The Possible Roles of Glucosamine-6-Phosphate Deaminases in Ammonium Metabolism in Cancer
Abstract
1. Introduction
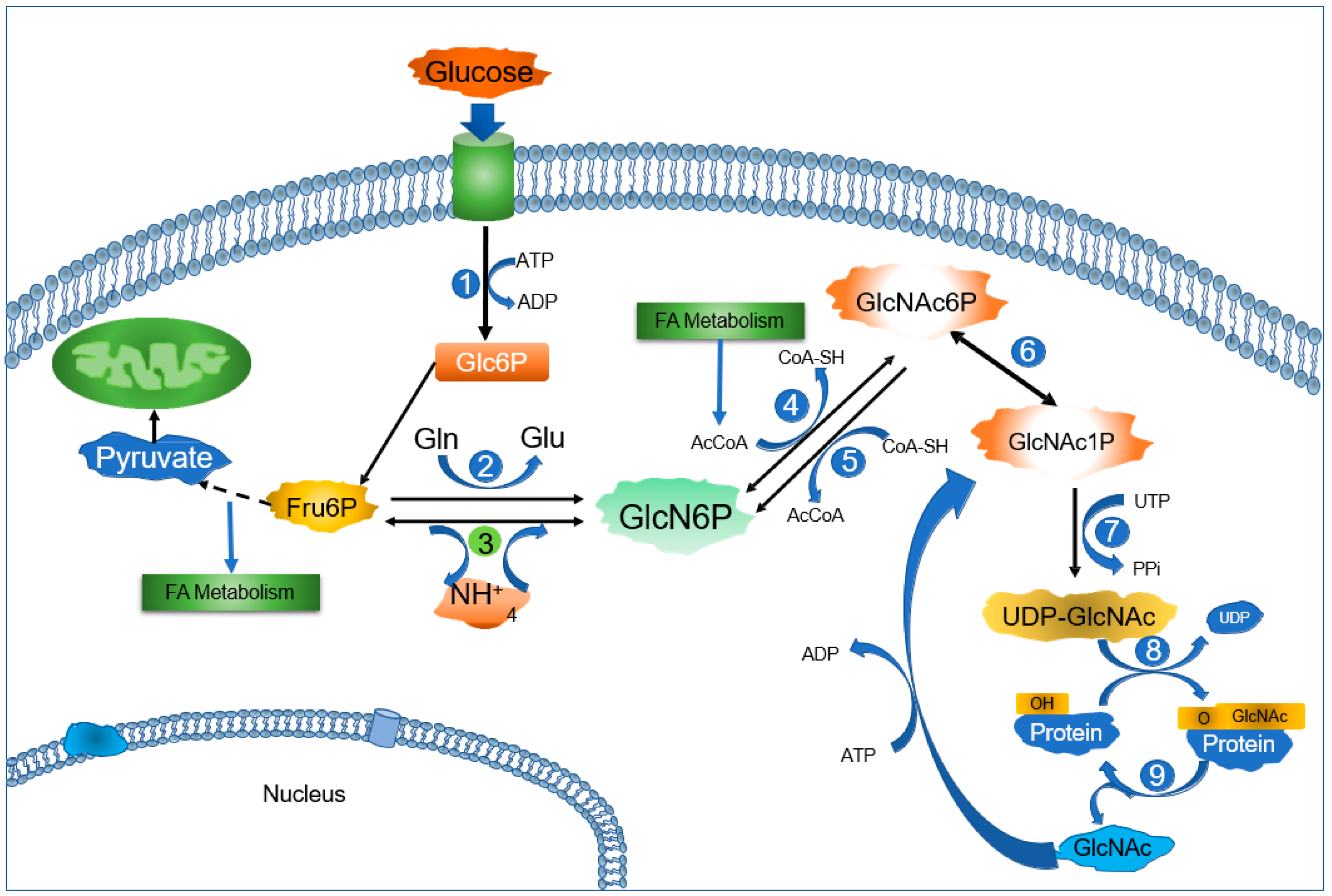
2. Glucosamine-6-Phosphate Deaminases: A Short Story
3. GNPDAs Show Relevant Differences in Enzyme Kinetics
4. GNPDAs and Disease
5. GNPDA1 and Cancer
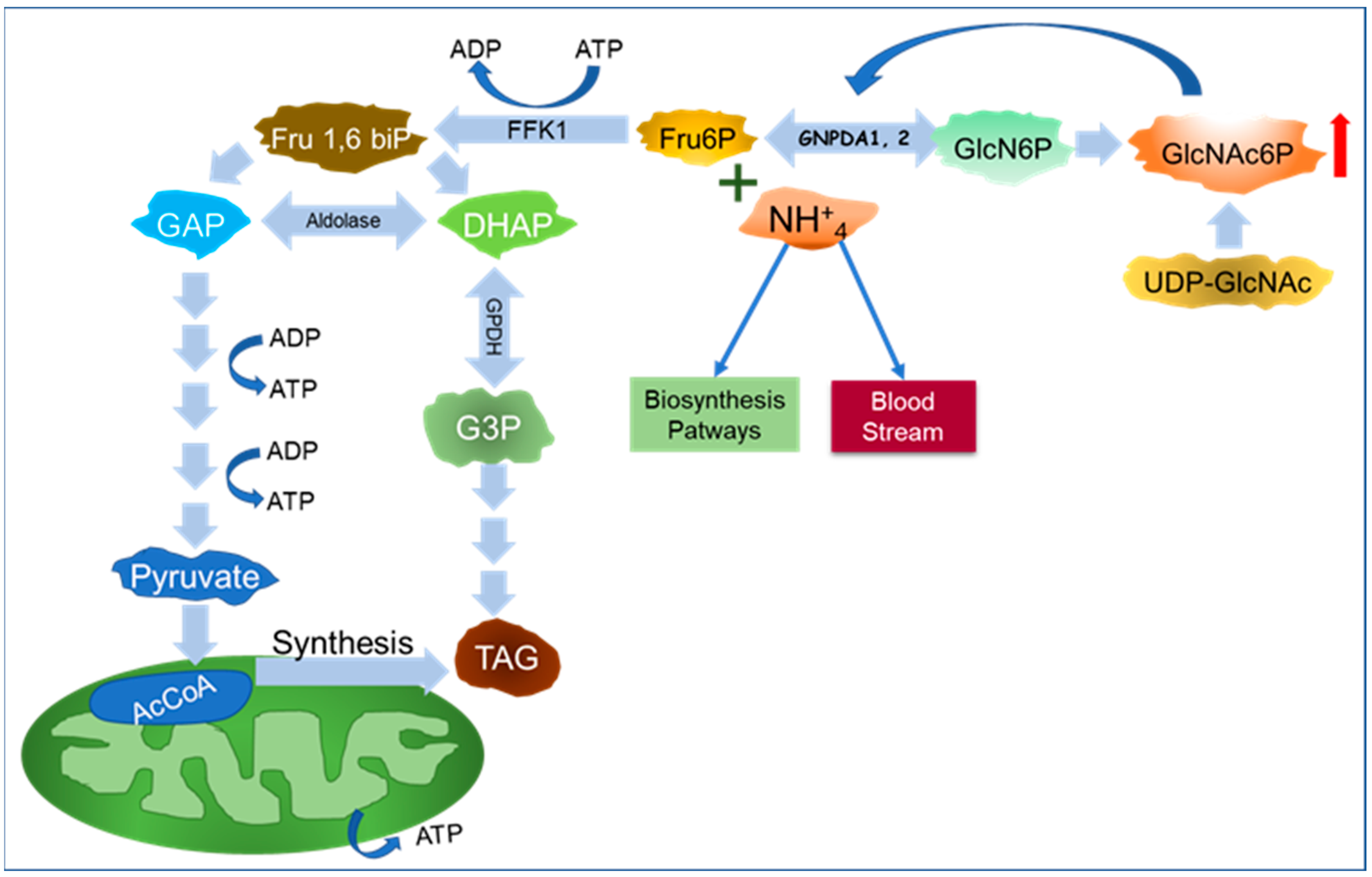
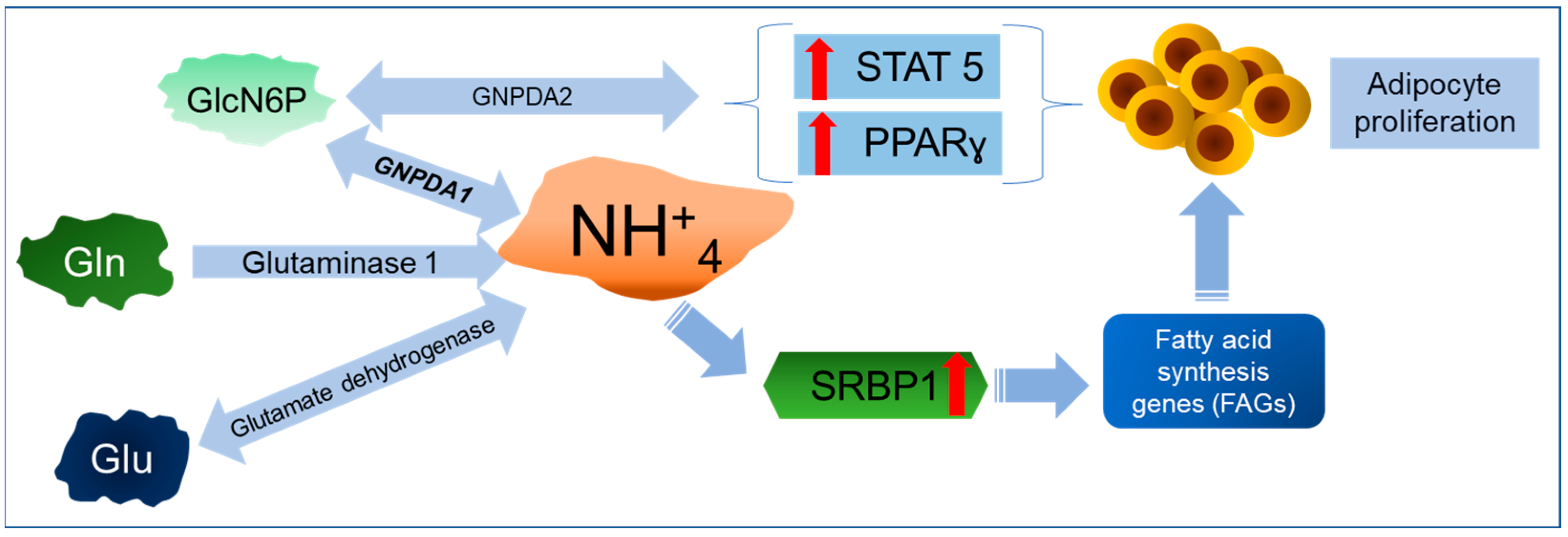
6. A New Perspective on GNPDAs, O-GlcNAcylation, and Ammonium
Importance of GNPDA in Ammonium Metabolism
7. Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Itano, N.; Iwamoto, S. Dysregulation of Hexosamine Biosynthetic Pathway Wiring Metabolic Signaling Circuits in Cancer. Biochim. Biophys. Acta Gen. Subj. 2023, 1867, 130250. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos-Dos-Santos, A.; de Queiroz, R.M.; da Costa Rodrigues, B.; Todeschini, A.R.; Dias, W.B. Hyperglycemia and Aberrant O-GlcNAcylation: Contributions to Tumor Progression. J. Bioenerg. Biomembr. 2018, 50, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Akella, N.M.; Ciraku, L.; Reginato, M.J. Fueling the Fire: Emerging Role of the Hexosamine Biosynthetic Pathway in Cancer. BMC Biol. 2019, 17, 52. [Google Scholar] [CrossRef]
- Leloir, L.F.; Cardini, C.E. Enzymes Acting on Glucosamine Phosphates. Biochim. Biophys. Acta 1956, 20, 33–42. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, W.; Zou, D.; Chen, G.; Wan, T.; Li, N.; Cao, X. Cloning and Functional Characterization of GNPI2, a Novel Human Homolog of Glucosamine-6-Phosphate Isomerase/Oscillin. J. Cell. Biochem. 2003, 88, 932–940. [Google Scholar] [CrossRef]
- Arreola, R.; Valderrama, B.; Morante, M.L.; Horjales, E. Two Mammalian Glucosamine-6-Phosphate Deaminases: A Structural and Genetic Study. FEBS Lett. 2003, 551, 63–70. [Google Scholar] [CrossRef]
- Oikari, S.; Makkonen, K.; Deen, A.J.; Tyni, I.; Kärnä, R.; Tammi, R.H.; Tammi, M.I. Hexosamine Biosynthesis in Keratinocytes: Roles of GFAT and GNPDA Enzymes in the Maintenance of UDP-GlcNAc Content and Hyaluronan Synthesis. Glycobiology 2016, 26, 710–722. [Google Scholar] [CrossRef] [PubMed]
- Faulkner, P.; Quastel, J.H. Anaerobic Deamination of D-glucosamine by Bacterial and Brain Extracts. Nature 1956, 177, 1216–1218. [Google Scholar] [CrossRef]
- Pattabiraman, T.N.; Bachhawat, B.K. Purification of Glucosamine 6-phosphate Deaminase from Human Brain. Biochim. Biophys. Acta 1961, 54, 273–283. [Google Scholar] [CrossRef]
- Leloir, L.F.; Cardini, C.E. Glucosamine-6-Phosphate Deaminase from Pig Kidney. Methods Enzymol. 1962, 5, 418–422. [Google Scholar]
- Kikuchi, K.; Kikuchi, H.; Tsuiki, S. Stabilization and Purification of Glucosamine 6-Phosphate Isomerase from Rat Kidney. Sci. Rep. Res. Inst. Tohoku Univ. Ser. C Med. 1979, 26, 92–98. [Google Scholar]
- Comb, D.G.; Roseman, S. Glucosamine Metabolism. IV. Glucosamine-6-Phosphate Deaminase. J. Biol. Chem. 1958, 232, 807–827. [Google Scholar] [CrossRef] [PubMed]
- Benson, R.; Friedman, S. Allosteric Control of Glucosamine Phosphate Isomerase from the Adult Housefly and Its Role in the Synthesis of Glucosamine 6-Phosphate. J. Biol. Chem. 1970, 245, 2219–2228. [Google Scholar] [CrossRef] [PubMed]
- Enghofer, E.; Kress, H. Glucosamine Metabolism in Drosophila virilis Salivary Glands: Ontogenetic Changes of Enzyme Activities and Metabolite Synthesis. Dev. Biol. 1980, 78, 63–75. [Google Scholar] [CrossRef]
