Nitrilase GiNIT from Gibberella intermedia Efficiently Degrades Nitriles Derived from Rapeseed Meal Glucosinolate
Abstract
1. Introduction
2. Results
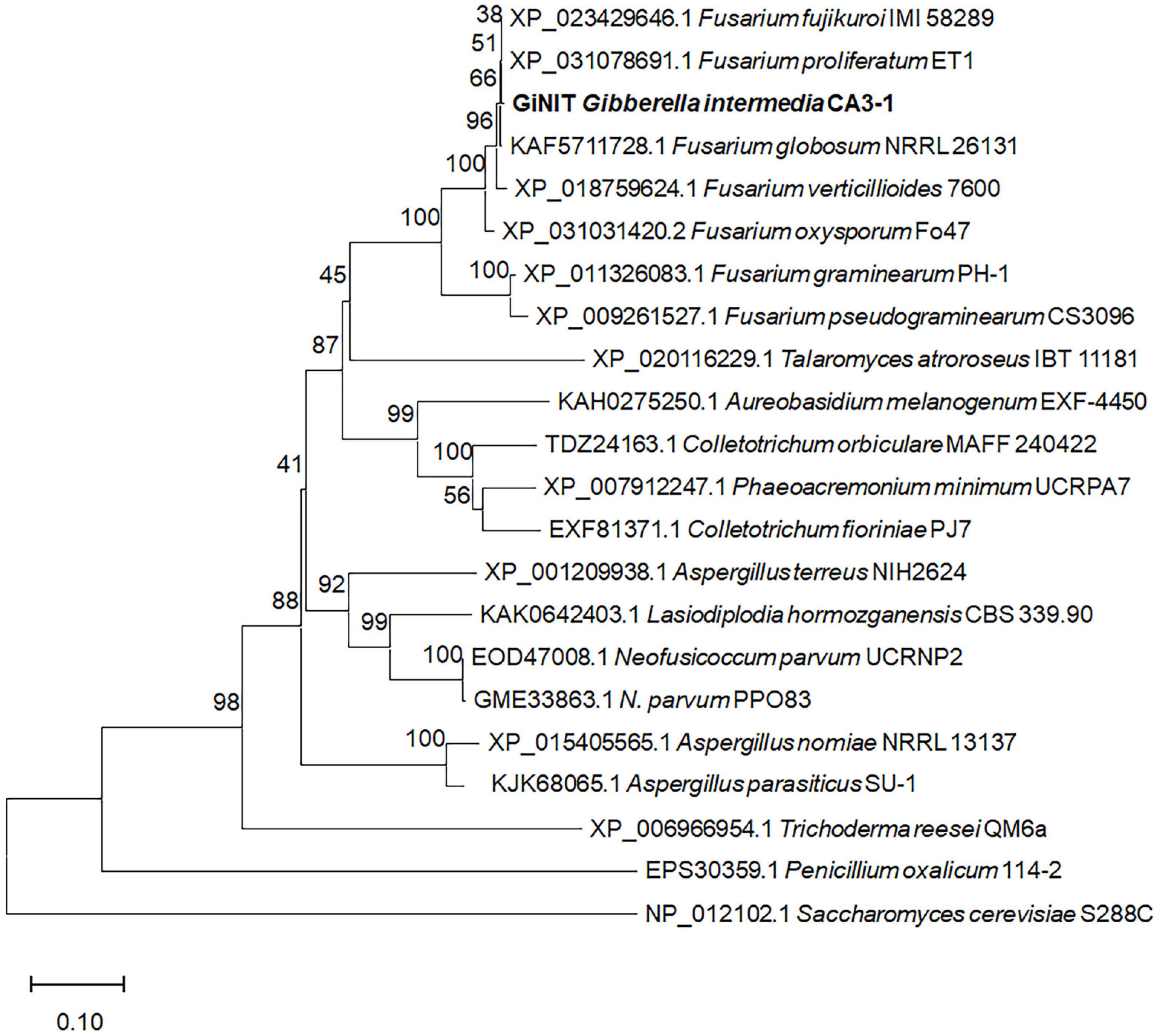
2.1. Discovery of Nitrilase with High Hydrolytic Activity Against 3-Butenenitrile and 4-Pentenenitrile
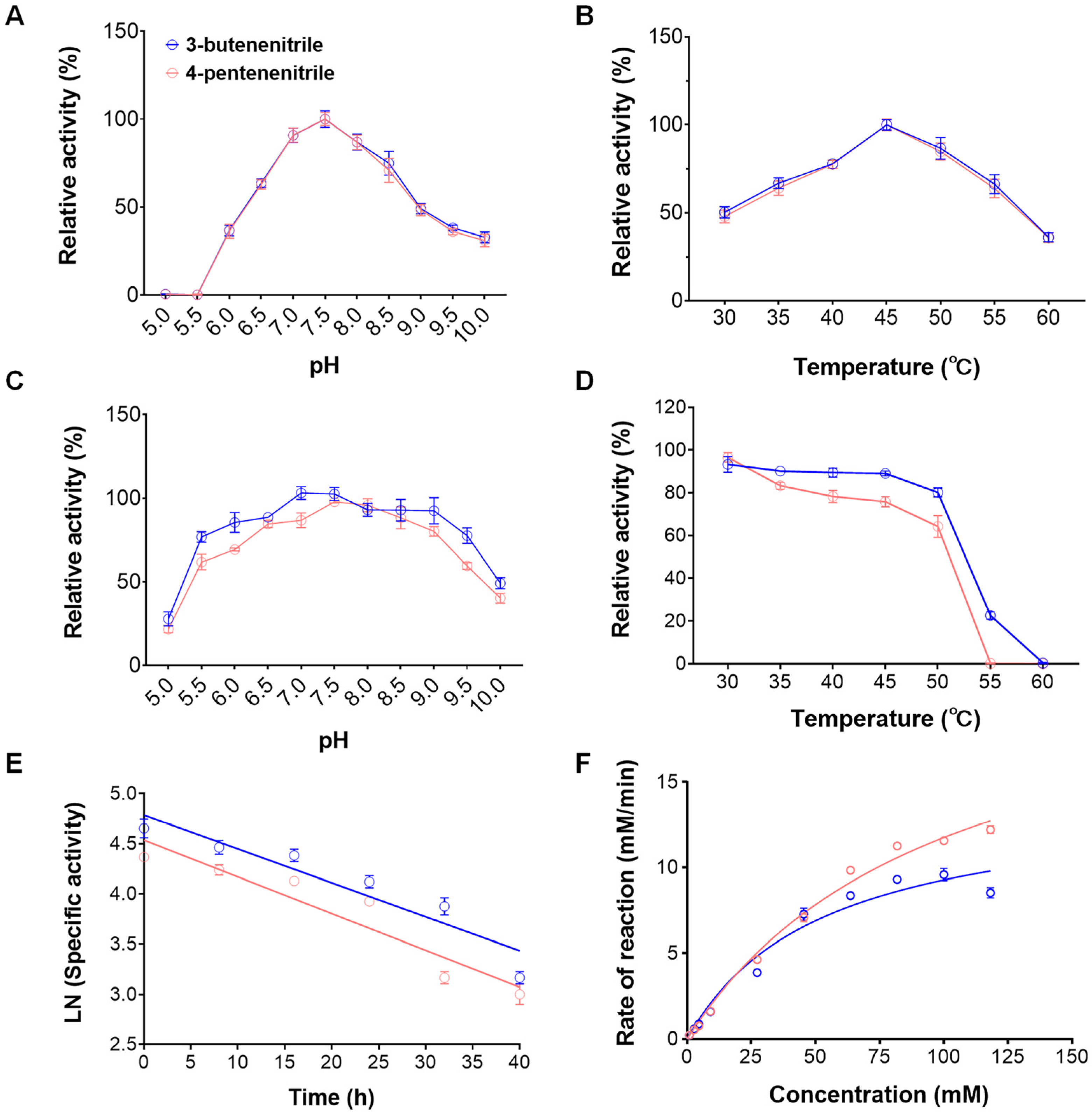
2.2. Biochemical Characterization and Kinetic Parameters of Nitrilase rGiNIT
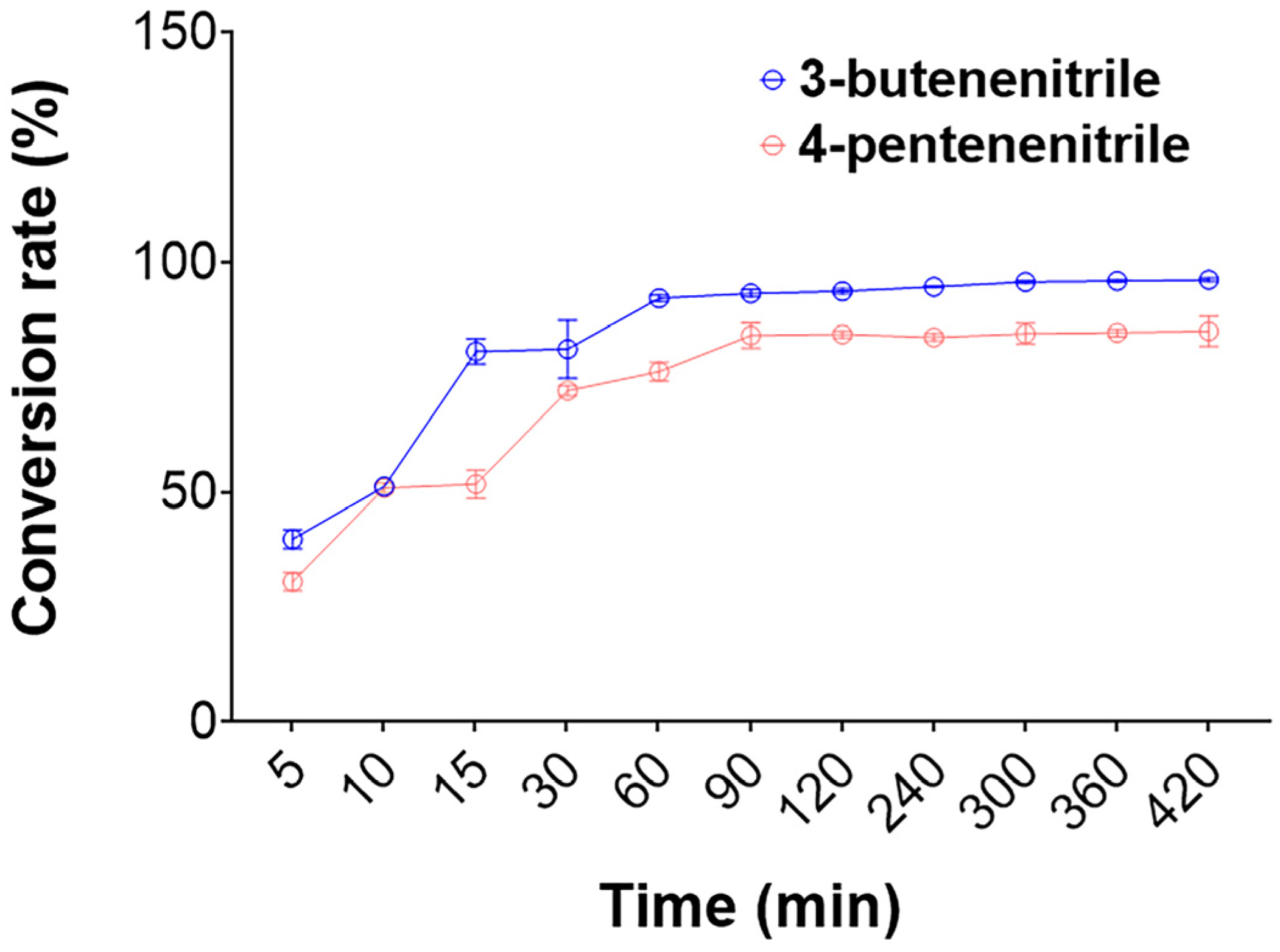
2.3. Efficient Degradation of 3-Butenenitrile and 4-Pentenenitrile by the rGiNIT
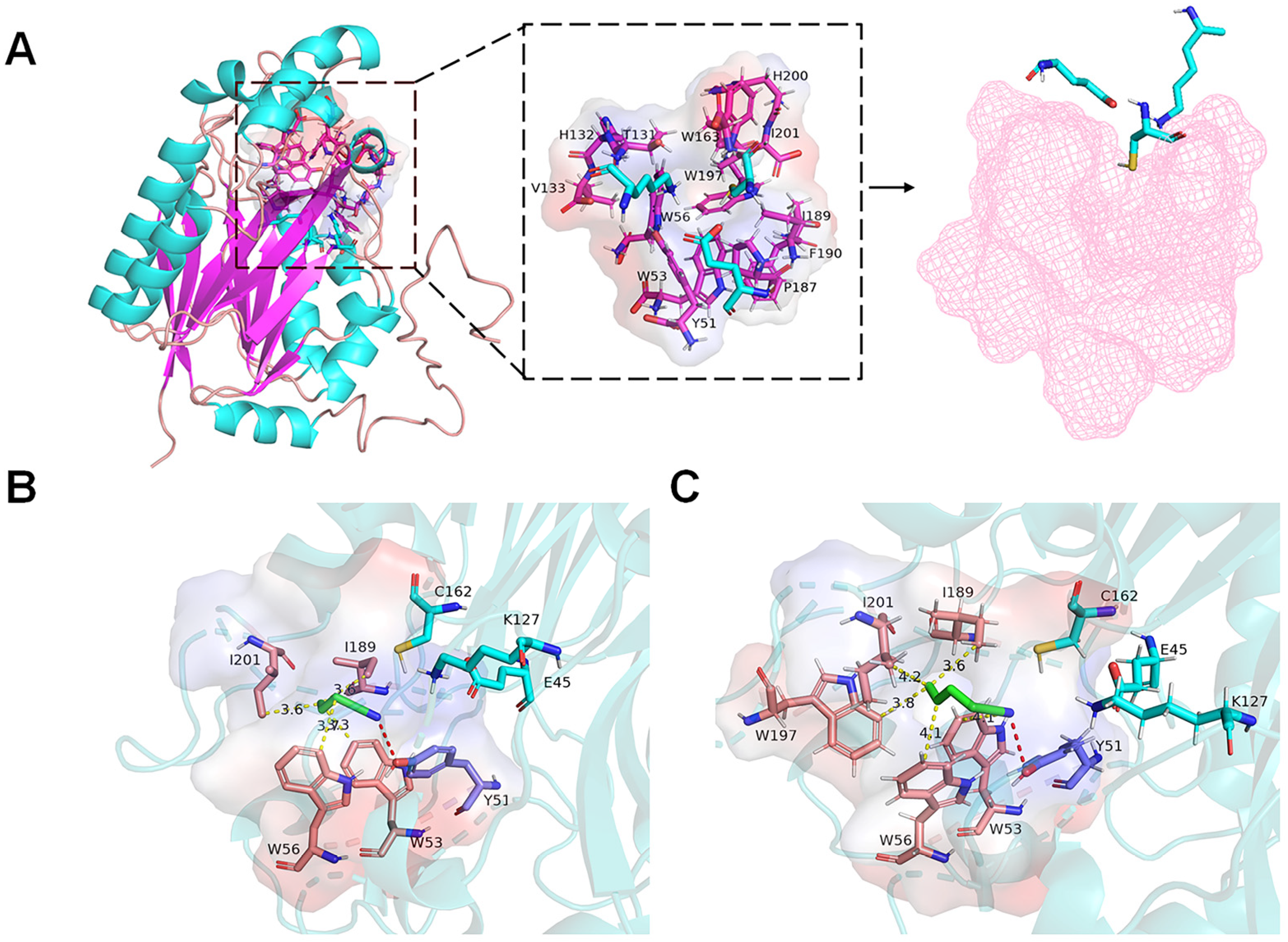
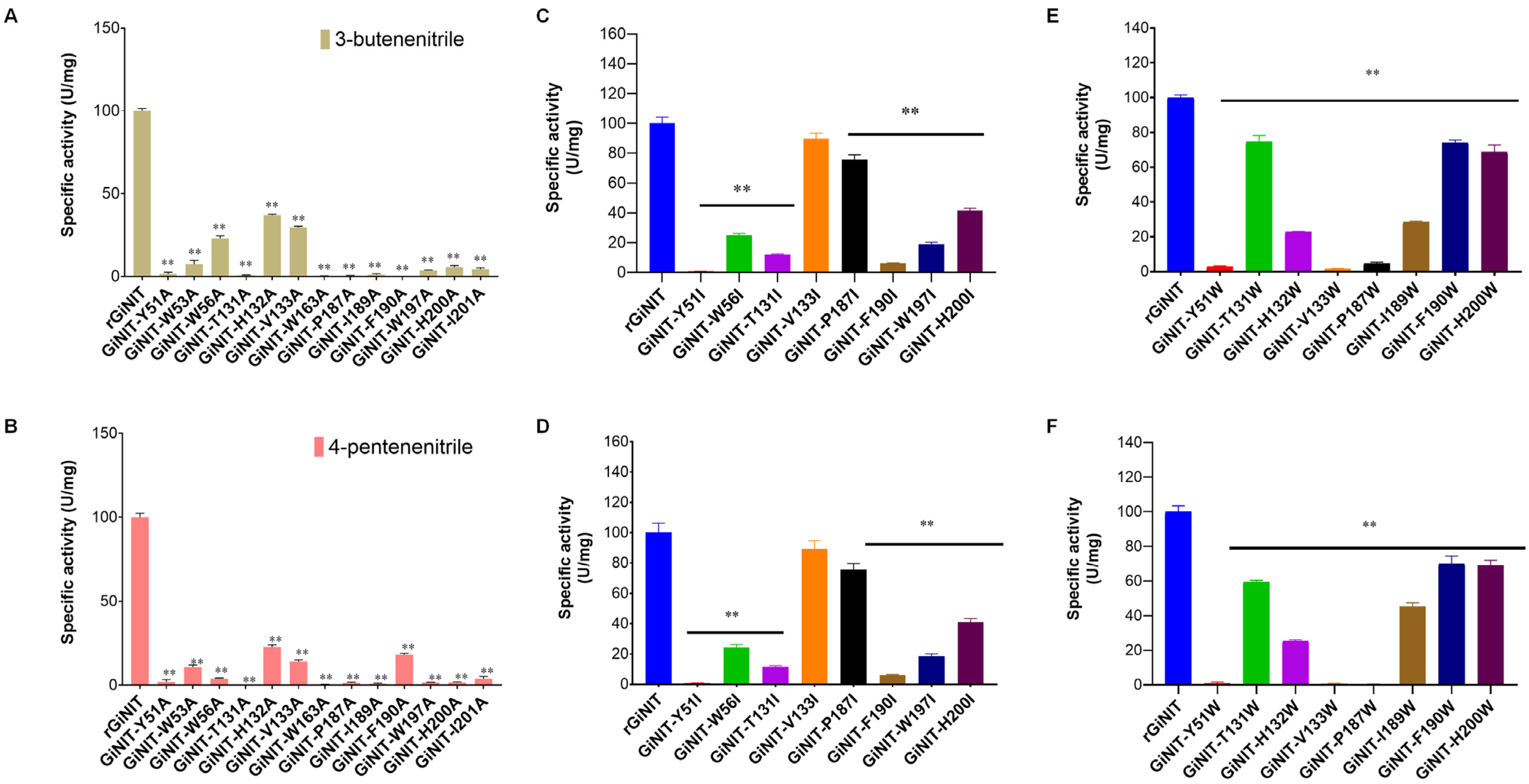
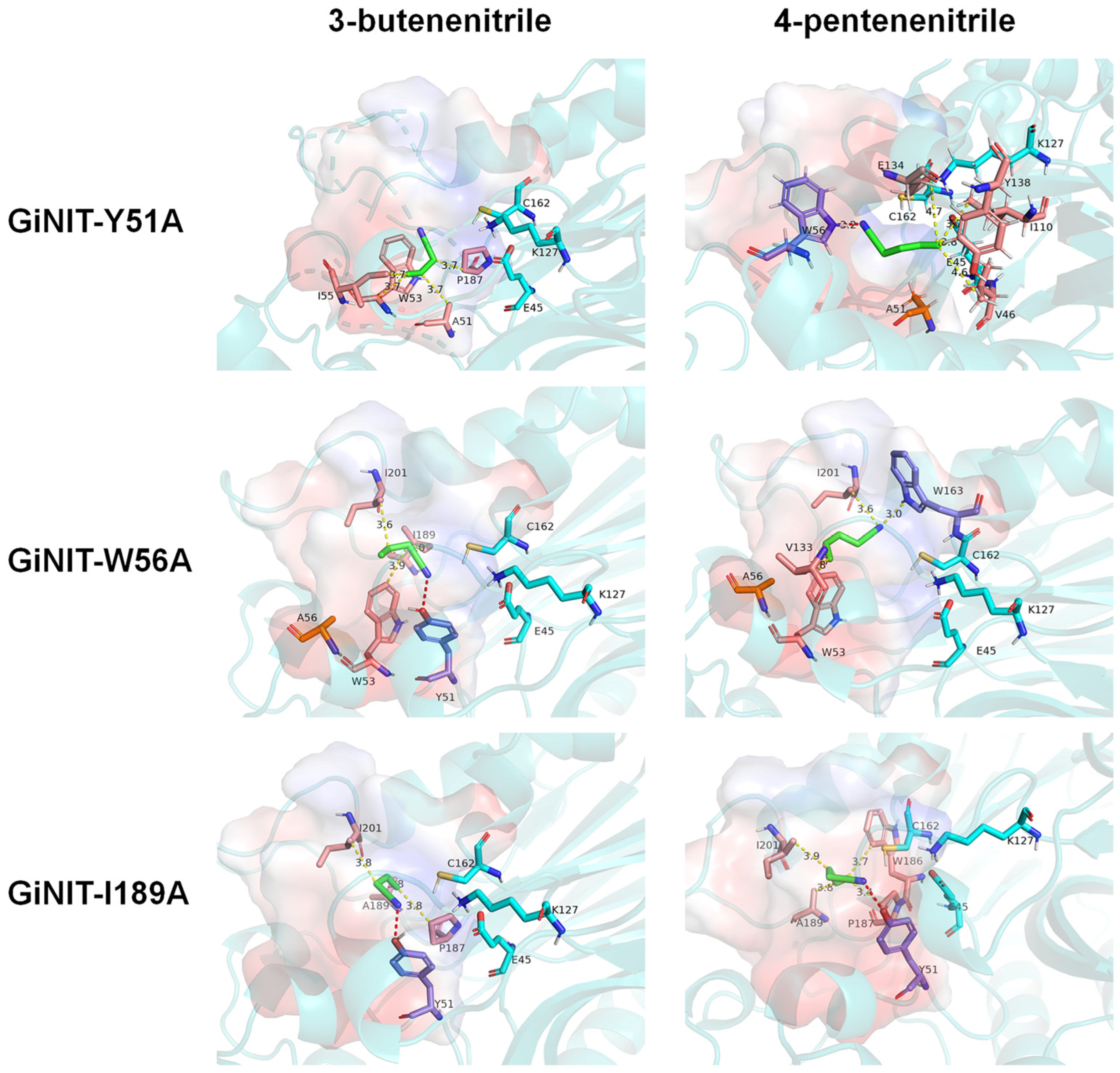
2.4. Molecular Docking Analysis of the rGiNIT with Substrates and Determination of Substrate Binding Pocket
3. Discussion
4. Materials and Methods
4.1. Microorganism Strains and Cultivation
4.2. Expression and Purification of the Recombinant rGiNIT
4.3. Determination of Nitrilase Activity
4.4. Biochemical Characterization of Nitrilase
4.5. Effect of Metal Ions and Organic Reagents
4.6. Bioinformatics Analysis of rGiNIT
