Innate Immunity and Synovitis: Key Players in Osteoarthritis Progression
Abstract
1. Background
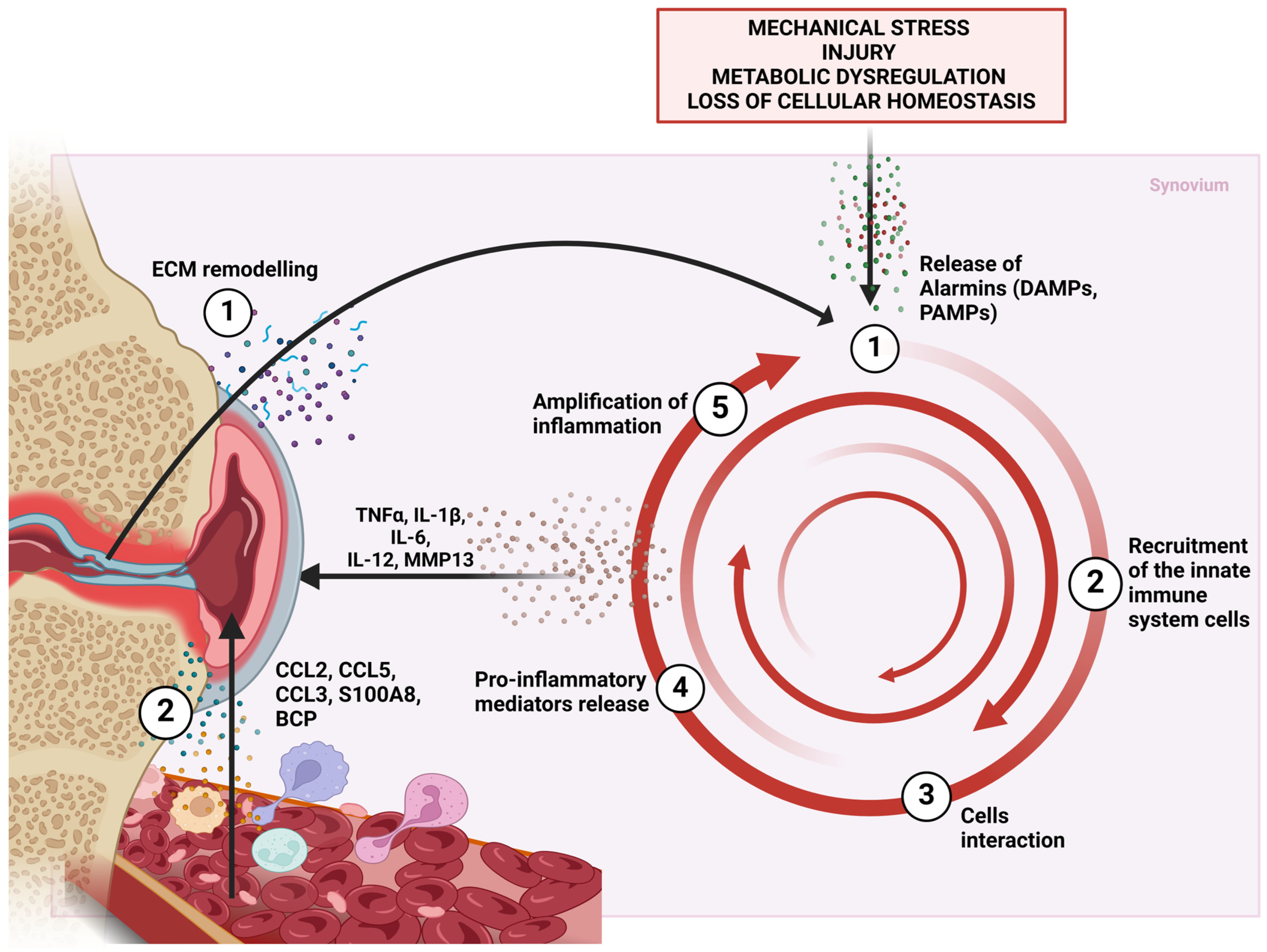
2. Synovitis in Osteoarthritis
3. Innate Immunity in Osteoarthritis
4. Materials and Methods
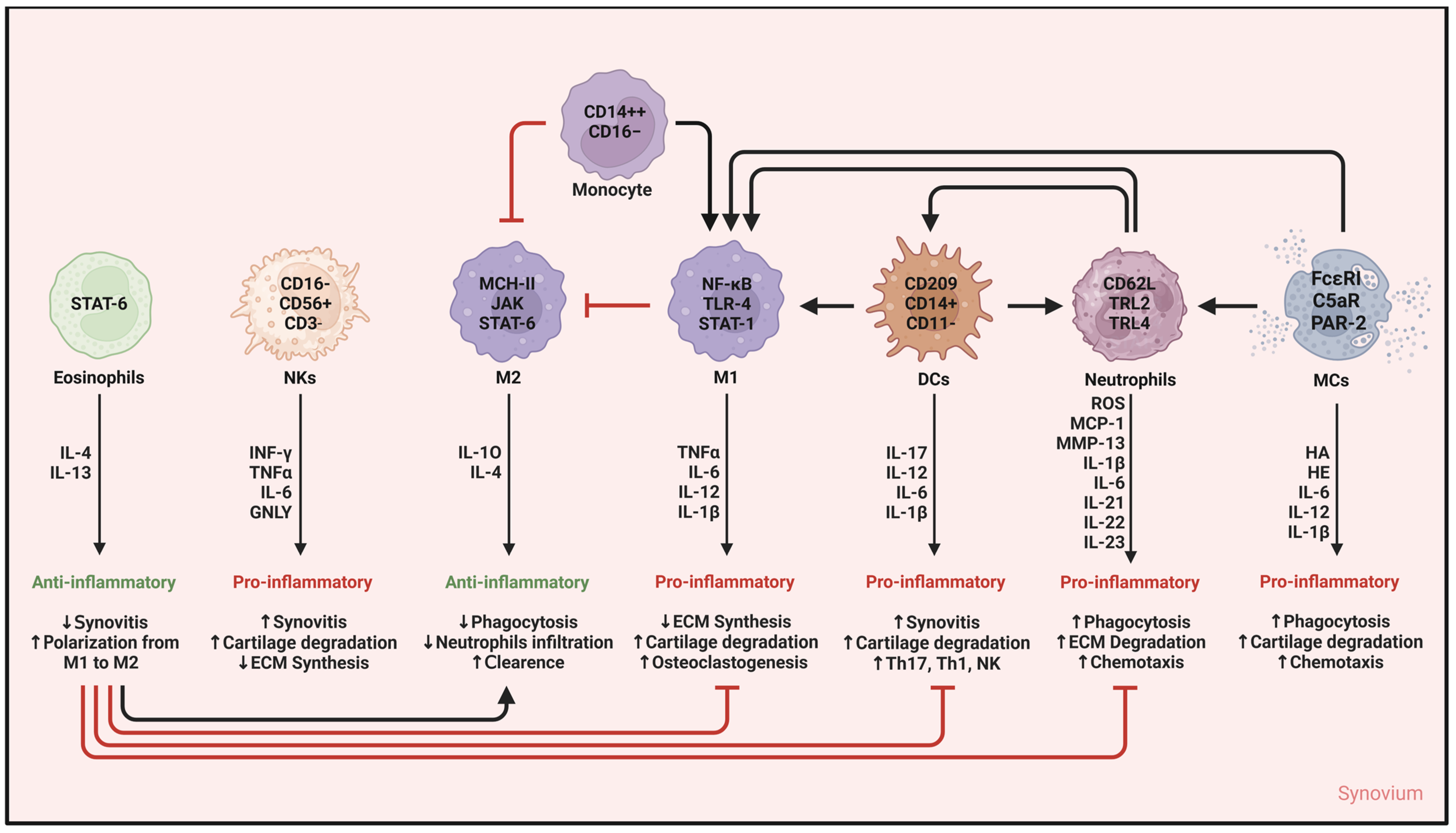
5. Innate Immunity Cells in Synovitis in OA
5.1. Myeloid-Derived Suppressor Cells
5.2. Monocytes
5.3. Macrophages
5.4. Dendritic Cells
5.5. Neutrophils
5.6. Eosinophils
5.7. Basophils
5.8. Mast Cells
5.9. Natural Killer Cells
5.10. A Final Glance at Innate Immunity Cells and Synovitis
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Long, H.; Liu, Q.; Yin, H.; Wang, K.; Diao, N.; Zhang, Y.; Lin, J.; Guo, A. Prevalence Trends of Site-Specific Osteoarthritis From 1990 to 2019: Findings From the Global Burden of Disease Study 2019. Arthritis Rheumatol. 2022, 74, 1172–1183. [Google Scholar] [CrossRef] [PubMed]
- Wong, A.Y.; Samartzis, D.; Maher, C. The global burden of osteoarthritis: Past and future perspectives. Lancet Rheumatol. 2023, 5, e496–e497. [Google Scholar] [CrossRef]
- Nelson, A.E. Multiple joint osteoarthritis (mjoa): What’s in a name? Osteoarthr. Cartil. 2024, 32, 234–240. [Google Scholar] [CrossRef] [PubMed]
- Palazzo, C.; Nguyen, C.; Lefevre-Colau, M.-M.; Rannou, F.; Poiraudeau, S. Risk factors and burden of osteoarthritis. Ann. Phys. Rehabil. Med. 2016, 59, 134–138. [Google Scholar] [CrossRef]
- Dorio, M.; Deveza, L.A. Phenotypes in osteoarthritis: Why do we need them and where are we at? Clin. Geriatr. Med. 2022, 38, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Deveza, L.A.; Nelson, A.E.; Loeser, R.F. Phenotypes of osteoarthritis: Current state and future implications. Clin. Exp. Rheumatol. 2019, 37, 64–72. [Google Scholar]
- Zhuo, Q.; Yang, W.; Chen, J.; Wang, Y. Metabolic syndrome meets osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 729–737. [Google Scholar] [CrossRef]
- Mobasheri, A.; Rayman, M.P.; Gualillo, O.; Sellam, J.; van der Kraan, P.; Fearon, U. The role of metabolism in the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2017, 13, 302–311. [Google Scholar] [CrossRef]
- Goldring, M.B.; Goldring, S.R. Osteoarthritis. J. Cell Physiol. 2007, 213, 626–634. [Google Scholar] [CrossRef]
- Glyn-Jones, S.; Palmer, A.J.; Agricola, R.; Price, A.J.; Vincent, T.L.; Weinans, H.; Carr, A.J. Osteoarthritis. Lancet 2015, 386, 376–387. [Google Scholar] [CrossRef]
- van Doormaal, M.C.; Meerhoff, G.A.; Vlieland, T.P.V.; Peter, W.F. A clinical practice guideline for physical therapy in patients with hip or knee osteoarthritis. Musculoskelet. Care 2020, 18, 575–595. [Google Scholar] [CrossRef] [PubMed]
- Mao, L.; Wu, W.; Wang, M.; Guo, J.; Li, H.; Zhang, S.; Xu, J.; Zou, J. Targeted treatment for osteoarthritis: Drugs and delivery system. Drug Deliv. 2021, 28, 1861–1876. [Google Scholar] [CrossRef]
- Cooper, C.; Chapurlat, R.; Al-Daghri, N.; Herrero-Beaumont, G.; Bruyere, O.; Rannou, F.; Roth, R.; Uebelhart, D.; Reginster, J.Y. Safety of oral non-selective non-steroidal anti-inflammatory drugs in osteoarthritis: What does the literature say? Drugs Aging 2019, 36, 15–24. [Google Scholar] [CrossRef]
- da Costa, B.R.; Pereira, T.V.; Saadat, P.; Rudnicki, M.; Iskander, S.M.; Bodmer, N.S.; Bobos, P.; Gao, L.; Kiyomoto, H.D.; Montezuma, T.; et al. Effectiveness and safety of non-steroidal anti-inflammatory drugs and opioid treatment for knee and hip osteoarthritis: Network meta-analysis. BMJ 2021, 375, n2321. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, X.; Xu, L.; Prasadam, I.; Duan, L.; Xiao, Y.; Xia, J. Non-surgical osteoarthritis therapy, intra-articular drug delivery towards clinical applications. J. Drug Target. 2021, 29, 609–616. [Google Scholar] [CrossRef]
- Ma, L.; Zheng, X.; Lin, R.; Sun, A.R.; Song, J.; Ye, Z.; Liang, D.; Zhang, M.; Tian, J.; Zhou, X.; et al. Knee Osteoarthritis Therapy: Recent Advances in Intra-Articular Drug Delivery Systems. Drug Des. Dev. Ther. 2022, 16, 1311–1347. [Google Scholar] [CrossRef]
- Szwedowski, D.; Szczepanek, J.; Paczesny, Ł.; Zabrzyński, J.; Gagat, M.; Mobasheri, A.; Jeka, S. The Effect of Platelet-Rich Plasma on the Intra-Articular Microenvironment in Knee Osteoarthritis. Int. J. Mol. Sci. 2021, 22, 5492. [Google Scholar] [CrossRef] [PubMed]
- Gan, X.; Wang, X.; Huang, Y.; Li, G.; Kang, H. Applications of Hydrogels in Osteoarthritis Treatment. Biomedicines 2024, 12, 923. [Google Scholar] [CrossRef] [PubMed]
- Farinelli, L.; Riccio, M.; Gigante, A.; De Francesco, F. Pain Management Strategies in Osteoarthritis. Biomedicines 2024, 12, 805. [Google Scholar] [CrossRef]
- Roelofs, A.J.; De Bari, C. Osteoarthritis year in review 2023: Biology. Osteoarthr. Cartil. 2024, 32, 148–158. [Google Scholar] [CrossRef]
- Scanzello, C.R.; Loeser, R.F. Editorial: Inflammatory Activity in Symptomatic Knee Osteoarthritis: Not All Inflammation Is Local. Arthritis Rheumatol. 2015, 67, 2797–2800. [Google Scholar] [CrossRef]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef]
- Loeser, R.F.; Collins, J.A.; Diekman, B.O. Ageing and the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 412–420. [Google Scholar] [CrossRef]
- Franceschi, C.; Bonafe, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging: An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Otin, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [PubMed]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef] [PubMed]