- Tesoriere, G.; Vento, R.; Magistro, D.; Dones, F. Glucosamine 6-Phosphate Deaminase from Rabbit Erythrocytes. Ital. J. Biochem. 1970, 19, 139–154. [Google Scholar]
- Weidanz, J.A.; Campbell, P.; De Lucas, L.J.; Jin, J.; Moore, D.; Rodén, L.; Yu, H.; Heilmann, E.; Vezza, A.C. Glucosamine 6-Phosphate Deaminase in Normal Human Erythrocytes. Br. J. Haematol. 1995, 91, 72–79. [Google Scholar] [CrossRef]
- Cayli, A.; Hirschmann, F.; Wirth, M.; Hauser, H.; Wagner, R. Cell Lines with Reduced UDP-N Acetylhexosamine Pool in the Presence of Ammonium. Biotechnol. Bioeng. 1999, 65, 192–200. [Google Scholar] [CrossRef]
- Calcagno, M.; Campos, P.J.; Mulliert, G.; Suastegui, J. Purification, Molecular and Kinetic Properties of Glucosamine-6-Phosphate Isomerase (Deaminase) from Escherichia coli. Biochim. Biophys. Acta 1984, 787, 165–173. [Google Scholar] [CrossRef]
- Oliva, G.; Fontes, M.R.; Garratt, M.C.; Altamirano, M.M.; Calcagno, M.L.; Horjales, E. Structure and Catalytic Mechanism of Glucosamine 6-Phosphate Deaminase from Escherichia coli at 2.1 A Resolution. Structure 1995, 3, 1323–1332. [Google Scholar] [CrossRef]
- Plumbridge, J.A.; Cochet, O.; Souza, J.M.; Altamirano, M.M.; Calcagno, M.L.; Badet, B. Coordinated Regulation of Amino Sugar-Synthesizing and -Degrading Enzymes in Escherichia coli K-12. J. Bacteriol. 1993, 175, 4951–4956. [Google Scholar] [CrossRef]
- Marcos-Viquez, J.; Rodríguez-Hernández, A.; Álvarez-Añorve, L.I.; Medina-García, A.; Plumbridge, J.; Calcagno, M.L.; Rodríguez-Romero, A.; Bustos-Jaimes, I. Substrate Binding in the Allosteric Site Mimics Homotropic Cooperativity in the SIS-fold Glucosamine-6-Phosphate Deaminases. Protein Sci. 2023, 32, e4651. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Añorve, L.I.; Gaugué, I.; Link, H.; Marcos-Viquez, J.; Díaz-Jiménez, D.M.; Zonszein, S.; Bustos-Jaimes, I.; Schmitz-Afonso, I.; Calcagno, M.L.; Plumbridge, J. Allosteric Activation of Escherichia coli Glucosamine-6-Phosphate Deaminase (NagB) In Vivo Justified by Intracellular Amino Sugar Metabolite Concentrations. J. Bacteriol. 2016, 198, 1610–1620. [Google Scholar] [CrossRef]
- Sosa-Peinado, A.; González-Andrade, M. Site-directed Fluorescence Labeling Reveals Differences on the R-Conformer of Glucosamine 6-Phosphate Deaminase of Escherichia coli Induced by Active or Allosteric Site Ligands at Steady State. Biochemistry 2005, 44, 15083–15092. [Google Scholar] [CrossRef] [PubMed]
- Lara-Lemus, R.; Libreros-Minotta, C.A.; Altamirano, M.M.; Calcagno, M.L. Purification and Characterization of Glucosamine-6-Phosphate Deaminase from Dog Kidney Cortex. Arch. Biochem. Biophys. 1992, 297, 213–220. [Google Scholar] [CrossRef]
- Parrington, J.; Swann, K.; Shevchenko, V.I.; Sesay, A.K.; Lai, F.A. Calcium Oscillations in Mammalian Eggs Triggered by a Soluble Sperm Protein. Nature 1996, 379, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Wolosker, H.; Kline, D.; Bian, Y.; Blackshaw, S.; Cameron, A.M.; Fralich, T.J.; Schnaar, R.L.; Snyder, S.H. Molecularly Cloned Mammalian Glucosamine-6-Phosphate Deaminase Localizes to Transporting Epithelium and Lacks Oscillin Activity. FASEB J. 1998, 12, 91–99. [Google Scholar] [PubMed]