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, J.; Li, F.; Na, R.; Bai, X.; Ma, Y.; Yang, Y.; Ma, Y.; Wang, X. The effect of replacing whole-plant corn silage with daylily on the growth performance, slaughtering performance, muscle amino acid composition, and blood composition of tan sheep. Animals 2023, 13, 3493. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Fang, X.; Feng, Z.; Chen, M.; Qiu, X.; Sun, J.; Wu, M.; He, J. Protein from rapeseed for food applications: Extraction, sensory quality, functional and nutritional properties. Food Chem. 2024, 439, 138109. [Google Scholar] [CrossRef] [PubMed]
- Tie, Y.; Li, L.; Liu, J.; Liu, C.; Fu, J.; Xiao, X.; Wang, G.; Wang, J. Two-step biological approach for treatment of rapeseed meal. J. Food Sci. 2020, 85, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.P.; Xue, Y.P.; Zheng, Y.G. Maximizing the potential of nitrilase: Unveiling their diversity, catalytic proficiency, and versatile applications. Biotechnol. Adv. 2024, 72, 108352. [Google Scholar] [CrossRef]
- Xia, Y.; Zhao, J.; Saeed, M.; Hussain, N.; Chen, X.; Guo, Z.; Yong, Y.; Chen, H. Molecular modification strategies of nitrilase for its potential application in agriculture. J. Agric. Food Chem. 2024, 72, 15106–15121. [Google Scholar] [CrossRef]