- Loeser, R.F.; Goldring, S.R.; Scanzello, C.R.; Goldring, M.B. Osteoarthritis: A disease of the joint as an organ. Arthritis Rheum. 2012, 64, 1697–1707. [Google Scholar] [CrossRef] [PubMed]
- Dilley, J.E.; Bello, M.A.; Roman, N.; McKinley, T.; Sankar, U. Post-traumatic osteoarthritis: A review of pathogenic mechanisms and novel targets for mitigation. Bone Rep. 2023, 18, 101658. [Google Scholar] [CrossRef]
- Martel-Pelletier, J.; Barr, A.J.; Cicuttini, F.M.; Conaghan, P.G.; Cooper, C.; Goldring, M.B.; Goldring, S.R.; Jones, G.; Teichtahl, A.J.; Pelletier, J.P. Osteoarthritis. Nat. Rev. Dis. Primers 2016, 2, 16072. [Google Scholar] [CrossRef]
- Ilas, D.C.; Churchman, S.M.; McGonagle, D.; Jones, E. Targeting Subchondral Bone Mesenchymal Stem Cell Activities for Intrinsic Joint Repair in Osteoarthritis. Futur. Sci. OA 2017, 3, FSO228. [Google Scholar] [CrossRef]
- Bianco, D.; Todorov, A.; Čengić, T.; Pagenstert, G.; Schären, S.; Netzer, C.; Hügle, T.; Geurts, J. Alterations of Subchondral Bone Progenitor Cells in Human Knee and Hip Osteoarthritis Lead to a Bone Sclerosis Phenotype. Int. J. Mol. Sci. 2018, 19, 475. [Google Scholar] [CrossRef] [PubMed]
- Goldring, S.R.; Goldring, M.B. Changes in the osteochondral unit during osteoarthritis: Structure, function and cartilage–bone crosstalk. Nat. Rev. Rheumatol. 2016, 12, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Zeng, N.; Yan, Z.-P.; Chen, X.-Y.; Ni, G.-X. Infrapatellar Fat Pad and Knee Osteoarthritis. Aging Dis. 2020, 11, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Paduszynski, W.; Jeskiewicz, M.; Uchanski, P.; Gackowski, S.; Radkowski, M.; Demkow, U. Hoffa’s fat pad abnormality in the development of knee osteoarthritis. Adv. Exp. Med. Biol. 2018, 1039, 95–102. [Google Scholar]
- Wenham, C.Y.J.; Conaghan, P.G. The role of synovitis in osteoarthritis. Ther. Adv. Musculoskelet. Dis. 2010, 2, 349–359. [Google Scholar] [CrossRef]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Benito, M.J.; Veale, D.J.; FitzGerald, O.; van den Berg, W.B.; Bresnihan, B. Synovial tissue inflammation in early and late osteoarthritis. Ann. Rheum. Dis. 2005, 64, 1263–1267. [Google Scholar] [CrossRef]
- Manferdini, C.; Paolella, F.; Gabusi, E.; Silvestri, Y.; Gambari, L.; Cattini, L.; Filardo, G.; Fleury-Cappellesso, S.; Lisignoli, G. From osteoarthritic synovium to synovial-derived cells characterization: Synovial macrophages are key effector cells. Arthritis Res. Ther. 2016, 18, 83. [Google Scholar] [CrossRef] [PubMed]
- Hugle, T.; Geurts, J. What drives osteoarthritis?-synovial versus subchondral bone pathology. Rheumatology 2017, 56, 1461–1471. [Google Scholar]
- Sellam, J.; Berenbaum, F. The role of synovitis in pathophysiology and clinical symptoms of osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef]
- Carmona-Rivera, C.; Carlucci, P.M.; Moore, E.; Lingampalli, N.; Uchtenhagen, H.; James, E.; Liu, Y.; Bicker, K.L.; Wahamaa, H.; Hoffmann, V.; et al. Synovial fibroblast-neutrophil interactions promote pathogenic adaptive immunity in rheumatoid arthritis. Sci. Immunol. 2017, 2, eaag3358. [Google Scholar] [CrossRef] [PubMed]
- Da, R.-R.; Qin, Y.; Baeten, D.; Zhang, Y. B Cell Clonal Expansion and Somatic Hypermutation of Ig Variable Heavy Chain Genes in the Synovial Membrane of Patients with Osteoarthritis. J. Immunol. 2007, 178, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Tu, J.; Huang, W.; Zhang, W.; Mei, J.; Zhu, C. A Tale of Two Immune Cells in Rheumatoid Arthritis: The Crosstalk Between Macrophages and T Cells in the Synovium. Front. Immunol. 2021, 12, 655477. [Google Scholar] [CrossRef] [PubMed]
- Orlowsky, E.W.; Kraus, V.B. The Role of Innate Immunity in Osteoarthritis: When Our First Line of Defense Goes On the Offensive. J. Rheumatol. 2015, 42, 363–371. [Google Scholar] [CrossRef]
- Kuang, G.; Tan, X.; Liu, X.; Li, N.; Yi, N.; Mi, Y.; Shi, Q.; Zeng, F.; Xie, X.; Lu, M.; et al. The Role of Innate Immunity in Osteoarthritis and the Connotation of “Immune-joint” Axis: A Narrative Review. Comb. Chem. High Throughput Screen. 2024, 27, 2170–2179. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, Y.; Li, X.; Deng, Z.; Zheng, M.; Zheng, Q. The Immune Cell Landscape in Different Anatomical Structures of Knee in Osteoarthritis: A Gene Expression-Based Study. BioMed Res. Int. 2020, 2020, 9647072. [Google Scholar] [CrossRef]
- Veglia, F.; Perego, M.; Gabrilovich, D. Myeloid-derived suppressor cells coming of age. Nat. Immunol. 2018, 19, 108–119. [Google Scholar] [CrossRef]
- Damuzzo, V.; Pinton, L.; Desantis, G.; Solito, S.; Marigo, I.; Bronte, V.; Mandruzzato, S. Complexity and challenges in defining myeloid-derived suppressor cells. Cytom. Part B Clin. Cytom. 2015, 88, 77–91. [Google Scholar] [CrossRef] [PubMed]
- Condamine, T.; Dominguez, G.A.; Youn, J.-I.; Kossenkov, A.V.; Mony, S.; Alicea-Torres, K.; Tcyganov, E.; Hashimoto, A.; Nefedova, Y.; Lin, C.; et al. Lectin-type oxidized LDL receptor-1 distinguishes population of human polymorphonuclear myeloid-derived suppressor cells in cancer patients. Sci. Immunol. 2016, 1, aaf8943. [Google Scholar] [CrossRef]
- Ling, Z.; Yang, C.; Tan, J.; Dou, C.; Chen, Y. Beyond immunosuppressive effects: Dual roles of myeloid-derived suppressor cells in bone-related diseases. Cell. Mol. Life Sci. 2021, 78, 7161–7183. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, S.; Huang, Y.; Wang, H.; Zhao, J.; Gaskin, F.; Yang, N.; Fu, S.M. Myeloid-derived suppressor cells are proinflammatory and regulate collagen-induced arthritis through manipulating Th17 cell differentiation. Clin. Immunol. 2015, 157, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-J.; Lee, S.-H.; Kim, E.-K.; Lee, E.-J.; Baek, J.-A.; Park, S.-H.; Kwok, S.-K.; Cho, M.-L. Interleukin-10 produced by myeloid-derived suppressor cells is critical for the induction of Tregs and attenuation of rheumatoid inflammation in mice. Sci. Rep. 2018, 8, 3753. [Google Scholar] [CrossRef] [PubMed]
