A Honeycomb Film Template-Based Method for High-Throughput Preparation of Anti-Salmonella typhimurium 14,028 Phage Microgels
Abstract
:1. Introduction
2. Results and Discussion
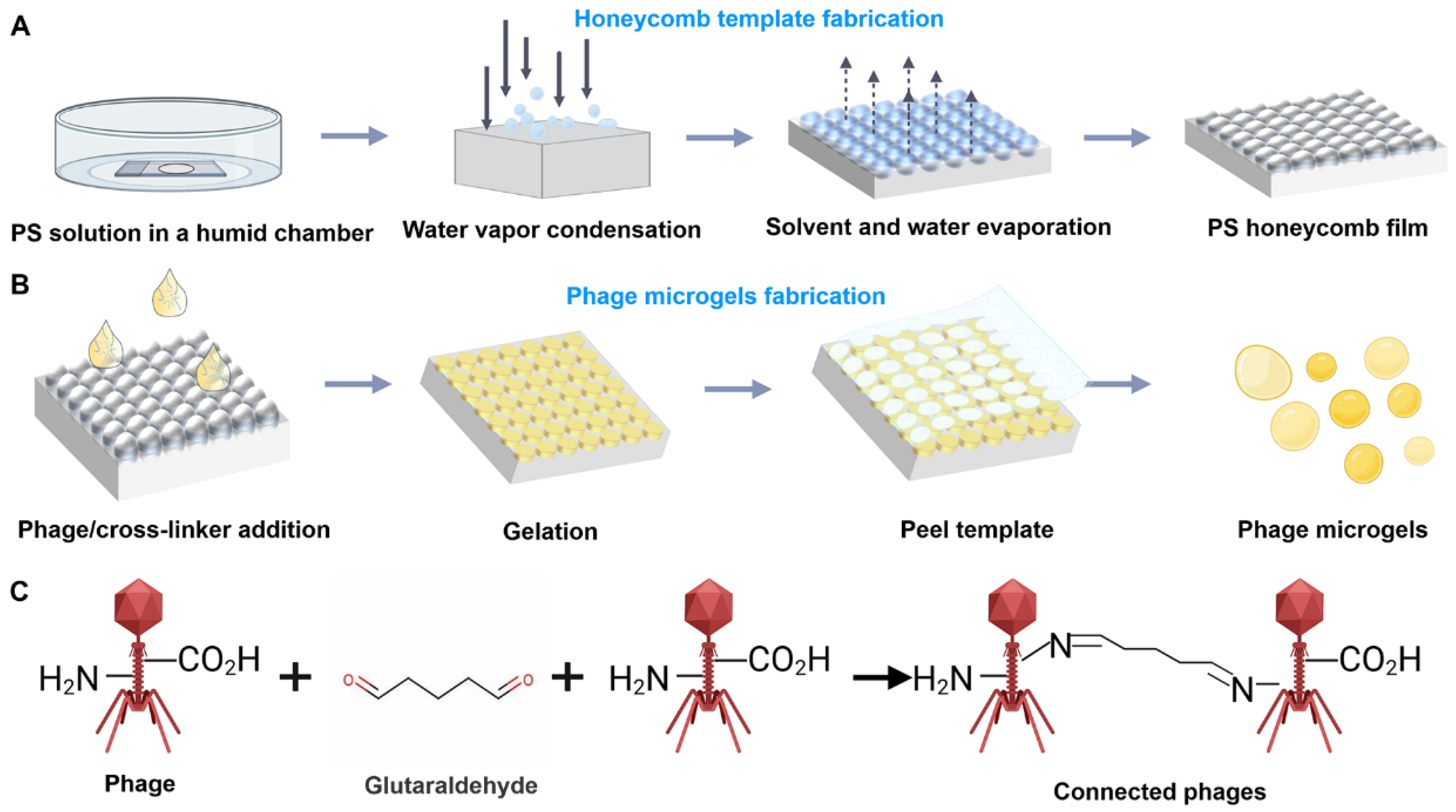
2.1. Scheme of the Preparation of Bacteriophage Microgels
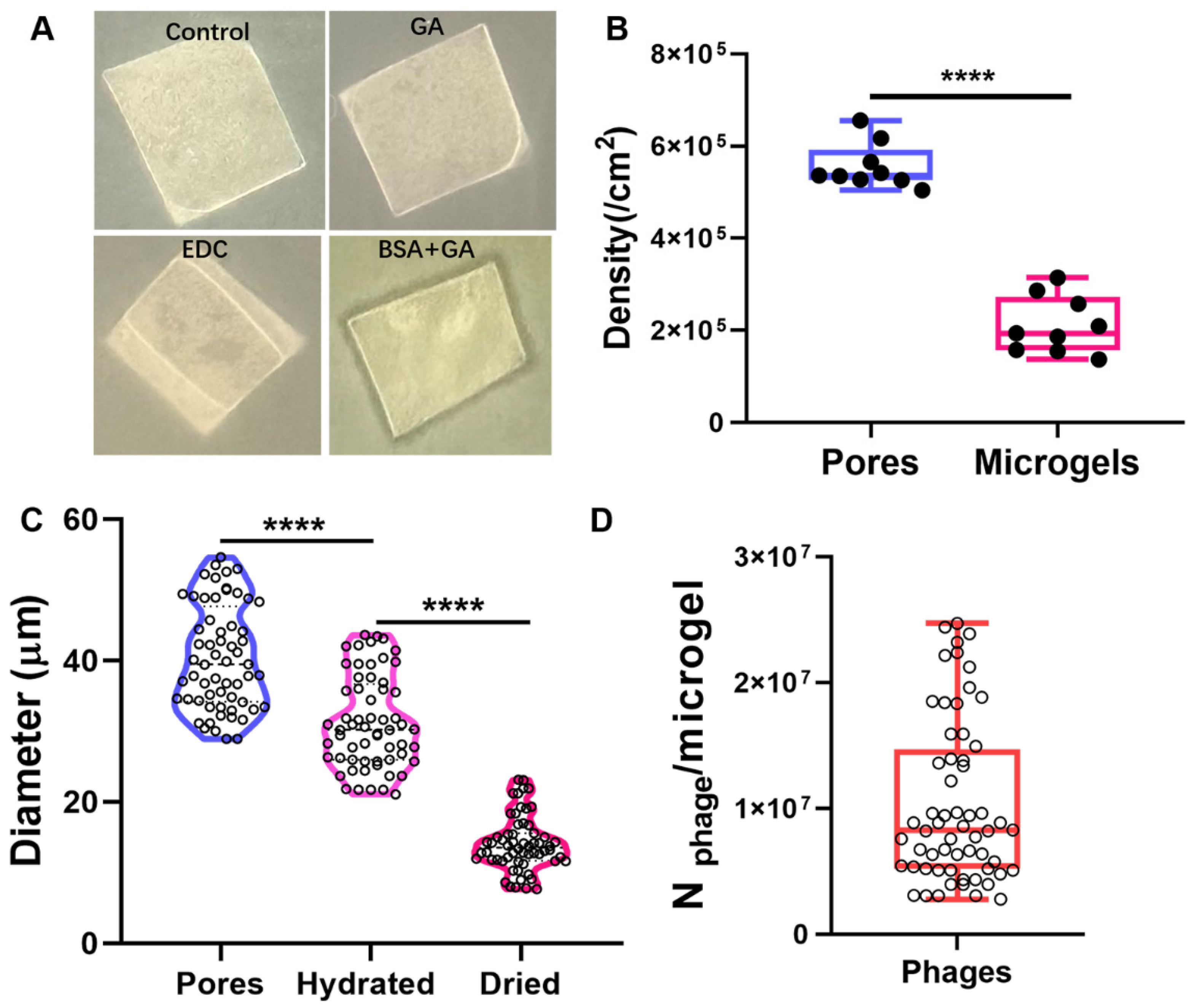
2.2. Characterizations of the Prepared Honeycomb Film and Phage Microgels
2.3. Preparation Efficiency of Phage Microgels
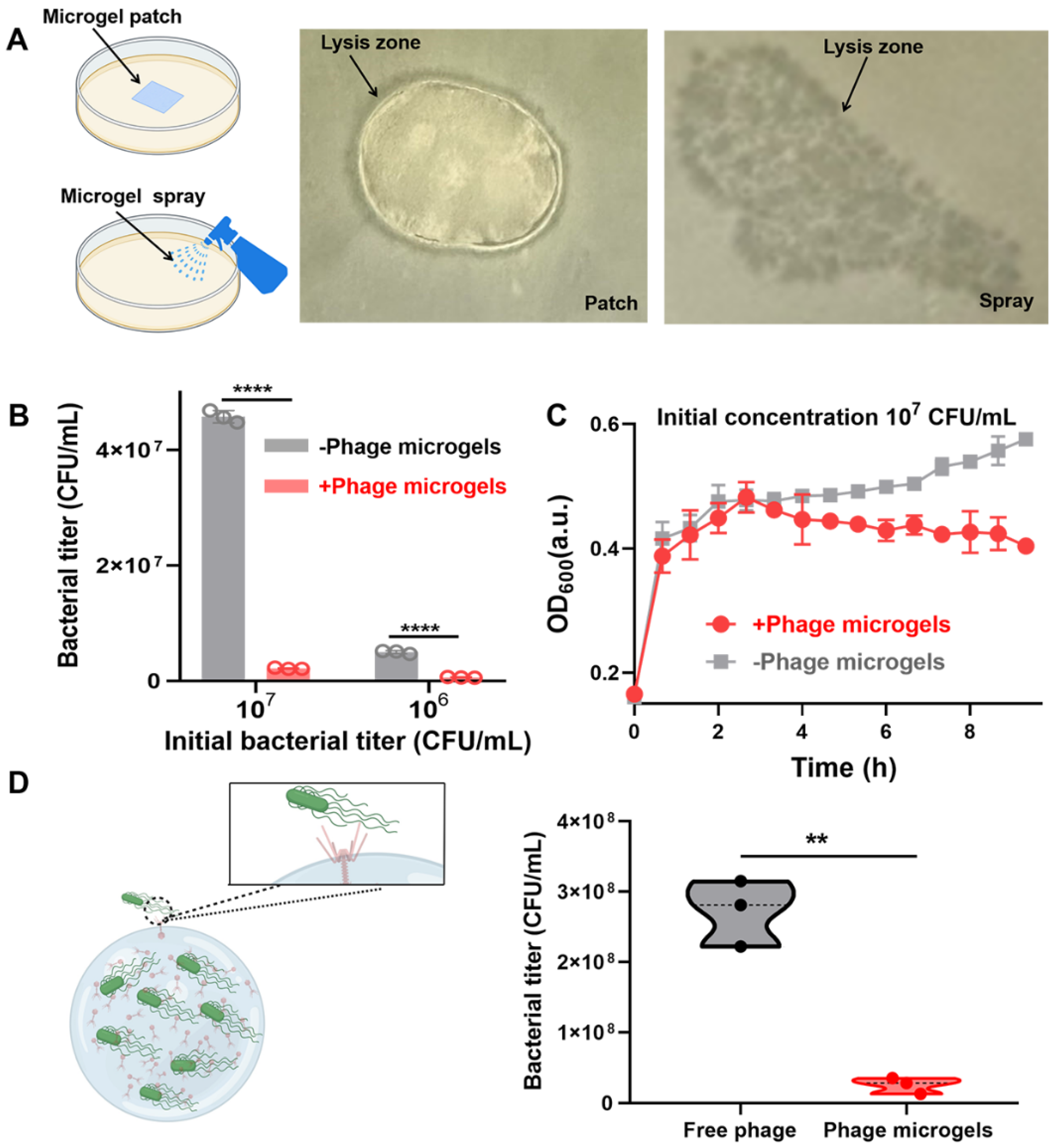
2.4. Targeted Anti-Microbial Functions of Phage Microgel Patches and Sprays
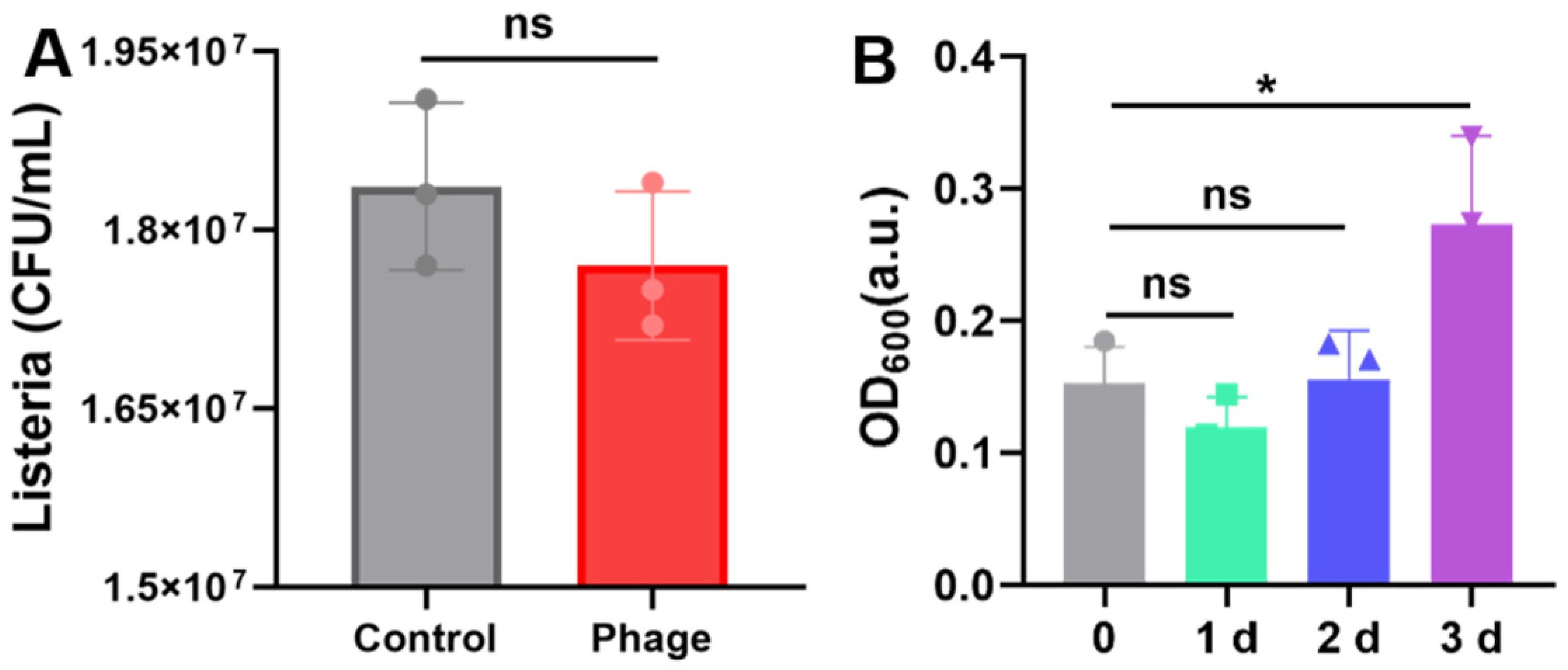
2.5. Specificity and Storage Stability of Phage Microgels
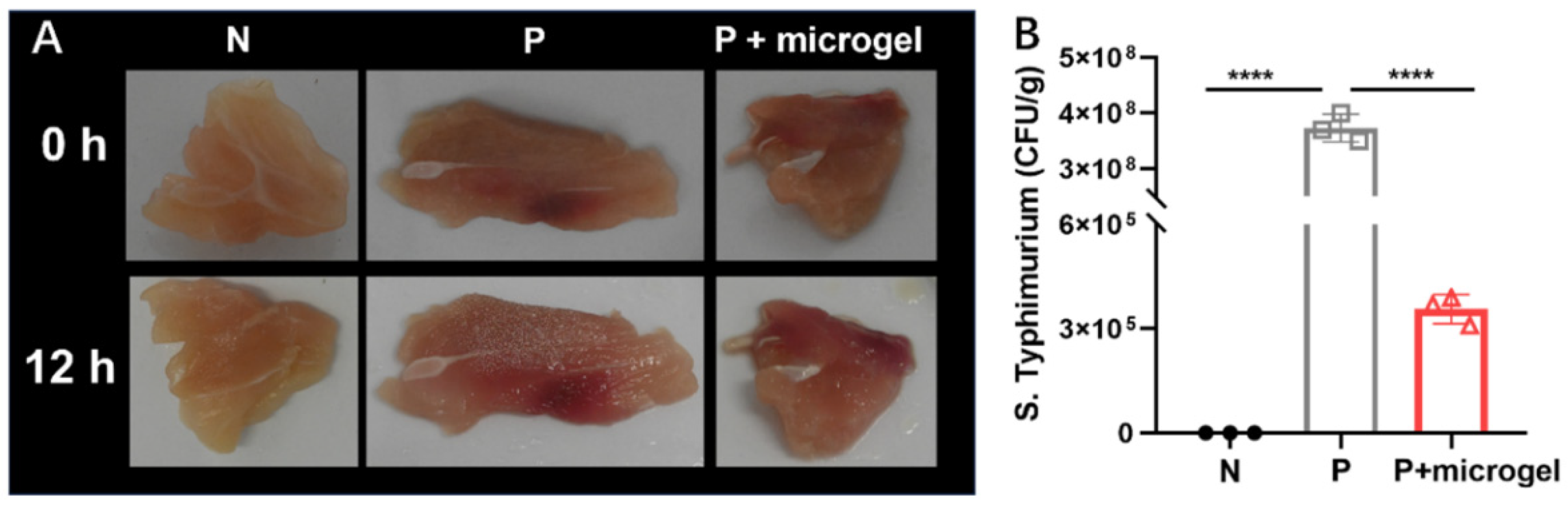
2.6. Anti-Salmonella Efficacy of Phage Microgel Spray on Chicken Meat
3. Methods
3.1. Phage Propagation, Purification, and Concentration
- (1)
- Phage enrichment
- (2)
- Phage isolation and purification
- (3)
- Phage propagation
- (4)
- Liquid amplification
3.2. Preparing Polystyrene Honeycomb Film
3.3. Phage Microgel Preparation
3.4. Separation of Microgels from the Honeycomb Membrane
3.5. Microgel Preparation Efficiency