- Wolny, Y.M.; Fissore, R.A.; Wu, H.; Reis, M.M.; Colombero, L.T.; Ergün, B.; Rosenwaks, Z.; Palermo, G.D. Human Glucosamine-6-Phosphate Isomerase, a Homologue of Hamster oscillin, Does Not Appear To Be Involved in Ca2+ Release in Mammalian Oocytes. Mol. Rep. Dev. 1999, 52, 277–287. [Google Scholar] [CrossRef]
- Shevchenko, V.; Hogben, M.; Ekong, R.; Parrington, J.; Lai, F.A. The Human Glucosamine-6-Phosphate Deaminase Gene: cDNA Cloning and Expression, Genomic Organization and Chromosomal Localization. Gene 1998, 216, 31–38. [Google Scholar] [CrossRef]
- Amireault, P.; Dube, F. Cloning, Sequencing, and Expression Analysis of Mouse Glucosamine-6-Phosphate Deaminase (GNPDA/Oscillin). Mol. Rep. Dev. 2000, 56, 424–435. [Google Scholar] [CrossRef]
- Lara-Lemus, R.; Calcagno, M.L. Glucosamine-6-Phosphate Deaminase from Beef Kidney Is an Allosteric System of the V-type. Biochim. Biophys. Acta 1998, 1388, 1–9. [Google Scholar] [CrossRef]
- Davies, J.S.; Coombes, D.; Horne, C.R.; Pearce, F.G.; Friemann, R.; North, R.A.; Dobson, R.A.J. Functional and Solution Structure Studies of Amino Sugar Deacetylase and Deaminase Enzymes from Staphylococcus aureus. FEBS Lett. 2019, 593, 52–66. [Google Scholar] [CrossRef] [PubMed]
- Moye, Z.D.; Burne, R.A.; Zeng, L. Uptake and Metabolism of N-acetylglucosamine and Glucosamine by Streptococcus mutans. Appl. Environ. Microbiol. 2014, 80, 5053–5067. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Takahashi, F.; Fukui, T.; Fujiwara, S.; Atomi, H.; Imanaka, T. Characterization of a Novel Glucosamine-6-Phosphate Deaminase from a Hyperthermophilic Archaeon. J. Bacteriol. 2005, 187, 7038–7044. [Google Scholar] [CrossRef]
- Solar, T.; Tursic, J.; Legisa, M. The Role of Glucosamine-6-Phosphate Deaminase at the Early Stages of Aspergillus niger Growth in a High-Citric-Acid-Yielding Medium. Appl. Microbiol. Biotechnol. 2008, 78, 613–619. [Google Scholar] [CrossRef]
- Aguilar-Díaz, H.; Díaz-Gallardo, M.; Laclette, J.P.; Carrero, J.C. In vitro Induction of Entamoeba histolytica Cyst-Like Structures from Trophozoites. PLoS Negl. Trop. Dis. 2010, 4, e607. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Álvarez-Añorve, L.I.; Alonzo, D.A.; Mora-Lugo, R.; Lara-González, S.; Bustos-Jaimes, I.; Plumbridge, J.; Calcagno, M.L. Allosteric Kinetics of the Isoform 1 of Human Glucosamine-6-Phosphate Deaminase. Biochim. Biophys. Acta 2011, 1814, 1846–1853. [Google Scholar] [CrossRef]
- Melén, E.; Himes, B.E.; Brehm, J.M.; Boutaoui, N.; Klanderman, E.J.; Sylvia, J.S.; Lasky-Su, J. Analyses of Shared Genetic Factors Between Asthma and Obesity in Children. J. Allergy Clin. Immunol. 2010, 126, e1–e8. [Google Scholar] [CrossRef]
- Colak, Y.; Afzal, S.; Lange, P.; Nordestgaard, B.G. Obese Individuals Experience Wheezing Without Asthma but not Asthma Without wheezing: A Mendelian Randomisation Study of 85 437 Adults from the Copenhagen General Population Study. Thorax 2016, 71, 247–254. [Google Scholar] [CrossRef]
- Ng, M.C.Y.; Tam, C.H.T.; So, W.Y.; Ho, J.S.; Chan, A.W.; Lee, H.M.; Wang, Y.; Lam, V.K.L.; Chan, J.C.N.; Ma, R.C.W. Implication of Genetic Variants Near NEGR1, SEC16B, TMEM18, ETV5/DGKG, GNPDA2, LIN7C/BDNF, MTCH2, BCDIN3D/FAIM2, SH2B1, FTO, MC4R, and KCTD15 with Obesity and Type 2 Diabetes in 7705 Chinese. J. Clin. Endocrinol. Metab. 2010, 95, 2418–2425. [Google Scholar] [CrossRef]
