Galectin-1 Induces the Production of Immune-Suppressive Cytokines in Human and Mouse T Cells
Abstract
1. Introduction
2. Results and Discussion
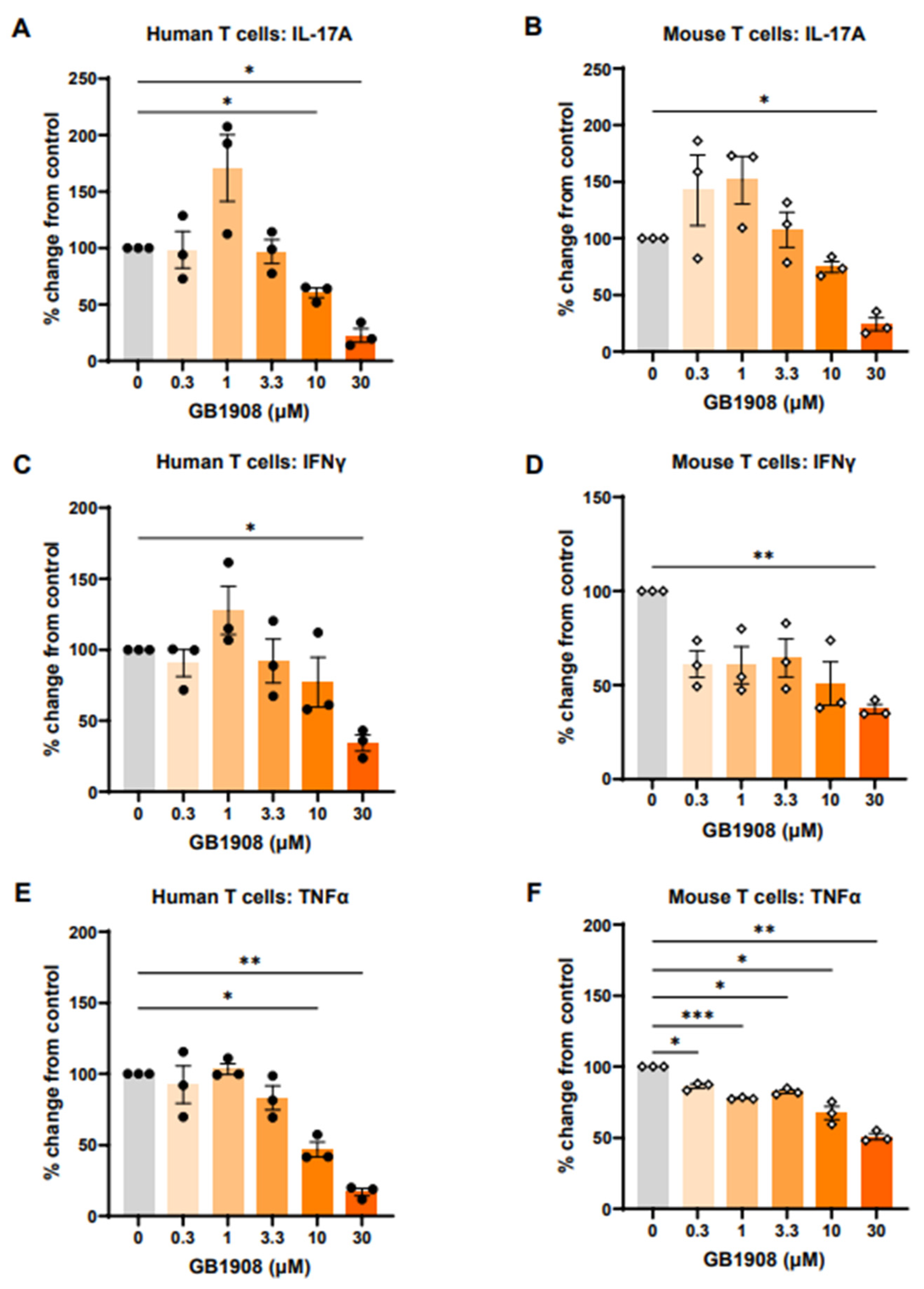
2.1. Galectin-1 Inhibition Reduces Immune-Suppressive Cytokines in Both Mouse and Human Stimulated T Cells
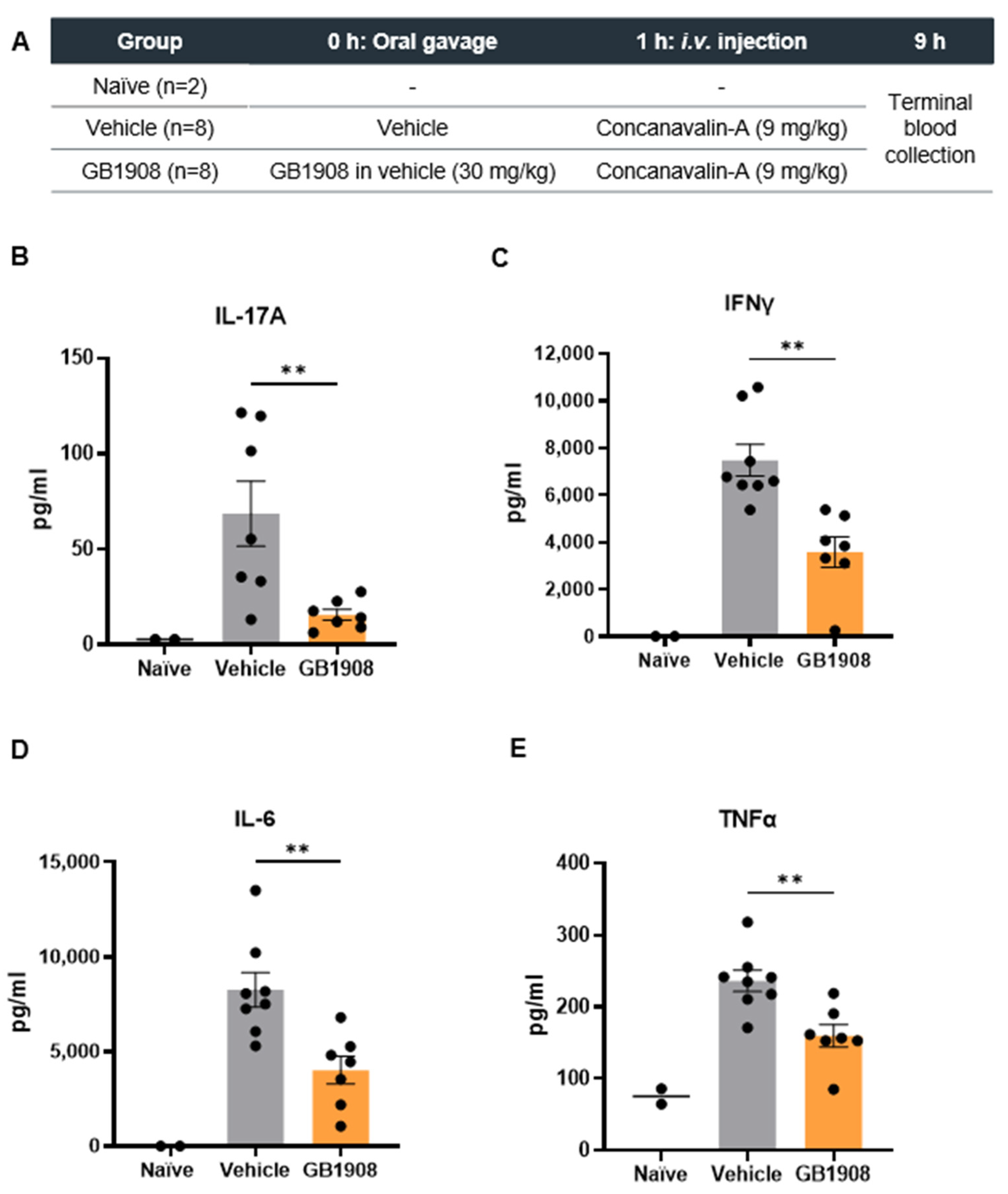
2.2. Galectin-1 Inhibition Reduces Immune-Suppressive Cytokines in an Acute T Cell Driven Inflammatory Mouse Model
3. Materials and Methods
3.1. Human and Mouse T Cell Assays
3.2. Concanavalin-A Mouse Models
3.3. Data Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rabinovich, G.A.; Toscano, M.A. Turning “sweet” on immunity: Galectin–glycan interactions in immune tolerance and inflammation. Nat. Rev. Immunol. 2009, 9, 338–352. [Google Scholar] [CrossRef]
- Liu, F.-T.; Stowell, S.R. The role of galectins in immunity and infection. Nat. Rev. Immunol. 2023, 23, 479–494. [Google Scholar] [CrossRef]
- Cousin, J.M.; Cloninger, M.J. The Role of Galectin-1 in Cancer Progression, and Synthetic Multivalent Systems for the Study of Galectin-. Int. J. Mol. Sci. 2016, 17, 1566. [Google Scholar] [CrossRef]
- Rabinovich, G.A.; Ariel, A.; Hershkoviz, R.; Hirabayashi, K.; Kasai, K.-I.; Lider, O. Specific inhibition of T-cell adhesion to extracellular matrix and proinflammatory cytokine secretion by human recombinant galectin-1. Immunology 1999, 97, 100–106. [Google Scholar] [CrossRef]
- Liu, S.D.; Tomassian, T.; Bruhn, K.W.; Miller, J.F.; Poirier, F.; Miceli, M.C. Galectin-1 Tunes TCR Binding and Signal Transduction to Regulate CD8 Burst Size. J. Immunol. 2009, 182, 5283–5295. [Google Scholar] [CrossRef]
- Cedeno-Laurent, F.; Watanabe, R.; Teague, J.E.; Kupper, T.S.; Clark, R.A.; Dimitroff, C.J. Galectin-1 inhibits the viability, proliferation, and Th1 cytokine production of nonmalignant T cells in patients with leukemic cutaneous T-cell lymphoma. Blood 2012, 119, 3534–3538. [Google Scholar] [CrossRef]