- Sharma, M.; Akhter, Y.; Chatterjee, S. A review on remediation of cyanide containing industrial wastes using biological systems with special reference to enzymatic degradation. World J. Microbiol. Biotechnol. 2019, 35, 70. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Ge, Y.; Meng, Q.; Zhang, Y. Constitutive secretory expression and characterization of nitrilase from Alcaligenes faecalis in Pichia pastoris for production of R-mandelic acid. Biotechnol. Appl. Biochem. 2022, 69, 587–595. [Google Scholar] [CrossRef]
- Martínková, L.; Rucká, L.; Nešvera, J.; Pátek, M. Recent advances and challenges in the heterologous production of microbial nitrilases for biocatalytic applications. World J. Microbiol. Biotechnol. 2017, 33, 8. [Google Scholar] [CrossRef]
- Khatik, A.G.; Jain, A.K.; Muley, A.B. Preparation, characterization and stability of cross linked nitrilase aggregates (nitrilase-CLEAs) for hydroxylation of 2-chloroisonicotinonitrile to 2-chloroisonicotinic acid. Bioprocess Biosyst. Eng. 2022, 45, 1559–1579. [Google Scholar] [CrossRef]
- Tang, X.L.; Li, Y.Y.; Mao, Y.; Zheng, R.C.; Zheng, Y.G. Improvement of multicatalytic properties of nitrilase from Paraburkholderia graminis for efficient biosynthesis of 2-chloronicotinic acid. Biotechnol. Bioeng. 2022, 119, 3421–3431. [Google Scholar] [CrossRef]