- Hebbar, P.; Abubaker, J.A.; Abu-Farha, M.; Tuomilehto, J.; Al-Mulla, F.; Thanaraj, T.A. A Perception on Genome-Wide Genetic Analysis of Metabolic Traits in Arab Populations. Front. Endocrinol. 2019, 10, 8. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, X.; Zhao, Q.; He, J.; Chen, L.; Zhao, Z.; Li, Q.; Ge, J.; Chen, G.; Guo, X.; et al. Obesity-Related Genomic Loci Are Associated with Type 2 Diabetes in a Han Chinese Population. PLoS ONE 2014, 9, e104486. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Guan, R.-C.; Zhao, Y.; Chen, Y. Obesity-Related Loci in TMEM18, CDKAL1 and FAIM2 Are Associated with Obesity and Type 2 Diabetes in Chinese Han patients. BMC Med. Genet. 2020, 21, 65. [Google Scholar] [CrossRef]
- Kong, X.; Xing, X.; Zhang, X.; Hong, J.; Yang, W. Sexual Dimorphism of a Genetic Risk Score for Obesity and Related Traits among Chinese Patients with Type 2 Diabetes. Obes. Facts 2019, 12, 328–343. [Google Scholar] [CrossRef] [PubMed]
- Hotta, K.; Nakamura, M.; Nakamura, T.; Matsuo, T.; Nakata, Y.; Kamohara, S.; Miyatake, N.; Kotani, K.; Komatsu, R.; Itoh, N.; et al. Association Between Obesity and Polymorphisms in SEC16B, TMEM18, GNPDA2, BDNF, FAIM2 and MC4R in a Japanese Population. J. Hum. Genet. 2009, 54, 727–731. [Google Scholar] [CrossRef]
- Robiou-du-Pont, S.; Bonnefond, A.; Yengo, L.; Vaillant, E.; Lobbens, S.; Durand, E.; Weill, J.; Lantieri, O.; Balkau, B.; Charpentier, G.; et al. Contribution of 24 Obesity-Associated Genetic Variants to Insulin Resistance, Pancreatic Beta-Cell Function and Type 2 Diabetes Risk in the French Population. Int. J. Obes. 2013, 37, 980–985. [Google Scholar] [CrossRef]
- Mejía-Benítez, A.; Klünder-Klünder, M.; Yengo, L.; Meyre, D.; Aradillas, C.; Cruz, E.; Pérez-Luque, E.; Malacara, J.M.; Garay, M.E.; Peralta-Romero, J.; et al. Analysis of the Contribution of FTO, NPC1, ENPP1, NEGR1, GNPDA2 and MC4R Genes to Obesity in Mexican Children. BMC Med. Genet. 2013, 14, 21. [Google Scholar] [CrossRef]
- Takeuchi, F.; Yamamoto, K.; Katsuya, T.; Nabika, T.; Sugiyama, T.; Fujioka, A.; Isono, M.; Ohnaka, K.; Fujisawa, T.; Nakashima, E.; et al. Association of Genetic Variants for Susceptibility to Obesity with Type 2 Diabetes in Japanese Individuals. Diabetologia 2011, 54, 1350–1359. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xi, B.; Zhang, M.; Shen, Y.; Zhao, X.; Cheng, H.; Hou, D.; Sun, D.; Ott, J.; Wang, X.; et al. Associations of Six Single Nucleotide Polymorphisms in Obesity-Related Genes with BMI and Risk of Obesity in Chinese Children. Diabetes 2010, 59, 3085–3089. [Google Scholar] [CrossRef]
- De, R.; Hu, T.; Moore, J.H.; Gilbert-Diamond, D. Characterizing Gene-Gene Interactions in a Statistical Epistasis Network of Twelve Candidate Genes for Obesity. BioData Min. 2015, 8, 45. [Google Scholar] [CrossRef][Green Version]
- Costa-Urrutia, P.; Abud, C.; Franco-Trecu, V.; Colistro, V.; Rodríguez-Arellano, M.E.; Alvarez-Fariña, R.; Acuña-Alonso, V.; Bertoni, B.; Granados, J. Effect of 15 BMI-Associated Polymorphisms, Reported for Europeans, Across Ethnicities and Degrees of Amerindian Ancestry in Mexican Children. Int. J. Mol. Sci. 2020, 21, 374. [Google Scholar] [CrossRef]