- (1)
- Template hole and microgel size measurement
- (2)
- Microgel preparation efficiency
3.6. Anti-Microbial Test of Phage Microgel Patches on the Bacterial Lawn
3.7. Anti-Microbial Test of Phage Microgel Sprays on the Bacterial Lawn
3.8. Food Decontamination Test of Phage Microgels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ilhan, H.; Tayyarcan, E.K.; Caglayan, M.G.; Boyaci, I.H.; Saglam, N.; Tamer, U. Replacement of antibodies with bacteriophages in lateral flow assay of Salmonella Enteritidis. Biosens. Bioelectron. 2021, 189, 113383. [Google Scholar] [CrossRef] [PubMed]
- Zakaria, Z.; Hassan, L.; Sharif, Z.; Ahmad, N.; Ali, R.; Husin, S.A.; Sohaimi, N.F.M.; Bakar, S.A.; Garba, B. Analysis of Salmonella enterica serovar Enteritidis isolates from chickens and chicken meat products in Malaysia using PFGE, and MLST. BMC Vet. Res. 2020, 16, 393. [Google Scholar] [CrossRef] [PubMed]
- Du, T.; Wang, S.; Li, X.; Liu, L.; Wang, J.; Zhang, W. Hydrogen-bonded self-assembly coating as GRAS sprayable preservatives for fresh food safety. Food Hydrocoll. 2023, 145, 109089. [Google Scholar] [CrossRef]