- Jin, Y.Z.; Shen, J.D.; Wang, Y.X.; Shao, H.M.; Xia, H.J.; Lin, J.H.; Liu, Z.Q.; Zheng, Y.G. Modification of nitrilase based on computer screening and efficient biosynthesis of 4-cyanobenzoic acid. Biotechnol. J. 2024, 19, e2300706. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, H.; Qin, X.; Wang, X.; Wang, Y.; Tu, T.; Wang, Y.; Yao, B.; Huang, H.; Luo, H. Biodegradation of nitriles derived from glucosinolates in rapeseed meal by BnNIT2: A nitrilase from Brassica napus with wide substrate specificity. Appl. Microbiol. Biotechnol. 2022, 106, 2445–2454. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Yanaka, N.; Nagasawa, T.; Yamada, H. Primary structure of an aliphatic nitrile-degrading enzyme, aliphatic nitrilase, from Rhodococcus rhodochrous K22 and expression of its gene and identification of its active site residue. Biochemistry 1992, 31, 9000–9007. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, G.; Li, M.; Wei, D.; Wang, X. A novel nitrilase from Rhodobacter sphaeroides LHS-305: Cloning, heterologous expression and biochemical characterization. World J. Microbiol. Biotechnol. 2014, 30, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.S. Cloning, Identification and Molecular Modification of Gibberella intermedia Nitrilase. Ph.D. Thesis, Jiangnan University, Wuxi, China, 2013. [Google Scholar]