- Hong, J.; Shi, J.; Qi, L.; Cui, B.; Gu, W.; Zhang, Y.; Li, L.; Xu, M.; Wang, L.; Zhai, Y.; et al. Genetic Susceptibility, Birth Weight and Obesity Risk in Young Chinese. Int. J. Obes. 2013, 37, 673–677. [Google Scholar] [CrossRef]
- Wu, L.; Ma, F.; Zhao, X.; Zhang, M.-X.; Wu, J.; Wu, J.; Jie, M. GNPDA2 Gene Affects Adipogenesis and Alters the Transcriptome Profile of Human Adipose-Derived Mesenchymal Stem Cells. Int. J. Endocrinol. 2019, 2019, 9145452. [Google Scholar] [CrossRef] [PubMed]
- Renström, F.; Payne, F.; Nordström, A.; Brito, E.C.; Rolandsson, O.; Hallmans, G.; Barroso, I.; Nordström, P.; Franks, P.W. Replication and Extension of Genome-Wide Association Study Results for Obesity in 4923 Adults from Northern Sweden. Hum. Mol. Genet. 2009, 18, 1489–1496. [Google Scholar] [CrossRef] [PubMed]
- Dorajoo, R.; Blakemore, A.I.F.; Sim, X.; Ong, R.T.-H.; Ng, D.P.K.; Seielstad, M.; Wong, T.-Y.; Saw, S.-M.; Froguel, P.; Liu, J.; et al. Replication of 13 Obesity Loci Among Singaporean Chinese, Malay and Asian-Indian Populations. Int. J. Obes. 2011, 36, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Yılmaz, B.; Karadağ, M.G. The Current Review of Adolescent Obesity: The Role of Genetic Factors. J. Pediatr. Endocrinol. Metab. 2020, 34, 151–162. [Google Scholar] [CrossRef] [PubMed]
- Graff, M.; Gordon-Larsen, P.; Lim, U.; Fowke, J.H.; Love, S.-A.; Fesinmeyer, M.; Wilkens, L.R.; Vertilus, S.; Ritchie, M.D.; Prentice, R.L.; et al. The Influence of Obesity-Related Single Nucleotide Polymorphisms on BMI Across the Life Course: The PAGE study. Diabetes 2013, 62, 1763–1767. [Google Scholar] [CrossRef]
- Felix, J.F.; Bradfield, J.P.; Monnereau, C.; van der Valk, R.J.P.; Stergiakouli, E.; Chesi, A.; Gaillard, R.; Feenstra, B.; Thiering, E.; Kreiner-Møller, E.; et al. Genome-Wide Association Analysis Identifies Three New Susceptibility Loci for Childhood Body Mass Index. Hum. Mol. Genet. 2016, 25, 389–403. [Google Scholar] [CrossRef]
- Zhao, J.; Bradfield, J.P.; Zhang, H.; Sleiman, P.M.; Kim, C.E.; Glessner, J.T.; Deliard, S.; Thomas, K.A.; Frackelton, E.C.; Li, M.; et al. Role of BMI-Associated Loci Identified in GWAS Meta-Analyses in the Context of Common Childhood Obesity in European Americans. Obesity 2011, 19, 2436–2439. [Google Scholar] [CrossRef]
- Willer, C.J.; Speliotes, E.K.; Loos, R.J.F.; Li, S.; Lindgren, C.M.; Heid, I.M.; Berndt, S.I.; Elliott, A.L.; Jackson, A.U.; Lamina, C.; et al. Six New Loci Associated with Body Mass Index Highlight a Neuronal Influence on Body Weight Regulation. Nat. Genet. 2009, 41, 25–34. [Google Scholar] [CrossRef]
- Flores-Dorantes, M.T.; Díaz-López, Y.E.; Gutiérrez-Aguilar, R. Environment and Gene Association with Obesity and Their Impact on Neurodegenerative and Neurodevelopmental Diseases. Front. Neurosci. 2020, 14, 863. [Google Scholar] [CrossRef]
- Gutierrez-Aguilar, R.; Grayson, B.E.; Kim, D.-H.; Yalamanchili, S.; Calcagno, M.L.; Woods, S.C.; Seeley, R.J. CNS GNPDA2 Does Not Control Appetite, but Regulates Glucose Homeostasis. Frnt. Nutr. 2021, 8, 787470. [Google Scholar] [CrossRef] [PubMed]
- Lachén-Montes, M.; González-Morales, A.; Iloro, I.; Elortza, F.; Ferrer, I.; Gveric, D.; Fernández-Irigoyen, J.; Santamaría, E. Unveiling the Olfactory Proteostatic Disarrangement in Parkinson’s Disease by Proteome-Wide Profiling. Neurobiol. Aging 2019, 73, 123–134. [Google Scholar] [CrossRef]
