PD-L1 in Myeloid Derived Suppressor Cells: A Case Study in Lung Cancer to Discover New Drugs with Specific on-Target Efficacy. Transl. Oncol. 2021, 14, 2020–2022. [Google Scholar] [CrossRef]
- Hellmann, M.D.; Ciuleanu, T.-E.; Pluzanski, A.; Lee, J.S.; Otterson, G.A.; Audigier-Valette, C.; Minenza, E.; Linardou, H.; Burgers, S.; Salman, P.; et al. Nivolumab plus Ipilimumab in Lung Cancer with a High Tumor Mutational Burden. N. Engl. J. Med. 2018, 378, 2093–2104. [Google Scholar] [CrossRef]
- Socinski, M.A.; Jotte, R.M.; Cappuzzo, F.; Orlandi, F.; Stroyakovskiy, D.; Nogami, N.; Rodríguez-Abreu, D.; Moro-Sibilot, D.; Thomas, C.A.; Barlesi, F.; et al. Atezolizumab for First-Line Treatment of Metastatic Nonsquamous NSCLC. N. Engl. J. Med. 2018, 378, 2288–2301. [Google Scholar] [CrossRef]
- Nindra, U.; Shahnam, A.; Stevens, S.; Pal, A.; Nagrial, A.; Lee, J.; Yip, P.Y.; Adam, T.; Boyer, M.; Kao, S.; et al. Elevated Neutrophil-to-Lymphocyte Ratio (NLR) Is Associated with Poorer Progression-Free Survival in Unresectable Stage III NSCLC Treated with Consolidation Durvalumab. Thorac. Cancer 2022, 13, 3058–3062. [Google Scholar] [CrossRef]
- Mandaliya, H.; Jones, M.; Oldmeadow, C.; Nordman, I.I.C. Prognostic Biomarkers in Stage IV Non-Small Cell Lung Cancer (NSCLC): Neutrophil to Lymphocyte Ratio (NLR), Lymphocyte to Monocyte Ratio (LMR), Platelet to Lymphocyte Ratio (PLR) and Advanced Lung Cancer Inflammation Index (ALI). Transl. Lung Cancer Res. 2019, 8, 886–894. [Google Scholar] [CrossRef] [PubMed]
- Mezquita, L.; Preeshagul, I.; Auclin, E.; Saravia, D.; Hendriks, L.; Rizvi, H.; Park, W.; Nadal, E.; Martin-Romano, P.; Ruffinelli, J.C.; et al. Predicting Immunotherapy Outcomes under Therapy in Patients with Advanced NSCLC Using DNLR and Its Early Dynamics. Eur. J. Cancer 2021, 151, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Aldea, M.; Benitez, J.C.; Mezquita, L. The Lung Immune Prognostic Index (LIPI) Stratifies Prognostic Groups in Advanced Non-Small Cell Lung Cancer (NSCLC) Patients. Transl. Lung. Cancer Res. 2020, 9, 967–970. [Google Scholar] [CrossRef] [PubMed]
- Mezquita, L.; Auclin, E.; Ferrara, R.; Charrier, M.; Remon, J.; Planchard, D.; Ponce, S.; Ares, L.P.; Leroy, L.; Audigier-Valette, C.; et al. Association of the Lung Immune Prognostic Index with Immune Checkpoint Inhibitor Outcomes in Patients With Advanced Non–Small Cell Lung Cancer. JAMA Oncol. 2018, 4, 351–357. [Google Scholar] [CrossRef]
- Banna, G.L.; Cortellini, A.; Cortinovis, D.L.; Tiseo, M.; Aerts, J.G.J.V.; Barbieri, F.; Giusti, R.; Bria, E.; Grossi, F.; Pizzutilo, P.; et al. The Lung Immuno-Oncology Prognostic Score (LIPS-3): A Prognostic Classification of Patients Receiving First-Line Pembrolizumab for PD-L1 ≥ 50% Advanced Non-Small-Cell Lung Cancer. ESMO Open 2021, 6, 100078. [Google Scholar] [CrossRef]