- Han, J.W.; Ruiz-Garcia, L.; Qian, J.P.; Yang, X.T. Food packaging: A comprehensive review and future trends. Compr. Rev. Food Sci. Food Saf. 2018, 17, 860–877. [Google Scholar] [CrossRef]
- Sangbin, K.; Bong Sun, K.; Jaewoo, B.; Yoonjee, C. Antibacterial κ-carrageenan/konjac glucomannan-based edible hydrogel film containing Salmonella phage PBSE191 and its application in chicken meat. LWT-Food Sci. Technol. 2023, 180, 114707. [Google Scholar] [CrossRef]
- Alves, D.; Marques, A.; Milho, C.; Costa, M.J.; Pastrana, L.M.; Cerqueira, M.A.; Sillankorva, S.M. Bacteriophage ϕIBB-PF7A loaded on sodium alginate-based films to prevent microbial meat spoilage. Int. J. Food Microbiol. 2019, 291, 121–127. [Google Scholar] [CrossRef]
- Shiue, S.J.; Syu, F.S.; Lin, H.Y. Two types of bacteriophage-modified alginate hydrogels as antibacterial coatings for implants. J. Taiwan Inst. Chem. Eng. 2022, 134, 104353. [Google Scholar] [CrossRef]
- Salmond, G.; Fineran, P. A century of the phage: Past, present and future. Nat. Rev. Microbiol. 2015, 13, 777–786. [Google Scholar] [CrossRef]
