Metabolic, Inflammatory, and Molecular Impact of Cancer Cachexia on the Liver
Abstract
1. Introduction
2. Literature Search Strategy
3. Inflammatory Aspects of Cachexia
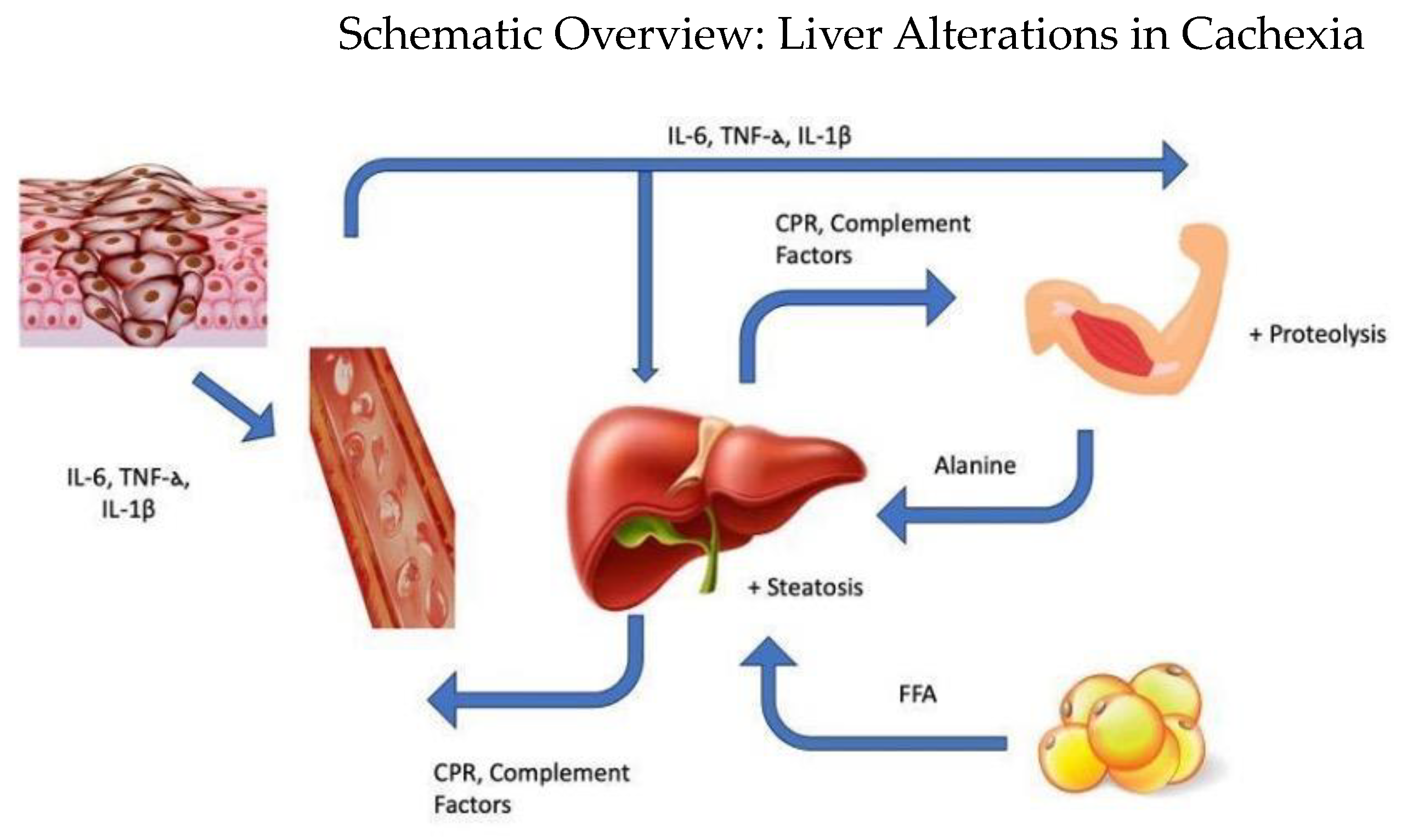
4. Hepatic Metabolic Alterations in Cachexia
5. Hepatic Alterations in Protein Metabolism
6. Hepatic Alterations in Lipid Metabolism
7. Pathophysiology of Cancer-Associated Cachexia, Inflammation, and Drug Resistance
8. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Baracos, V.E.; Martin, L.; Korc, M.; Guttridge, D.C.; Fearon, K.C.H. Cancer-associated cachexia. Nat. Rev. Dis. Primers 2018, 4, 17105. [Google Scholar] [CrossRef] [PubMed]
- Bing, C. Lipid mobilization in cachexia: Mechanisms and mediators. Curr. Opin. Support. Palliat. Care 2011, 5, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Argilés, J.M.; López-Soriano, F.J.; Stemmler, B.; Busquets, S. Cancer-associated cachexia—Understanding the tumour macroenvironment and microenvironment to improve management. Nat. Rev. Clin. Oncol. 2023, 20, 250–264. [Google Scholar] [CrossRef] [PubMed]
- Yule, M.S.; Thompson, J.; Leesahatsawat, K.; Sousa, M.S.; Anker, S.D.; Arends, J.; Balstad, T.R.; Brown, L.R.; Bye, A.; Dajani, O.; et al. Cancer Cachexia Endpoints Working Group. Biomarker endpoints in cancer cachexia clinical trials: Systematic Review 5 of the cachexia endpoint series. J. Cachexia Sarcopenia Muscle 2024, 15, 853–867. [Google Scholar] [CrossRef] [PubMed]
- Roeland, E.J.; Bohlke, K.; Baracos, V.E.; Bruera, E.; Del Fabbro, E.; Dixon, S.; Fallon, M.; Herrstedt, J.; Lau, H.; Platek, M.; et al. Management of Cancer Cachexia: ASCO Guideline. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2020, 38, 2438–2453. [Google Scholar] [CrossRef]
- Baba, M.R.; Buch, S.A. Revisiting Cancer Cachexia: Pathogenesis, Diagnosis, and Current Treatment Approaches. Asia-Pac. J. Oncol. Nurs. 2021, 8, 508–518. [Google Scholar] [CrossRef]
- Sato, R.; Vatic, M.; Peixoto da Fonseca, G.W.; Anker, S.D.; von Haehling, S. Biological basis and treatment of frailty and sarcopenia. Cardiovasc. Res. 2024, 120, 982–998. [Google Scholar] [CrossRef]
- Fearon, K.C.; Glass, D.J.; Guttridge, D.C. Cancer cachexia: Mediators, signaling, and metabolic pathways. Cell Metab. 2012, 16, 153–166. [Google Scholar] [CrossRef]
- Tisdale, M.J. Mechanisms of cancer cachexia. Physiol. Rev. 2009, 89, 381–410. [Google Scholar] [CrossRef]
- Siddiqui, J.A.; Pothuraju, R.; Jain, M.; Batra, S.K.; Nasser, M.W. Advances in cancer cachexia: Intersection between affected organs, mediators, and pharmacological interventions. Biochim. Biophys. Acta. Rev. Cancer 2020, 1873, 188359. [Google Scholar] [CrossRef]
- Fonseca, G.W.P.D.; Farkas, J.; Dora, E.; von Haehling, S.; Lainscak, M. Cancer Cachexia and Related Metabolic Dysfunction. Int. J. Mol. Sci. 2020, 21, 2321. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, T.; Sari, I.N.; Wijaya, Y.T.; Julianto, N.M.; Muhammad, J.A.; Lee, H.; Chae, J.H.; Kwon, H.Y. Cancer cachexia: Molecular mechanisms and treatment strategies. J. Hematol. Oncol. 2023, 16, 54. [Google Scholar] [CrossRef] [PubMed]