- Lang, D.; Brauner, A.; Huemer, F.; Rinnerthaler, G.; Horner, A.; Wass, R.; Brehm, E.; Kaiser, B.; Greil, R.; Lamprecht, B. Sex-Based Clinical Outcome in Advanced NSCLC Patients Undergoing PD-1/PD-L1 Inhibitor Therapy-A Retrospective Bi-Centric Cohort Study. Cancers 2021, 14, 93. [Google Scholar] [CrossRef]
- Liang, J.; Hong, J.; Tang, X.; Qiu, X.; Zhu, K.; Zhou, L.; Guo, D. Sex difference in response to non-small cell lung cancer immunotherapy: An updated meta-analysis. Ann. Med. 2022, 54, 2605–2615. [Google Scholar] [CrossRef]
- Suay, G.; Garcia-Cañaveras, J.C.; Aparisi, F.; Lahoz, A.; Juan-Vidal, O. Sex Differences in the Efficacy of Immune Checkpoint Inhibitors in Neoadjuvant Therapy of Non-Small Cell Lung Cancer: A Meta-Analysis. Cancers 2023, 15, 4433–4447. [Google Scholar] [CrossRef]
- Dunn, S.E.; Perry, W.A.; Klein, S.L. Mechanisms and Consequences of Sex Differences in Immune Responses. Nat. Rev. Nephrol. 2024, 20, 37–55. [Google Scholar] [CrossRef]
- Zak, K.M.; Grudnik, P.; Magiera, K.; Dömling, A.; Dubin, G.; Holak, T.A. Structural Biology of the Immune Checkpoint Receptor PD-1 and Its Ligands PD-L1/PD-L2. Structure 2017, 25, 1163–1174. [Google Scholar] [CrossRef]
- Zhou, L.; Chai, F.; He, Y.; Zhou, Z.; Guo, S.; Li, P.; Sun, Q.; Zu, X.; Liu, X.; Huang, Q.; et al. Homodimerized Cytoplasmic Domain of PD-L1 Regulates Its Complex Glycosylation in Living Cells. Commun. Biol. 2022, 5, 887. [Google Scholar] [CrossRef] [PubMed]
- Salvia, R.; Rico, L.G.; Ward, M.D.; Bradford, J.A.; Petriz, J. Functional Flow Cytometry to Predict PD-L1 Conformational Changes. Curr. Protoc. 2023, 3, e944. [Google Scholar] [CrossRef] [PubMed]
- Petriz, J.; Bradford, J.A.; Ward, M.D. No Lyse No Wash Flow Cytometry for Maximizing Minimal Sample Preparation. Methods 2018, 134–135, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Rico, L.G.; Salvia, R.; Ward, M.D.; Bradford, J.A.; Petriz, J. Flow-Cytometry-Based Protocols for Human Blood/Marrow Immunophenotyping with Minimal Sample Perturbation. STAR Protoc. 2021, 2, 100883. [Google Scholar] [CrossRef]
- Youden, W.J. Index for Rating Diagnostic Tests. Cancer 1950, 3, 32–35. [Google Scholar] [CrossRef]
- Kaplan, E.L.; Meier, P. Nonparametric Estimation from Incomplete Observations. J. Am. Stat. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
| Characteristic | Value |
|---|---|
| Patients enrolled—no. (%) | 37 (100%) |
| Male sex—no. (%) | 27 (73%) |
| Median age—year (range) | 68 (50–83) |
| Histology—no. (%) | |
| Adenocarcinoma | 24 (64.9%) |
| Squamous cell carcinoma | 13 (35.1%) |
| Driver or actionable mutations—no. (%) | |
| TP53 | 3 (8.1%) |
| KRAS | 9 (24.3%) |
| BRAF | 3 (8.1%) |
| None | 22 (59.5%) |
| Tumor PD-L1 expression—no. (%) | |
| 0–20% | 18 (48.7%) |
| 21–49% | 0 (0%) |
| 50–60% | 4 (10.8%) |
| 61–70% | 6 (16.2%) |
| 71–80% | 7 (18.9%) |
| 81–90% | 2 (5.4%) |
| Stage at diagnosis—no. (%) | |
| IIIA | 3 (8.1%) |
| IIIB | 9 (24.3%) |
| IV | 25 (67.6%) |
| ECOG performance-status score—no. (%) | |
| 0 | 11 (29.7%) |
| 1 | 24 (64.9%) |
| 2 | 2 (5.4%) |