- Vincent, L.; Logan, G.; Alexandra, S.; Zeinab, H.; Carlos, D.M.F. Long-term antimicrobial activity of phage-sugar glasses is closely tied to the processing conditions. ACS Omega 2018, 3, 18295–18303. [Google Scholar] [CrossRef]
- Bryan, H.; Isaac, P.; Lorena, L.; Frances, A.; Jeffrey, W.; Pamela, S. In situ reprogramming of gut bacteria by oral delivery. Nat. Commun. 2020, 11, 1. [Google Scholar] [CrossRef]
- Tian, L.; He, L.; Jackson, K.; Saif, A.; Khan, S.; Wan, Z.; Didar, T.; Hosseinidoust, Z. Self-assembling nanofibrous bacteriophage microgels as sprayable antimicrobials targeting multidrug-resistant bacteria. Nat. Commun. 2022, 13, 7158. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Jackson, K.; He, L.; Khan, S.; Thirugnanasampanthar, M.; Gomez, M.; Bayat, F.; Didar, T.; Hosseinidoust, Z. High-throughput fabrication of antimicrobial phage microgels and example applications in food decontamination. Nat. Protoc. 2024, 19, 1591–1622. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Chen, Y.; Wang, X.; Zhang, Z.; Wang, D.; Li, Y. Chemical synthesis of single atomic site catalysts. Chem. Rev. 2019, 31, 5442–5449. [Google Scholar] [CrossRef] [PubMed]