- Kalantar-Zadeh, K.; Rhee, C.; Sim, J.J.; Stenvinkel, P.; Anker, S.D.; Kovesdy, C.P. Why cachexia kills: Examining the causality of poor outcomes in wasting conditions. J. Cachexia Sarcopenia Muscle 2013, 4, 89–94. [Google Scholar] [CrossRef] [PubMed]
- de Matos-Neto, E.M.; Lima, J.D.; de Pereira, W.O.; Figuerêdo, R.G.; Riccardi, D.M.; Radloff, K.; das Neves, R.X.; Camargo, R.G.; Maximiano, L.F.; Tokeshi, F.; et al. Systemic Inflammation in Cachexia—Is Tumor Cytokine Expression Profile the Culprit? Front. Immunol. 2015, 6, 629. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J.; Morley, J.E.; Argilés, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A new definition. Clin. Nutr. 2008, 27, 793–799. [Google Scholar] [CrossRef]
- Wang, Y.F.; An, Z.Y.; Lin, D.H.; Jin, W.L. Targeting cancer cachexia: Molecular mechanisms and clinical study. MedComm 2022, 3, e164. [Google Scholar] [CrossRef]
- Rohm, M.; Zeigerer, A.; Machado, J.; Herzig, S. Energy metabolism in cachexia. EMBO Rep. 2019, 20, e47258. [Google Scholar] [CrossRef]
- Schmitt, T.L.; Martignoni, M.E.; Bachmann, J.; Fechtner, K.; Friess, H.; Kinscherf, R.; Hildebrandt, W. Activity of the Akt-dependent anabolic and catabolic pathways in muscle and liver samples in cancer-related cachexia. J. Mol. Med. 2007, 85, 647–654. [Google Scholar] [CrossRef]
- Argilés, J.M.; Stemmler, B.; López-Soriano, F.J.; Busquets, S. Nonmuscle Tissues Contribution to Cancer Cachexia. Mediat. Inflamm. 2015, 2015, 182872. [Google Scholar] [CrossRef]
- Prokopchuk, O.; Steinacker, J.M.; Nitsche, U.; Otto, S.; Bachmann, J.; Schubert, E.C.; Friess, H.; Martignoni, M.E. IL-4 mRNA Is Downregulated in the Liver of Pancreatic Cancer Patients Suffering from Cachexia. Nutr. Cancer 2017, 69, 84–91. [Google Scholar] [CrossRef]
- Tisdale, M.J. Cachexia in cancer patients. Nat. Rev. Cancer 2002, 2, 862–871. [Google Scholar] [CrossRef] [PubMed]
- Dallavalasa, S.; Beeraka, N.M.; Basavaraju, C.G.; Tulimilli, S.V.; Sadhu, S.P.; Rajesh, K.; Aliev, G.; Madhunapantula, S.V. The Role of Tumor Associated Macrophages (TAMs) in Cancer Progression, Chemoresistance, Angiogenesis and Metastasis—Current Status. Curr. Med. Chem. 2021, 28, 8203–8236. [Google Scholar] [CrossRef] [PubMed]
- Richlitzki, C.; Wiesweg, M.; Metzenmacher, M.; Guberina, N.; Pöttgen, C.; Hautzel, H.; Eberhardt, W.E.E.; Darwiche, K.; Theegarten, D.; Aigner, C.; et al. C-reactive protein as robust laboratory value associated with prognosis in patients with stage III non-small cell lung cancer (NSCLC) treated with definitive radiochemotherapy. Sci. Rep. 2024, 14, 13765. [Google Scholar] [CrossRef] [PubMed]