- Cedeno-Laurent, F.; Opperman, M.; Barthel, S.R.; Kuchroo, V.K.; Dimitroff, C.J. Galectin-1 triggers an immunoregulatory signature in Th cells functionally defined by IL-10 expression. J. Immunol. 2012, 188, 3127–3137. [Google Scholar] [CrossRef]
- Zetterberg, F.R.; Peterson, K.; Nilsson, U.J.; Andréasson Dahlgren, K.; Diehl, C.; Holyer, I.; Håkansson, M.; Khabut, A.; Kahl-Knutson, B.; Leffler, H.; et al. Discovery of the Selective and Orally Available Galectin-1 Inhibitor GB1908 as a Potential Treatment for Lung Cancer. J. Med. Chem. 2024, 67, 9374–9388. [Google Scholar] [CrossRef]
- Heymann, F.; Hamesch, K.; Weiskirchen, R.; Tacke, F. The concanavalin A model of acute hepatitis in mice. Lab. Anim. 2015, 49, 12–20. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, X.; Herjan, T.; Li, X. The role of interleukin-17 in tumor development and progression. J. Exp. Med. 2019, 217, e20190297. [Google Scholar] [CrossRef]
- Cui, G.; Li, Z.; Florholmen, J.; Goll, R. Dynamic stromal cellular reaction throughout human colorectal adenoma-carcinoma sequence: A role of TH17/IL-17A. Biomed. Pharmacother. 2021, 140, 111761. [Google Scholar] [CrossRef] [PubMed]
- Liao, H.; Chang, X.; Gao, L.; Ye, C.; Qiao, Y.; Xie, L.; Lin, J.; Cai, S.; Dong, H. IL-17A promotes tumorigenesis and upregulates PD-L1 expression in non-small cell lung cancer. J. Transl. Med. 2023, 21, 828. [Google Scholar] [CrossRef]
- Liu, C.; Liu, R.; Wang, B.; Lian, J.; Yao, Y.; Sun, H.; Zhang, C.; Fang, L.; Guan, X.; Shi, J.; et al. Blocking IL-17A enhances tumor response to anti-PD-1 immunotherapy in microsatellite stable colorectal cancer. J. Immunother. Cancer 2021, 9, e001895. [Google Scholar] [CrossRef]