- Gong, J.S.; Li, H.; Zhu, X.Y.; Lu, Z.M.; Wu, Y.; Shi, J.S.; Xu, Z.H. Fungal His-tagged nitrilase from Gibberella intermedia: Gene cloning, heterologous expression and biochemical properties. PLoS ONE 2012, 7, e50622. [Google Scholar] [CrossRef]
- Zhu, D.; Mukherjee, C.; Yang, Y.; Rios, B.E.; Gallagher, D.T.; Smith, N.N.; Biehl, E.R.; Hua, L. A new nitrilase from Bradyrhizobium japonicum USDA 110. Gene cloning, biochemical characterization and substrate specificity. J. Biotechnol. 2008, 133, 327–333. [Google Scholar] [CrossRef]
- Wu, Y.; Gong, J.S.; Lu, Z.M.; Li, H.; Zhu, X.Y.; Li, H.; Shi, J.S.; Xu, Z.H. Isolation and characterization of Gibberella intermedia CA3-1, a novel and versatile nitrilase-producing fungus. J. Basic Microbiol. 2013, 53, 934–941. [Google Scholar] [CrossRef]
- Wang, Z.K.; Gong, J.S.; Feng, D.T.; Su, C.; Li, H.; Rao, Z.M.; Lu, Z.M.; Shi, J.S.; Xu, Z.H. Geometric remodeling of nitrilase active pocket based on ALF-scanning strategy to enhance aromatic nitrile substrate preference and catalytic efficiency. Appl. Environ. Microbiol. 2023, 89, e0022023. [Google Scholar] [CrossRef]
- Jhingan, S.; Harloff, H.J.; Abbadi, A.; Welsch, C.; Blümel, M.; Tasdemir, D.; Jung, C. Reduced glucosinolate content in oilseed rape (Brassica napus L.) by random mutagenesis of BnMYB28 and BnCYP79F1 genes. Sci. Rep. 2023, 13, 2344. [Google Scholar] [CrossRef]