- Lachén-Montes, M.; González-Morales, A.; Fernández-Irigoyen, J.; Santamaría, E. Deployment of Label-Free Quantitative Olfactory Proteomics to Detect Cerebrospinal Fluid Biomarker Candidates in Synucleinopathies. Methods Mol. Biol. 2019, 2044, 273–289. [Google Scholar] [CrossRef] [PubMed]
- Sukeno, T.; Kikuchi, H.; Saeki, H.; Tsuiki, S. Transformation of Glucosamine to Glycogen and Lactate by Ascites Tumor Cells. Biochim. Biophys. Acta 1971, 244, 19–29. [Google Scholar] [CrossRef]
- Kikuchi, K.; Tsuiki, S. Metabolism of Exogenous N-Acetylglucosamine in Extracts of Rat Kdney, Liver and Hepatoma. Biochim. Biophys. Acta 1979, 584, 246–253. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Cheng, X.; Zheng, W.; Chen, J. Glucosamine-6-Phosphate Isomerase 1 Promotes Tumor Progression and Indicates Poor Prognosis in Hepatocellular Carcinoma. Cancer Manag. Res. 2020, 12, 4923–4935. [Google Scholar] [CrossRef]
- Zhou, W.; Zhang, S.; Cai, Z.; Gao, F.; Deng, W.; Wen, Y.; Qiu, Z.-W.; Hou, Z.-K.; Chen, X.-L. A Glycolysis-Related Gene Pairs Signature Predicts Prognosis in Patients with Hepatocellular Carcinoma. Peer J. 2020, 8, e9944. [Google Scholar] [CrossRef] [PubMed]
- Xia, R.; Tang, H.; Shen, J.; Xu, S.; Liang, Y.; Zhang, Y.; Gong, X.; Min, Y.; Zhang, D.; Tao, C.; et al. Prognostic Value of a Novel Glycolysis-Related Gene Expression Signature for Gastrointestinal Cancer in the Asian Population. Cancer Cell Int. 2021, 21, 154. [Google Scholar] [CrossRef]
- He, Y.-J.; Li, W.-L.; Liu, B.-H.; Dong, H.; Mou, Z.-R.; Wu, Y.-Z. Identification of Differential Proteins in Colorectal Cancer Cells Treated with Caffeic Acid Phenethyl Ester. World J. Gastroenterol. 2014, 33, 11840–11849. [Google Scholar] [CrossRef]
- Tarze, A.; Deniaud, A.; Le Bras, M.; Maillier, E.; Molle, D.; Larochette, N.; Zamzami, N.; Jan, G.; Kroemer, G.; Brenner, C. GAPDH, a Novel Regulator of the pro-Apoptotic Mitochondrial Membrane Permeabilization. Oncogene 2007, 26, 2606–2620. [Google Scholar] [CrossRef]
- Carvalho-Cruz, P.; Alisson-Silva, F.; Todeschini, A.R.; Dias, W.B. Cellular Glycosylation Senses Metabolic Changes and Modulates Cell Plasticity During Epithelial to Mesenchymal Transition. Dev. Dyn. 2018, 247, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Piccioni, F.; Malvicini, M.; Garcia, M.G.; Rodriguez, A.; Atorrasagasti, C.; Kippes, N.; Piedra Buena, I.T.; Rizzo, M.M.; Bayo, J.; Aquino, J.; et al. Antitumor effects of Hyaluronic Acid Inhibitor 4-methylumbelliferone in an Orthotopic HepatoCellular Carcinoma Model in Mice. Glycobiology 2012, 22, 400–410. [Google Scholar] [CrossRef] [PubMed]
- Lara-Lemus, R. On The Role of Myelin and Lymphocyte Protein (MAL) In Cancer: A Puzzle with Two Faces. J. Cancer 2019, 10, 2312–2318. [Google Scholar] [CrossRef]
- Yang, X.Y.; Qian, K.V. Protein O-GlcNAcylation: Emerging Mechanisms and Functions. Nat. Rev. Mol. Cell Biol. 2017, 18, 452–465. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, Y. Emerging Roles of O-GlcNAcylation in Protein Trafficking and Secretion. J. Biol. Chem. 2024, 300, 105677. [Google Scholar] [CrossRef]
- Dupas, T.; Lauzier, B.; McGraw, S. O-GlcNAcylation: The Sweet Side of Epigenetics. Epigenet. Chromatin 2023, 16, 49. [Google Scholar] [CrossRef]