- Rajabinejad, M.; Salari, F.; Gorgin Karaji, A.; Rezaiemanesh, A. The role of myeloid-derived suppressor cells in the pathogenesis of rheumatoid arthritis; anti- or pro-inflammatory cells? Artif. Cells Nanomed. Biotechnol. 2019, 47, 4149–4158. [Google Scholar] [CrossRef]
- Tang, C.; Liu, Q.; Zhang, Y.; Liu, G.; Shen, G. Identification of CIRBP and TRPV4 as Immune-Related Diagnostic Biomarkers in Osteoarthritis. Int. J. Gen. Med. 2021, 14, 10235–10245. [Google Scholar] [CrossRef]
- Danilin, S.; Merkel, A.R.; Johnson, J.R.; Johnson, R.W.; Edwards, J.R.; Sterling, J.A. Myeloid-derived suppressor cells expand during breast cancer progression and promote tumor-induced bone destruction. OncoImmunology 2012, 1, 1484–1494. [Google Scholar] [CrossRef]
- Edgington-Mitchell, L.E.; Rautela, J.; Duivenvoorden, H.M.; Jayatilleke, K.M.; van der Linden, W.A.; Verdoes, M.; Bogyo, M.; Parker, B.S. Cysteine cathepsin activity suppresses osteoclastogenesis of myeloid-derived suppressor cells in breast cancer. Oncotarget 2015, 6, 27008–27022. [Google Scholar] [CrossRef]
- Zhuang, J.; Zhang, J.; Lwin, S.T.; Edwards, J.R.; Edwards, C.M.; Mundy, G.R.; Yang, X. Osteoclasts in Multiple Myeloma Are Derived from Gr-1+CD11b+Myeloid-Derived Suppressor Cells. PLoS ONE 2012, 7, e48871. [Google Scholar] [CrossRef] [PubMed]
- Sawant, A.; Ponnazhagan, S. Myeloid-Derived Suppressor Cells as Osteoclast Progenitors: A Novel Target for Controlling Osteolytic Bone Metastasis. Cancer Res. 2013, 73, 4606–4610. [Google Scholar] [CrossRef] [PubMed]
- Kirkwood, K.L.; Zhang, L.; Thiyagarajan, R.; Seldeen, K.L.; Troen, B.R. Myeloid-Derived Suppressor Cells at the Intersection of Inflammaging and Bone Fragility. Immunol. Investig. 2018, 47, 844–854. [Google Scholar] [CrossRef]
- Kwack, K.H.; Maglaras, V.; Thiyagarajan, R.; Zhang, L.; Kirkwood, K.L. Myeloid-derived suppressor cells in obesity-associated periodontal disease: A conceptual model. Periodontology 2000 2021, 87, 268–275. [Google Scholar] [CrossRef]
- Kwack, K.H.; Zhang, L.; Kirkwood, K.L. In vitro osteoclastogenesis assessment using murine myeloid-derived suppressor cells. Methods Cell Biol. 2024, 184, 133–147. [Google Scholar] [PubMed]
- Zhang, L.; Kirkwood, C.L.; Sohn, J.; Lau, A.; Bayers-Thering, M.; Bali, S.K.; Rachala, S.; Marzo, J.M.; Anders, M.J.; Beier, F.; et al. Expansion of myeloid-derived suppressor cells contributes to metabolic osteoarthritis through subchondral bone remodeling. Arthritis Res. Ther. 2021, 23, 287. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, H.L.; Passlick, B.; Flieger, D. The Monoclonal Antimonocyte Antibody My4 Stains B Lymphocytes and Two Distinct Monocyte Subsets in Human Peripheral Blood. Hybridoma 1988, 7, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Passlick, B.; Flieger, D.; Ziegler-Heitbrock, H.W. Identification and characterization of a novel monocyte subpopulation in human peripheral blood. Blood 1989, 74, 2527–2534. [Google Scholar] [CrossRef]
- Ożańska, A.; Szymczak, D.; Rybka, J. Pattern of human monocyte subpopulations in health and disease. Scand. J. Immunol. 2020, 92, e12883. [Google Scholar] [CrossRef]
- Guillem-Llobat, P.; Marín, M.; Rouleau, M.; Silvestre, A.; Blin-Wakkach, C.; Ferrándiz, M.L.; Guillén, M.I.; Ibáñez, L. New Insights into the Pro-Inflammatory and Osteoclastogenic Profile of Circulating Monocytes in Osteoarthritis Patients. Int. J. Mol. Sci. 2024, 25, 1710. [Google Scholar] [CrossRef]
- Lee, H.-R.; Lee, S.; Yoo, I.S.; Yoo, S.-J.; Kwon, M.-H.; Joung, C.-I.; Park, J.A.; Kang, S.W.; Kim, J. CD14+ monocytes and soluble CD14 of synovial fluid are associated with osteoarthritis progression. Arch. Rheumatol. 2022, 37, 335–343. [Google Scholar] [CrossRef]
- Evers, T.M.; Sheikhhassani, V.; Haks, M.C.; Storm, C.; Ottenhoff, T.H.; Mashaghi, A. Single-cell analysis reveals chemokine-mediated differential regulation of monocyte mechanics. iScience 2021, 25, 103555. [Google Scholar] [CrossRef]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef]
- Raghu, H.; Lepus, C.M.; Wang, Q.; Wong, H.H.; Lingampalli, N.; Oliviero, F.; Punzi, L.; Giori, N.J.; Goodman, S.B.; Chu, C.R.; et al. Ccl2/ccr2, but not ccl5/ccr5, mediates monocyte recruitment, inflammation and cartilage destruction in osteoarthritis. Ann. Rheum. Dis. 2017, 76, 914–922. [Google Scholar] [CrossRef]
- Mondadori, C.; Palombella, S.; Salehi, S.; Talò, G.; Visone, R.; Rasponi, M.; Redaelli, A.; Sansone, V.; Moretti, M.; Lopa, S. Recapitulating monocyte extravasation to the synovium in an organotypic microfluidic model of the articular joint. Biofabrication 2021, 13, 045001. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Gu, M.; Xu, X.; Wen, X.; Yang, G.; Li, L.; Sheng, P.; Meng, F. Ccl3/ccr1 mediates cd14(+)cd16(-) circulating monocyte recruitment in knee osteoarthritis progression. Osteoarthr. Cartil. 2020, 28, 613–625. [Google Scholar] [CrossRef] [PubMed]