- Aguirre-Sampieri, S.; Casañal, A.; Emsley, P.; Garza-Ramos, G. Cryo-EM structure of bacterial nitrilase reveals insight into oligomerization, substrate recognition, and catalysis. J. Struct. Biol. 2024, 216, 108093. [Google Scholar] [CrossRef]
- Gerdes, S.Y.; Kurnasov, O.V.; Shatalin, K.; Polanuyer, B.; Sloutsky, R.; Vonstein, V.; Overbeek, R.; Osterman, A.L. Comparative genomics of NAD biosynthesis in cyanobacteria. J. Bacteriol. 2006, 188, 3012–3023. [Google Scholar] [CrossRef] [PubMed]
- Ungerfeld, E.M.; Rust, S.R.; Burnett, R. Use of some novel alternative electron sinks to inhibit ruminal methanogenesis. Reprod. Nutr. Dev. 2003, 43, 189–202. [Google Scholar] [CrossRef]
- Api, A.M.; Belsito, D.; Botelho, D.; Bruze, M.; Burton, G.A., Jr.; Buschmann, J.; Dagli, M.L.; Date, M.; Dekant, W.; Deodhar, C.; et al. RIFM fragrance ingredient safety assessment, 4-pentenoic acid, 2-acetyl-4-methyl-, ethyl ester, CAS Registry Number 20962-70-3. Food Chem. Toxicol. 2018, 122, S123–S128. [Google Scholar] [CrossRef]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Stecher, G.; Kumar, S. MEGA11: Molecular evolutionary genetics analysis version 11. Mol. Biol. Evol. 2021, 38, 3022–3027. [Google Scholar] [CrossRef]