- Bennani-Baiti, N.; Walsh, D. Animal models of the cancer anorexia-cachexia syndrome. Support. Care Cancer Off. J. Multinatl. Assoc. Support. Care Cancer 2011, 19, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Lira, F.S.; Yamashita, A.; Carnevali, L.C., Jr.; Gonçalves, D.C.; Lima, W.P.; Rosa, J.C.; Caperuto, E.C.; Rosa, L.F.; Seelaender, M. Exercise training reduces PGE2 levels and induces recovery from steatosis in tumor-bearing rats. Horm. Metab. Res. 2010, 42, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Miyaguti, N.A.D.S.; Chiocchetti, G.M.E.; Salgado, C.M.; Lopes-Aguiar, L.; Viana, L.R.; Blanchard, L.; Santos, R.W.D.; Gomes-Marcondes, M.C.C. Walker-256 Tumour-Induced Cachexia Altered Liver Metabolomic Profile and Function in Weanling and Adult Rats. Metabolites 2021, 11, 831. [Google Scholar] [CrossRef]
- Seelaender, M.C.; Curi, R.; Colquhoun, A.; Williams, J.F.; Zammitt, V.A. Carnitine palmitoyltransferase II activity is decreased in liver mitochondria of cachectic rats bearing the Walker 256 carcinosarcoma: Effect of indomethacin treatment. Biochem. Mol. Biol. Int. 1998, 44, 185–193. [Google Scholar] [CrossRef]
- Wyart, E.; Bindels, L.B.; Mina, E.; Menga, A.; Stanga, S.; Porporato, P.E. Cachexia, a Systemic Disease beyond Muscle Atrophy. Int. J. Mol. Sci. 2020, 21, 8592. [Google Scholar] [CrossRef]
- Williams, J.A.; Shacter, E. Regulation of macrophage cytokine production by prostaglandin E2. Distinct roles of cyclooxygenase-1 and -2. J. Biol. Chem. 1997, 272, 25693–25699. [Google Scholar] [CrossRef]
- Tustumi, F.; Xavier das Neves, R.; Pereira, M.A.; Coelho, F.F.; Andraus, W. Editorial: Liver cancer awareness month 2023: Current progress and future prospects on advances in primary liver cancer investigation and treatment. Front. Oncol. 2024, 14, 1453709. [Google Scholar] [CrossRef]
- das Neves, R.X.; Yamashita, A.S.; Riccardi, D.M.R.; Köhn-Gaone, J.; Camargo, R.G.; Neto, N.I.; Caetano, D.; Gomes, S.P.; Santos, F.H.; Lima, J.D.C.C.; et al. Cachexia causes time-dependent activation of the inflammasome in the liver. J. Cachexia Sarcopenia Muscle 2023, 14, 1621–1630. [Google Scholar] [CrossRef] [PubMed]
- Liang, K. Mitochondrial CPT1A: Insights into structure, function, and basis for drug development. Front. Pharmacol. 2023, 14, 1160440. [Google Scholar] [CrossRef] [PubMed]
- Silvério, R.; Laviano, A.; Rossi Fanelli, F.; Seelaender, M. L-Carnitine induces recovery of liver lipid metabolism in cancer cachexia. Amino Acids 2012, 42, 1783–1792. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, D.C.; Lira, F.S.; Yamashita, A.S.; Carnevali Junior, L.C.; Eder, R.; Laviano, A.; Seelaender MC, L. Liver lipid metabolism disruption in cancer cachexia is aggravated by cla supplementation -induced inflammation. Clin. Nutr. 2019, 38, 2219–2230. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.C.; Moses, A.G. Cancer cachexia. Int. J. Cardiol. 2002, 85, 73–81. [Google Scholar] [CrossRef]
- Tacke, F.; Luedde, T.; Trautwein, C. Inflammatory pathways in liver homeostasis and liver injury. Clin. Rev. Allergy Immunol. 2009, 36, 4–12. [Google Scholar] [CrossRef]