- Wojdasiewicz, P.; Poniatowski, A.; Kotela, A.; Deszczyński, J.; Kotela, I.; Szukiewicz, D. The Chemokine CX3CL1 (Fractalkine) and its Receptor CX3CR1: Occurrence and Potential Role in Osteoarthritis. Arch. Immunol. Ther. Exp. 2014, 62, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Endres, M.; Andreas, K.; Kalwitz, G.; Freymann, U.; Neumann, K.; Ringe, J.; Sittinger, M.; Häupl, T.; Kaps, C. Chemokine profile of synovial fluid from normal, osteoarthritis and rheumatoid arthritis patients: CCL25, CXCL10 and XCL1 recruit human subchondral mesenchymal progenitor cells. Osteoarthr. Cartil. 2010, 18, 1458–1466. [Google Scholar] [CrossRef]
- Fantuzzi, L.; Tagliamonte, M.; Gauzzi, M.C.; Lopalco, L. Dual ccr5/ccr2 targeting: Opportunities for the cure of complex disorders. Cell. Mol. Life Sci. 2019, 76, 4869–4886. [Google Scholar] [CrossRef] [PubMed]
- Ni, F.; Zhang, Y.; Peng, X.; Li, J. Correlation between osteoarthritis and monocyte chemotactic protein-1 expression: A meta-analysis. J. Orthop. Surg. Res. 2020, 15, 516. [Google Scholar] [CrossRef]
- Loukov, D.; Karampatos, S.; Maly, M.; Bowdish, D. Monocyte activation is elevated in women with knee-osteoarthritis and associated with inflammation, BMI and pain. Osteoarthr. Cartil. 2018, 26, 255–263. [Google Scholar] [CrossRef]
- Yunna, C.; Mengru, H.; Lei, W.; Weidong, C. Macrophage M1/M2 polarization. Eur. J. Pharmacol. 2020, 877, 173090. [Google Scholar] [CrossRef]
- Wood, M.J.; Leckenby, A.; Reynolds, G.; Spiering, R.; Pratt, A.G.; Rankin, K.S.; Isaacs, J.D.; Haniffa, M.A.; Milling, S.; Hilkens, C.M. Macrophage proliferation distinguishes 2 subgroups of knee osteoarthritis patients. J. Clin. Investig. 2019, 4, e125325. [Google Scholar] [CrossRef]
- Kurowska-Stolarska, M.; Alivernini, S. Synovial tissue macrophages: Friend or foe? RMD Open 2017, 3, e000527. [Google Scholar] [CrossRef]
- Shi, C.; Pamer, E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef] [PubMed]
- Luukkonen, J.; Huhtakangas, J.; Palosaari, S.; Tuukkanen, J.; Vuolteenaho, O.; Lehenkari, P. Preliminary Report: Osteoarthritis and Rheumatoid Arthritis Synovial Fluid Increased Osteoclastogenesis In Vitro by Monocyte Differentiation Pathway Regulating Cytokines. Mediat. Inflamm. 2022, 2022, 2606916. [Google Scholar] [CrossRef]
- Hirose, S.; Lin, Q.; Ohtsuji, M.; Nishimura, H.; Verbeek, J.S. Monocyte subsets involved in the development of systemic lupus erythematosus and rheumatoid arthritis. Int. Immunol. 2019, 31, 687–696. [Google Scholar] [CrossRef] [PubMed]
- Monibi, F.; Roller, B.L.; Stoker, A.; Garner, B.; Bal, S.; Cook, J.L. Identification of Synovial Fluid Biomarkers for Knee Osteoarthritis and Correlation with Radiographic Assessment. J. Knee Surg. 2015, 29, 242–247. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Aristizábal, A.; Gandhi, R.; Mahomed, N.N.; Marshall, K.W.; Viswanathan, S. Synovial fluid monocyte/macrophage subsets and their correlation to patient-reported outcomes in osteoarthritic patients: A cohort study. Arthritis Res. Ther. 2019, 21, 26. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, W.; Ying, H.; Du, J.; Chen, J.; Chen, S.; Shen, B. The relationship of platelet to lymphocyte ratio and neutrophil to monocyte ratio to radiographic grades of knee osteoarthritis. Z. Fur Rheumatol. 2017, 77, 533–537. [Google Scholar] [CrossRef]
- Gao, K.; Zhu, W.; Liu, W.; Ma, D.; Li, H.; Yu, W.; Wang, L.; Cao, Y.; Jiang, Y. Diagnostic value of the blood monocyte–lymphocyte ratio in knee osteoarthritis. J. Int. Med. Res. 2019, 47, 4413–4421. [Google Scholar] [CrossRef]
- Lendeckel, U.; Venz, S.; Wolke, C. Macrophages: Shapes and functions. ChemTexts 2022, 8, 12. [Google Scholar] [CrossRef]
- Zhao, K.; Ruan, J.; Nie, L.; Ye, X.; Li, J. Effects of synovial macrophages in osteoarthritis. Front. Immunol. 2023, 14, 1164137. [Google Scholar] [CrossRef]
- Murray, P.J. Macrophage polarization. Annu. Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, X.-H.; Jin, L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front. Immunol. 2019, 10, 792. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Lin, C.; Zeng, C.; Wang, Z.; Wang, H.; Lu, J.; Liu, X.; Shao, Y.; Zhao, C.; Pan, J.; et al. Synovial macrophage M1 polarisation exacerbates experimental osteoarthritis partially through R-spondin-2. Ann. Rheum. Dis. 2018, 77, 1524–1534. [Google Scholar] [CrossRef] [PubMed]
- Pessler, F.; Chen, L.X.; Dai, L.; Gomez-Vaquero, C.; Diaz-Torne, C.; Paessler, M.E.; Scanzello, C.; Çakir, N.; Einhorn, E.; Schumacher, H.R. A histomorphometric analysis of synovial biopsies from individuals with Gulf War Veterans’ Illness and joint pain compared to normal and osteoarthritis synovium. Clin. Rheumatol. 2008, 27, 1127–1134. [Google Scholar] [CrossRef]
- Zhang, H.; Cai, D.; Bai, X. Macrophages regulate the progression of osteoarthritis. Osteoarthr. Cartil. 2020, 28, 555–561. [Google Scholar] [CrossRef]
- van Lent, P.L.; Blom, A.B.; van der Kraan, P.; Holthuysen, A.E.; Vitters, E.; van Rooijen, N.; Smeets, R.L.; Nabbe, K.C.; van den Berg, W.B. Crucial role of synovial lining macrophages in the promotion of transforming growth factor beta-mediated osteophyte formation. Arthritis Rheum. 2004, 50, 103–111. [Google Scholar] [CrossRef]
- Blom, A.B.; van Lent, P.L.; Holthuysen, A.E.; van der Kraan, P.M.; Roth, J.; van Rooijen, N.; van den Berg, W.B. Synovial lining macrophages mediate osteophyte formation during experimental osteoarthritis. Osteoarthr. Cartil. 2004, 12, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Topoluk, N.; Steckbeck, K.; Siatkowski, S.; Burnikel, B.; Tokish, J.; Mercuri, J. Amniotic mesenchymal stem cells mitigate osteoarthritis progression in a synovial macrophage-mediated in vitro explant coculture model. J. Tissue Eng. Regen. Med. 2017, 12, 1097–1110. [Google Scholar] [CrossRef]