- Vuckovic, S.; Minnie, S.A.; Smith, D.; Gartlan, K.H.; Watkins, T.S.; Markey, K.A.; Mukhopadhyay, P.; Guillerey, C.; Kuns, R.D.; Locke, K.R.; et al. Bone marrow transplantation generates T cell–dependent control of myeloma in mice. J. Clin. Investig. 2019, 129, 106–121. [Google Scholar] [CrossRef]
- Coffelt, S.B.; Kersten, K.; Doornebal, C.W.; Weiden, J.; Vrijland, K.; Hau, C.-S.; Verstegen, N.J.M.; Ciampricotti, M.; Hawinkels, L.J.A.C.; Jonkers, J.; et al. IL-17-producing γδ T cells and neutrophils conspire to promote breast cancer metastasis. Nature 2015, 522, 345–348. [Google Scholar] [CrossRef]
- Johnson, D.E.; O’Keefe, R.A.; Grandis, J.R. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat. Rev. Clin. Oncol. 2018, 15, 234–248. [Google Scholar] [CrossRef]
- Huseni, M.A.; Wang, L.; Klementowicz, J.E.; Yuen, K.; Breart, B.; Orr, C.; Liu, L.; Li, Y.; Gupta, V.; Li, C.; et al. CD8+ T cell-intrinsic IL-6 signaling promotes resistance to anti-PD-L1 immunotherapy. Cell Rep. Med. 2023, 4, 100878. [Google Scholar] [CrossRef]
- Hailemichael, Y.; Johnson, D.H.; Abdel-Wahab, N.; Foo, W.C.; Bentebibel, S.-E.; Daher, M.; Haymaker, C.; Wani, K.; Saberian, C.; Ogata, D.; et al. Interleukin-6 blockade abrogates immunotherapy toxicity and promotes tumor immunity. Cancer Cell 2022, 40, 509–523.e6. [Google Scholar] [CrossRef]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in tumor progression and regression: A review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Gocher, A.M.; Workman, C.J.; Vignali, D.A.A. Interferon-γ: Teammate or opponent in the tumour microenvironment? Nat. Rev. Immunol. 2022, 22, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Spranger, S.; Spaapen, R.M.; Zha, Y.; Williams, J.; Meng, Y.; Ha, T.T.; Gajewski, T.F. Up-Regulation of PD-L1, IDO, and Tregs in the Melanoma Tumor Microenvironment Is Driven by CD8+ T Cells. Sci. Transl. Med. 2013, 5, 200ra116. [Google Scholar] [CrossRef] [PubMed]