- Widawski, G.; Rawiso, M. Self-organized honeycomb morphology of star-polymer polystyrene films. Nature 1994, 369, 387–389. [Google Scholar] [CrossRef]
- Zhu, C.; Tian, L.; Liao, J.; Zhang, X.; Gu, Z. Fabrication of bioinspired hierarchical functional structures by using honeycomb films as templates. Adv. Funct. Mater. 2018, 28, 1803194. [Google Scholar] [CrossRef]
- Grabarek, Z.; Gergely, J. Zero-length crosslinking procedure with the use of active esters. Anal. Biochem. 1990, 185, 131–135. [Google Scholar] [CrossRef]
- Timkovich, R. Detection of the stable addition of carbodiimide to proteins. Anal. Biochem. 1977, 79, 135–143. [Google Scholar] [CrossRef]
- Chen, P.Y.; Chen, P.Y.; Dang, X.; Matthew, T.K.; Qi, J.; Noémie-Manuelle, D.C.; Fred, J.B.; Nicholas, F.; Paula, T.H.; Angela, M.B. Versatile three-dimensional virus-based template for dye-sensitized solar cells with improved electron transport and light harvesting. ACS Nano 2013, 7, 6563–6657. [Google Scholar] [CrossRef]
- Ohmura, J.F.; Burpo, F.J.; Lescott, C.J.; Ransil, A.; Yoon, Y.; Records, W.C.; Belcher, A.M. Highly adjustable 3D nano-architectures and chemistries: Via assembled 1D biological templates. Nanoscale 2019, 11, 1091–1101. [Google Scholar] [CrossRef]
- Wei, W.; Wang, L.; Yuan, L.; Wei, Q.; Yang, X.; Su, Z.; Ma, G. Preparation and application of novel microspheres possessing autofluorescent properties. Adv. Funct. Mater. 2007, 17, 3153–3158. [Google Scholar] [CrossRef]
- Kenney, P.O.; Gómez-Duarte, O.G. Low-volume enrichment method supports high throughput bacteriophage screening and isolation from wastewater. PLoS ONE 2024, 19, e0298833. [Google Scholar] [CrossRef]
- Gencay, Y.E.; Birk, T.; Sørensen, M.C.H.; Brøndsted, L. Methods for Isolation, Purification, and Propagation of Bacteriophages of Campylobacter jejuni; Humana Press: New York, NY, USA, 2017; pp. 19–28. [Google Scholar] [CrossRef]
- Ali, Z.; Dishisha, T.; El-Gendy, A.O.; Azmy, A.F. Isolation and phenotypic characterization of bacteriophage SA14 with lytic- and anti-biofilm activity against multidrug-resistant. Beni-Suef Univ. J. Basic Appl. 2023, 12, 21. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.; An, T.; Song, Y.; Wang, S. A Honeycomb Film Template-Based Method for High-Throughput Preparation of Anti-Salmonella typhimurium 14,028 Phage Microgels. Int. J. Mol. Sci. 2024, 25, 11911. https://doi.org/10.3390/ijms252211911
Wu J, An T, Song Y, Wang S. A Honeycomb Film Template-Based Method for High-Throughput Preparation of Anti-Salmonella typhimurium 14,028 Phage Microgels. International Journal of Molecular Sciences. 2024; 25(22):11911. https://doi.org/10.3390/ijms252211911
Chicago/Turabian StyleWu, Jing, Tingtao An, Yaxiong Song, and Shuo Wang. 2024. "A Honeycomb Film Template-Based Method for High-Throughput Preparation of Anti-Salmonella typhimurium 14,028 Phage Microgels" International Journal of Molecular Sciences 25, no. 22: 11911. https://doi.org/10.3390/ijms252211911
APA StyleWu, J., An, T., Song, Y., & Wang, S. (2024). A Honeycomb Film Template-Based Method for High-Throughput Preparation of Anti-Salmonella typhimurium 14,028 Phage Microgels. International Journal of Molecular Sciences, 25(22), 11911. https://doi.org/10.3390/ijms252211911