- Takano, S.; Uchida, K.; Inoue, G.; Miyagi, M.; Aikawa, J.; Iwase, D.; Iwabuchi, K.; Matsumoto, T.; Satoh, M.; Mukai, M.; et al. Nerve growth factor regulation and production by macrophages in osteoarthritic synovium. Clin. Exp. Immunol. 2017, 190, 235–243. [Google Scholar] [CrossRef]
- Kraus, V.; McDaniel, G.; Huebner, J.; Stabler, T.; Pieper, C.; Shipes, S.; Petry, N.; Low, P.; Shen, J.; McNearney, T.; et al. Direct in vivo evidence of activated macrophages in human osteoarthritis. Osteoarthr. Cartil. 2016, 24, 1613–1621. [Google Scholar] [CrossRef]
- Utomo, L.; Bastiaansen-Jenniskens, Y.; Verhaar, J.; van Osch, G. Cartilage inflammation and degeneration is enhanced by pro-inflammatory (M1) macrophages in vitro, but not inhibited directly by anti-inflammatory (M2) macrophages. Osteoarthr. Cartil. 2016, 24, 2162–2170. [Google Scholar] [CrossRef]
- Culemann, S.; Grüneboom, A.; Nicolás-Ávila, J.Á.; Weidner, D.; Lämmle, K.F.; Rothe, T.; Quintana, J.A.; Kirchner, P.; Krljanac, B.; Eberhardt, M.; et al. Locally renewing resident synovial macrophages provide a protective barrier for the joint. Nature 2019, 572, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, V.; Timofte, D.V.; Zară-Dănceanu, C.-M.; Labusca, L. Obesity, Metabolic Syndrome, and Osteoarthritis Require Integrative Understanding and Management. Biomedicines 2024, 12, 1262. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhang, M.; Zhao, J.; Zheng, M.; Yang, H. Imbalance of m1/m2 macrophages is linked to severity level of knee osteoarthritis. Exp. Ther. Med. 2018, 16, 5009–5014. [Google Scholar] [CrossRef]
- Batoon, L.; Hawse, J.R.; McCauley, L.K.; Weivoda, M.M.; Roca, H. Efferocytosis and Bone Dynamics. Curr. Osteoporos. Rep. 2024, 22, 471–482. [Google Scholar] [CrossRef]
- Luo, H.; Li, L.; Han, S.; Liu, T. The role of monocyte/macrophage chemokines in pathogenesis of osteoarthritis: A review. Int. J. Immunogenetics 2024, 51, 130–142. [Google Scholar] [CrossRef]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.M.; Liu, Y.-J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80. [Google Scholar] [CrossRef] [PubMed]
- Lande, R.; Giacomini, E.; Serafini, B.; Rosicarelli, B.; Sebastiani, G.D.; Minisola, G.; Tarantino, U.; Riccieri, V.; Valesini, G.; Coccia, E.M. Characterization and Recruitment of Plasmacytoid Dendritic Cells in Synovial Fluid and Tissue of Patients with Chronic Inflammatory Arthritis. J. Immunol. 2004, 173, 2815–2824. [Google Scholar] [CrossRef] [PubMed]
- Collin, M.; Bigley, V. Human dendritic cell subsets: An update. Immunology 2018, 154, 3–20. [Google Scholar] [CrossRef]
- Moret, F.M.; Hack, C.E.; van der Wurff-Jacobs, K.M.; de Jager, W.; Radstake, T.R.; Lafeber, F.P.; van Roon, J.A. Intra-articular cd1c-expressing myeloid dendritic cells from rheumatoid arthritis patients express a unique set of t cell-attracting chemokines and spontaneously induce th1, th17 and th2 cell activity. Arthritis Res. Ther. 2013, 15, R155. [Google Scholar] [CrossRef]
- Marzaioli, V.; Canavan, M.; Floudas, A.; Flynn, K.; Mullan, R.; Veale, D.J.; Fearon, U. Cd209/cd14(+) dendritic cells characterization in rheumatoid and psoriatic arthritis patients: Activation, synovial infiltration, and therapeutic targeting. Front. Immunol. 2021, 12, 722349. [Google Scholar] [CrossRef]
- E, X.; Cao, Y.; Meng, H.; Qi, Y.; Du, G.; Xu, J.; Bi, Z. Dendritic Cells of Synovium in Experimental Model of Osteoarthritis of Rabbits. Cell. Physiol. Biochem. 2012, 30, 23–32. [Google Scholar] [CrossRef] [PubMed]
- Nie, F.; Ding, F.; Chen, B.; Huang, S.; Liu, Q.; Xu, C. Dendritic cells aggregate inflammation in experimental osteoarthritis through a toll-like receptor (TLR)-dependent machinery response to challenges. Life Sci. 2019, 238, 116920. [Google Scholar] [CrossRef]
- Segura, E.; Touzot, M.; Bohineust, A.; Cappuccio, A.; Chiocchia, G.; Hosmalin, A.; Dalod, M.; Soumelis, V.; Amigorena, S. Human Inflammatory Dendritic Cells Induce Th17 Cell Differentiation. Immunity 2013, 38, 336–348. [Google Scholar] [CrossRef]
- Bertola, A.; Ciucci, T.; Rousseau, D.; Bourlier, V.; Duffaut, C.; Bonnafous, S.; Blin-Wakkach, C.; Anty, R.; Iannelli, A.; Gugenheim, J.; et al. Identification of Adipose Tissue Dendritic Cells Correlated With Obesity-Associated Insulin-Resistance and Inducing Th17 Responses in Mice and Patients. Diabetes 2012, 61, 2238–2247. [Google Scholar] [CrossRef]
- Chou, C.-H.; Jain, V.; Gibson, J.; Attarian, D.E.; Haraden, C.A.; Yohn, C.B.; Laberge, R.-M.; Gregory, S.; Kraus, V.B. Synovial cell cross-talk with cartilage plays a major role in the pathogenesis of osteoarthritis. Sci. Rep. 2020, 10, 10868. [Google Scholar] [CrossRef] [PubMed]
- Nefla, M.; Holzinger, D.; Berenbaum, F.; Jacques, C. The danger from within: Alarmins in arthritis. Nat. Rev. Rheumatol. 2016, 12, 669–683. [Google Scholar] [CrossRef]
- Corr, E.M.; Cunningham, C.C.; Helbert, L.; McCarthy, G.M.; Dunne, A. Osteoarthritis-associated basic calcium phosphate crystals activate membrane proximal kinases in human innate immune cells. Arthritis Res. Ther. 2017, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Kraus, V.; Burnett, B.; Coindreau, J.; Cottrell, S.; Eyre, D.; Gendreau, M.; Gardiner, J.; Garnero, P.; Hardin, J.; Henrotin, Y.; et al. Application of biomarkers in the development of drugs intended for the treatment of osteoarthritis. Osteoarthr. Cartil. 2011, 19, 515–542. [Google Scholar] [CrossRef] [PubMed]