| Metal Ions (5 mM) | Relative Activity with 3-Butenenitrile as Substrate (%) | Relative Activity with 4-Pentenenitrile as Substrate (%) |
|---|---|---|
| Control | 100 ± 0.63 | 100 ± 0.53 |
| Ag+ | 2.30 ± 0.10 | 4.20 ± 0.68 |
| Fe3+ | 57.98 ± 0.39 | 54.18 ± 0.98 |
| Fe2+ | 50.68 ± 1.72 | 50.98 ± 1.67 |
| Mg2+ | 102.51 ± 1.72 | 103.14 ± 0.38 |
| Ca2+ | 88.29 ± 1.31 | 79.16 ± 0.07 |
| Zn2+ | 71.35 ± 1.56 | 76.73 ± 1.69 |
| Mn2+ | 88.57 ± 2.51 | 91.13 ± 0.61 |
| Cu2+ | 11.28 ± 0.68 | 8.31 ± 0.83 |
| Co2+ | 91.65 ± 1.28 | 89.45 ± 2.77 |
| K+ | 100.80 ± 0.31 | 96.75 ± 1.27 |
| Organic Reagents | Relative Activity with 3-Butenenitrile as Substrate (%) | Relative Activity with 4-Pentenenitrile as Substrate (%) | |
|---|---|---|---|
| control | 100 ± 0.96 | 100 ± 0.98 | |
| methanol | 5% | 50.05 ± 1.70 | 57.08 ± 0.87 |
| 20% | 3.48 ± 0.06 | 2.71 ± 0.15 | |
| ethanol | 5% | 40.07 ± 3.35 | 55.53 ± 2.01 |
| 20% | 3.20 ± 0.21 | 2.59 ± 0.31 | |
| ether | 5% | 61.63 ± 0.79 | 93.85 ± 1.19 |
| 20% | 62.48 ± 1.19 | 71.85 ± 0.86 | |
| chloroform | 5% | 65.03 ± 3.37 | 88.01 ± 0.72 |
| 20% | 27.59 ± 3.18 | 27.91 ± 0.35 | |
| isopropanol | 5% | 34.46 ± 1.50 | 55.53 ± 0.69 |
| 20% | 3.58 ± 0.26 | 9.74 ± 0.12 | |
| normal butanol | 5% | 7.02 ± 0.12 | 2.59 ± 0.23 |