- He, X.-F.; Hu, X.; Wen, G.-J.; Wang, Z.; Lin, W.-J. O-GlcNAcylation in Cancer Development and Immunotherapy. Cancer Lett. 2023, 566, 216258. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qu, S.; Jin, H.; Jia, Q.; Li, M. Role of O-GlcNAcylation in Cancer Biology. Pathol. Res. Pract. 2024, 253, 155001. [Google Scholar] [CrossRef]
- Chen, L.; Hu, M.; Chen, L.; Peng, Y.; Zhang, C.; Wang, X.; Li, X.; Yao, Y.; Song, Q.; Li, J.; et al. Targeting O-GlcNAcylation in Cancer Therapeutic Resistance: The Sugar Saga Continues. Cancer Lett. 2024, 588, 216742. [Google Scholar] [CrossRef]
- Lin, C.-H.; Liao, C.-C.; Chen, M.-Y.; Chou, T.-Y. Feedback Regulation of O-GlcNAc Transferase through Translation Control to Maintain Intracellular O-GlcNAc Homeostasis. Int. J. Mol. Sci. 2021, 22, 3463. [Google Scholar] [CrossRef]
- Ciraku, L.; Esquea, E.M.; Reginato, M.J. O-GlcNAcylation Regulation of Cellular Signaling in Cancer. Cell. Signal. 2022, 90, 110201. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Sheng, X.; Zhao, T.; Zhang, L.; Ruan, Y.; Lu, H. O-GlcNAcylation of MEK2 Promotes the Proliferation and Migration of Breast Cancer Cells. Glycobiology 2021, 31, 571–581. [Google Scholar] [CrossRef] [PubMed]
- Eng, C.H.; Yu, K.; Lucas, J.; White, E.; Abraham, R.T. Ammonia Derived from Glutaminolysis is a Diffusible Regulator of Autophagy. Sci. Signal. 2010, 3, ra31. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hu, Z.; Cai, L.; Li, K.; Choi, E.; Faubert, B.; Bezwada, D.; Rodriguez-Canales, J.; Villalobos, P.; Lin, Y.F.; et al. CPS1 Maintains Pyrimidine Pools and DNA Synthesis in KRAS/LKB1-mutant Lung Cancer Cells. Nature 2017, 546, 168–172. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, J.S.; Yoon, H.; Ringel, A.E.; Jeanfavre, S.; Clish, C.B.; Haigis, M.C. Metabolic Recycling of Ammonia via Glutamate Dehydrogenase Supports Breast Cancer Biomass. Science 2017, 358, 941–946. [Google Scholar] [CrossRef]
- Cheng, C.; Geng, F.; Li, Z.; Zhong, Y.; Wang, H.; Cheng, X.; Zhao, Y.; Mo, X.; Horbinski, C.; Duan, W.; et al. Ammonia Stimulates SCAP/Insig Dissociation and SREBP-1 Activation to Promote Lipogenesis and Tumour Growth. Nat. Metab. 2022, 4, 575–588. [Google Scholar] [CrossRef]
- Shimano, H.; Sato, R. SREBP-Regulated Lipid Metabolism: Convergent Physiology-Divergent Pathophysiology. Nat. Rev. Endocrinol. 2017, 13, 710–730. [Google Scholar] [CrossRef]
- Lee, H.M.; Nayak, A.; Kim, J. A new role for ammonia in tumorigenesis? Cell Metab. 2022, 34, 944–946. [Google Scholar] [CrossRef]
- Swinnen, J.V.; Brusselmans, K.; Verhoeven, G. Increased lipogenesis in cancer cells: New players, novel targets. Curr. Opin. Clin. Nutr. Metab. Care 2006, 9, 358–365. [Google Scholar] [CrossRef]
- Moreno-Sánchez, R.; Marín-Hernández, A.; Gallardo-Pérez, J.C.; Pacheco-Velázquez, S.C.; Robledo-Cadena, D.X.; Padilla-Flores, J.A.; Saavedra, E.; Rodríguez-Enríquez, S. Role of Glutamate Dehydrogenase in Cancer Cells. Front. Oncol. 2020, 10, 429. [Google Scholar] [CrossRef]
- McClain, D.A.; Hazel, M.; Parker, G.; Cooksey, R.C. Adipocytes with Increased Hexosamine Flux Exhibit Insulin Resistance, Increased Glucose Uptake, and Increased Synthesis and Storage of Lipid. Am. J. Physiol. Metab. 2005, 288, E973–E979. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rumberger, J.M.; Wu, T.; Hering, M.A.; Marshall, S. Role of Hexosamine Biosynthesis in Glucose-Mediated Up-Regulation of Lipogenic Enzyme mRNA Levels. Effects of Glucose, Glutamine, and Glucosamine on Glycerophosphate Dehydrogenase, Fatty Acid Synthase, and Acetyl-coA Carboxylase mRNA Levels. J. Biol. Chem. 2003, 278, 28547–28552. [Google Scholar] [CrossRef] [PubMed]