- Arden, N.B., F.; Cooper, C.; Guermazi, A.; Hayashi, D.; Hunter, D.; Javaid, M.K.; Rannou, F.; Roemer, F.; Reginsteret, J.-Y. Atlas of Osteoarthritis; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar]
- Rollet-Labelle, E.; Vaillancourt, M.; Marois, L.; Newkirk, M.M.; E Poubelle, P.; Naccache, P.H. Cross-linking of IgGs bound on circulating neutrophils leads to an activation of endothelial cells: Possible role of rheumatoid factors in rheumatoid arthritis-associated vascular dysfunction. J. Inflamm. 2013, 10, 27. [Google Scholar] [CrossRef]
- Hsueh, M.; Zhang, X.; Wellman, S.S.; Bolognesi, M.; Kraus, V.B. Synergistic Roles of Macrophages and Neutrophils in Osteoarthritis Progression. Arthritis Rheumatol. 2020, 73, 89–99. [Google Scholar] [CrossRef]
- Haraden, C.A.; Huebner, J.L.; Hsueh, M.-F.; Li, Y.-J.; Kraus, V.B. Synovial fluid biomarkers associated with osteoarthritis severity reflect macrophage and neutrophil related inflammation. Arthritis Res. Ther. 2019, 21, 146. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, D.J.; Falconer, A.M.D.; Wright, H.L.; Lin, H.; Yamamoto, K.; Cheung, K.; Charlton, S.H.; Arques, M.D.C.; Janciauskiene, S.; Refaie, R.; et al. Matrix metalloproteinase-13 is fully activated by neutrophil elastase and inactivates its serpin inhibitor, alpha-1 antitrypsin: Implications for osteoarthritis. FEBS J. 2022, 289, 121–139. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Jing, W.; Bi, Y.; Li, Y.; Ma, L.; Yang, H.; Zhang, Y. Neutrophil Elastase Induces Chondrocyte Apoptosis and Facilitates the Occurrence of Osteoarthritis via Caspase Signaling Pathway. Front. Pharmacol. 2021, 12, 666162. [Google Scholar] [CrossRef]
- Manukyan, G.; Gallo, J.; Mikulkova, Z.; Trajerova, M.; Savara, J.; Slobodova, Z.; Fidler, E.; Shrestha, B.; Kriegova, E. Phenotypic and functional characterisation of synovial fluid-derived neutrophils in knee osteoarthritis and knee infection. Osteoarthr. Cartil. 2022, 31, 72–82. [Google Scholar] [CrossRef]
- Molnar, V.; Matišić, V.; Kodvanj, I.; Bjelica, R.; Jeleč, Ž.; Hudetz, D.; Rod, E.; Čukelj, F.; Vrdoljak, T.; Vidović, D.; et al. Cytokines and Chemokines Involved in Osteoarthritis Pathogenesis. Int. J. Mol. Sci. 2021, 22, 9208. [Google Scholar] [CrossRef] [PubMed]
- Tamassia, N.; Bianchetto-Aguilera, F.; Arruda-Silva, F.; Gardiman, E.; Gasperini, S.; Calzetti, F.; Cassatella, M.A. Cytokine production by human neutrophils: Revisiting the “dark side of the moon”. Eur. J. Clin. Investig. 2018, 48, e12952. [Google Scholar] [CrossRef]
- Benigni, G.; Dimitrova, P.; Antonangeli, F.; Sanseviero, E.; Milanova, V.; Blom, A.; van Lent, P.; Morrone, S.; Santoni, A.; Bernardini, G. Cxcr3/cxcl10 axis regulates neutrophil-nk cell cross-talk determining the severity of experimental osteoarthritis. J. Immunol. 2017, 198, 2115–2124. [Google Scholar] [CrossRef]
- Simon, D.; Simon, H.U. Eosinophilic disorders. J. Allergy Clin. Immunol. 2007, 119, 1291–1300. [Google Scholar] [CrossRef]
- Anthony, R.M.; Rutitzky, L.I.; Urban, J.F., Jr.; Stadecker, M.J.; Gause, W.C. Protective immune mechanisms in helminth infection. Nat. Rev. Immunol. 2007, 7, 975–987. [Google Scholar] [CrossRef]
- Shamri, R.; Xenakis, J.J.; Spencer, L.A. Eosinophils in innate immunity: An evolving story. Cell Tissue Res. 2010, 343, 57–83. [Google Scholar] [CrossRef]
- Iwaszko, M.; Biały, S.; Bogunia-Kubik, K. Significance of Interleukin (IL)-4 and IL-13 in Inflammatory Arthritis. Cells 2021, 10, 3000. [Google Scholar] [CrossRef] [PubMed]
- Swain, S.L.; Mckonzie, D.T.; Dutton, R.W.; Tonkonogy, S.L.; English, M. The Role of IL4 and IL5: Characterization of a Distinct Helper T Cell Subset that makes IL4 and IL5 (Th2) and Requires Priming before Induction of Lymphokine Secretion. Immunol. Rev. 1988, 102, 77–105. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, J.C.; Van Dyken, S.J.; Von Moltke, J.; Cheng, L.E.; Mohapatra, A.; Molofsky, A.B.; Thornton, E.E.; Krummel, M.F.; Chawla, A.; Liang, H.-E.; et al. Type 2 innate lymphoid cells control eosinophil homeostasis. Nature 2013, 502, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Andreev, D.; Oeser, K.; Krljanac, B.; Hueber, A.; Kleyer, A.; Voehringer, D.; Schett, G.; Bozec, A. Th2 and eosinophil responses suppress inflammatory arthritis. Nat. Commun. 2016, 7, 11596. [Google Scholar] [CrossRef] [PubMed]
- Finnegan, A.; Mikecz, K.; Tao, P.; Glant, T.T. Proteoglycan (Aggrecan)-Induced Arthritis in BALB/c Mice Is a Th1-Type Disease Regulated by Th2 Cytokines. J. Immunol. 1999, 163, 5383–5390. [Google Scholar] [CrossRef]
- Horsfall, A.C.; Butler, D.M.; Marinova, L.; Warden, P.J.; O Williams, R.; Maini, R.N.; Feldmann, M. Suppression of collagen-induced arthritis by continuous administration of IL-4. J. Immunol. 1997, 159, 5687–5696. [Google Scholar] [CrossRef]
- Cao, Y.; Brombacher, F.; Tunyogi-Csapo, M.; Glant, T.T.; Finnegan, A. Interleukin-4 regulates proteoglycan-induced arthritis by specifically suppressing the innate immune response. Arthritis Rheum. 2007, 56, 861–870. [Google Scholar] [CrossRef]
- Chen, Z.; Bozec, A.; Ramming, A.; Schett, G. Anti-inflammatory and immune-regulatory cytokines in rheumatoid arthritis. Nat. Rev. Rheumatol. 2018, 15, 9–17. [Google Scholar] [CrossRef]
- Yamada, T.; Tani, Y.; Nakanishi, H.; Taguchi, R.; Arita, M.; Arai, H. Eosinophils promote resolution of acute peritonitis by producing proresolving mediators in mice. FASEB J. 2010, 25, 561–568. [Google Scholar] [CrossRef]