- Ben-Baruch, A. The Tumor-Promoting Flow of Cells Into, Within and Out of the Tumor Site: Regulation by the Inflammatory Axis of TNFα and Chemokines. Cancer Microenviron. 2012, 5, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Monfort, A.; Colacios, C.; Levade, T.; Andrieu-Abadie, N.; Meyer, N.; Ségui, B. The TNF Paradox in Cancer Progression and Immunotherapy. Front. Immunol. 2019, 10, 1818. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herman, K.D.; Holyer, I.; Humphries, D.C.; Adamska, A.; Roper, J.A.; Peterson, K.; Zetterberg, F.R.; Pedersen, A.; MacKinnon, A.C.; Slack, R.J. Galectin-1 Induces the Production of Immune-Suppressive Cytokines in Human and Mouse T Cells. Int. J. Mol. Sci. 2024, 25, 11948. https://doi.org/10.3390/ijms252211948
Herman KD, Holyer I, Humphries DC, Adamska A, Roper JA, Peterson K, Zetterberg FR, Pedersen A, MacKinnon AC, Slack RJ. Galectin-1 Induces the Production of Immune-Suppressive Cytokines in Human and Mouse T Cells. International Journal of Molecular Sciences. 2024; 25(22):11948. https://doi.org/10.3390/ijms252211948
Chicago/Turabian StyleHerman, Kimberly D., Ian Holyer, Duncan C. Humphries, Anna Adamska, James A. Roper, Kristoffer Peterson, Fredrik R. Zetterberg, Anders Pedersen, Alison C. MacKinnon, and Robert J. Slack. 2024. "Galectin-1 Induces the Production of Immune-Suppressive Cytokines in Human and Mouse T Cells" International Journal of Molecular Sciences 25, no. 22: 11948. https://doi.org/10.3390/ijms252211948
APA StyleHerman, K. D., Holyer, I., Humphries, D. C., Adamska, A., Roper, J. A., Peterson, K., Zetterberg, F. R., Pedersen, A., MacKinnon, A. C., & Slack, R. J. (2024). Galectin-1 Induces the Production of Immune-Suppressive Cytokines in Human and Mouse T Cells. International Journal of Molecular Sciences, 25(22), 11948. https://doi.org/10.3390/ijms252211948