- Gupta, S.C.; Kim, J.H.; Kannappan, R.; Reuter, S.; Dougherty, P.M.; Aggarwal, B.B. Role of nuclear factor κB-mediated inflammatory pathways in cancer-related symptoms and their regulation by nutritional agents. Exp. Biol. Med. 2011, 236, 658–671. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Du, X.; Ma, H.; Yao, J. The Anti-Cancer Mechanisms of Berberine: A Review. Cancer Manag. Res. 2020, 12, 695–702. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Argilés, J.M.; Busquets, S.; Toledo, M.; López-Soriano, F.J. The role of cytokines in cancer cachexia. Curr. Opin. Support. Palliat. Care 2009, 3, 263–268. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, Q.; Wang, J.; Harnish, D.C. Suppression of interleukin-6-induced C-reactive protein expression by FXR agonists. Biochem. Biophys. Res. Commun. 2009, 379, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Argilés, J.M.; Alvarez, B.; López-Soriano, F.J. The metabolic basis of cancer cachexia. Med. Res. Rev. 1997, 17, 477–498. [Google Scholar] [CrossRef]
- Inui, A. Cancer anorexia-cachexia syndrome: Current issues in research and management. CA A Cancer J. Clin. 2002, 52, 72–91. [Google Scholar] [CrossRef] [PubMed]
- Agustsson, T.; Ryden, M.; Hoffstedt, J.; van Harmelen, V.; Dicker, A.; Laurencikiene, J.; Isaksson, B.; Permert, J.; Arner, P. Mechanism of increased lipolysis in cancer cachexia. Cancer Res. 2007, 67, 5531–5537. [Google Scholar] [CrossRef] [PubMed]
- Bing, C.; Russell, S.; Becket, E.; Pope, M.; Tisdale, M.J.; Trayhurn, P.; Jenkins, J.R. Adipose atrophy in cancer cachexia: Morphologic and molecular analysis of adipose tissue in tumour-bearing mice. Br. J. Cancer 2006, 95, 1028–1037. [Google Scholar] [CrossRef] [PubMed]
- Bing, C.; Brown, M.; King, P.; Collins, P.; Tisdale, M.J.; Williams, G. Increased gene expression of brown fat uncoupling protein (UCP)1 and skeletal muscle UCP2 and UCP3 in MAC16-induced cancer cachexia. Cancer Res. 2000, 60, 2405–2410. [Google Scholar]
- Trayhurn, P.; Bing, C. Appetite and energy balance signals from adipocytes. Philos. Trans. R. Soc. Lond. Ser. B Biol. Sci. 2006, 361, 1237–1249. [Google Scholar] [CrossRef]
- Ryden, M.; Agustsson, T.; Laurencikiene, J.; Britton, T.; Sjolin, E.; Isaksson, B.; Permert, J.; Arner, P. Lipolysis–not inflammation, cell death, or lipogenesis–is involved in adipose tissue loss in cancer cachexia. Cancer 2008, 113, 1695–1704. [Google Scholar] [CrossRef]
- Morigny, P.; Zuber, J.; Haid, M.; Kaltenecker, D.; Riols, F.; Lima, J.D.C.; Simoes, E.; Otoch, J.P.; Schmidt, S.F.; Herzig, S.; et al. High levels of modified ceramides are a defining feature of murine and human cancer cachexia. J. Cachexia Sarcopenia Muscle 2020, 11, 1459–1475. [Google Scholar] [CrossRef]
- Kisková, T.; Kassayová, M. Resveratrol Action on Lipid Metabolism in Cancer. Int. J. Mol. Sci. 2019, 20, 2704. [Google Scholar] [CrossRef]