- Isobe, Y.; Kato, T.; Arita, M. Emerging Roles of Eosinophils and Eosinophil-Derived Lipid Mediators in the Resolution of Inflammation. Front. Immunol. 2012, 3, 32988. [Google Scholar] [CrossRef]
- Evans, C.H.; Ghivizzani, S.C.; Robbins, P.D. Osteoarthritis gene therapy in 2022. Curr. Opin. Rheumatol. 2022, 35, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Andreev, D.; Kachler, K.; Liu, M.; Chen, Z.; Krishnacoumar, B.; Ringer, M.; Frey, S.; Krönke, G.; Voehringer, D.; Schett, G.; et al. Eosinophils preserve bone homeostasis by inhibiting excessive osteoclast formation and activity via eosinophil peroxidase. Nat. Commun. 2024, 15, 1067. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Du, H.; Lv, H.; Lu, J.; Li, J.; Yao, J. Identification of the osteoarthritis signature gene PDK1 by machine learning and its regulatory mechanisms on chondrocyte autophagy and apoptosis. Front. Immunol. 2023, 13, 1072526. [Google Scholar] [CrossRef] [PubMed]
- Moussa, K.; Gurung, P.; Adams-Huet, B.; Devaraj, S.; Jialal, I. Increased eosinophils in adipose tissue of metabolic syndrome. J. Diabetes Its Complicat. 2019, 33, 535–538. [Google Scholar] [CrossRef]
- Liu, Y.M.; Jiang, W.M.; Huang, J.M.; Zhong, L.M. Bioinformatic analysis combined with immune infiltration to explore osteoarthritis biomarkers and drug prediction. Medicine 2024, 103, e38430. [Google Scholar] [CrossRef]
- Yellin, M.J.; Winikoff, S.; Fortune, S.M.; Baum, D.; Crow, M.K.; Lederman, S.; Chess, L. Ligation of CD40 on fibroblasts induces CD54 (ICAM-1) and CD106 (VCAM-1) up-regulation and IL-6 production and proliferation. J. Leukoc. Biol. 1995, 58, 209–216. [Google Scholar] [CrossRef]
- Permin, H.; Skov, P.S.; Norn, S. Basophil Histamine Release Induced by Leukocyte Nuclei in Patients with Rheumatoid Arthritis. Allergy 1983, 38, 273–281. [Google Scholar] [CrossRef]
- Urb, M.; Sheppard, D.C. The Role of Mast Cells in the Defence against Pathogens. PLOS Pathog. 2012, 8, e1002619. [Google Scholar] [CrossRef]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2016, 6, 620. [Google Scholar] [CrossRef]
- Ioan-Facsinay, A. Initiating pain in osteoarthritis (OA): Is it the mast cell? Osteoarthr. Cartil. 2017, 26, 1–3. [Google Scholar] [CrossRef]
- Yu, Y.; Blokhuis, B.R.; Garssen, J.; Redegeld, F.A. Non-IgE mediated mast cell activation. Eur. J. Pharmacol. 2016, 778, 33–43. [Google Scholar] [CrossRef] [PubMed]
- Redegeld, F.A.; Yu, Y.; Kumari, S.; Charles, N.; Blank, U. Non-IgE mediated mast cell activation. Immunol. Rev. 2018, 282, 87–113. [Google Scholar] [CrossRef] [PubMed]
- Palmer, H.S.; Kelso, E.B.; Lockhart, J.C.; Sommerhoff, C.P.; Plevin, R.; Goh, F.G.; Ferrell, W.R. Protease-activated receptor 2 mediates the proinflammatory effects of synovial mast cells. Arthritis Rheum. 2007, 56, 3532–3540. [Google Scholar] [CrossRef] [PubMed]
- Klein-Wieringa, I.R.; de Lange-Brokaar, B.J.; Yusuf, E.; Andersen, S.N.; Kwekkeboom, J.C.; Kroon, H.M.; van Osch, G.J.; Zuurmond, A.-M.; Stojanovic-Susulic, V.; Nelissen, R.G.; et al. Inflammatory Cells in Patients with Endstage Knee Osteoarthritis: A Comparison between the Synovium and the Infrapatellar Fat Pad. J. Rheumatol. 2016, 43, 771–778. [Google Scholar] [CrossRef]
- de Lange-Brokaar, B.; Ioan-Facsinay, A.; van Osch, G.; Zuurmond, A.-M.; Schoones, J.; Toes, R.; Huizinga, T.; Kloppenburg, M. Synovial inflammation, immune cells and their cytokines in osteoarthritis: A review. Osteoarthr. Cartil. 2012, 20, 1484–1499. [Google Scholar] [CrossRef]
- Nigrovic, P.A.; Lee, D.M. Synovial mast cells: Role in acute and chronic arthritis. Immunol. Rev. 2007, 217, 19–37. [Google Scholar] [CrossRef]
- de Lange-Brokaar, B.J.; Kloppenburg, M.; Andersen, S.N.; Dorjée, A.L.; Yusuf, E.; Herb-van Toorn, L.; Kroon, H.M.; Zuurmond, A.M.; Stojanovic-Susulic, V.; Bloem, J.L.; et al. Characterization of synovial mast cells in knee osteoarthritis: Association with clinical parameters. Osteoarthr. Cartil. 2016, 24, 664–671. [Google Scholar] [CrossRef]
- Uchida, K.; Takano, S.; Inoue, G.; Iwase, D.; Aikawa, J.; Takata, K.; Tazawa, R.; Kawakubo, A.; Sekiguchi, H.; Takaso, M. Increase in mast cell marker expression in the synovium of obese patients with osteoarthritis of the knee. Diabetes Metab. Syndr. Obes. Targets Ther. 2019, 12, 377–382. [Google Scholar] [CrossRef]
- Sousa-Valente, J.; Calvo, L.; Vacca, V.; Simeoli, R.; Arévalo, J.; Malcangio, M. Role of TrkA signalling and mast cells in the initiation of osteoarthritis pain in the monoiodoacetate model. Osteoarthr. Cartil. 2018, 26, 84–94. [Google Scholar] [CrossRef]
- Pitcher, T.; Sousa-Valente, J.; Malcangio, M. The monoiodoacetate model of osteoarthritis pain in the mouse. J. Vis. Exp. 2016, 53746. [Google Scholar]
- Huss, R.S.; Huddleston, J.I.; Goodman, S.B.; Butcher, E.C.; Zabel, B.A. Synovial tissue–infiltrating natural killer cells in osteoarthritis and periprosthetic inflammation. Arthritis Rheum. 2010, 62, 3799–3805. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zeng, Y.; Zhang, M.; Ma, H.; Xu, B.; Jiang, H.; Wang, J.; Li, G. Cd56(bright)cd16(-) natural killer cells are shifted toward an ifn-gamma-promoting phenotype with reduced regulatory capacity in osteoarthritis. Hum. Immunol. 2019, 80, 871–877. [Google Scholar] [CrossRef] [PubMed]
- Dogra, P.; Rancan, C.; Ma, W.; Toth, M.; Senda, T.; Carpenter, D.J.; Kubota, M.; Matsumoto, R.; Thapa, P.; Szabo, P.A.; et al. Tissue Determinants of Human NK Cell Development, Function, and Residence. Cell 2020, 180, 749–763.e13. [Google Scholar] [CrossRef]