| Therapeutic Strategies (a)—ICI type, no. (%) | |
| First-line ICI | Pembrolizumab, 15 (40.5%) |
| First-line chemotherapy plus ICI | Pembrolizumab, 6 (16.2%) |
| Second-line ICI | Pembrolizumab, 2 (5.4%) Nivolumab, 6 (16.2%) Atezolizumab, 1 (2.7%) |
| Consolidation ICI after chemoradiation | Durvalumab, 7 (18.9%) |
| History of tobacco use—no. (%) | |
| Never | 2 (5.4%) |
| Former | 24 (64.9%) |
| Current | 11 (29.7%) |
| Characteristic | Value |
|---|---|
| Best response (RECIST criteria)—no. (%) | |
| Partial response | 21 (56.7%) |
| Stable disease | 4 (10.8%) |
| Progressive disease | 8 (21.6%) |
| Nonassessable | 4 (10.8%) |
| Median ICI duration—months (range) | |
| First-line ICI | 7.5 (0–24.8) |
| First-line chemotherapy plus ICI | 9.5 (0.8–21) |
| Second-line ICI | 0.8 (0–2.8) |
| Consolidation ICI after chemoradiation | 11 (1.4–12.7) |
| Disease progression—no. (%) | |
| No progression | 22 (59.5%) |
| Progression | 15 (40.5%) |
| Survival status—no. (%) | |
| Alive | 20 (54%) |
| Deceased | 17 (46%) |
| Death causes—no. (%) | |
| Disease progression | 10 (27%) |
| Respiratory disease (a) | 3 (8.1%) |
| Cardiac failure | 2 (5.4%) |
| ICI-related adverse events (b) | 1 (2.7%) |
| Tumor-related disease (c) | 1 (2.7%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salvia, R.; Rico, L.G.; Morán, T.; Bradford, J.A.; Ward, M.D.; Drozdowskyj, A.; Climent-Martí, J.; Martínez-Cáceres, E.M.; Rosell, R.; Petriz, J. Prognostic Significance of PD-L1 Expression on Circulating Myeloid-Derived Suppressor Cells in NSCLC Patients Treated with Anti-PD-1/PD-L1 Checkpoint Inhibitors. Int. J. Mol. Sci. 2024, 25, 12269. https://doi.org/10.3390/ijms252212269
Salvia R, Rico LG, Morán T, Bradford JA, Ward MD, Drozdowskyj A, Climent-Martí J, Martínez-Cáceres EM, Rosell R, Petriz J. Prognostic Significance of PD-L1 Expression on Circulating Myeloid-Derived Suppressor Cells in NSCLC Patients Treated with Anti-PD-1/PD-L1 Checkpoint Inhibitors. International Journal of Molecular Sciences. 2024; 25(22):12269. https://doi.org/10.3390/ijms252212269
Chicago/Turabian StyleSalvia, Roser, Laura G. Rico, Teresa Morán, Jolene A. Bradford, Michael D. Ward, Ana Drozdowskyj, Joan Climent-Martí, Eva M. Martínez-Cáceres, Rafael Rosell, and Jordi Petriz. 2024. "Prognostic Significance of PD-L1 Expression on Circulating Myeloid-Derived Suppressor Cells in NSCLC Patients Treated with Anti-PD-1/PD-L1 Checkpoint Inhibitors" International Journal of Molecular Sciences 25, no. 22: 12269. https://doi.org/10.3390/ijms252212269
APA StyleSalvia, R., Rico, L. G., Morán, T., Bradford, J. A., Ward, M. D., Drozdowskyj, A., Climent-Martí, J., Martínez-Cáceres, E. M., Rosell, R., & Petriz, J. (2024). Prognostic Significance of PD-L1 Expression on Circulating Myeloid-Derived Suppressor Cells in NSCLC Patients Treated with Anti-PD-1/PD-L1 Checkpoint Inhibitors. International Journal of Molecular Sciences, 25(22), 12269. https://doi.org/10.3390/ijms252212269