- Chance, W.T.; Cao, L.; Nelson, J.L.; Foley-Nelson, T.; Fischer, J.E. Hyperammonemia in Anorectic Tumor-Bearing Rats. Life Sci. 1988, 43, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Phang, J.M.; Liu, W.; Hancock, C.N.; Fischer, J.W. Proline Metabolism and Cancer: Emerging Links to Glutamine and Collagen. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 71–77. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.W.; Lee, D.H.; Jeon, Y.H.; Yoo, J.; Kim, S.Y.; Lee, S.W.; Cho, H.Y.; Kwon, S.H. Glutamine Synthetase as a Therapeutic Target for Cancer Treatment. Int. J. Mol. Sci. 2021, 22, 1701. [Google Scholar] [CrossRef]
- Dai, W.; Shen, J.; Yan, J.; Bott, A.J.; Maimouni, S.; Daguplo, H.Q.; Wang, Y.; Khayati, K.; Guo, J.Y.; Zhang, L.; et al. Glutamine Synthetase Limits β-Catenin–Mutated Liver Cancer Growth by Maintaining Nitrogen Homeostasis and Suppressing mTORC1. J. Clin. Investig. 2022, 132, e161408. [Google Scholar] [CrossRef]
- Chavira-Suárez, E.; Ramírez-Mendieta, A.J.; Martínez-Gutiérrez, S.; Zárate-Segura, P.; Beltrán-Montoya, J.; Espinosa-Maldonado, N.C.; de la Cerda-Ángeles, J.C.; Vadillo-Ortega, F. Influence of Pre-Pregnancy Body Mass Index (p-BMI) and Gestational Weight Gain (GWG) on DNA Methylation and Protein Expression of Obesogenic Genes in Umbilical Vein. PLoS ONE 2019, 14, e0226010. [Google Scholar] [CrossRef]

| Enzyme | Related Diseases | Kind of Study | References |
|---|---|---|---|
| GNPDA2 | Obesity and asthma | Epidemiologic a; no experimental data | [37,38] |
| GNPDA2 | Obesity and type 2 diabetes | Epidemiologic and experimental b | [39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59] |
| GNPDA2 | Obesity and neurologic diseases | Meta-analysis | [59,60] |
| GNPDA2 | Metabolic control and neurologic diseases | Experimental c | [61,62,63] |
| GNPDA1 | Gastrointestinal cancer, hepatocellular cancer, and melanoma | Experimental d | [64,65,66,67,68,69] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lara-Lemus, R.; Castillejos-López, M.; Aquino-Gálvez, A. The Possible Roles of Glucosamine-6-Phosphate Deaminases in Ammonium Metabolism in Cancer. Int. J. Mol. Sci. 2024, 25, 12054. https://doi.org/10.3390/ijms252212054
Lara-Lemus R, Castillejos-López M, Aquino-Gálvez A. The Possible Roles of Glucosamine-6-Phosphate Deaminases in Ammonium Metabolism in Cancer. International Journal of Molecular Sciences. 2024; 25(22):12054. https://doi.org/10.3390/ijms252212054
Chicago/Turabian StyleLara-Lemus, Roberto, Manuel Castillejos-López, and Arnoldo Aquino-Gálvez. 2024. "The Possible Roles of Glucosamine-6-Phosphate Deaminases in Ammonium Metabolism in Cancer" International Journal of Molecular Sciences 25, no. 22: 12054. https://doi.org/10.3390/ijms252212054
APA StyleLara-Lemus, R., Castillejos-López, M., & Aquino-Gálvez, A. (2024). The Possible Roles of Glucosamine-6-Phosphate Deaminases in Ammonium Metabolism in Cancer. International Journal of Molecular Sciences, 25(22), 12054. https://doi.org/10.3390/ijms252212054