- Vivier, E.; Tomasello, E.; Baratin, M.; Walzer, T.; Ugolini, S. Functions of natural killer cells. Nat. Immunol. 2008, 9, 503–510. [Google Scholar] [CrossRef] [PubMed]
- Mace, E.M. Human natural killer cells: Form, function, and development. J. Allergy Clin. Immunol. 2022, 151, 371–385. [Google Scholar] [CrossRef]
- Regis, S.; Dondero, A.; Spaggiari, G.M.; Serra, M.; Caliendo, F.; Bottino, C.; Castriconi, R. miR-24-3p down-regulates the expression of the apoptotic factors FasL and BIM in human natural killer cells. Cell. Signal. 2022, 98, 110415. [Google Scholar] [CrossRef]
- Siew, Y.-Y.; Neo, S.-Y.; Yew, H.-C.; Lim, S.-W.; Ng, Y.-C.; Lew, S.-M.; Seetoh, W.-G.; Seow, S.-V.; Koh, H.-L. Oxaliplatin regulates expression of stress ligands in ovarian cancer cells and modulates their susceptibility to natural killer cell-mediated cytotoxicity. Int. Immunol. 2015, 27, 621–632. [Google Scholar] [CrossRef]
- Wu, J.; He, B.; Miao, M.; Han, X.; Dai, H.; Dou, H.; Li, Y.; Zhang, X.; Wang, G. Enhancing Natural Killer Cell-Mediated Cancer Immunotherapy by the Biological Macromolecule Nocardia rubra Cell-Wall Skeleton. Pathol. Oncol. Res. 2022, 28, 1610555. [Google Scholar] [CrossRef]
- Freud, A.G.; Mundy-Bosse, B.L.; Yu, J.; Caligiuri, M.A. The Broad Spectrum of Human Natural Killer Cell Diversity. Immunity 2017, 47, 820–833. [Google Scholar] [CrossRef]
- Björkström, N.K.; Ljunggren, H.-G.; Michaëlsson, J. Emerging insights into natural killer cells in human peripheral tissues. Nat. Rev. Immunol. 2016, 16, 310–320. [Google Scholar] [CrossRef]
- Cooper, M.A.; Elliott, J.M.; Keyel, P.A.; Yang, L.; Carrero, J.A.; Yokoyama, W.M. Cytokine-induced memory-like natural killer cells. Proc. Natl. Acad. Sci. USA 2009, 106, 1915–1919. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Yu, H.T.; Hwang, I.; Park, S.; Park, S.H.; Kim, S.; Shin, E.C. Phenotypic and functional analysis of human nk cell subpopulations according to the expression of fcepsilonrigamma and nkg2c. Front. Immunol. 2019, 10, 2865. [Google Scholar] [CrossRef]
- E Belizário, J.; Neyra, J.M.; Rodrigues, M.F.S.D. When and how NK cell-induced programmed cell death benefits immunological protection against intracellular pathogen infection. J. Endotoxin Res. 2018, 24, 452–465. [Google Scholar] [CrossRef]
- Drvar, V.E.D.R.A.N.A.; Curko-Cofek, B.O.Z.E.N.A.; Karleusa, L.J.E.R.K.A.; Aralica, M.E.R.I.C.A.; Rogoznica, M.A.R.I.J.A.; Kehler, T.A.T.J.A.N.A.; Legovic, D.A.L.E.N.; Rukavina, D.A.N.I.E.L.; Laskarin, G.O.R.D.A.N.A. Granulysin expression and granulysin-mediated apoptosis in the peripheral blood of osteoarthritis patients. Biomed. Rep. 2022, 16, 44. [Google Scholar] [CrossRef]
- Jaime, P.; Garcia-Guerrero, N.; Estella, R.; Pardo, J.; Garcia-Alvarez, F.; Martinez-Lostao, L. Cd56(+)/cd16(-) natural killer cells expressing the inflammatory protease granzyme a are enriched in synovial fluid from patients with osteoarthritis. Osteoarthr. Cartil. 2017, 25, 1708–1718. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Fan, Y.; Liu, S. ATF3 as a potential diagnostic marker of early-stage osteoarthritis and its correlation with immune infiltration through bioinformatics analysis. Bone Jt. Res. 2022, 11, 679–689. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Liu, Y.; Liu, K.; Tu, F.; Zhang, C.; Wang, H. Synovial fibroblasts regulate the cytotoxicity and osteoclastogenic activity of synovial natural killer cells through the RANKL-RANK axis in osteoarthritis. Scand. J. Immunol. 2021, 94, e13069. [Google Scholar] [CrossRef]
- Mostafa, R.E.; Salama, A.A. Eplerenone modulates the inflammatory response in monosodium iodoacetate-induced knee osteoarthritis in rats: Involvement of RANKL/OPG axis. Life Sci. 2023, 316, 121405. [Google Scholar] [CrossRef] [PubMed]
- Groh, V.; Brühl, A.; El-Gabalawy, H.; Nelson, J.L.; Spies, T. Stimulation of T cell autoreactivity by anomalous expression of NKG2D and its MIC ligands in rheumatoid arthritis. Proc. Natl. Acad. Sci. USA 2003, 100, 9452–9457. [Google Scholar] [CrossRef]
- Malafoglia, V.; Ilari, S.; Gioia, C.; Vitiello, L.; Tenti, M.; Iannuccelli, C.; Cristiani, C.M.; Garofalo, C.; Passacatini, L.C.; Viglietto, G.; et al. An observational study on chronic pain biomarkers in fibromyalgia and osteoarthritis patients: Which role for mu opioid receptor’s expression on NK cells? Biomedicines 2023, 11, 931. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panichi, V.; Costantini, S.; Grasso, M.; Arciola, C.R.; Dolzani, P. Innate Immunity and Synovitis: Key Players in Osteoarthritis Progression. Int. J. Mol. Sci. 2024, 25, 12082. https://doi.org/10.3390/ijms252212082
Panichi V, Costantini S, Grasso M, Arciola CR, Dolzani P. Innate Immunity and Synovitis: Key Players in Osteoarthritis Progression. International Journal of Molecular Sciences. 2024; 25(22):12082. https://doi.org/10.3390/ijms252212082
Chicago/Turabian StylePanichi, Veronica, Silvia Costantini, Merimma Grasso, Carla Renata Arciola, and Paolo Dolzani. 2024. "Innate Immunity and Synovitis: Key Players in Osteoarthritis Progression" International Journal of Molecular Sciences 25, no. 22: 12082. https://doi.org/10.3390/ijms252212082
APA StylePanichi, V., Costantini, S., Grasso, M., Arciola, C. R., & Dolzani, P. (2024). Innate Immunity and Synovitis: Key Players in Osteoarthritis Progression. International Journal of Molecular Sciences, 25(22), 12082. https://doi.org/10.3390/ijms252212082