| 20% | 4.14 ± 0.06 | 2.94 ± 0.32 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.-Z.; Liu, M.-Y.; Wang, Y.-Y.; Luo, X.-M.; Feng, J.-X.; Zhao, S. Nitrilase GiNIT from Gibberella intermedia Efficiently Degrades Nitriles Derived from Rapeseed Meal Glucosinolate. Int. J. Mol. Sci. 2024, 25, 11986. https://doi.org/10.3390/ijms252211986
Li H-Z, Liu M-Y, Wang Y-Y, Luo X-M, Feng J-X, Zhao S. Nitrilase GiNIT from Gibberella intermedia Efficiently Degrades Nitriles Derived from Rapeseed Meal Glucosinolate. International Journal of Molecular Sciences. 2024; 25(22):11986. https://doi.org/10.3390/ijms252211986
Chicago/Turabian StyleLi, Han-Zhi, Ming-Yu Liu, Yu-Yue Wang, Xue-Mei Luo, Jia-Xun Feng, and Shuai Zhao. 2024. "Nitrilase GiNIT from Gibberella intermedia Efficiently Degrades Nitriles Derived from Rapeseed Meal Glucosinolate" International Journal of Molecular Sciences 25, no. 22: 11986. https://doi.org/10.3390/ijms252211986
APA StyleLi, H.-Z., Liu, M.-Y., Wang, Y.-Y., Luo, X.-M., Feng, J.-X., & Zhao, S. (2024). Nitrilase GiNIT from Gibberella intermedia Efficiently Degrades Nitriles Derived from Rapeseed Meal Glucosinolate. International Journal of Molecular Sciences, 25(22), 11986. https://doi.org/10.3390/ijms252211986