- Guzmán, M.; Castro, J. Zonation of fatty acid metabolism in rat liver. Biochem. J. 1989, 264, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Dumas, J.F.; Goupille, C.; Julienne, C.M.; Pinault, M.; Chevalier, S.; Bougnoux, P.; Servais, S.; Couet, C. Efficiency of oxidative phosphorylation in liver mitochondria is decreased in a rat model of peritoneal carcinosis. J. Hepatol. 2011, 54, 320–327. [Google Scholar] [CrossRef]
- VanderVeen, B.N.; Cardaci, T.D.; Bullard, B.M.; Madden, M.; Li, J.; Velazquez, K.T.; Kubinak, J.L.; Fan, D.; Murphy, E.A. Involvement of the gut microbiota in cancer cachexia. Am. J. Physiol. Cell Physiol. 2024, 327, C661–C670. [Google Scholar] [CrossRef]
- Trobec, K.; Kos, M.K.; von Haehling, S.; Springer, J.; Anker, S.D.; Lainscak, M. Pharmacokinetics of Drugs in Cachectic Patients: A Systematic Review. PLoS ONE 2013, 8, e79603. [Google Scholar] [CrossRef]
| Inflammatory Mediator | Producing Cells | Function | Levels in Cancer Cachexia |
|---|---|---|---|
| Interleukin 6 (IL-6) | Infiltrated Macrophages, TAMs, CD-68-Postive Monocytes | Muscle proteolysis, insulin resistance, increase in CRP, systemic inflammation | increased |
| Interleukin 1β (IL-1β) | Infiltrated Macrophages, TAMs, CD-68-Postive Monocytes | Muscle proteolysis, insulin resistance, increase in CRP, systemic inflammation | increased |
| Interleukin 4 (IL-4) | Anti-inflammatory properties | decreased | |
| Interleukin 8 (IL-8) | Infiltrated Macrophages, TAMs, CD-68-Postive Monocytes | Pro-inflammatory properties, systemic inflammation | increased |
| Tumor Necrosis Factor α (TNF-α) | Infiltrated Macrophages, TAMs, CD-68-Postive Monocytes | Muscle proteolysis, insulin resistance, increase in CRP, systemic inflammation | increased |
| Interferon γ (IFN-γ) | Infiltrated Macrophages, TAMs, CD-68-Postive Monocytes | Pro-inflammatory properties, systemic inflammation | increased |
| C-Reactive Protein (CRP) | Hepatic Cells | Immune system activation | increased |
| Prostaglandin E2 (PGE 2) | Kupffer Cells | Muscle mass loss, anorexia, decreased protein synthesis in liver and muscle, liver steatosis, decreased CPT II maximal activity | increased |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, D.C.; Gomes, S.P.; Seelaender, M. Metabolic, Inflammatory, and Molecular Impact of Cancer Cachexia on the Liver. Int. J. Mol. Sci. 2024, 25, 11945. https://doi.org/10.3390/ijms252211945
Gonçalves DC, Gomes SP, Seelaender M. Metabolic, Inflammatory, and Molecular Impact of Cancer Cachexia on the Liver. International Journal of Molecular Sciences. 2024; 25(22):11945. https://doi.org/10.3390/ijms252211945
Chicago/Turabian StyleGonçalves, Daniela Caetano, Silvio Pires Gomes, and Marília Seelaender. 2024. "Metabolic, Inflammatory, and Molecular Impact of Cancer Cachexia on the Liver" International Journal of Molecular Sciences 25, no. 22: 11945. https://doi.org/10.3390/ijms252211945
APA StyleGonçalves, D. C., Gomes, S. P., & Seelaender, M. (2024). Metabolic, Inflammatory, and Molecular Impact of Cancer Cachexia on the Liver. International Journal of Molecular Sciences, 25(22), 11945. https://doi.org/10.3390/ijms252211945







